Abstract
HrcA, a negative control repressor for chaperone expression from the obligate thermophile Bacillus thermoglucosidasius KP1006, was purified in a His-tagged form in the presence of 6 M urea but hardly renatured to an intact state due to extreme insolubility. Renaturation trials revealed that the addition of DNA to purified B. thermoglucosidasius HrcA can result in solubilization of HrcA free from the denaturing agent urea. Results from band shift and light scattering assays provided three new findings: (i) any species of DNA can serve to solubilize B. thermoglucosidasius HrcA, but DNA containing the CIRCE (controlling inverted repeat of chaperone expression) element is far more effective than other nonspecific DNA; (ii) B. thermoglucosidasius HrcA renatured with nonspecific DNA bound the CIRCE element in the molecular ratio of 2.6:1; and (iii) B. thermoglucosidasius HrcA binding to the CIRCE element was stable at below 50°C whereas the complex was rapidly denatured at 70°C, suggesting that the breakdown of HrcA is induced by heat stress and HrcA may act as a thermosensor to affect the expression of heat shock regulatory genes. These results will help to determine the nature of HrcA protein molecules.
In the heat shock response of gram-positive bacteria and α-proteobacteria, the CIRCE (controlling inverted repeat of chaperone expression)-HrcA systems play an important role in the mechanism for regulation of expression of heat shock genes (14, 15, 29). HrcA, a repressor protein encoded by the hrcA gene, is responsible for negative control of heat shock genes by binding to the CIRCE element located in the region controlling transcription. The CIRCE element in Bacillus species is composed of a pair of conserved 9-bp inverted repeats and intervening nonconservative 9-bp spacers. The groE and dnaK operons of Bacillus species have been demonstrated to be under this control system (28, 29). Indeed, analyses of the entire genome sequence of Bacillus subtilis revealed that the strictly conserved inverted repeat is restricted to these two chaperone operons (15). Further studies on B. subtilis HrcA and Bacillus stearothermophilus HrcA indicated that the GroE chaperonin machine modulates the activity of the HrcA repressor (12). In Clostridium acetobutylicum DSM1731, however, the HrcA protein can be refolded by the chaperones DnaK, DnaJ, and GrpE (17). Furthermore, in Bradyrhizobium japonicum 110spc4, elevated levels of GroESL in an hrcA mutant had no influence on free-living growth and symbiotic nitrogen fixation (11). The many examples of cooperation between HrcA and these chaperones for proper function of HrcA in diverse bacteria (1) are consistent with the common and stable characteristics that HrcA is hardly soluble and easily forms aggregates when expressed and purified from Escherichia coli. This unfavorable characteristic was reported for B. subtilis HrcA (19), B. stearothermophilus HrcA (12), Staphylococcus aureus HrcA (16), and C. acetobutylicum HrcA (17). Although in vitro experiments performed with B. stearothermophilus HrcA revealed the participation of GroEL in modulating HrcA function, the purification process and band shift assays still required the presence of 8 and 0.5 M urea, respectively (12). The insolubility of HrcA has thus far impeded in vitro analyses aimed at elucidating the nature of HrcA. On the other hand, many DNA-binding proteins tend to form inclusion bodies upon overproduction, which is a serious obstacle to purification of the proteins after gene cloning (4, 6).
Based on our interest in protein thermostability, we characterized the dnaK operon genes from the obligate thermophile Bacillus thermoglucosidasius KP1006 and showed that there are significant differences in the proline content of the dnaK operon proteins (DnaK, DnaJ, and GrpE) which are closely correlated with the thermostability of enzyme proteins (25, 26). B. thermoglucosidasius KP1006 is an obligate thermophile that can grow at temperatures between 42 and 69°C, with optimum growth between 61 and 63°C (21). B. thermoglucosidasius strains show considerable phenotypic resemblance to B. stearothermophilus. The stress-inducible proteins from B. thermoglucosidasius and B. stearothermophilus are proposed to share a high degree of identity (26). We are now shifting our focus to B. thermoglucosidasius HrcA with the aim of investigating its thermostability and function. To study the native features of B. thermoglucosidasius HrcA, it is necessary to solubilize the purified protein in the absence of the denaturing agent urea. In many trials, we devised a convenient method to renature B. thermoglucosidasius HrcA in a soluble form, excluding urea even in the absence of the chaperonin GroEL. Here we show that the presence of DNA is crucial to the solubilization and thermostability of HrcA. Later, we found that the method we devised is essentially the same as that previously reported by Egan and Schleif (5). On the basis of results of our in vitro studies, we discuss the possibility that HrcA binding to the CIRCE element may act as a thermosensor for regulation of the two chaperone operons in B. thermoglucosidasius KP1006.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains used in this study were B. thermoglucosidasius KP1006 (DSM2542) (21), E. coli MV1184 {ara Δ(lac-proAB) rpsL thi (φ80 lacZΔM15) Δ(srl-recA)306::Tn10(Tetr)/F′ [traD306 proAB+ lacIq lacZΔM15]} (23), and E. coli BL21(DE3) [F− ompT hsdSB(rB− mB−) gal dem(DE3)] (20). Plasmids pUC119 (23) and pSTV29 (Takara Shuzo, Kyoto, Japan) were used as cloning vectors, and pET15-b (Novagen, Madison, Wis.) was used as a His-tagging fusion vector. B. thermoglucosidasius KP1006 was grown as previously described (22). The E. coli cells were aerobically grown at 37°C in L broth (1% polypeptone, 0.5% yeast extract, 0.5% NaCl, 0.1% glucose [pH 7.2]) supplemented with ampicillin (50 μg/ml).
DNA manipulations.
Extraction of genomic DNA from B. thermoglucosidasius KP1006 was carried out as described before (24). Two primers, HF (CGGAATTCGAAGAAATGTTTTTTGGG) and HR (AGGCTGCAGTTTTCCATT), were used to amplify the DNA fragment containing the B. thermoglucosidasius hrcA gene and its flanking region by PCR with B. thermoglucosidasius genomic DNA as the template. The latter corresponds to the nucleotide sequence of a part of B. thermoglucosidasius HrcA (amino acid residues 276 to 294) (26), and the former was designed from the nucleotide sequences for the 5′-flanking regions of B. subtilis hrcA (9) and B. stearothermophilus hrcA (13). PCR was performed with LA-Taq DNA polymerase (Takara Shuzo) for 30 cycles according to the manufacturer's directions. Denaturation, annealing, and extension temperatures were 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min, respectively. Other DNA manipulations followed protocols described before (2).
Purification and renaturation of B. thermoglucosidasius HrcA.
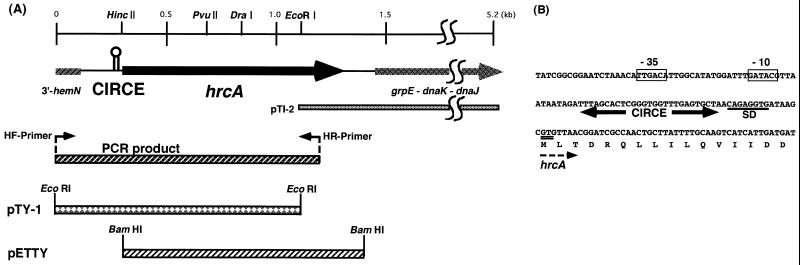
To purify B. thermoglucosidasius HrcA overexpressed in E. coli, the hrcA gene was tagged with a stretch of six His residues at its N terminus. A DNA fragment containing the gene was amplified by PCR using primer 1 (CGGGATCCCATGTTAACGGATCGCCAACT, corresponding to the region for the initiation codon of hrcA) and primer 2 (CGGGATCCAAATAGTCACAAGGA, corresponding to the sequence between hrcA and grpE), both with BamHI sites at their termini, with B. thermoglucosidasius genomic DNA as the template. The amplified DNA fragment was digested with BamHI and cloned into a BamHI site of the His-tagging vector pET15-b. The resulting plasmid was named as pETTY (Fig. 1A) and used for transformation of E. coli BL21(DE3). His-tagged B. thermoglucosidasius HrcA (His-HrcA) was produced in the transformant cells by induction with isopropyl-β-d-thiogalactopyranoside (0.5 mM) and purified essentially according to the protocol supplied by Clontech (Palo Alto, Calif.) under denaturing conditions in the presence of 6 M urea with metal affinity chromatography. All procedures were done at 4°C unless otherwise stated. After passing through the affinity resin, the eluate containing pure His-HrcA in the presence of 6 M urea was extensively dialyzed against 20 mM Tris-HCl–5 mM EDTA (pH 7.5) containing 3 M urea. The protein concentration was then adjusted to 200 μg/ml with dialyzing buffer. To 1-ml aliquots, 50 μg of plasmid DNA (pUC119) dissolved in dialyzing buffer (50 μl) was added and then extensively dialyzed against 20 mM Tris-HCl–5 mM EDTA (pH 7.5). The dialysate was ultracentrifuged at 100,000 × g for 15 min, and the supernatant was saved as a final sample. Plasmid DNA used for renaturation was purified by ultracentrifugation to equilibrium in cesium chloride-ethidium density gradients (18).
FIG. 1.
(A) Schematic representation and physical map of the hrcA-dnaK operon. The CIRCE element and the hrcA gene are indicated with a hairpin and thick arrow, respectively. A 1.1-kbp PCR product obtained from primers HF and HR, the EcoRI fragment cloned in pTY-1, and the BamHI fragment in pETTY are shown as bars, as are pTI-2 (26) containing the C terminus of the hrcA gene with the grpE, dnaK, and dnaJ genes. (B) DNA sequence of the N terminus of the hrcA gene and its 5′-flanking region. The vegetative promoter (−35 and −10 consensus sequence) is boxed. The CIRCE element is indicated with thick arrows. A Shine-Dalgarno sequence (SD) for ribosome binding is underlined.
Band shift assay.
The CIRCE-containing probe was a 330-bp DNA fragment carrying the region upstream of the start codon of B. thermoglucosidasius hrcA and synthesized by PCR with primers 1 and HrcA-5 (CGGGATCCAAAATAAGCAGTTGG, corresponding to amino acid residues 5 to 10 of B. thermoglucosidasius HrcA). The probe used as nonspecific DNA was a 340-bp fragment carrying B. thermoglucosidasius dnaK from amino acid residues 505 to the stop codon (26). The nonspecific DNA probe was generated by PCR with primers DnaK505 (CGGGATCCGGAAAGAAGCGGCAGAACTC) and DnaK-Rev (CGGGATCCTTATTTGTTGTCGTCTTTCAC). Each probe was internally labeled by PCR in the presence of 60 kBq of [α-32P]dCTP. The mixture for binding assay reactions contained binding buffer (20 mM Tris-HCl–5 mM EDTA [pH 7.5]), 0.2 μg of labeled probe, and 1.0 μg of purified His-HrcA. Binding was performed as described before (12, 28). The samples were loaded onto a 7% polyacrylamide gel and run at 20 mA with 1× Tris-borate-EDTA buffer (18). After polyacrylamide gel electrophoresis (PAGE), gels were wrapped with Saran Wrap and exposed to X-ray film for 3 to 15 h at room temperature.
Light scattering photometry.
Light scattering was carried out using a Shimadzu RF5300PC fluorescence spectrophotometer equipped with a temperature-controlled cell holder and a magnetic stirrer. The excitation and emission wavelengths were set at 450 nm; the excitation and emission slits were set at 5 nm. The data were sent to a computer every 20 s. The reaction was started by adding 25 μg of purified His-HrcA (0.1 ml) to a solution (2.9 ml) containing the appropriate amount of DNA in 20 mM Tris-HCl–5 mM EDTA (pH 7.5). Purified B. thermoglucosidasius DnaK (26) and GroEL (unpublished result) proteins used for the assay were prepared from E. coli MV1184 harboring plasmids carrying the B. thermoglucosidasius dnaK and groEL genes, respectively.
Protein concentration assay.
Protein concentration was determined by the Bradford method (3) to eliminate false-positive signals associated with urea.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases with accession number AB039833.
RESULTS
Cloning of B. thermoglucosidasius hrcA.
Since the dnaK, grpE, and dnaJ genes were cloned from B. thermoglucosidasius KP1006 together with the C terminus of the hrcA gene in the same operon (26), we tried to clone the N terminus of the hrcA gene and its 5′-flanking region, using colony hybridization on the genomic library of various restriction enzyme-digested fragments. However, we were unable to obtain the fragment encompassing the desired region of the hrcA gene. This had also been the case for B. stearothermophilus hrcA (13). Thus, we switched to a method based on cloning by PCR, essentially as used for B. stearothermophilus hrcA. By this PCR-based method, a 1.1-kbp DNA fragment was amplified as a single band. The fragment was cleaved with EcoRI, and the larger fragment was ligated to the vector plasmid pUC119 at the EcoRI site (pTY-1 [Fig. 1A]). The entire DNA sequence of the cloned fragment in pTY-1 was determined. As expected, the fragment contained the region coding for the N terminus of B. thermoglucosidasius hrcA and 300 bp of its 5′-flanking region. The coding region further upstream of hrcA showed high sequence similarity to the hemN gene of B. subtilis (9). This indicates that the gene organization is identical to that found in B. subtilis MB11 (10) and B. stearothermophilus NUB36 (7, 13). Between the stop codon of the hemN gene and the start codon of the hrcA gene, there were 83-bp-long intervening sequences. A set of vegetative promoters (TTGACA for the −35 region and TACGTT for the −10 region) and a genetic element consisting of a pair of 9-bp inverted repeats separated by 9-bp spacers were also found in the intervening region (Fig. 1B). The inverted repeats here are the CIRCE element of B. thermoglucosidasius KP1006, which precisely matches the CIRCE elements of B. subtilis MB11 (29) and B. stearothermophilus NUB36 (13).
The predicted amino acid sequence of the putative B. thermoglucosidasius HrcA protein implied that HrcA consists of 344 amino acids and exhibits 63.1, 92.4, and 29.4% identity with the HrcA proteins of B. subtilis MB11, B. stearothermophilus NUB36, and B. japonicum 110spc4, respectively. As it has been reported that B. stearothermophilus hrcA can complement B. subtilis hrcA deletion (13), it seems highly likely that B. thermoglucosidasius hrcA would also be able to complement a B. subtilis hrcA mutant. In gross features, the B. thermoglucosidasius KP1006 dnaK operon genes resemble that of B. stearothermophilus NUB36.
Purification and renaturation of B. thermoglucosidasius HrcA.
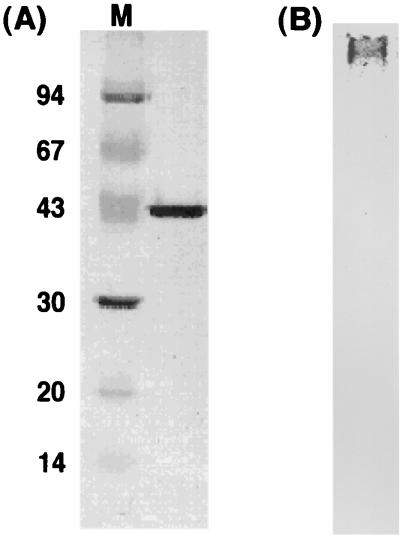
Although intact B. thermoglucosidasius HrcA was overexpressed in E. coli cells, the HrcA protein was hardly detected in the soluble cell-free fraction by sodium dodecyl sulfate (SDS)-PAGE analyses. The band for the HrcA protein was seen at the position of the predicted molecular mass (39 kDa) only for the whole-cell fraction, not for the cell extract (data not shown). This indicates that B. thermoglucosidasius HrcA can be expressed but is almost entirely insoluble. This characteristic also applies for HrcA proteins from other bacteria. Thus, we modified the B. thermoglucosidasius hrcA gene to add a six-His tag at its N terminus, using pET15-b plasmid. His-HrcA was then purified to homogeneity by metal affinity chromatography under denaturing conditions containing 6 M urea (Fig. 2A). After purification, the effects of stepwise decreases in the urea concentration were determined. Results from these analyses indicated that His-HrcA could be hardly solubilized in less than 3 M urea.
FIG. 2.
(A) SDS-PAGE pattern of B. thermoglucosidasius HrcA purified by metal chromatography; (B) native-PAGE pattern of B. thermoglucosidasius HrcA renatured with nonspecific DNA (pUC119). The gel concentrations used were 10% for SDS-PAGE and 7.5% for native PAGE. Each lane contained 2 μg of B. thermoglucosidasius HrcA protein. Molecular masses of the protein markers (M) in panel A are indicated in kilodaltons.
To renature purified His-HrcA in the absence of urea, various additives, such as glycerol, detergents, and plasmid DNA, were added at different concentrations to the His-HrcA solution containing 3 M urea, followed by dialysis. Eventually, we found that DNA served to solubilize His-HrcA in the absence of urea. The addition of additives other than DNA caused the formation of visible aggregates rapidly after the start of dialysis. In contrast, the addition of more than 50 μg of plasmid DNA (pUC119, 3.2 kbp) to 200 μg of purified His-HrcA in 1 ml of 20 mM Tris-HCl–5 mM EDTA–3 M urea (pH 7.5) effectively renatured His-HrcA. We note that the DNA added should be dissolved in advance in the same buffer (20 mM Tris-HCl, 5 mM EDTA, 3 M urea [pH 7.5]) to prevent slight aggregation of HrcA. Furthermore, effective plasmid DNA is not restricted to pUC119; any other vector plasmid DNAs that are also nonspecific for HrcA are as effective for His-HrcA renaturation as pUC119. However, plasmid pTY-1, which contains the CIRCE element, was much more effective than nonspecific plasmid DNAs. The amount of plasmid DNA added could be decreased to less than 30 μg. Moreover, we investigated the His-HrcA renaturation with sonicated plasmid DNA. When DNA fragments prepared by sonication of plasmid pUC119 (resulting lengths were 200 to 500 bp) were used, more than 200 μg DNA was necessary for renaturation of the same amount of His-HrcA indicated above. The increase in the requirement for DNA may be due to excessive disruption of plasmid DNA by sonication. At any rate, these results suggest that the His-HrcA protein binds to nonspecific DNA that is favorable for renaturation and that binding of the protein to DNA containing the CIRCE element is much stronger than that with nonspecific DNA. The native-PAGE pattern (Fig. 2B) showed that HrcA renatured with DNA hardly migrated, presumably due to the apparent increase of molecular weight by binding to plasmid DNA, which has a rather high molecular mass (3.2 kbp = 2.1 × 106 Da). The native-PAGE pattern was independent of whether DNA used for HrcA solubilization contained the CIRCE element. In addition, it should be noted that the His-HrcA solution does not precipitate from solution for more than several weeks at 4°C.
Renatured HrcA protein binds to the CIRCE element without urea and GroEL.
To provide evidence that HrcA proteins bind to the CIRCE element, excessive amounts of HrcA proteins from B. stearothermophilus NUB36 (12) and B. subtilis 168 (28) were used for the band shift assay for the DNA fragment containing the CIRCE element. However, these reaction mixtures still contained 0.5 M urea and/or GroEL to prevent further aggregation of HrcA. Therefore, we carried out band shift assays to examine whether B. thermoglucosidasius HrcA renatured with DNA can efficiently interact with the CIRCE element under in the absence of urea and GroEL.
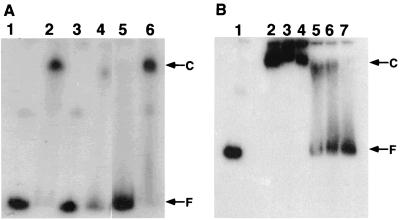
His-HrcA protein was incubated at room temperature with a 330-bp DNA fragment containing the CIRCE element and end labeled with 32P. As seen in Fig. 3A, His-HrcA protein (0.10 μg, 2.4 pmol) retarded the entire probe DNA fragment (0.20 μg, 0.92 pmol) containing the CIRCE element (lane 2). If it is assumed that the renatured protein is 100% active, the molar ratio of HrcA and DNA would be 2.6:1. A lower amount of His-HrcA protein failed to induce a complete band shift for the probe. This result indicates that two to three HrcA molecules bind to the fragment. As reported previously, HrcA protein renatured by GroEL and ATP retarded at most only approximately 0.3 equivalents of the probe fragment (12). Therefore, the His-HrcA protein used here was demonstrated to be renatured to a more functional state and thus to interact with the fragment containing the CIRCE element more efficiently. On the other hand, His-HrcA protein retarded the nonspecific probe DNA with poor efficiency (lane 4). In competition assays (lanes 5 and 6), the excess amount of nonlabeled probe DNA containing the CIRCE element significantly inhibited the interaction of His-HrcA with the labeled specific probe DNA, while nonlabeled nonspecific probe DNA slightly affected retardation of the probe DNA. These results indicated that His-HrcA protein interacts far more strongly with DNA containing the CIRCE element than with any other DNA.
FIG. 3.
(A) Band shift assays of the CIRCE probe with B. thermoglucosidasius HrcA renatured with nonspecific DNA (pUC119). Positions of the radiolabeled free DNA probe (F) and HrcA-probe complex (C) are marked. B. thermoglucosidasius HrcA samples contained no urea. Lane 1, labeled CIRCE probe (0.2 μg) alone; lane 2, labeled CIRCE probe (0.2 μg) with renatured HrcA (0.1 μg); lane 3, labeled nonspecific probe (0.2 μg) alone; lane 4, labeled nonspecific probe (0.2 μg) with renatured HrcA (0.1 μg); lane 5, same as lane 2 with nonlabeled CIRCE probe (1 μg); lane 6, same as lane 2 with nonlabeled nonspecific probe (1 μg). (B) Thermostability analyses of HrcA and probe complex by band shift assays. The binding reaction of B. thermoglucosidasius HrcA to the CIRCE probe was done at room temperature for 10 min; then each mixture was incubated at the indicated temperature for 1 min. Immediately after heat shock treatment, the samples were loaded into wells of a polyacrylamide gel. Lane 1, labeled CIRCE probe (0.2 μg) alone; lanes 2 to 7, the labeled CIRCE probe (0.2 μg) with renatured HrcA (0.1 μg); lane 2, no heat shock treatment after the binding reaction (control); lanes 3 to 7, heat shock treatment at 40, 50, 60, 65, and 70°C, respectively. Positions of the radiolabeled free CIRCE probe (F) and HrcA-probe complex (C) are marked.
Next we examined the thermostability of His-HrcA bound to the probe DNA containing the CIRCE element. After formation of a complex of His-HrcA and the probe DNA at room temperature, the mixture was incubated at various temperatures (40, 50, 60, 65 and 70°C) for 1 min. Then the reaction mixtures were immediately applied to the gel (Fig. 3B). Although His-HrcA continued to bind to the specific probe DNA after heat treatment below 50°C, the binding complex of His-HrcA and the probe diminished in quantity after a temperature shift to above 60°C. At 70°C, the interaction of His-HrcA and the probe DNA was abrogated, and no band retardation was observed. Similar results were observed in the case of the upshift to 70°C even after formation of a complex of His-HrcA and the probe at 50°C.
Light scattering assays revealed that DNA prevents HrcA from aggregation without GroEL.
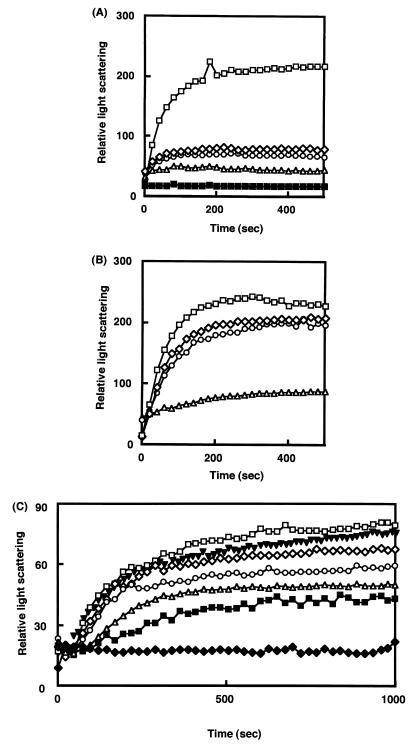
To confirm the effect of DNA on renaturation of B. thermoglucosidasius HrcA, we investigated the light scattering change of His-HrcA solution containing DNA. Light scattering increases with time if the protein is aggregated. As seen in Fig. 4A, plasmids pUC119 and pTY-1 suppressed the increase of light scattering. Addition of 50 μg of pUC119 (7.4 nM) or 15 μg of pTY-1 (1.66 nM) to 25 μg of His-HrcA protein (0.20 μM) prevented HrcA aggregation. The stationary level of light scattering was dependent on the amount and species of DNA added. Amounts of DNA lower than those indicated above could suppress aggregation, but the stationary level of light scattering was elevated. However, the level of light scattering did not increase even if the reaction time was extended. These results indicate that HrcA has a stronger interaction with the CIRCE element than with nonspecific DNA and that the HrcA-DNA complex is stable under these conditions.
FIG. 4.
Light scattering patterns of B. thermoglucosidasius HrcA in the presence of DNA (A) and other proteins (B) and effect of temperature on light scattering patterns (C). Incubation was at 15°C throughout the experiments in panels A and B. (A) 25 μg of B. thermoglucosidasius HrcA (final concentration, 0.2 μM) in 0.1 ml of 20 mM Tris-HCl–5 mM EDTA–3 M urea (pH 7.5) was added to the solution (2.9 ml) as follows: no DNA (□), 50 μg of plasmid DNA pUC119 (◊), 100 μg of pUC119 (○), 20 μg of plasmid DNA pTY-1 containing the CIRCE element (▵), and 50 μg of pTY-1 (■). (B) The solution contained HrcA (final concentration, 0.2 μM) alone (◊) or with bovine serum albumin (0.2 μM; □); B. thermoglucosidasius DnaK (0.2 μM; ○) or B. thermoglucosidasius GroEL (0.2 μM; ▵). (C) The solution (3.0 ml) containing HrcA (25 μg) and plasmid DNA pTY-1 (20 μg) was preincubated with stirring at 15°C for 3 min; then the cuvette was transferred into the cell holder of a fluorescence spectrophotometer set at 15°C (⧫), 40°C (■), 50°C (▵), 60°C (○), 65°C (◊), or 70°C (□). In the experiment to investigate the effect of B. thermoglucosidasius GroEL (▾), the GroEL protein (final concentration, 0.2 μM) was added to the solution mixture in the cuvette, which was then transferred into the cell holder set at 70°C. Data collection always started when the cuvette was transferred into the cell holder.
It has been reported that GroEL modulates HrcA activity (12). Therefore, B. thermoglucosidasius GroEL was used in place of DNA to examine the effect of GroEL on renaturation of HrcA. As shown in Fig. 4B, GroEL but not other proteins (bovine serum albumin and DnaK) prevented aggregation of HrcA. However, light scattering for HrcA in the presence of GroEL increased slightly and did not show a stationary level like that in the presence of DNA. This indicates that the complex of HrcA and GroEL is not so stable and that the potential function of GroEL to prevent aggregation of HrcA might be insufficient compared with DNA.
Next we tested the thermostability of the HrcA-DNA complex in a light scattering assay. His-HrcA renatured with plasmid pTY-1 containing the CIRCE element was incubated in a cuvette maintained at various temperatures (15, 40, 50, 60, 65, and 70°C) (Fig. 4C). No increase in light scattering was observed during extended incubation at 15°C, while only a slow increase was detected during incubation for several minutes below 50°C. However, the increase became faster with the elevation of temperature. At 70°C, HrcA protein was mostly aggregated within 750 s, while some portions of the protein were still soluble below this temperature. This result, which is consistent with that obtained by band shift assays (Fig. 3B), suggested that heat treatment above the optimum growth temperature range for B. thermoglucosidasius KP1006 may subject the cells to heat stress, and hence HrcA is easily denatured above that temperature. Furthermore, we examined whether GroEL prevents the aggregation of HrcA in the presence of DNA at 70°C (Fig. 4C). The presence of GroEL had no protective effect against the aggregation of HrcA.
DISCUSSION
This work presents a convenient method to renature and solubilize B. thermoglucosidasius KP1006 HrcA with DNA. Previous attempts to perform in vitro studies for bacillary HrcA proteins have been impeded by the insolubility of the protein (12, 19). The presence of the denaturing agent urea and the chaperonin GroEL, which are somehow effective for renaturation of B. stearothermophilus HrcA, prevents direct analysis of the native nature of HrcA repressors (12). Therefore, using B. thermoglucosidasius KP1006 HrcA, which has features similar to those of B. stearothermophilus HrcA, we devised a method to renature and solubilize the protein in the absence of urea and GroEL. During assays with various reagents, we found that DNA can efficiently renature and solubilize B. thermoglucosidasius HrcA in a functional state that is free from urea and GroEL. The method utilizes the inherent capacity of B. thermoglucosidasius HrcA to bind to DNA.
Early work on DNA-dependent renaturation of an insoluble DNA-binding protein was reported by Egan and Schleif (5), who used sheared salmon sperm or calf thymus DNA in large excess to AraC and RhaS proteins. In contrast, we found here that widely available and nonspecific plasmid DNA can promote the refolding of B. thermoglucosidasius HrcA in quantitative amounts. We also found that DNA sheared by sonication (to about 200 to 500 bp in length) was less effective for renaturation, probably due to undetectable damage to the DNA that we prepared. While any species of DNA can renature B. thermoglucosidasius HrcA, DNA containing the CIRCE element is far more effective for renaturation than nonspecific DNA. This finding was also confirmed by the band shift and light scattering assays. Many DNA-binding proteins in addition to HrcA tend to aggregate during purification processes (4, 6). The types of protocols represented by our method using plasmid DNA and the work reported by Egan and Schleif (5) should prove useful for eliminating or minimizing solubility problems during the process of solubilization and renaturation of insoluble DNA-binding proteins.
The band shift assays shown in Fig. 3A provide a clue to the molecular relationships between B. thermoglucosidasius HrcA and the CIRCE element. Judging from the molecular ratio obtained (2.6:1), two or three B. thermoglucosidasius HrcA molecules bound to the CIRCE element. Previous studies on bacillary HrcA proteins failed to determine the molecular relationships with the CIRCE element since a large excess amount of HrcA was necessary for band shift assays due to the extreme insolubility (11, 12, 28). B. thermoglucosidasius HrcA is more likely to have such an oligomeric structure since DNA-binding proteins tend to be oligomeric (4, 27). In contrast to our observation, Ohta et al. visualized the binding of presumably monomeric S. aureus HrcA to the CIRCE element by atomic force microscopy (16). The low identity (31.2%) between B. thermoglucosidasius HrcA and S. aureus HrcA may explain the difference in the subunit structures of the proteins. More stoichiometric analysis is required to elucidate the precise molecular relationship. In addition, the results of light scattering assays (Fig. 4B) demonstrated that DNA containing the CIRCE element can keep B. thermoglucosidasius HrcA in the folded state more effectively and stably than GroEL. This suggests that the fate of HrcA molecules present in a cell should be dependent on whether the protein molecules bind to the CIRCE element under nonstress conditions.
While preparing this report, we became aware of a report that B. japonicum HrcA remained in a soluble form upon overproduction and during purification processes (11). The identity between B. japonicum HrcA and B. thermoglucosidasius HrcA was as low as 29.4% despite their common function to bind to the CIRCE element. We do not know the reason for the comparatively high solubility of B. japonicum HrcA. However, the fact that an excess amount of B. japonicum HrcA versus DNA was necessary for a complete band shift indicates that the repressor protein may precipitate from solution over time. By contrast, B. thermoglucosidasius HrcA solubilized with DNA does not precipitate for several weeks at 4°C.
How can we explain the implication of HrcA binding to nonspecific DNA? Two plausible explanations may be proposed based on two different viewpoints. From the viewpoint of repression, HrcA may possess two modes of binding to DNA. The first mode would be preliminary binding to nonspecific DNA, which would serve to assist the second mode binding to the CIRCE element for negative control of heat shock genes. This seems reasonable since there are only two copies of the CIRCE element in the genomic DNA of B. subtilis (15), while nonspecific DNA is abundant in a cell. From the viewpoint of protein refolding, nonspecific DNA may act as a ligand to promote refolding of HrcA. In this case, the DNA would prevent correctly folded HrcA from unfolding and aggregating just like chaperonin GroEL (12).
The thermostability of the B. thermoglucosidasius HrcA-DNA complex containing the CIRCE element was examined in band shift and light scattering assays. Its thermostability should be crucial in investigating whether HrcA plays a role as a thermosensor for chaperone expression. It is natural that B. thermoglucosidasius HrcA exists on genomic DNA in cells to repress gene expression by binding to the CIRCE element under nonstress conditions. Therefore, it seems reasonable to assume that B. thermoglucosidasius HrcA renatured with DNA containing the CIRCE element in vitro would be comparable to the native form of the repressor in vivo. As seen in Fig. 3B and 4C, B. thermoglucosidasius HrcA is stable below 50°C during a few minutes of incubation, but much of the protein is denatured by treatment above 65°C. The susceptibility of B. thermoglucosidasius HrcA at higher temperatures suggests that the protein may be detached from the CIRCE element after denaturation caused by heat stress. The susceptibility is not affected by the presence of GroEL (Fig. 4C), as seen in yeast α-glucosidase (8). However, the denaturation of HrcA in our experiments was mostly irreversible since thermally denatured HrcA failed to re-form the complex with probe DNA upon incubation at the lower temperature (data not shown). This irreversibility of denaturation suggests that HrcA inactivated by heat stress is less likely to be regenerated by chaperonin GroEL (Fig. 4C), and de novo HrcA synthesis should be required when cells have recovered. This implies that B. thermoglucosidasius HrcA may act as a thermosensor, and the thermal inactivation of HrcA protein may affect heat stress gene expression as an alternative. Most probably, de novo HrcA synthesis and effective refolding of newly synthesized HrcA by GroEL are key for initiation of the negative control of heat shock genes (28).
We suggest that HrcA may be a thermosensor to affect the expression of heat shock genes, while the overall flow of negative gene regulation is dependent on how HrcA can be effectively refolded by GroEL. We plan to analyze more native features of B. thermoglucosidasius HrcA and determine the basis of its physical interaction with GroEL.
REFERENCES
- 1.Ahmad S, Selvapandiyan A, Bhantnagar R K. A protein-based phylogenetic tree for gram-positive bacteria derived from hrcA, a unique heat-shock regulatory gene. Int J Syst Bacteriol. 1999;49:1387–1394. doi: 10.1099/00207713-49-4-1387. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Dorman C J, Hinton J C, Free A. Domain organization and oligomerization among H-NS-like nucleoid-associated proteins in bacteria. Trends Microbiol. 1999;7:124–128. doi: 10.1016/s0966-842x(99)01455-9. [DOI] [PubMed] [Google Scholar]
- 5.Egan S M, Schleif R F. DNA-dependent renaturation of an insoluble DNA binding protein. Identification of the RhaA binding site at rhaBAD. J Mol Biol. 1994;243:821–829. doi: 10.1006/jmbi.1994.1684. [DOI] [PubMed] [Google Scholar]
- 6.Grob P, Guiney D G. In vitro binding of the Salmonella dublin virulence plasmid regulatory protein SpvR to the promoter regions of spvA and spvR. J Bacteriol. 1996;178:81–84. doi: 10.1128/jb.178.7.1813-1820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbort M, Schön U, Angermann K, Lang J, Schumann W. Cloning and sequencing of the dnaK operon of Bacillus stearothermophilus. Gene. 1996;170:81–84. doi: 10.1016/0378-1119(95)00859-4. [DOI] [PubMed] [Google Scholar]
- 8.Höll-Neugenbauer B, Rudolph R, Schmidt M, Buchner J. Reconstitution of a heat shock effect in vitro: influence of GroE on the thermal aggregation α-glucosidase from yeast. Biochemistry. 1991;30:11609–11614. doi: 10.1021/bi00114a001. [DOI] [PubMed] [Google Scholar]
- 9.Homuth G, Heineman M, Zuber U, Schumann W. The genes lepA and hemN form a bicistronic operon in Bacillus subtilis. Microbiology. 1996;142:1641–1649. doi: 10.1099/13500872-142-7-1641. [DOI] [PubMed] [Google Scholar]
- 10.Homuth G, Masuda S, Mogk A, Kobayashi Y, Schumann W. The dnaK operon of Bacillus subtilis is heptacistronic. J Bacteriol. 1997;179:1153–1164. doi: 10.1128/jb.179.4.1153-1164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minder A, Fischer H, Hennecke H, Narberhaus F. Role of HrcA and CIRCE in the heat shock regulatory network of Bradyrhizobium japonicum. J Bacteriol. 2000;182:14–22. doi: 10.1128/jb.182.1.14-22.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogk A, Homuth G, Scholz C, Kim L, Schmid F X, Schumann W. The GroE chaperonine machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 1997;16:4579–4590. doi: 10.1093/emboj/16.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogk A, Schumann W. Cloning and sequencing of the hrcA gene of Bacillus stearothermophilus. Gene. 1997;194:133–136. doi: 10.1016/s0378-1119(97)00000-0. [DOI] [PubMed] [Google Scholar]
- 14.Nakahigashi K, Ron E Z, Yanagi H, Yura T. Differential and independent roles of a ς32 homolog (RpoH) and an HrcA repressor in the heat shock response of Agrobacterium tumefaciens. J Bacteriol. 1999;181:7509–7515. doi: 10.1128/jb.181.24.7509-7515.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narberhaus F. Negative regulation of bacterial heat shock genes. Mol Microbiol. 1999;31:1–8. doi: 10.1046/j.1365-2958.1999.01166.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohta T, Nettikandan S, Tokumasu F, Ideno H, Abe Y, Kuroda M, Hayashi H, Takeyasu K. Atomic force microscopy proposes a novel model for stem-loop structure that binds a heat shock protein in the Staphylococcus aureus HSP70 operon. Biochem Biophys Res Commun. 1996;226:730–734. doi: 10.1006/bbrc.1996.1421. [DOI] [PubMed] [Google Scholar]
- 17.Rüngeling E, Laufen T, Bahl H. Functional characterization of the chaperones DnaK, DnaJ and GrpE from Clostridium acetobutylicum. FEMS Microbiol Lett. 1999;170:119–123. doi: 10.1111/j.1574-6968.1999.tb13363.x. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 19.Schulz A, Schumann W. hrcA, the first gene of the Bacillus subtilis dnaK operon, encoding a negative regulator of class I heat shock genes. J Bacteriol. 1996;178:1088–1093. doi: 10.1128/jb.178.4.1088-1093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studier F W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991;219:37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Kishigami T, Inoue K, Mizouchi Y, Eto N, Takagi M, Abe S. Bacillus thermoglucosidasius sp nov., a new species of obligately thermophilic bacilli. Syst Appl Microbiol. 1983;4:487–495. doi: 10.1016/S0723-2020(83)80006-X. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Yuki T, Kishigami T, Abe S. Purification and properties of extracellular α-glucosidase of a thermophile, Bacillus thermoglucosidius KP1006. Biochim Biophys Acta. 1976;445:386–397. doi: 10.1016/0005-2744(76)90092-9. [DOI] [PubMed] [Google Scholar]
- 23.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Iha H, Ohashi A, Suzuki Y. Cloning and expression in Escherichia coli of an extremely thermostable oligo-1,6-glucosidase gene from Bacillus thermoglucosidasius KP1006. J Bacteriol. 1989;171:1219–1222. doi: 10.1128/jb.171.2.1219-1222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe K, Suzuki Y. Protein thermostabilization by proline substitutions. J Mol Catal B Enzym. 1998;4:167–180. [Google Scholar]
- 26.Watanabe K, Iwashiro T, Suzuki Y. Features of dnaK operon genes of the obligate thermophile Bacillus thermoglucosidasius KP1006. Antonie Leeuwenhoek. 2000;77:241–250. doi: 10.1023/a:1002483620374. [DOI] [PubMed] [Google Scholar]
- 27.Weigel, Schmidt C A, Seitz H, Tungler D, Welzeck M, Messer W. The N-terminus promotes oligomerization of the Escherichia coli initiator protein DnaA. Mol Microbiol. 1999;34:53–66. doi: 10.1046/j.1365-2958.1999.01568.x. [DOI] [PubMed] [Google Scholar]
- 28.Yuan G, Wong S L. Isolation and characterization of Bacillus subtilis groE regulatory mutants: evidence for orf39 in the dnaK operon as a repressor gene in regulating the expression of both groE and dnaK. J Bacteriol. 1995;177:6462–6468. doi: 10.1128/jb.177.22.6462-6468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuber U, Schumann W. CIRCE, a novel heat shock element involvement in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]