Summary
Background
The lack of detectable precancerous lesions poses challenges to the early detection of human papillomavirus-driven oropharyngeal cancer (HPV-OPC). Antibodies against HPV16 early proteins, especially E6, are uniquely sensitive and specific biomarkers detectable years prior to HPV-OPC diagnosis. Thus, HPV16 early protein serology warrants clinical investigation for HPV-OPC screening.
Methods
Using multiplex serology, we analyzed HPV16 serum antibodies of the first 5000 participants (n=4,424 sera, recruited 2016-2017) of the Hamburg City Health Study, a population-based prospective cohort (45-74 years). Participants seropositive for HPV16 E6 and at least one additional early protein (E1, E2, E7) were considered at high risk for HPV-OPC development and invited to six-monthly non-invasive head and neck follow-up (FU) examinations (visual inspection, endoscopy, ultrasonography, performed 2019-2020). Participants with suspicious lesions were examined by magnetic resonance imaging and panendoscopy with biopsy. Histologically confirmed OPC cases were treated according to standard of care.
Findings
In total, 35 out of 4,424 study participants (0·8%, 95% confidence interval (CI) 0·6-1·1%) were HPV16 E6 seropositive. Among these, eleven (0·3%, 95%CI 0·1-0·5%) were considered at high risk for HPV-OPC of which nine were successfully re-contacted and invited to regular clinical FU examinations. Two males and one female were diagnosed with stage I HPV-OPC within 1·3 years of clinical FU (3-4 years after initial blood draw), representing one diagnosis of prevalent advanced disease, one incident diagnosis of advanced disease, and one incident diagnosis of early disease. The remaining participants showed no detectable signs of cancer, and undergo regular examinations (median clinical FU: 1·0 years, median total FU from blood draw to last clinical FU visit: 4·7 years).
Interpretation
HPV16 early antibodies allowed identifying three asymptomatic stage I HPV-OPC patients, out of eleven participants considered at high risk. However, two of the three cases already showed signs of advanced disease at diagnosis. Targeting multiple early proteins may considerably improve the positive predictive value of HPV16 serology and may have clinical utility for HPV-OPC screening.
Funding
This work was funded by DKFZ and UKE intramural funding.
Keywords: HPV-driven oropharyngeal cancer, Secondary prevention, HPV16 early antigen serology, Screening and early detection
Research in context.
Evidence before this study
Antibodies against HPV16 early proteins, especially the oncoprotein E6, have been shown to be promising biomarkers for HPV-driven oropharyngeal cancer (HPV-OPC) in multiple studies. HPV16 E6 seropositivity is highly sensitive (≥90%) and specific (99%) for HPV-OPC compared to molecular tumor HPV status, i.e. the presence of HPV DNA and RNA in tumor tissue, and can be detected several years prior to diagnosis. Thus, HPV16 E6 seropositivity is a biomarker with unique characteristics and with the potential to identify individuals at risk for developing HPV-OPC. Despite low HPV16 E6 seroprevalence in the general population (<1%), a solely HPV16 E6 serology-based HPV-OPC screening would detect many false-positives due to the rarity of this cancer. Thus, further risk stratification in HPV16 E6 seropositives is warranted to enable a serology-based screening approach with balanced risk-benefit ratio. At diagnosis, most individuals with HPV-OPC (>80%) are seropositive for HPV16 E6 and at least one additional early protein (E1, E2, E7) while multiple seropositivity is virtually absent in the general population. First evidence for the feasibility of an HPV16 early protein serology-based HPV-OPC screening approach was generated in an Australian study, by prospectively identifying an asymptomatic stage I HPV-OPC case seropositive for HPV16 E6 and E2 prior to diagnosis.
Added value of this study
This is the first proof of principle study conducting a comprehensive HPV16 E6 serology-based HPV-OPC screening approach in the general population aged ≥45 years, and the first utilizing seropositivity for additional HPV16 early proteins (E1, E2, E7) as risk stratification tool. We identified eleven individuals at high risk for HPV-OPC based on their HPV16 antibody profile, and invited them to regular non-invasive head and neck examinations; two were lost to follow-up. Within less than two years of clinical follow-up, three out of nine individuals were diagnosed with stage I HPV16-OPC, ranging from diagnosis of prevalent advanced disease to incident diagnosis of early disease.
Implications of all the available evidence
Our findings support an improved positive predictive value of the HPV16 serology-based HPV-OPC screening approach when taking additional early proteins into account. However, so far we did not observe a uniform shift towards earlier diagnosis of HPV-OPC which may reduce treatment-related morbidity and improve survivors’ quality of life.
Alt-text: Unlabelled box
Introduction
Oropharyngeal cancer (OPC) is a rare head and neck cancer, albeit with strongly increasing incidence rates in many countries. Risk factors for OPC are infection with oncogenic human papillomavirus (HPV) types, and tobacco and/or alcohol consumption. In the US and some European countries, more than 70% of OPC are attributable to HPV, predominantly type 16 (HPV16), causing 80–90% of all HPV-driven OPC (HPV-OPC).1,2 Although HPV-OPC is often characterized by favorable survival, diagnosis typically occurs after the primary tumor has metastasized to the neck, thus sometimes necessitating the use of multimodality treatment.3 However, combined therapy with primary chemoradiation or surgery with adjuvant treatment often results in severe treatment related morbidity. To date, no precursor lesion for OPC has been identified rendering early detection challenging.
Serum antibodies against HPV16 proteins, especially the oncoprotein E6, are promising biomarkers for identifying individuals at risk for HPV-OPC development. HPV16 E6 antibodies have been shown to be present in approximately 90% of HPV-OPC patients at the time of diagnosis and are often detectable more than ten years prior to diagnosis.4, 5, 6, 7 At the same time, HPV16 E6 seropositivity is also highly specific to distinguish between non-HPV-driven and HPV-driven OPC (>99%).5 HPV16 E6 seroprevalence in the general population and in cancer-free controls ranges between 0·5% and 0·8%.6, 7, 8, 9 However, mainly due to the rarity of HPV-OPC, the positive predictive value of a positive HPV16 E6 screening test in the general population is low, and the majority of HPV16 E6 seropositive individuals (about 80-90%) is not expected to develop an HPV16-driven cancer.4,9 Thus, further risk stratification of HPV16 E6 seropositives is required to develop an efficient screening strategy for secondary prevention of HPV-OPC. The majority (>80%) of HPV16 E6 seropositive HPV-OPC patients are also seropositive for at least one additional early protein (E1, E2, E7) at diagnosis, while seropositivity for multiple early proteins is virtually absent in individuals without HPV-associated malignancies.9, 10, 11 Thus, we hypothesized that individuals seropositive for HPV16 E6 and at least one additional early protein (E1, E2, or E7) are at high risk of developing HPV-OPC.
We recently conducted a small HPV-OPC screening study nested within the Study of Prevention of Anal Cancer (SPANC) encompassing 603 gay and bisexual Australian men.12 Thirteen HPV16 E6 seropositive individuals were invited for a head and neck exam, of which nine accepted. This led to the diagnosis of an asymptomatic stage I HPV-OPC case with high HPV16 E6 antibody levels who was also seropositive for HPV16 E2. A second participant was seropositive for all four HPV16 early proteins (E6, E1, E2, E7) with high antibody levels for E6, E2 and E7. He had died of metastatic lung cancer from his primary tonsillar cancer by the time of attempted re-contact.
We have expanded upon these prior findings by conducting the first proof of principle HPV16 E6 serology-based screening study for HPV-OPC in a population-based setting. The Hamburg City Health Study (HCHS) is a prospective cohort study which started enrolling 45,000 participants between 45 and 74 years of age from the general population of the city of Hamburg (Germany) in 2016.13 We measured serum antibodies against HPV16 early proteins (E6, E1, E2, E7) in the first 5,000 HCHS participants, and identified individuals with a high-risk HPV16 antibody profile for the development of HPV-OPC. These individuals were monitored with regular, non-invasive head and neck exams every six months to investigate whether risk stratification using antibodies against additional HPV16 early proteins is a successful strategy for HPV-OPC screening and early detection.
Materials and methods
Study population
The HCHS is a prospective, population-based cohort study established to investigate risk and prognostic factors for major diseases.13 Recruitment began in February 2016 with the goal of enrolling 45,000 participants aged between 45 and 74 years from the general population of the city of Hamburg (Germany, about 1·8 Mio. inhabitants). By early 2022, more than 15,000 participants have been recruited. At the enrolment visit, participants completed self-report questionnaires regarding lifestyle, environmental and medical conditions, and provided blood. The HCHS was approved by the local ethics committee of the State of Hamburg Chamber of Medical Practitioners (PV5131), the Data Protection Commissioner of the University Medical Center of the University Hamburg-Eppendorf (UKE), and the Data Protection Commissioner of the Free Hanseatic City of Hamburg. All participants provided written informed consent. The first 5,000 participants enrolled by February 2017 were investigated as part of this study.
HPV16 serology
Available sera (n=4,424) were shipped on dry ice to the German Cancer Research Center (DKFZ) in Heidelberg, Germany, and tested for HPV16 serum antibodies using multiplex serology as previously described.14,15 Briefly, HPV16 early antigens E1, E2, E6, E7, and the major capsid protein L1 were bacterially expressed as Glutathione S-transferase (GST) fusion proteins and affinity purified on one fluorescence labelled glutathione-casein coupled polystyrene bead set per antigen. Antigen-loaded beads were combined into one bead mix and simultaneously presented to primary serum antibodies at a final serum dilution of 1:100. Formed immunocomplexes were detected using a triple-specific biotinylated goat-α-human IgG/IgM/IgA secondary antibody and streptavidin-R-phycoerythrin as reporter dye on a Luminex 200 instrument. Per bead set and serum, 100 beads were measured and antibody levels determined as median fluorescence intensities (MFI). Previously published standard cut-offs were used to determine HPV16 seropositivity: 1000 MFI (E6), 200 MFI (E1), 679 MFI (E2), 548 MFI (E7), and 422 MFI (L1).7,16 Given the 1000 MFI cut-off for HPV16 E6 is primarily defined for incident HPV-OPC cases and not for a screening population, we performed a sensitivity analysis with the previously established 484 MFI cut-off (Supplementary Tables 2-4).16 The results of the sensitivity analysis did not substantially differ from the main analysis.
Clinical exam and treatment
Individuals were considered at high risk for HPV-OPC development if they were seropositive for HPV16 E6 and at least one additional HPV16 early protein (E1, E2, E7). These individuals were re-contacted via telephone by the HCHS study center and invited to undergo regular non-invasive head and neck follow-up (FU) examinations at the Department of Otorhinolaryngology (UKE). Invited participants had their first clinical FU visit between March 2019 and June 2020, i.e. 2·3 to 3·9 years after initial blood draw in 2016-2017. All participants received flexible videoendoscopy of the nasal cavity, naso-, oro-, hypopharynx and larynx. Clinical examination of the oral cavity and neck included palpation and ultrasonography (via the neck). In case of clinically unremarkable findings, participants were scheduled for six-monthly FU visits. This report includes FU visits that took place by June 2021, i.e. a median 1·0 years after onset of clinical FU (median time of total FU from blood draw to last clinical examination: 4·7 years). Suspicious lesions were further examined via magnetic resonance imaging (MRI) of the neck. In case of participant 7, narrow band imaging was performed to inform further decisions (Supplementary Table 6). As clinically indicated, panendoscopy was conducted under general anesthesia with excisional biopsies of the suspicious lesion. In the event of histologically proven oropharyngeal cancer, the main outcome of this study, the patient underwent further staging and treatment according to standard of care. TNM stage was determined according to UICC version 8. Patients were advised to see a urologist/gynaecologist and proctologist to exclude anogenital HPV-associated cancer.
Tumor analysis
All tissue samples taken during panendoscopy were formalin fixed, paraffin embedded and subjected to pathological examination at the Department of Pathology (UKE). To determine the HPV status, all tissues were analyzed by p16 immunohistochemistry and PCR as described before.17 In all cases with a positive result for HPV, PCR products were sequenced for confirmation of HPV type.
Additionally, one FFPE biopsy of the chronic ulcer of participant 1 taken at the first FU examination, and one FFPE block of participant 7 taken at diagnosis (Supplementary Table 6) were tested for HPV16 DNA using Multiplex Papillomavirus Genotyping and HPV16 E6*I mRNA at DKFZ as previously described.18,19
Statistical analysis
Missing questionnaire information and the participant answers “I don't know” or “I don't want to answer” were summarized as “not available” (NA). Age at blood draw was categorized into three categories based on the age range at recruitment, i.e. at time of blood draw (46-55 years, 56-65 years, and 66-76 years). In total, n=107 individuals were >74 years-old by the time of recruitment due to delays between sampling of the study population and recruitment into the study. Ethnic groups “Hispanic”, “Asian”, “Black”, “Black mixed” and “others” were classified as “other” due to the limited number of participants of non-Caucasian ethnicity. As measures of socioeconomic status, education and monthly household net income were investigated. Education was classified into low, intermediate and high according to the International Standard Classification of Education (ISCED).13 Monthly household net income was categorized into <2500 €, 2500- <4000 € and ≥4000 € creating approximately equally sized groups of individuals. Alcohol consumption was assessed by frequency of alcohol consumption, amount of alcohol consumed per day and frequency of drinking more than six alcoholic drinks per day within the last 12 months according to the alcohol use disorders identification test (AUDIT-C).13,20 Alcohol consumption was categorized as harmful with a score ≥4 for women and ≥5 for men. Smoking status was assessed in the categories current, former and never. Same-sex intercourse was categorized into ever and never according to the reported number of male and female sex partners. Age at sexual debut (ASD) was categorized into two categories above and below the median (<18 years, ≥18 years). The number of overall lifetime sex partners (LSP) and lifetime vaginal sex partners (LVSP) were categorized into 0-1, 2-5, and ≥6. Sexual behavior with regard to oral and anal sex partners was categorized into 0, 1, 2-5 and ≥6 lifetime oral sex partners (LOSP), and 0, 1, ≥2 lifetime anal sex partners (LASP). Previous anal or oral sex were categorized as ever or never. HPV16 antigen-specific seroprevalences were calculated among the full study cohort and stratified by demographic and lifestyle variables. Significance testing was conducted using Pearson's chi-squared test or Fisher's exact test if five or less individuals were included in at least one category. Chi-squared tests for trends in proportions were used to test for significant trends excluding NA's (education, LSP, LVSP, LOSP, LASP). Risk factor analyses were conducted using univariate and multivariate logistic regression models adjusting for age categories and sex to estimate odds ratios (OR) and 95% confidence intervals (CI). Raw data verification was performed by CJB and NiB. All analyses were performed with R version 4.0.3.
Role of the funding source
Funding sources did not have a role in study design, collection, analysis and interpretation of data, in writing the report nor the decision to submit the paper for publication.
Results
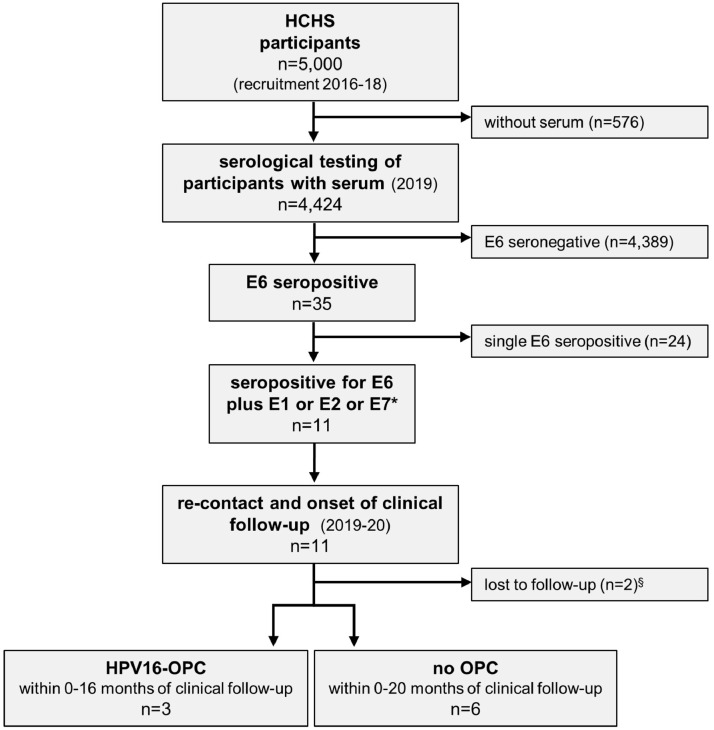
Available serum samples from the first 5,000 HCHS participants (n=4,424; 89%) were serologically tested for HPV16 antibodies (Figure 1). Of 35 HPV16 E6 seropositive individuals (0·8%, 95% CI 0·6-1·1%), n=11 (0·3%, 95% CI 0·1-0·5%) were additionally seropositive for HPV16 E1, E2 and/or E7, and thus considered at high risk for HPV-OPC. These 11 individuals were invited to regular non-invasive head and neck examinations. Two individuals were lost to FU, three were diagnosed with HPV16-OPC 3-4 years after initial blood draw, and six asymptomatic individuals undergo regular FU.
Figure 1.
Consort chart. The first 5000 HCHS study participants were included in this study and serologically tested for serum antibodies against HPV16 early proteins E6, E1, E2 and E7. *Individuals seropositive for HPV16 E6 and any other early protein (E1, E2 and / or E7) were considered at high risk for HPV-driven oropharyngeal cancer (HPV-OPC), re-contacted and invited for clinical follow-up by regular six-monthly non-invasive head and neck examinations. §Two individuals were lost to follow-up; one could not be contacted and one had deceased by the time of re-contact. HCHS: Hamburg City Health Study; OPC: oropharyngeal cancer; HPV16-OPC: HPV16-driven OPC.
HCHS study population
No significant lifestyle and demographic differences between the individuals who provided a serum sample (n=4,424) and the full study cohort (n=5,000) were observed (Table 1, Supplementary Table 1). The study population comprised almost exclusively Caucasian participants (98·2%) with an approximately equal gender distribution (49·4% males; Table 1). The median age at recruitment was 62 years (inter-quartile range (IQR): 54-69 years). Most participants (90·5%) reported intermediate or high education. Approximately half (51·8%) reported to have smoked in their life and 25·7% reported harmful alcohol consumption. The median age at sexual debut (ASD) was 18 years (IQR: 17-20 years). In total, 47·8% and 19·8% reported to have had oral or anal sex, respectively. Previous same-sex intercourse was reported by 3·7% of the study population.
Table 1.
Demographic and lifestyle characteristics of the study population with available serum samples (n=4,424).
| Variable | Category | n | % |
|---|---|---|---|
| Sex | Male | 2,185 | 49·4 |
| Female | 2,239 | 50·6 | |
| Age [years] | 46-55 | 1,236 | 27·9 |
| 56-65 | 1,503 | 34·0 | |
| 66-76 | 1,685 | 38·1 | |
| Education | Low | 181 | 4·1 |
| Intermediate | 2,198 | 49·7 | |
| High | 1,806 | 40·8 | |
| NA | 239 | 5·4 | |
| Ethnicity | Caucasian | 4,343 | 98·2 |
| Other | 65 | 1·5 | |
| NA | 16 | 0·4 | |
| Household net income / month [€] | <2500 | 1,100 | 24·9 |
| 2500- <4000 | 1,011 | 22·8 | |
| ≥4000 | 1,181 | 26·7 | |
| NA | 1,132 | 25·6 | |
| Smoking status | Never | 1,323 | 29·9 |
| Former | 1,639 | 37·0 | |
| Current | 656 | 14·8 | |
| NA | 806 | 18·2 | |
| Harmful alcohol consumption | Yes | 1,136 | 25·7 |
| No | 2,060 | 46·6 | |
| NA | 1,228 | 27·7 | |
| Age at sexual debut (ASD) [years] | <18 | 1,460 | 33·0 |
| ≥18 | 1,742 | 39·4 | |
| NA | 1,222 | 27·6 | |
| Same-sex intercourse | Never | 2,723 | 61·6 |
| Ever | 164 | 3·7 | |
| NA | 1,537 | 34·7 | |
| Lifetime number of sex partners (LSP) | 0-1 | 538 | 12·2 |
| 2-5 | 1,284 | 29·0 | |
| ≥6 | 1,290 | 29·2 | |
| NA | 1,312 | 29·7 | |
| Lifetime number of vaginal sex partners (LVSP) | 0-1 | 586 | 13·2 |
| 2-5 | 1,175 | 26·6 | |
| ≥6 | 1,178 | 26·6 | |
| NA | 1,485 | 33·6 | |
| Oral sex | Never | 717 | 16·2 |
| Ever | 2,115 | 47·8 | |
| NA | 1,592 | 36·0 | |
| Lifetime number of oral sex partners (LOSP) | 0 | 607 | 13·7 |
| 1 | 561 | 12·7 | |
| 2-5 | 830 | 18·8 | |
| ≥6 | 454 | 10·3 | |
| NA | 1,972 | 44·6 | |
| Anal sex | Never | 2,052 | 46·4 |
| Ever | 875 | 19·8 | |
| NA | 1,497 | 33·8 | |
| Lifetime number of anal sex partners (LASP) | 0 | 1,751 | 39·6 |
| 1 | 439 | 9·9 | |
| ≥2 | 355 | 8·0 | |
| NA | 1,879 | 42·5 |
NA: not available.
Characterization of HPV16 E6 seropositives
We measured serum antibodies against HPV16 E1, E2, E6, E7 and L1 in all available samples. The seroprevalences were 0·8% for E6, 2·1% for both E1 and E2, 3·5% for E7, and 3·1% for L1. No significant differences in participant characteristics were observed between HPV16 E6 seropositives and seronegatives (Table 2). Yet, HPV16 E6 seropositive participants were more likely to be male, and to report a higher number of LSP, LSVP and LOSP, and previous oral or anal sex (Table 2, Supplementary Tables 3 and 4). None reported a previous history of HPV-associated cancer (cervical, head and neck or anal cancer). Mostly insignificant trends of higher seroprevalences with increasing levels of sexual exposure were also observed for the other HPV16 antibodies (Supplementary Tables 2-4).
Table 2.
Demographic and lifestyle characteristics of HPV16 E6 seronegatives and -positives, and HPV16 E6 seroprevalence in the study population (n=4,424).
| variable | Category | n E6− | % | n E6+ | % | E6 sero-prevalence [%] | p-value |
|---|---|---|---|---|---|---|---|
| Sex | Male | 2,162 | 49·3 | 23 | 65·7 | 1·1 | 0·08 |
| Female | 2,227 | 50·7 | 12 | 34·3 | 0·5 | ||
| Age | 46-55 | 1,226 | 27·9 | 10 | 28·6 | 0·8 | 0·77 |
| 56-65 | 1,493 | 34·0 | 10 | 28·6 | 0·7 | ||
| 66-76 | 1,670 | 38·1 | 15 | 42·9 | 0·9 | ||
| Education | Low | 179 | 4·1 | 2 | 5·7 | 1·1 | 0·75 |
| Intermediate | 2,180 | 49·7 | 18 | 51·4 | 0·8 | ||
| High | 1,793 | 40·9 | 13 | 37·1 | 0·7 | ||
| NA | 237 | 5·4 | 2 | 5·7 | 0·8 | ||
| Ethnicity | Caucasian | 4,308 | 98.2 | 35 | 100.0 | 0.8 | 1·0 |
| Other | 65 | 1.5 | 0 | 0.0 | 0.0 | ||
| NA | 16 | 0.4 | 0 | 0.0 | 0.0 | ||
| Household net income / month [€] | <2500 | 1,092 | 24·9 | 8 | 22·9 | 0·7 | 0·79 |
| 2500- <4000 | 1,001 | 22·8 | 10 | 28·6 | 1·0 | ||
| ≥4000 | 1,171 | 26·7 | 10 | 28·6 | 0·8 | ||
| NA | 1,125 | 25·6 | 7 | 20·0 | 0·6 | ||
| Smoking status | Never | 1,315 | 30·0 | 8 | 22·9 | 0·6 | 0·42 |
| Former | 1,623 | 37·0 | 16 | 45·7 | 1·0 | ||
| Current | 649 | 14·8 | 7 | 20·0 | 1·1 | ||
| NA | 802 | 18·3 | 4 | 11·4 | 0·5 | ||
| Harmful alcohol consumption | Yes | 1,127 | 25·7 | 9 | 25·7 | 0·8 | 0·96 |
| No | 2,043 | 46·6 | 17 | 48·6 | 0·8 | ||
| NA | 1,219 | 27·8 | 9 | 25·7 | 0·7 | ||
| Age at sexual debut (ASD) | <18 | 1,448 | 33·0 | 12 | 34·3 | 0·8 | 0·81 |
| ≥18 | 1,727 | 39·3 | 15 | 42·9 | 0·9 | ||
| NA | 1,214 | 27·7 | 8 | 22·9 | 0·7 | ||
| Same-sex intercourse | Never | 2,701 | 61·5 | 22 | 62·9 | 0·8 | 0·63 |
| ever | 162 | 3·7 | 2 | 5·7 | 1·2 | ||
| NA | 1,526 | 34·8 | 11 | 31·4 | 0·7 | ||
| Lifetime number of sex partners (LSP) | 0-1 | 535 | 12·2 | 3 | 8·6 | 0·6 | 0·46 |
| 2-5 | 1,273 | 29·0 | 11 | 31·4 | 0·9 | ||
| ≥6 | 1,278 | 29·1 | 12 | 34·3 | 0·9 | ||
| NA | 1,303 | 29·7 | 9 | 25·7 | 0·7 | ||
| Lifetime number of vaginal sex partners (LVSP) | 0-1 | 582 | 13·3 | 4 | 11·4 | 0·7 | 0·32 |
| 2-5 | 1,166 | 26·6 | 9 | 25·7 | 0·8 | ||
| ≥6 | 1,165 | 26·5 | 13 | 37·1 | 1·1 | ||
| NA | 1,476 | 33·6 | 9 | 25·7 | 0·6 | ||
| Oral sex | Never | 713 | 16·2 | 4 | 11·4 | 0·6 | 0·58 |
| Ever | 2,095 | 47·7 | 20 | 57·1 | 0·9 | ||
| NA | 1,581 | 36·0 | 11 | 31·4 | 0·7 | ||
| Lifetime number of oral sex partners (LOSP) | 0 | 604 | 13·8 | 3 | 8·6 | 0·5 | 0·16 |
| 1 | 555 | 12·6 | 6 | 17·1 | 1·1 | ||
| 2-5 | 824 | 18·8 | 6 | 17·1 | 0·7 | ||
| ≥6 | 447 | 10·2 | 7 | 20·0 | 1·5 | ||
| NA | 1,959 | 44·6 | 13 | 37·1 | 0·7 | ||
| Anal sex | Never | 2,036 | 46·4 | 16 | 45·7 | 0·8 | 0·63 |
| Ever | 866 | 19·7 | 9 | 25·7 | 1·0 | ||
| NA | 1,487 | 33·9 | 10 | 28·6 | 0·7 | ||
| Lifetime number of anal sex partners (LASP) | 0 | 1,737 | 39·6 | 14 | 40·0 | 0·8 | 0·75 |
| 1 | 434 | 9·9 | 5 | 14·3 | 1·1 | ||
| ≥2 | 352 | 8·0 | 3 | 8·6 | 0·8 | ||
| NA | 1,866 | 42·5 | 13 | 37·1 | 0·7 |
NA: not available.
E6-: seronegative for HPV16 E6.
E6+: seropositive for HPV16 E6.
HPV16 serology-based risk stratification
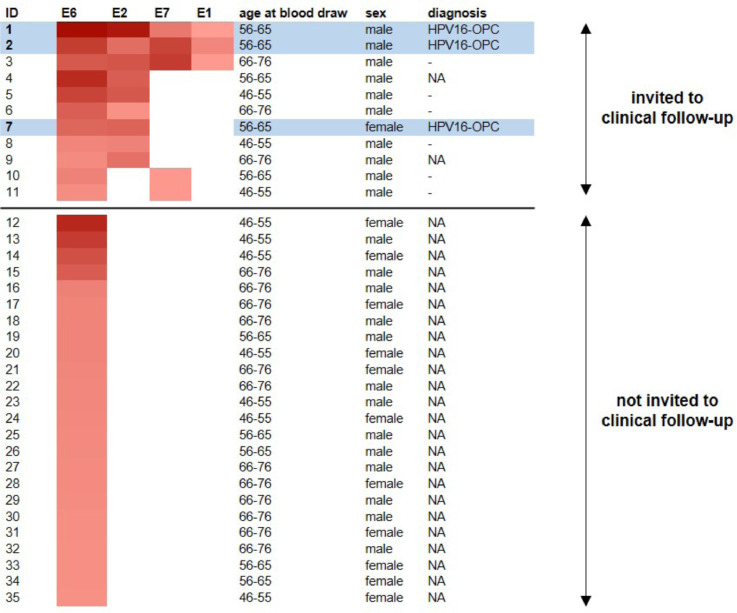
We further investigated HPV16 antibody patterns among the 35 E6 seropositive participants (Figure 2, Supplementary Table 5). Eleven (31%) were seropositive for at least one additional early protein (E2, E7, E1). Of these, nine (82%) were seropositive for E2, five (46%) for E7 and three (27%) for E1. Three of these 11 high-risk participants were seropositive for all three additional early proteins (Figure 2). Compared to participants seropositive for HPV16 E6 only, participants with high-risk serologic profiles tended to be male (91% versus 54%), and slightly younger (median age (IQR): 60 years (58-66 years) versus 65 years (55-71 years)).
Figure 2.
Heatmap of HPV16 early protein antibody reactivity in individuals seropositive for HPV16 E6 including 11 individuals (IDs 1-11) with high-risk HPV16 antibody profile who were invited to clinical follow-up. Positive antibody responses against HPV16 proteins E6, E2, E7 and E1 are marked in red. The color code ranging between light red and dark red indicates increasing antibody levels among seropositives. The individuals highlighted in light blue were diagnosed with HPV16-OPC during clinical follow-up. Individuals invited to clinical follow-up without OPC diagnosis were marked with a dash. NA: not available because individuals were either not invited to clinical follow-up (IDs 12-35) or lost to follow-up (IDs 4 and 9).
Clinical follow-up of individuals with high risk antibody profile
The 11 high-risk participants were invited to six-monthly non-invasive head and neck examinations. Re-contact was successful for nine individuals (Supplementary Table 6). One participant had deceased prior to re-contact (ID 9; cause of death unrelated to HPV) and one was lost to FU despite multiple re-contact attempts (ID 4). The first head and neck exams of the remaining nine participants occurred between 2·3 and 3·9 years after blood draw. Within 0 to 1·3 years of clinical FU, i.e. 3-4 years after initial blood draw, three participants (two males, one female) were diagnosed with HPV16-OPC. Disease stage was pT2 pN1 cM0 for both males and pT1 pN0 cM0 for the female patient. The two males were fourfold seropositive with high antibody levels for HPV16 E6, E1, E2 and E7 while the female patient was seropositive for HPV16 E6 and E2 (Figure 2). All underwent treatment, currently undergo post-treatment surveillance and are currently free of recurrence.21 Clinical diagnosis and treatment are described in detail in the supplement (Supplementary Table 6 and Supplementary Figure 1). In essence, one male participant was diagnosed at his first study visit with a prevalent tumor showing signs of advanced disease (i.e., extracapsular extension, ECE); the other male participant had an incident diagnosis of an ECE-positive tumor at his third study visit; and the female participant presented with an incident diagnosis of a small tumor without lymph node involvement at her third study visit. The remaining six participants were free of detectable symptoms and continue to undergo six-monthly non-invasive head and neck exams (median clinical FU: 1·0 years, range: 0·0-1·7 years; median total FU: 4·7 years, range: 3·9-5·0 years). For these patients, the available office-based clinical investigations targeting potential anogenital HPV-associated disease retrieved no findings. Details of the non-invasive head and neck examinations, HPV16 antibody profiles and clinical findings are summarized in Supplementary Table 6.
Discussion
This study is the first population-based proof of principle study providing evidence for the feasibility of a serology-based HPV-OPC screening approach. We identified HPV16 E6 seropositive individuals in the HCHS, a population-based study enrolling participants in the age range with the highest HPV-OPC incidence rate (≥45 years). To improve risk stratification, E6 seropositive individuals additionally seropositive for at least one other HPV16 early protein (E1, E2, E7) were considered at high risk for HPV-OPC, and invited to non-invasive head and neck FU exams. Using this approach, we diagnosed three high-risk participants with stage I HPV-OPC after 0 to 1.3 years of clinical FU, i.e. 3-4 years after blood draw. A fundamental question is: Is this early detection? Our approach clearly is not comparable to cervical cancer screening and detection of precursor lesions, i.e. cervical intraepithelial neoplasia. The best case scenario of our approach is the diagnosis of small tumors without lymph node involvement in asymptomatic individuals. Even in case of an incident diagnosis, tumorigenesis and possibly yet undefined precursor lesions have likely been present for months or years, but were clinically undetectable. However, according to WHO, screening aims at identifying asymptomatic individuals with either risk factors or early stage disease by using simple tests to reduce i) mortality by early detection and treatment, ii) disease incidence by identifying and treating precursor lesions, or iii) disease severity by offering effective treatment.22,23 Whether screening for a disease may be recommended and implemented depends on multiple characteristics of the disease, the screening test, and available diagnosis and treatment options. A comprehensive summary of these considerations specific for OPC screening, including disease incidence, requirements for a screening biomarker, methods of detection, treatment of early-stage patients, and the potential improvement of clinical outcomes was described by Kreimer et al.24 Briefly, the main obstacles for OPC screening are currently i) the identification and description of, as well as diagnostic and prophylactic treatment options for the yet-unknown precursor lesion to HPV-OPC, and ii) the need to improve screening algorithms to identify those individuals at highest risk.24 To address these questions, especially the latter, we performed a proof-of-concept study nested within a cohort study of the potential target population (men and women above 45 years of age). Previous studies often focused on HPV16 E6 seropositivity as the sole, and most promising biomarker for HPV-OPC.6,7,24 However, despite high specificity for HPV-OPC, only a minority of HPV16 E6 seropositive individuals is expected to eventually develop HPV-OPC.7,9,25 Recently, Robbins et al. investigated the potential of this biomarker in a setting of high OPC incidence rate and high HPV attributable fraction (i.e., the US) by modelling the risk for OPC by HPV16 E6 serostatus.25 The 5-year absolute risk in 50 and 60 years-old HPV16 E6 seropositive females and males for OPC ranged between 2.0 and 13.3%, and was thus comparable to the risk associated with established screening interventions in the US including breast, cervix, colorectal and lung cancer screening.25 To further improve absolute risk estimates in a screening setting, additional biomarkers among HPV16 E6 seropositives identifying individuals at immediate risk for OPC are needed. We investigated HPV16 antibody patterns as risk stratification markers. The rationale for this approach is based on previous observations of specific HPV16 early protein antibody patterns in HPV-OPC cases versus the general population.4,9, 10, 11, 12 More than 80% of HPV-OPC cases show multiple seropositivity for HPV16 E6 and other early HPV16 proteins (E1, E2, E7) indicating accumulation of the breadth of the antibody response towards diagnosis; in contrast, this pattern is extremely rare in seemingly healthy individuals.7, 8, 9, 10, 11 The first study providing evidence for this approach was nested within the SPANC study and identified i) an incident asymptomatic stage I HPV-OPC case seropositive for HPV16 E6 and E2, with high HPV16 E6 antibody levels, and ii) a prevalent HPV-OPC patient with high antibody levels for all four HPV16 early antigens.12 In our study, about one third (11 of 35, 31%) of HPV16 E6 seropositives were seropositive for at least one other early protein. We invited these individuals to non-invasive head and neck examinations to minimize harm introduced by the diagnostic work-up, and were able to diagnose three asymptomatic stage I HPV-OPC cases. Based on OPC incidence rates in Germany of 15 per 100,000 person-years in the corresponding age groups in 2017, and an HPV attributable fraction of 50%,26,27 we expected two to three HPV-OPC diagnoses in our study population. Two individuals seropositive for all HPV16 early proteins (E6, E1, E2, E7) were diagnosed with pT2 pN1 HPV-OPC within one year of clinical FU, i.e. approximately three years after blood draw. These were two male participants with very high HPV16 E6 (>7000 MFI) antibody levels. The third participant was seropositive for HPV16 E6 and E2 (both >4000 MFI) and was diagnosed with a very small tumor (pT1) without lymph node involvement after 1.3 years of clinical FU (about 4 years after blood draw). Our findings suggest the utility of HPV16 early antigen antibody patterns beyond HPV16 E6 seropositivity, as well as antibody levels, as risk stratification markers for HPV-OPC in seemingly healthy individuals. We observed a trend of higher HPV16 E6 seroprevalence in individuals reporting previous oral and/or anal sex, and increased number of vaginal and oral sex partners. This confirms previous observations from the UK Biobank where antibodies against HPV16 E6 and other HPV16 proteins tended to be more common in individuals reporting more risky sexual behaviour, i.e. same-sex intercourse and a higher number of sexual partners.9 Thus, the potential of demographic and life style factors such as age and sexual behaviour as risk stratification tools for a serology-based screening approach requires further investigation in larger studies.
To date, only one other study based on HPV16 early antigen serology to screen for HPV-OPC was conducted, the HOUSTON trial.28 Of 553 middle-aged men, Dahlstrom et al. invited 47 individuals seropositive for HPV16 early antibodies and/or oral HPV16 DNA, and matched negative controls for regular, six-monthly FU visits. After a median of 13 months of FU, no HPV-OPC was identified.
A major strength of our study is the large number of individuals from the general population (n=4,424) screened for HPV16 E6 antibodies with the serology assay that has been utilized in most prospective epidemiological studies of HPV-OPC.4,6,7 In addition, our study covers up to five years of total FU time in order to account for the long lead times of HPV16 E6 serology in the prediction of HPV-OPC.4,6,7 The invited individuals underwent six-monthly non-invasive head and neck examinations to detect HPV-OPC as early as technically possible without putting the participants at unnecessary risk of adverse events during the examination, and none were observed. Thus, our study showed that routine exams such as visual inspection, palpation and ultrasound, may be sufficient for early detection of HPV-OPC. In addition, high compliance to undergo these examinations every six months suggests these procedures are acceptable for the study participants on a recurring basis. Despite the unparalleled size of our screening population, the main limitation of our study remains the small number of identified cancer patients. One female patient was diagnosed with a small tumor without lymph node involvement (pT1, pN0), allowing for a curative approach with single modality treatment. Thus, this study suggests that screening for HPV-OPC holds the potential to reduce treatment morbidity at least in some cases. However, we consider this the only case of early detection in our study. The other two patients were diagnosed after lymph node involvement with extracapsular extension (ECE), a sign for more advanced disease. The diagnosis of an asymptomatic, but advanced tumor (pT2, pN1 with ECE) at the first study visit of one of the male study participant clearly represents a case of disease diagnosis. More interestingly, the other male study participant was incidently diagnosed with an advanced tumor (pT2, pN1 with ECE) at his third study visit, after two previous six-monthly visits that did not support a cancer diagnosis. This raises concerns about the distinction of asymptomatic advanced disease from early disease in our approach, and warrants further investigation. Our focus on participants with a high-risk antibody profile also constitutes a limitation, as it is currently unknown whether any of the non-invited participants developed HPV-OPC, and the study thus lacks a control group. However, all participants of the HCHS cohort undergo passive FU through cancer registry linkage, and the negative predictive value of HPV16 E6 serology for HPV-OPC exceeds 99.9%4,25 making it extremely unlikely that an HPV16 E6 seronegative study participant develops HPV-OPC. Other limitations of our current study are the lack of other HPV biomarkers to complement HPV early antigen serology (e.g. liquid biopsies for the detection of cell-free HPV DNA), the lack of organized clinical FU for anogenital HPV-associated lesions, and the delayed initiation of clinical FU between two and four years after blood draw.
Despite these limitations, our study provides valuable contributions regarding the screening algorithm, additional risk stratification, and clinical detection methods. However, for the introduction of an HPV-OPC screening program, several important questions remain unanswered, and need to be addressed in further and larger studies. These include i) the positive predictive value of the high-risk serological signature, ii) the improvement of clinical detection methods to identify HPV-OPC precursor lesions and/or very small tumors, e.g. by regular imaging (MRI, PET-CT), iii) the onset and frequency of clinical FU in order to distinguish long-term from imminent risk for HPV-OPC, iv) investigations of age and stage migration of cases as a result of early detection, v) the impact of an HPV-OPC screening intervention with regard to clinical and public health outcomes (e.g. survival, quality of life), and vi) cost-effectiveness estimations. In future studies, the application of other, potentially more invasive methods such as tonsillectomy and/or tongue base mucosectomy may also be investigated to prevent lymph node metastasis from very small tumors, and to improve the outcome in individuals with very high risk of developing HPV-OPC.
It remains speculative when the three HPV-OPC cases would have been diagnosed without our study. There is no dedicated head and neck cancer screening in Germany, and general health checks do not include a detailed examination of the oropharynx or palpation of the head and neck, thus questioning if and when the asymptomatic individuals diagnosed in this study would have been diagnosed by routine check-ups. It appears more likely that months or years later, the participants would have detected enlarged lymph nodes due to a visible cervical mass or other symptoms themselves.
We plan to extend our screening approach for HPV-OPC to the next 10,000 HCHS participants also incorporating additional HPV biomarkers (oral HPV DNA, blood-based cell-free HPV DNA).29,30 The additional biomarkers may further improve the positive predictive value of our screening approach, and may inform treatment decisions while the tumor is still small and prior to lymph node involvement. In addition, we plan to invite HPV16 E6 single seropositives to regular non-invasive head and neck examinations and blood draws to monitor their HPV16 antibody patterns, reduce the time between initial blood draw and initiation of clinical FU, and include organized anogenital examinations in the FU procedure. In the long-term, we aim to incorporate the HPV16 serology-based HPV-OPC screening in the full HCHS cohort (45,000 individuals). This study will likely provide unique information to inform a population-based HPV-OPC screening approach.
Contributors
CJB formal analysis, data analysis, investigation, visualisation, writing – original draft.
ASH project administration, investigation, data curation, writing – review & editing.
DV methodology, formal analysis, data analysis, writing – review & editing.
BB project administration, investigation, data curation, writing – review & editing.
TR formal analysis, writing – review & editing.
CB conceptualisation, supervision, writing – review & editing.
NoB project administration, data analysis, writing – review & editing.
LS methodology, investigation, writing – review & editing.
YH project administration, resources, data collection, writing – review & editing.
EP data curation, project administration, data collection, writing – review & editing.
AJ conceptualisation, project administration, data collection, writing – review & editing.
IS project administration, resources, data collection, writing – review & editing.
KLK conceptualisation, writing – review & editing.
EB methodology, investigation, writing – review & editing.
MP conceptualisation, supervision, methodology, writing – review & editing.
TW conceptualisation, supervision, methodology, writing – original draft.
NiB data analysis, investigation, visualisation, conceptualisation, writing – original draft.
Declaration of interests
Dr. Betz reports personal fees from Brainlab AG, personal fees from Bristol Myers Squibb, personal fees from Sanofi Aventis, personal fees from Merck Serono, outside the submitted work. Dr. Busch reports grants and personal fees from Bristol-Myers Squibb, personal fees from GlaxoSmithKline, personal fees from Merck, personal fees from Merck Sharp & Dohme, outside the submitted work. Dr. Waterboer reports personal fees from MSD (Merck) Sharp & Dohme, outside the submitted work. All other authors have nothing to disclose.
Acknowledgements
We thank Ute Koch, Claudia Brandel, Monika Oppenländer and Evi Schyr for excellent technical support.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101659.
Appendix. Supplementary materials
References
- 1.Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreimer AR, Johansson M, Yanik EL, et al. Kinetics of the human papillomavirus type 16 E6 antibody response prior to oropharyngeal cancer. J Natl Cancer Inst. 2017;109(8) doi: 10.1093/jnci/djx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hibbert J, Halec G, Baaken D, Waterboer T, Brenner N. Sensitivity and specificity of human papillomavirus (HPV) 16 early antigen serology for HPV-driven oropharyngeal cancer: a systematic literature review and meta-analysis. Cancers (Basel) 2021;13(12) doi: 10.3390/cancers13123010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreimer AR, Ferreiro-Iglesias A, Nygard M, et al. Timing of HPV16-E6 antibody seroconversion before OPSCC: findings from the HPVC3 consortium. Ann Oncol. 2019;30(8):1335–1343. doi: 10.1093/annonc/mdz138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anantharaman D, Gheit T, Waterboer T, et al. Human papillomavirus infections and upper aero-digestive tract cancers: the ARCAGE study. J Natl Cancer Inst. 2013;105(8):536–545. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 9.Brenner N, Mentzer AJ, Hill M, et al. Characterization of human papillomavirus (HPV) 16 E6 seropositive individuals without HPV-associated malignancies after 10 years of follow-up in the UK Biobank. EBioMedicine. 2020;62 doi: 10.1016/j.ebiom.2020.103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzinger D, Wichmann G, Baboci L, et al. Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer. 2017;140(12):2748–2757. doi: 10.1002/ijc.30697. [DOI] [PubMed] [Google Scholar]
- 11.Whitmarsh A, Pring M, Thomas SJ, et al. Survival advantage in patients with human papillomavirus-driven oropharyngeal cancer and variation by demographic characteristics and serologic response: findings from head and neck 5000. Cancer. 2021;127(14):2442–2452. doi: 10.1002/cncr.33505. [DOI] [PubMed] [Google Scholar]
- 12.Waterboer T, Brenner N, Gallagher R, et al. Early detection of human papillomavirus-driven oropharyngeal cancer using serology from the study of prevention of anal cancer. JAMA Oncol. 2020;6(11):1806–1808. doi: 10.1001/jamaoncol.2020.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagodzinski A, Johansen C, Koch-Gromus U, et al. Rationale and design of the Hamburg City health study. Eur J Epidemiol. 2020;35(2):169–181. doi: 10.1007/s10654-019-00577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 15.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309(1-2):200–204. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Clifford GM, Shin HR, Oh JK, et al. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1874–1879. doi: 10.1158/1055-9965.EPI-07-0349. [DOI] [PubMed] [Google Scholar]
- 17.Grobe A, Hanken H, Kluwe L, et al. Immunohistochemical analysis of p16 expression, HPV infection and its prognostic utility in oral squamous cell carcinoma. J Oral Pathol Med. 2013;42(9):676–681. doi: 10.1111/jop.12086. [DOI] [PubMed] [Google Scholar]
- 18.Halec G, Schmitt M, Dondog B, et al. Biological activity of probable/possible high-risk human papillomavirus types in cervical cancer. Int J Cancer. 2013;132(1):63–71. doi: 10.1002/ijc.27605. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol. 2006;44(2):504–512. doi: 10.1128/JCM.44.2.504-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batra A, Muller CA, Mann K, Heinz A. Alcohol dependence and harmful use of alcohol. Dtsch Arztebl Int. 2016;113(17):301–310. doi: 10.3238/arztebl.2016.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussmann L, Laban S, Wittekindt C, et al. Comparative effectiveness trial of transoral head and neck surgery followed by adjuvant radio(chemo)therapy versus primary radiochemotherapy for oropharyngeal cancer (TopROC) BMC Cancer. 2020;20(1):701. doi: 10.1186/s12885-020-07127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson JMG, Jungner G & World Health Organization. (1968). Principles and practice of screening for disease / J. M. G. Wilson, G. Jungner. World Health Organization. https://apps.who.int/iris/handle/10665/37650 Public health papers; no. 34
- 23.WHO . Increase Effectiveness, Maximize Benefits and Minimize Harm: Copenhagen WHO Regional Office for Europe; 2020. Screening Programmes: a Short Guide. [Google Scholar]
- 24.Kreimer AR, Shiels MS, Fakhry C, et al. Screening for human papillomavirus-driven oropharyngeal cancer: considerations for feasibility and strategies for research. Cancer. 2018;124(9):1859–1866. doi: 10.1002/cncr.31256. [DOI] [PubMed] [Google Scholar]
- 25.Robbins HA, Ferreiro-Iglesias A, Waterboer T, et al. Absolute risk of oropharyngeal cancer after an HPV16-E6 serology test and potential implications for screening: results from the human papillomavirus cancer cohort consortium. J Clin Oncol. 2022, Jun 14:JCO2101785. 10.1200/JCO.21.01785. Epub ahead of print. PMID: 35700419. [DOI] [PMC free article] [PubMed]
- 26.Institut RK. Zentrum für Krebsregisterdaten im Robert Koch-Institut: Datenbankabfrage mit Schätzung der Inzidenz, Prävalenz und des Überlebens von Krebs in Deutschland auf Basis der epidemiologischen Landeskrebsregisterdaten 2021. Available from: www.krebsdaten.de/abfrage.
- 27.Reuschenbach M, Tinhofer I, Wittekindt C, Wagner S, Klussmann JP. A systematic review of the HPV-attributable fraction of oropharyngeal squamous cell carcinomas in Germany. Cancer Med. 2019;8(4):1908–1918. doi: 10.1002/cam4.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahlstrom KR, Anderson KS, Guo M, et al. Screening for HPV-related oropharyngeal, anal, and penile cancers in middle-aged men: initial report from the Houston clinical trial. Oral Oncol. 2021;120 doi: 10.1016/j.oraloncology.2021.105397. [DOI] [PubMed] [Google Scholar]
- 29.Agalliu I, Gapstur S, Chen Z, et al. Associations of oral alpha-, beta-, and gamma-human papillomavirus types with risk of incident head and neck cancer. JAMA Oncol. 2016;2(5):599–606. doi: 10.1001/jamaoncol.2015.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatfield-Reed K, Roche VP, Pan Q. cfDNA detection for HPV+ squamous cell carcinomas. Oral Oncol. 2021;115 doi: 10.1016/j.oraloncology.2020.104958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.