Abstract
Nitric oxide (NO) is a signal molecule and plays a critical role in the regulation of vascular tone, displays anti-platelet and anti-inflammatory properties. While our earlier and current studies found that low NO doses trigger a rapid heme insertion into immature heme-free soluble guanylyl cyclase β subunit (apo-sGCβ), resulting in a mature sGC-αβ heterodimer, more recent evidence suggests that low NO doses can also trigger heme-maturation of hemoglobin and myoglobin. This low NO phenomena was not only limited to sGC and the globins, but was also found to occur in all three nitric oxide synthases (iNOS, nNOS and eNOS) and Myeloperoxidase (MPO). Interestingly high NO doses were inhibitory to heme-insertion for these hemeproteins, suggesting that NO has a dose-dependent dual effect as it can act both ways to induce or inhibit heme-maturation of key hemeproteins. While low NO stimulated heme-insertion of globins required the presence of the NO-sGC-cGMP signal pathway, iNOS heme-maturation also required the presence of an active sGC. These effects of low NO were significantly diminished in the tissues of double (n/eNOS−/−) and triple (n/i/eNOS−/−) NOS knock out mice where lung sGC was found be heme-free and the myoglobin or hemoglobin from the heart/lungs were found be low in heme, suggesting that loss of endogenous NO globally impacts the whole animal and that this impact of low NO is both essential and physiologically relevant for hemeprotein maturation. Effects of low NO were also found to be protective against ischemia reperfusion injury on an ex vivo lung perfusion (EVLP) system prior to lung transplant, which further suggests that low NO levels are also therapeutic.
Keywords: Globins: hemeproteins, Heme, Myoblasts, Nitric oxide, Reperfusion
Graphical abstract
Highlights
-
•
Low levels of NO enable heme-maturation of the globins by a process that required an NO triggered heme-insertion into sGCβ.
•This effect of low NO was also found to occur for all three nitric oxide synthases (NOSs) and Myeloperoxidase (MPO).
•Tissues from n/eNOS−/− and n/i/eNOS−/− knock out mice had low heme levels in the globins, while sGC was largely heme-free.
•Low NO at ppm levels also manifests itself as a therapy during ischemic reperfusion injury of lungs on the EVLP.
1. Introduction
Ever since its benchmark discovery, nitric oxide (NO) has thus far been known to play important functional roles in a variety of physiological systems [1]. NO is a signal molecule and plays a critical role in the regulation of vascular tone, and within the vasculature NO induces vasodilation [[2], [3], [4], [5]], inhibits platelet aggregation [4,[6], [7], [8], [9]], displays anti-inflammatory properties [10], prevents neutrophil/platelet adhesion to endothelial cells [11], inhibits smooth muscle cell proliferation and migration [12], regulates apoptosis and endothelial cell barrier function [1,12]. NO is produced by many cell types in the body and plays important roles in controlling the normal function of cells as well as in regulating key processes in the nervous and immune systems. In the immune system, NO generated by macrophages acts as an antimicrobial agent. High amounts of NO produced by the macrophages is toxic to the bacteria or the pathogens and it causes their destruction [13]. However, too much NO has also been implicated in conditions where the immune system is overactive eg. in diseases like arthritis and the so-called autoimmune diseases [14]. Hence the balance between physiological to pathophysiological levels of cellular NO can dictate many of the cell functions.
NO generated by NOS enzymes [[15], [16], [17], [18]] or produced by noncanonical means [19], is a central component of the NO-sGC-cGMP signal pathway and works by activating the soluble guanylyl cyclase (sGC), an obligate heterodimer of an α-subunit (sGCα) and a β-subunits (sGCβ) [[20], [21], [22]]. Although much attention has been directed in the last two decades to study the physiological effects of NO-cGMP signaling [[23], [24], [25], [26], [27]], little is known about its hematologic effects and how this pathway contributes to erythropoiesis [28]. The only known hematologic effects of NO-cGMP signaling have revealed that it is capable of stimulating erythropoiesis at the transcriptional level both in vivo and in vitro [29]. NO increases cGMP via sGC activation, which increases Hbγ (fetal) levels in human erythroid cells [30] whereas inhibition of sGC prevents NO-induced increase in Hbγ gene expression [31]. While our earlier studies had established a role for chaperon hsp90 to be involved in the heme-maturation for adult (β) and fetal (γ) hemoglobin (Hb) [32] and for myoglobin (Mb), which also required an active sGC [33], our present findings suggest that cellular GAPDH is involved in heme-maturation of myoglobin and all three hemoglobins (Hb & Mb) [30]. In these studies we established that in all these globin maturation events GAPDH acts as a heme chaperon, allocating/providing mitochondria generated heme to the apo-Hsp90/AHSP-Hbα/β/γ/Mb complexes [30], where these globin heme maturations are influenced by iron provision and magnitude of expression of GAPDH, d-aminolevulinic acid, and FLVCR1b [30,34]. Together these finding imply that hsp90 and GAPDH play key roles in globin maturations and our current studies link the activation of sGC to maturation of the globins (Mb/Hb), thereby contributing to the formation of a novel NO-sGC-Globin axes.
NO is an uncharged radical and hence the cell redox milieu leads its biological functions [35]. While interactions of NO with marquee hemeproteins like soluble guanylate cyclase is known to cause activation [36], those with hemoglobin are known to cause scavenging of the NO [[37], [38], [39]]. While there are several other facets of NO hemeprotein relationship which are implicitly stated in the published literature, our earlier and current studies found that low NO doses trigger a rapid heme insertion into immature heme-free apo-sGCβ1, resulting in mature sGC heterodimer [[40], [41], [42]], and more examples of this low NO effect was clearly lacking. Whether this low NO phenomena is part of the NO-sGC signal pathway activation and whether other hemeproteins like globins also undergo such NO driven heme-insertion/maturation were largely unidentified. There is evidence that shows that high NO doses can block heme-insertion into hemeproteins [43] and there is implicit literature stating that proteins like MPO can also have their heme-dependent activities regulated by low and high NO levels, suggesting that NO may have a dual effect on hemeprotein maturation [44]. However a comprehensive study demonstrating the impact of the dual role of NO on hemeprotein maturation, including applications to highlight the role of low NO as a therapy was needed. In our current study we showcase the dual effect of NO in promoting or inhibiting heme-insertion/maturation in seven different hemeproteins. We then corroborate the impact of endogenous NO on sGC and globin maturations (Hb and Mb) using tissues from NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) knock out mice [45]. Finally to demonstrate the efficacy of low NO levels as an application towards therapy, we show it to be protective against ischemic reperfusion injury of porcine lungs on a ex vivo lung perfusion (EVLP) [[46], [47], [48]] system prior to lung transplant.
2. Material and methods
Reagents: All chemicals were purchased from Sigma (St. Louis, MO) and Fischer chemicals (New Jersey). NO donor, NOC-18 or DETA NONOate (2,2ʹ-{Hydroxynitrosohydrazino) bis-ethanamine}), phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX), NO inhibitor l-NAME (L-Nitro Arginine Methyl Ester), o-Dianisidine for heme-staining, sGC stimulator BAY 41–2272 (BAY 41) which is a heme & NO dependent sGC activator or BAY 60–2770 (BAY 60), a heme/NO independent sGC activator were all purchased from Sigma. Reagents such as l-Arginine, Sodium pyruvate, Lactate dehydrogenase (LDH), NADPH, flavins (FAD, FMN), Tetrahydrobiopterin (H4B) needed for NOS activity assays were also obtained from Sigma. Myc-DDK tagged human hemoglobin alpha (Hbα), beta (Hbβ), human skeletal muscle myoglobin expression construct, Myc-tagged Hbβ and DDK-tagged Hbα or DDK/Flag-tagged MPO construct were purchased from OriGene (Rockville, MD, USA). V5-tag sGC-β1 and Myc-tagged sGC-α1 expression constructs was a gift from Dr. Andreas Papadopoulos (Athens University, Athens, Greece). Mammalian expression construct of iNOS (pcdna3.1 iNOS) was obtained from Dr. Stuehr's lab (Cleveland Clinic). HEK 293T, RAW 264.7, A549, SH-SY5Y and C2C12 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). All HASMCs (human airway smooth muscle cells) explanted from normal donor lungs were provided by the laboratory of Dr. Rey Panettieri (Rutgers) [49]. Stable cell lines of HEK293 expressing eNOS or nNOS were obtained from Prof. Solomon Synder's lab (Johns Hopkins, Baltimore). Mice tissues including lungs and hearts from NOS double (n/eNOS−/−) and NOS triple (n/i/eNOS−/−) KOs were obtained from Prof. Masato Tsutsui's and Prof. Hiroaki Shimokawa's lab (University of Ryukyus, Japan). Pig blood, control pig lung tissues, while those obtained from the EVLP (Ex-Vivo Lung Perfusion) perfused with blood mixed with NO or via the airways were obtained from lung transplant surgeon Prof. Toshihiro Okamoto (Cleveland Clinic). MPO activity assay kit was purchased from Sigma, while cGMP ELISA assay kits were obtained from Cell Signaling Technology (Danvers, MA, USA). Protein G-sepharose beads were purchased from Sigma and molecular mass markers were purchased from Bio-Rad (Hercules, CA, USA).
Antibodies: Antibodies were purchased from different sources. Table S1 describes various types of antibodies used and its source.
Cell culture, transient transfection, growth/induction of cells: All cell lines were grown and harvested as previously described [32,50]. Cultures (50–60% confluent) of HEK 293T/COS-7 cells were transiently transfected with either Myc-Mb or Myc/DDK-Hb-αβ or Flag-MPO constructs, and after 28h of transient transfection, NO donor doses from NOC-18 were given for additional 18h before harvest. Cultures of HASMCs (human airway smooth muscle cells) or stable HEK lines expressing eNOS/nNOS were grown to confluency and then treated with NO doses for 18 h before harvest, and in most cultures where NO donor doses were given, the cells were also given sGC stimulator BAY 41 before cell harvest. In different experiments RAW cells were induced with combinations with interferon gamma (IFN-γ), LPS for variable time points between 0 and 24h −/+ l-NAME, before cell harvest. At the point of cell harvest, the monolayers were washed twice with 3 ml of cold PBS containing 1 mg/ml of glucose, and cells on each plate were collected by scraping in presence of 250 μl of classical lysis buffer (40 mM EPPS buffer pH 7.6, 10% Glycerol, 3 mM DTT, 150 mM NaCl and 1% NP40) or at times cell lysis buffer from cGMP estimation kit (Cell Signaling Technology) was used. The collected cells were lysed by 3 cycles of freeze-thawing (in liquid nitrogen and at 37 °C, respectively). The lysates were centrifuged for 30 min at 4 °C and the supernatants were collected and stored at −80 °C. Total protein contents of the supernatants was determined using the Bio-Rad protein assay kit. In all cases wherever applicable cell supernatants were assayed for protein expression by Western blot, binding assays by IPs, depiction of heme-maturation by heme staining as indicated.
Western blots, heme staining and immunoprecipitations (IPs): Western blots were performed using standard protocols as previously mentioned. For westerns, 50-80 μg of the supernatants were run on SDS-PAGE (8 or 15%), transferred to the same PVDF membrane, probed with a specific antibody and developed using ECL reagent. In all cases β-actin or GAPDH was used as a loading control. Multiple protein detection was achieved by stripping the membranes and re-probing with specific antibodies. Heme staining of cell supernatants either from C2C12 cells (250 μg) or induced RAW cells (200 μg) or from stable/transfected HEK/COS-7 cell supernatants (250 μg) cells was done as previously described [32,33]. For immunoprecipitations (IP), 600 μg of the total cell supernatant was precleared with 20 μl of protein G-sepharose beads (Amersham) for 1 h at 4 °C, beads were pelleted, and the supernatants incubated overnight at 4 °C with 3 μg of the indicated antibody. Protein G-sepharose beads (20 μL) were then added and incubated for 1 h at 4 °C. The beads were micro-centrifuged (6000 rpm), washed three times with wash buffer (50 mM HEPES pH 7.6, 100 mM NaCl, 1 mM EDTA and 0.5% NP-40) and then boiled with SDS-buffer and centrifuged. The supernatants were then loaded on SDS-PAGE gels and western blotted with specific antibodies. Band intensities on westerns were quantified using Image J quantification software (NIH).
UV–visible absorption spectra, Heme content of Hb estimation and Olis Clarity spectroscopy: UV–visible absorption spectra of blood tissue supernatants extracted from mice lungs were recorded at room temperature between 350 and 700 nm on a Shimadzu spectrophotometer (Shimadzu, Kyoto, Japan). Equal amounts (200 μg) of total protein supernatants were used for respective wavelength scans. The heme content for Hb was determined from the Soret absorption peak at 414 by using the extinction coefficient of 342500 M−1 cm−1 and a manipulation to account for the variable absorbance contributions that were attributable to sample turbidity. This involved creating a baseline for each scan by drawing a line that connected the absorbance values at 380 and 470 nm. The additional Soret absorbance at 414 nm above this baseline was then used to calculate the Hb heme content [30,33]. The analysis of Mb heme in the mice heart muscle was done by using the Olis Clarity spectrophotometer, which can record the heme spectra of suspensions or solutions in particulate form. Here the absorption spectra was collected with an integrating sphere detector. To segregate the Mb in the heart muscle from the mixed blood, IPs were performed on 600 μg of total protein with Mb antibody, beads washed with wash buffer and the bead bound Mb spectra from WT, double or triple NOS KO mice heart supernatants were recorded on the Olis Clarity.
Nitrite in the culture media: Nitrite was measured using ozone-based chemiluminescence with the triiodide method and using the Sievers NO analyzer (GE Analytical Instruments, Boulder, CO, USA) [51,52]. Briefly, the triiodide reagent was made using 1g of KCI and 0.65 g of iodine dissolved in 20 ml of distilled water, with the addition of 70 ml of acetic acid. A total of 6 ml of this solution was used in the reaction chamber for nitrite conversion into NO. A standard curve was generated using 0–50 μM of nitrite. Samples were used neat or diluted. These were injected into the reaction chamber. Since the volume of the reaction chamber would alter over time, we limited the number of injections but kept the samples from each set together. The concentration of nitrite was calculated using the standard curve. All samples were done in triplicate.
In vitro NOS reconstitution and Griess assays: iNOS, nNOS or eNOS activity was determined by measuring production of nitrite alone or nitrite plus nitrate (stable oxidation products of NO that accumulate quantitatively) in 30-min incubations run at 37 °C. Aliquots of HEK cell supernatants were transferred to microwells containing 40 mM Tris buffer (pH 7.8) supplemented with 3 mM DTT, 2 mM l-arginine, 1 mM NADPH, protease inhibitors, and a 4 mM concentration each of FAD, FMN, and H4biopterin, to give a final volume of 0.1 ml. Reactions were terminated by enzymatic depletion of the remaining NADPH [53]. In separate experiments the cell culture media from induced RAW cells or transiently expressing iNOS were taken out at specific time points to determine the accumulated nitrite by Griess assay [50].
Oxyhemoglobin assay to measure NO release rates at various NOC-18 concentrations: The NO-mediated conversion of oxyhemoglobin to methemoglobin was used to determine the rate of NO release from NOC-18 at 37 °C [40] (Fig. S1). Various concentrations of NOC-18 were added to cuvettes that contained DMEM culture media with 10 μM oxyhemoglobin. The absorbance gain at 401 nm was recorded over a 3h period. The rate of NO release was calculated using the difference extinction coefficient of 38 mM−1 cm−1.
MPO activity assay: Myeloperoxidase (MPO) is abundantly expressed in neutrophils and is a lysosomal protein stored in the azurophilic granules of the neutrophil [54]. MPO catalyzes the production of hypochlorous acid (HClO) from hydrogen peroxide (H2O2) and chloride anion, Cl− (or halide). In our study, the MPO enzyme activity of cell lysates were analyzed by BioVision's Myeloperoxidase (MPO) Activity Colorimetric Assay Kit (Catalog #K744-100), according to manufacturer's protocol. Briefly, HClO produced from H2O2 and Cl− reacts with taurine to generate taurine chloramine, which subsequently reacts with the TNB probe to eliminate the color (λ = 412 nm).
cGMP enzyme-linked immunosorbent assay: The cGMP concentration in various cell supernatants made from intact cells that were either given NO donor, NOC-18 or a combination of NOC18 doses and BAY 41 before harvest. RAW cells induced with interferon gamma (IFN-γ) and LPS −/+ l-NAME were estimated using the cGMP ELISA assay kit (Cell Signaling Technology). In other cases cGMP in pig lung tissue supernatants were also estimated using the cGMP ELISA assay kit. Control WT or NOS double (n/eNOS−/−) and (n/i/eNOS−/−) triple KOs mice lung tissue supernatants were assayed for sGC enzymatic activity [55] in reactions containing aliquots of the tissue supernatants supplemented by adding 250 μm GTP, 10 μm of sGC stimulator BAY 41 or activator BAY 60, 250 μM IBMX and then incubated for 20 min at 37 °C. Reactions were quenched by addition of 10 mm Na2CO3 and Zn (CH3CO3)2. The generated cGMP was then determined by ELISA.
Biotin Switch assays: The biotin switch assay on pig lung tissue supernatants was performed to determine S-nitrosated proteins as described previously [56], and the presence of the S-nitrosated target protein was assayed by immunoblotting with specific antibodies.
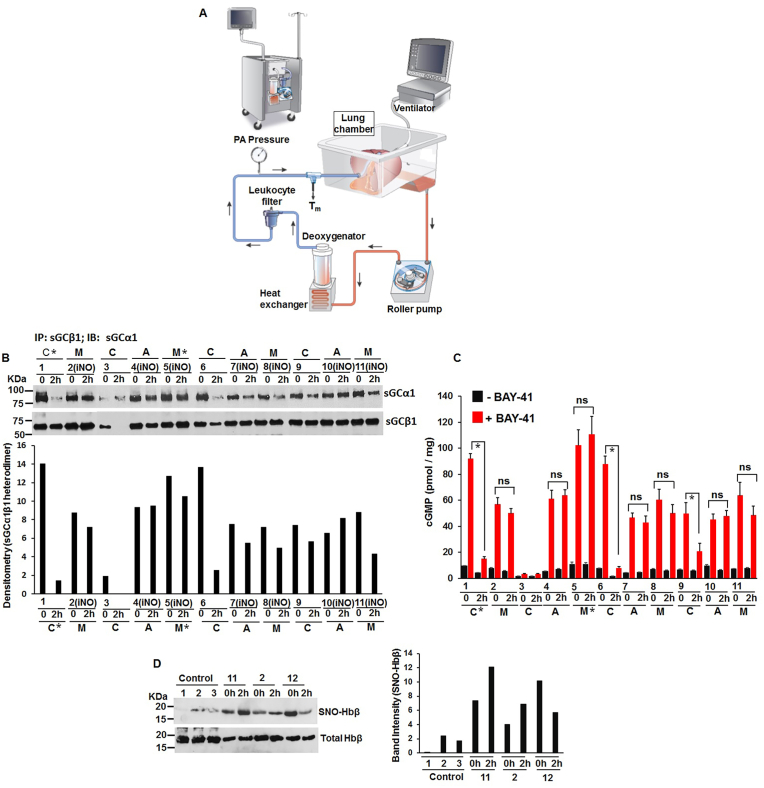
Ex Vivo Lung Perfusion (EVLP) Studies: These studies were done in collaboration by Dr. T. Okamoto (Cleveland Clinic). We received a total of 12 cases of EVLP (Table S3), out of which we got porcine lung tissues from 11 cases, and 3 cases had all relevant blood perfusates. Tissues and blood perfusates from Control group n = 4; iNO (Airways) n = 3; iNO (Membranes) n = 5 were the cases that were used for doing biochemical studies (Table S3). Lund-type EVLP was used and 2 h of perfusions was maintained. In iNO Airway group iNO (nitric oxide in ppm) was delivered via trachea tube and in iNO Membrane groups, iNO (nitric oxide in ppm) was delivered via membrane in the EVLP circuit (i.e. nitric oxide was mixed with the blood perfusate) by using INOVent (INOTherapeutics LLC, Hampton, NJ) (Fig. 10A). All nitric oxide doses for iNO Airways or iNO Membranes were 80 ppm, except for one iNO Airway and one iNO Membrane group which was 40 ppm. At 2 h, physiological data and transplant suitability was evaluated. The control groups were given warm ischemia as well as cold ischemia to have moderate lung injury during EVLP. The same ischemia time was given to the iNO groups. Instrumentation: The LS1 (XVIVO Perfusion Inc, Engwood, CO) consisted of a roller pump, reservoir, membrane oxygenator, heat exchanger, and leukocyte filter (Fig. 10A). The EVLP system was primed with 2.0 L of STEEN solution, heparin 10000 IU, and packed red blood cells 500–600 mL, whose hematocrit level was 10–15%. The pH in the solution was adjusted with isotonic trometamol. The pulmonary artery cannula was securedusing 2-0 silk, and the trachea was connected to the mechanical ventilator (Servo-i; Maquet Critical Care, Solna, Sweden) using an endotracheal tube. The left atrium was opened widely. Details of EVLP were previously reported [57].
Fig. 10.
Low NO doses are protective against ischemia reperfusion injury on a ex vivo lung perfusion (EVLP) system. (A) Diagramatic representation of the EVLP system. (B) Representative IPs depicting sGC-α1β1 heterodimerization in control (C) untreated, or NO treated via airways (A) or NO perfused via blood (M). Bottom panel represents the densitometry of sGCα1 bound to β1. * represents samples which were suitable for transplant. (C) Corresponding tissue homogenates estimated for cGMP by BAY-41 activation of sGC. All values depicted are mean n = 3, ±SD, ns is statistically non significant. *p < 0.05, by one-way ANOVA. (D) SNO-Hbβ levels in the control untreated and NO treated pig blood samples (left), with densitometry of SNO-Hbβ levels (right) normalized by total protein.
3. Results
NO causes heme-insertion into the sGCβ1 subunit inducing heterodimerization of sGC-α1β1 subunits: Endorsing our previous and current studies [40,42] we found that low doses of NO can trigger heme-insertion into sGCβ1 to build the sGC heterodimer. We first transiently expressed Myc-sGCα1 and V5-sGCβ1 in COS-7 cells for 28 h, followed by treatment with variable doses of NO from NO donor NOC-18 (0–100 μM) for 18 h before cell harvest. Performing immunoprecipitations (IPs) we found that there was a strong sGC-α1β1 heterodimer buildup at low NOC-18 concentrations (between 1 and 7.5 μM, Fig. 1A) and a concomitant drop in sGCβ1-hsp90 binding. We then repeated these experiments in three human airway smooth muscle cells (HASMCs) that were explanted from 3 healthy donors and then treated the cultures with variable doses of NOC-18 (0–100 μM) for 18 h. IPs performed on generated cell supernatants showed that the sGC-α1β1 heterodimer buildup occurred at extremely low doses of NO, i.e. between 0.1 and 10 μM concentrations of NOC-18 and this gradually dissociated at higher NO doses beyond concentrations of 25–100 μM (Fig. 1B, S1 and S2). On the contrary the sGCβ1-hsp90 interactions were inversely correlated with the heterodimer, decaying with buildup of the heterodimer and then rising as the heterodimer dissociated at higher NO doses (25–100 μM of NOC-18, Fig. 1B). The cGMP estimates from corresponding HASMC supernatants correlated with buildup of the sGC heterodimer at low NO doses and with the fall at higher doses (Fig. 1C). While we demarcate low and high NO levels mainly based on the drastic effects on heme-insertion we see here, the calculated values of NO release rates obtained for each increasing concentration of NOC-18 (0–250 μM, Fig. S1) shows a slender rise, which may suggest that these observed effects are based on subtle changes in molecular events which enable this NO-driven heme-insertion process. Together these data suggest that low NO doses induce heme-insertion into the β1 subunit, which subsequently heterodimerizes and this breaks apart at higher NO doses. Since this effect of NO obtained on transient expression of sGC-α1β1 subunits in cell lines (COS-7) and those obtained from three primary cultures of diverse HASMCs (Table S2, in terms of race and gender) are relatively similar, it further suggests that this low NO effect causing heme-insertion and subsequent sGC-α1β1 heterodimerization is a rather universal occurrence.
Fig. 1.
Low NO doses induced sGCα1β1 heterodimerization by causing heme-insertion into the sGCβ1 subunit. Myc-sGCα1 and V5-sGCβ1 constructs were either transiently transfected in COS-7 cells for 28h and then exposed to variable NO doses of NOC-18 for additional 18h before cell harvest or HASMCs were cultured to confluency and then treated with similar doses of NOC-18, followed by BAY 41–2272 treatment. (A) and (B) Immunoprecipitations (IPs) depicting build-up of sGC-α1β1 heterodimer at low NO doses with a concomitant drop in sGCβ1-hsp90 interaction. (C) Estimated cGMP values from experiments performed in panel B. All values depicted are mean n = 3, ±SD. *p < 0.05, by one-way ANOVA. Wherever applicable molecular weight markers (KDa) are depicted at the left of gel bands throughout the figure legends.
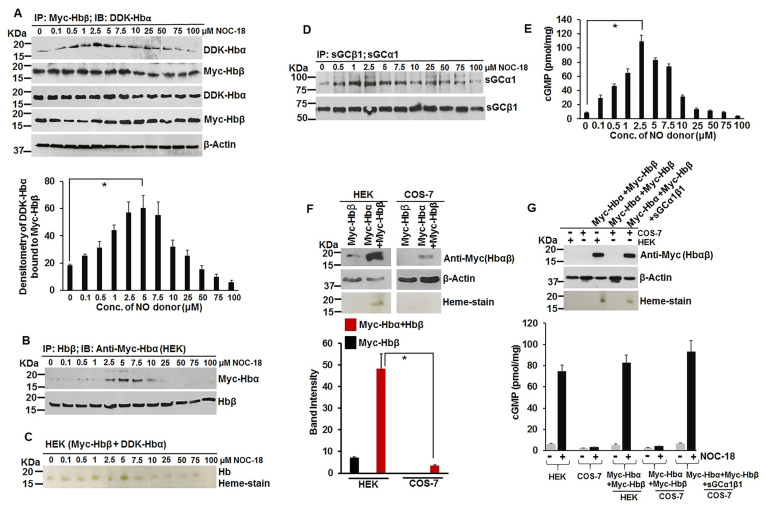
NO induces heme-insertion into the globins (Mb and Hb) and this occurs via the NO-sGC-cGMP pathway: Having determined the effect of NO on sGC heme-insertion, we wondered whether this process actually occurred for other hemeproteins including the all important globins (Mb or Hb). We used our novel find here to see whether treating by low NO doses can increase the Mb heme-insertion and what effect this would have on the Mb-hsp90 binding. We treated C2C12 cells with variable doses of NO for 18 h similar to that depicted in Fig. 1. As depicted in Fig. 2A, there was a gradual buildup of Mb heme in the low dosage range of NO (2.5–7.5 μM NOC-18), and this disappeared at higher NO dosage (>7.5 μM). Likewise there was also a gradual increase in Mb dimer which faded away at higher NO doses (>7.5 μM). While working with Mb heme-maturation we found that myoglobin also exists in a dimeric form in both mouse skeletal muscle (C2C12) as well as in human lung epithelial cells (A549) and in human lung tissue (Fig. S3). Dimeric myoglobin has been earlier reported in equine muscle but its significance or function is not fully understood [58]. Next we did IPs to access the level of hsp90 bound to Mb and how the Mb-hsp90 interaction changes with variable NO dosage. The Mb-hsp90 interactions were reciprocal with buildup of Mb heme and its dimer (Fig. 2B). This suggests that low NO doses caused heme-insertion into the Mb, this was correlated with a concomitant increase in the Mb heme bands (Fig. 1A) and with the loss of Mb-hsp90 interactions. Since Mb heme-maturation also involves an active sGC [33], we looked at the level of the sGC-α1β1 heterodimer under these conditions and found that the heterodimer peaked between 2.5 and 5 μM of NO and this was in accord with the accumulated cGMP (Fig. 2C and D). We then determined whether this NO induced heme-insertion into Mb goes via the NO-sGC pathway. For this we expressed Myc-Mb into HEK and in COS-7 (in the absence or presence of transfected sGCα1β1) cells and then gave similar NO doses from NOC-18. As depicted in Fig. 2E–G, Myc-Mb expressed well both at the protein and heme levels in HEK cells, but lost its expression at higher NO doses (>10 μM) and had no heme on the Mb in COS-7 cells. This loss in Mb expression at higher NO doses can be a direct effect of NO, which has been also implicated in proteosomal degradation of diverse proteins including iron regulatory protein-2 or certain hemeproteins like human cytochrome P450 [59,60]. However since COS-7 cells unlike the HEK are devoid of its endogenous NO receptor, sGC [33], overexpressing sGCα1β1 subunits along with Myc-Mb in COS-7 cells restored both heme and the protein levels on the Mb. Doing similar experiments of transfection in HEK cells with Myc-Hbβ and DDK-Hbα (or with Hbβ & Myc-Hbα), and treating with variable NO doses for 18 h before cell harvest, we performed IPs and heme-staining on the supernatants. As depicted in Fig. 3A–C there was a gradual increase in the Hb-αβ interaction at low NO doses, with the heme on the Hb (1-10μΜ), and this faded away at higher doses. The sGCα1β1 heterodimer as well as the generated cGMP peaked within this low NO dose (2.5 μM), suggesting that Hb heme-maturation also needed an active sGC (Fig. 3D–F). This was further supported by the fact that both expression and heme levels of Myc-Hbαβ were drastically reduced in COS-7 cells which lack sGC (Fig. 3F) [33], but were effectively rescued when sGCα1β1 was co-expressed with Myc-Hbαβ (Fig. 3G). The resurgence in the cGMP levels of COS-7 cells upon sGCα1β1 transfection followed by NO treatment (2.5 μM of NOC-18 for 18h) also showed that the NO-sGC axis was activated (Fig. 3G, lowermost panel). Together these data suggest that (Hb)-αβ also undergo a low NO dose driven heterodimerization following heme-insertion into the Hb-αβ subunits and this occurs via the NO-sGC-cGMP pathway.
Fig. 2.
Low NO doses also induced heme-insertion into the myoglobin (Mb) in C2C12 cells as well as in transiently expressed Mb and this heme-insertion/maturation occurs via the NO-sGC-cGMP pathway. C2C12 myoblasts were grown to confluency and then treated with variable doses of NOC-18 for 18h, and then treated with BAY 41–2272 before cell harvest. The cell supernatants were then assayed for IPs to determine the status of Mb-hsp90 interaction or sGC-α1β1 heterodimer, heme-stains to determine Mb heme levels and cGMP in cell supernatants was estimated by ELISA. In other experiments Myc-Mb was transiently expressed in HEK or COS-7 (−/+ sGC-α1β1) cells to determine the impact of sGC expression on Mb heme. (A) Mb expression by westerns (upper left panel), while lower left panel depicts Mb heme by heme-staining. Right panel depicts the mean densitometries of Mb heme-stains from n = 3 repeats. (B) IPs depicting Mb-hsp90 interaction. (C) IPs depicting sGC-α1β1 heterodimer and densitometry of bound sGCα1 under corresponding conditions of Mb heme-maturation. (D) Estimated cGMP values from experiments performed in panel A. All values depicted are mean n = 3, ±SD. *p < 0.05, by one-way ANOVA. (E–G) Expression and heme-stains of Myc-Mb in HEK or COS-7 (−/+ sGC-α1β1).
Fig. 3.
Transiently transfected hemoglobin (Hb)-αβ subunits also undergo a low NO dose dependent heterodimerization, driven by the NO-sGC-cGMP axis following heme-insertion into the globins. Myc-Hbβ/Hbβ and DDK-Hbα/Myc-Hbα constructs were transiently expressed in HEK cells for 28h and then treated with variable doses of NOC-18 for additional 18h before treatment with BAY 41–2272 and cell harvest. In other experiments Myc-Hbα and/-Hbβ were transiently expressed or in combination with sGCα1β1 in HEK/COS-7 cells for 28h followed by NOC-18 treatment for 18h before cell harvest. (A) IPs depicting formation of the DDK-Hbα-Myc-Hbβ heterodimer at low NO doses. Western expression of Myc-Hbβ and DDK-Hbα with β-actin used as a loading control. Lowermost panel depicts mean densitometries of DDK-Hbα bound to Myc-Hbβ from n = 3 repeats. (B) IPs depicting formation of the Myc-Hbα-Hbβ heterodimer at low NO doses. (C) Heme-stain of Myc-Hbβ and DDK-Hbα transfectants in HEK cells. (D) IPs depicting corresponding sGCα1β1 heterodimerization in HEK cells with (E) estimated cGMPs. (F) Western expression and heme-stains of Myc-Hbα/β and corresponding mean densitometries as indicated. (G) Western expression and heme-stains of Myc-Hbαβ in respective cell types. Lowermost panel depicts the estimated cGMP levels. All values depicted are mean n = 3, ±SD. *p < 0.05, by one-way ANOVA.
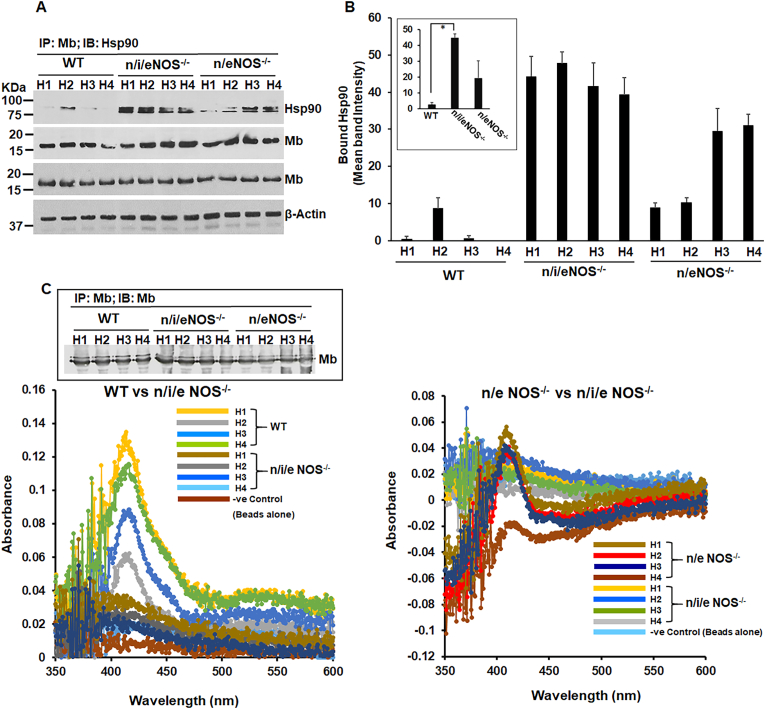
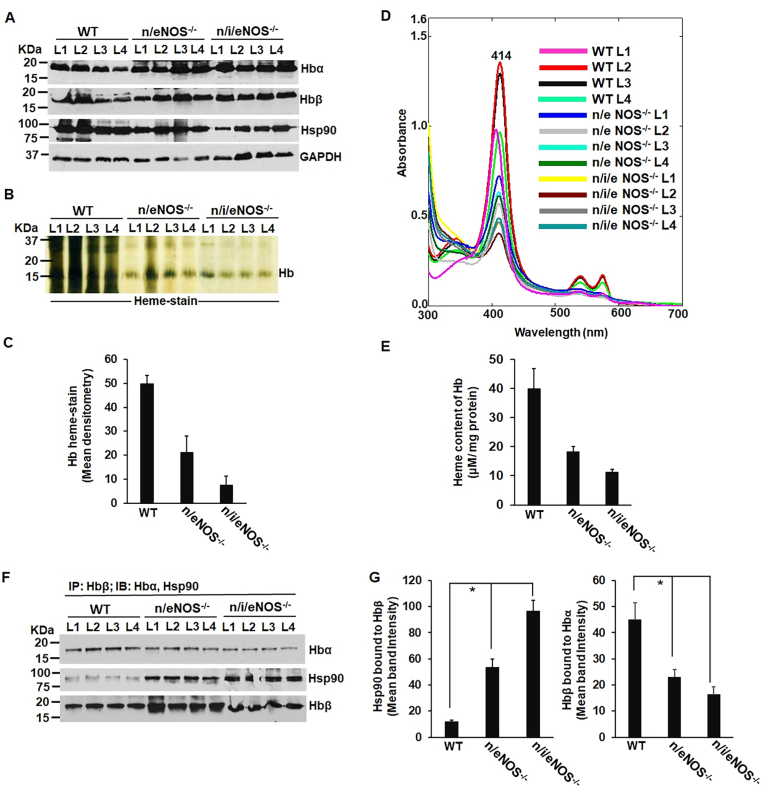
Lowering or loss of endogenous NO produced by NOS enzymes globally impacts sGC, Mb and Hb heme-maturations as evidenced in the tissues of NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) knock out mice: In order to determine the physiological semblance of NO induced heme-insertion of sGC and the globins we performed biochemical analyses on the lung, heart and blood tissues of NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) knock out (KO) mice that we obtained from Prof. Tsutsui's and Shimokawa's lab. Both these mice have a higher mortality rate relative to the wild-types [45] and the triple NOS (n/i/eNOS−/−) KO was recently shown to develop spontaneous pulmonary emphysema in juvenile mice [61] indicating a novel preventive role of the endogenous NO and this may also relate to lung sGC dysfunction. WT or NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) KO mice lung/heart/blood supernatants were analyzed for protein expression by westerns, IPs were performed to detect the strength of sGC-α1β1 heterodimer, or the strength of Mb-hsp90 or the Hb-αβ/Hbβ-hsp90 interactions and sGC activation assays were done using BAY-41 vs BAY-60, to detect formation of heme-free sGC. BAY-41 is a NO and heme dependent sGC activator, while BAY-60 is a heme/NO independent activator and use of these drugs causes preferential activation of the heme-containing and heme-free forms to estimate the relative populations. Western blots (Fig. 4A–B) revealed lowered expression levels of sGCα1 and β1 in the lung supernatants of NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) KOs relative to WT mice. Doing IPs on these lung supernatants (Fig. 4C), we found a weak (for n/eNOS−/−) to almost a non-existent (for n/i/eNOS−/−) sGC-α1β1 heterodimer for the double and triple NOS KOs respectively, indicating that lung sGC in these mice were heme-free. This was confirmed by doing sGC activation assays with BAY-41 and BAY-60 (Fig. 4E). Here that lack of heme in the lung sGC of double and triple NOS KOs is substituted by BAY-60, which like heme binds to sGCβ1 to increase the sGCα1-β1 heterodimer and thus rescues the sGC activity (Fig. 4E). These data suggest that endogenous NO produced by NOS enzymes are important for heme-maturation of the sGCβ1 and absence or lowering of endogenous NO in NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) KO mice results in the generation of heme-free sGC. Analyzing the mouse heart supernatants by IPs and determination of Mb heme on the Olis Clarity spectrophotometer we found that the Mb-hsp90 interactions were greatest for triple (n/i/eNOS−/−) where the Mb heme spectra was lowest, followed by double (n/eNOS−/−) KOs relative to the WT, indicating greater incidence of heme-free Mb in the KOs (Fig. 5A–C). For analysis of Mb heme we first segregated the Mb from the heart blood tissue (Hb) by IP with the Mb antibody and then suspended equal amounts of the bead bound Mb extracts for absorption spectra that was collected with an integrating sphere detector (Olis Clarity). Finally evaluating the blood tissue supernatants extracted from the mice lungs (Fig. 6) we found that the Hb from NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) KOs to be low in heme or the heme was loosely bound to the globin (heme-stain, Fig. 6B and C) relative to the WT. The results from the heme-stain corroborated with UV–visible spectra and also with the heme-content of Hb that was estimated from the corresponding spectral measures (Fig. 6D and E). IPs done on these supernatants showed that there was a corresponding lowering in the Hb-αβ interaction with a concomitant rise in Hbβ-hsp90 interactions which is indicative of an increase in heme-free Hb (Fig. 6F and G). Both the heme estimates and the IPs revealed that the Hb heme and the strength of Hb-αβ interactions were was lowest for the triple (n/i/eNOS−/−) KOs (Fig. 6B–G). Taken together our results show that inadequate NO levels can drastically impact the whole animal and can cause pathologic accumulation of heme-free sGC, can cause Mb to become heme-free and also trigger an increase in the heme-free Hb in these double (n/eNOS−/−) and triple (n/i/eNOS−/−) NOS KO mice. These data also corroborate our previous findings that low NO doses can trigger heme-insertion into the sGC and the globins (Fig. 1, Fig. 2, Fig. 3).
Fig. 4.
Endogenous NO produced by NOS enzymes are important for heme-maturation of the sGCβ1 and absence or lowering of endogenous NO in NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) knockout mice results in the generation of heme-free sGC. WT or NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) knockout mice lung supernatants were analyzed for protein expression by westerns, IPs were performed to detect the strength of sGC-α1β1 heterodimer and sGC activation assays were done by BAY 41–2272 or BAY 60–2770, with cGMP as the readout on an ELISA to detect formation of heme-free sGC. (A) Protein expression by westerns as indicated with (B) corresponding mean densitometries of sGCα1, sGCβ1 and hsp90. (C) IPs depicting sGC-α1β1 heterodimerization and its concomitant sGCβ1-hsp90 interactions. (D) Mean densitometries from interactions depicted in panel C. (E) Estimated cGMP by ELISA obtained by activating sGC in the lung supernatants with heme-dependent sGC stimulator BAY 41–2272 or with heme-independent sGC activator BAY 60–2770. Values depicted are mean n = 3 ± SD. *p < 0.05, by one-way ANOVA.
Fig. 5.
The absence or lowering of NO in NOS double (n/eNOS−/−) and (n/i/eNOS−/−) triple KOs makes the myoglobin (Mb) heme-deprived relative to wild-type controls. WT or NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) knockout mice heart supernatants were analyzed for protein expression by westerns, IPs were performed to detect the strength of Mb-hsp90 interaction which is a measure of heme-free Mb and Mb spectra the mice heart supernatants was estimated by absorption spectra collected with an integrating sphere detector (Olis Clarity), using bead bound Mb that was immunoprecipitated with Mb antibody. (A) IPs depicting Mb-hsp90 interactions in various mouse heart supernatants as indicated. Protein expression is depicted by westerns as indicated. (B) Corresponding mean cumulative densitometries of Mb-hsp90 interactions. All values depicted are mean n = 3, ±SD, *p < 0.05, by one-way ANOVA. (C) Corresponding absorption spectra of heart muscle Mb of WT, NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) KOs, collected with an integrating sphere detector. Inset shows the immunoprecipitated Mb from WT and the NOS KOs western blotted with Mb antibody.
Fig. 6.
Heme in the blood hemoglobin of NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) KOs mice is low in heme or is loosely bound with a corresponding lowering in Hb-αβ interaction. Blood extracted from the WT or NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) knockout mice lungs was homogenized and generated supernatants were subjected to protein expression by westerns, heme-stains to determine heme levels of Hb in the blood and IPs were performed to detect the strength of Hbα-β interactions and its concomitant Hbβ-hsp90 interactions which is a measure of heme-free Hb. (A) Protein expression by westerns as indicated. (B) Heme-stains of Hb in the generated supernatants as indicated. (C) Mean densitometry of Hb heme-stain depicted in panel B normalized by loading control GAPDH. (D) UV–visible absorption spectra of blood extracted from lungs of WT, double and triple KO with equal protein. (E) Calculated heme-content of Hb. (F) IPs depicting Hbα-β and its associated Hbβ-hsp90 interactions in various supernatants as indicated. (G) Corresponding mean densitometries of Hbα-hsp90 and Hbβ-hsp90 interactions. All values depicted are mean n = 4, ±SD. *p < 0.05, by one-way ANOVA.
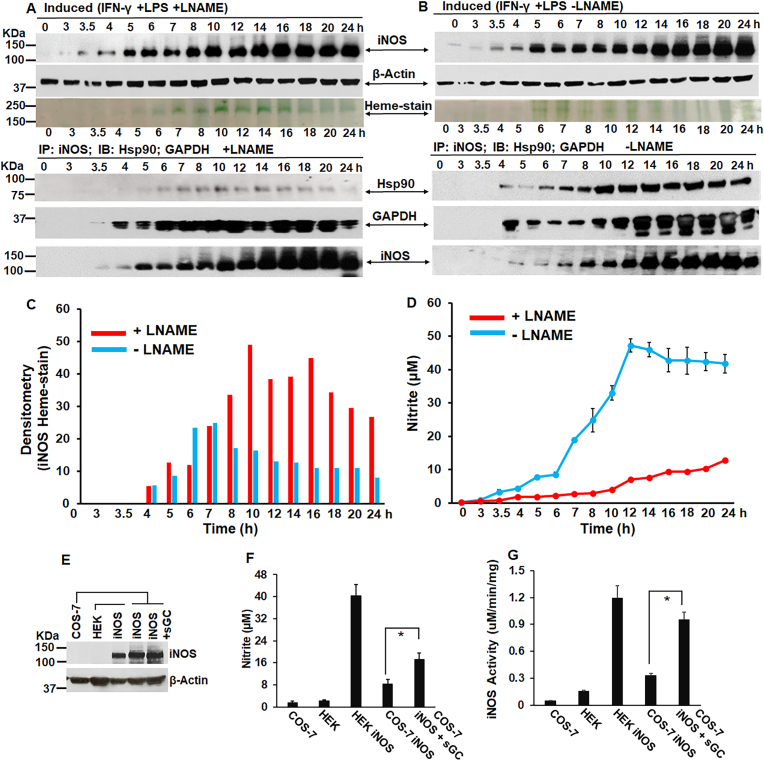
NOSs need low doses of NO for its own heme-maturation while higher NO doses are inhibitory to the process, and iNOS maturation additionally needs an active sGC: RAW 264.7 cells was induced for iNOS with interferon gamma (IFN-γ) and lipopolysaccharides (LPS) −/+ NOS inhibitor l-NAME and the expression of iNOS was followed over fifteen such time points between 0 and 24 h as depicted in Fig. 7. The incorporation of heme into the newly synthesized iNOS protein was followed by in-gel heme-stain, while IPs were performed at each time point to determine the strength of iNOS-hsp90 interactions which is a measure of heme-free iNOS or apo-iNOS (Fig. 7A–C) [50]. As shown in Fig. 7 there was gradual synthesis of iNOS protein and as it increased with time there was gradual heme-insertion into iNOS as depicted by the heme-stain. This heme-insertion was very distinct, and almost 2-fold greater in the l-NAME treated cells relative to the untreated ones (Fig. 7C), as in this case the NO synthesis was blocked, resulting in low NO levels as depicted in the nitrite values estimated by the ozone based chemiluminescence assay (Fig. 7D). However in the l-NAME untreated cells (Fig. 7B) there was less heme-insertion into the iNOS protein which declined post 10–12h of iNOS induction, as evident from the heme-stain and the decreasing nitrite values (Fig. 7C and D). Moreover IPs clearly showed that more hsp90 was bound to iNOS in the absence of l-NAME, suggesting that there was a gradual increase in the heme-free iNOS with the decline in iNOS heme (Fig. 7A and B). These results suggest that high levels of NO generated by iNOS inhibits its own heme-insertion and lowering the NO levels by using l-NAME is beneficial for iNOS heme-maturation as low NO levels are needed for iNOS heme-maturation. We also found by cGMP estimates (Fig. S4) that under time points of iNOS induction, the distribution of cGMP was largely greater under plus l-NAME relative to minus l-NAME conditions, when NO levels were low (+L-NAME). This promoted us to further determine the role of sGC in iNOS heme-maturation by estimating the nitrite values and doing in vitro iNOS reconstitution assay (Fig. 7E–G) on iNOS transfected HEK or COS-7 supernatants (−/+sGC). We found that the iNOS activity was increased nearly 3 fold in COS-7 cells expressing sGC (Fig. 7G). These findings suggest that both low NO and an active sGC are essential for iNOS heme-maturation. Next we analyzed HEK cultures stably expressing nNOS or eNOS or SH-SY5Y cells that express nNOS, that were treated with variable doses of NOC-18 (0-100μΜ) for 18h before cell harvest. As depicted in Fig. 8, we did protein expression by westerns, in-vitro NOS reconstitution assays and in-gel heme-stains at the indicated NO doses and determined that both nNOS and eNOS need low NO concentrations to heme-mature, while higher NO doses are inhibitory to the process (Fig. 8A–E).
Fig. 7.
Low doses of NO along with an active sGC promotes heme-maturation of iNOS, while iNOS heme-maturation is inhibitory at higher NO concentrations. (A, B) Expression profile and heme-stains of newly synthesized iNOS post induction of RAW cells with IFN-γ and LPS −/+ l-NAME between 0 and 24h. Lower three panels depict IPs under parallel conditions showing apo-iNOS-hsp90 or apo-iNOS-GAPDH interactions. (C) Densitometry of iNOS heme-stain as depicted in A & B. (D) Mean Nitrite (n = 3 repeats) from culture media estimated by ozone-based chemiluminescence method (using the Sievers NO analyzer) as a measure of generated NO during iNOS induction −/+ l-NAME. IPs and western blots are representative and Nitrite values are mean (n = 3). (E-G/) iNOS was transiently expressed in HEK or COS (−/+ sGC), with media collected after 42h for nitrite estimation by Griess assay and supernatants generated for protein expression by westerns and iNOS reconstitution assays. All values depicted are mean n = 3, ±SD. *p < 0.05, by one-way ANOVA.
Fig. 8.
Both eNOS and nNOS need low NO concentrations to heme-mature, while higher doses are inhibitory to the process. HEK cells stably expressing eNOS or nNOS, or SH-SY5Y cells which express nNOS endogenously were grown to confluency and then given variable doses of NO from NOC-18 for 18h before cell harvest. The generated cell supernatants were assayed for nNOS/eNOS expression by westerns, NOS heme by heme-staining and NOS activity assays by in-vitro reconstitution assays. (A–C) nNOS protein expression by westerns and nNOS heme depicted by heme-stainning under indicated NO doses. In-vitro NOS reconstitution was performed on HEK eNOS or SH-SY5Y to determine nNOS activity. (D, E) eNOS protein expression by westerns and eNOS heme depicted by heme-stainning under indicated NO doses. eNOS activity was determined by in-vitro NOS reconstitution assays. All values depicted are mean n = 3, ±SD. *p < 0.05, by one-way ANOVA.
NO also triggers heme-insertion into hemeproteins like MPO: In order to determine the universal nature of the low NO phenomena causing heme-insertion into apo-heme proteins, we tested this effect other hemeproteins, eg. MPO. RAW cells were induced from 0 to 24 h with IFN-γ and LPS −/+ l-NAME, similar to that depicted for Fig. 7. The generated cell supernatants were assayed for MPO activity assays and MPO expression was determined by westerns. As depicted in Fig. 9A–B, we found that MPO expression was largely constant from 0 to 10 h of IFN-γ and LPS induction but decreased from 12 to 24 h for both −/+ l-NAME conditions. Doing MPO activity assays and measuring the corresponding NO levels (Fig. 9C–D), we found that low concentration of NO (+L-NAME) promotes heme-maturation into MPO, as determined by its increased activity, while higher NO concentrations (-l-NAME) are inhibitory to MPO heme-insertion as seen by a drastic reduction in MPO activity. These drastic differences in MPO activities were however not due to the decreases in MPO expression as these occurred for both −/+ l-NAME conditions, but are a direct effect of the generated NO levels. Experiments involving transient expression of the MPO-β subunit in HEK cells (Fig. 9E and F) treated with variable NO doses (0–100 μM from NOC-18), also showed that MPO activity attained a maximum at low NO (5–10 μM), before falling at higher doses (>10 μM). Together these data suggests that heme-maturations are regulated similarly by NO for MPO, where low NO levels promote and high doses are inhibitory.
Fig. 9.
Low concentration of NO promotes heme-maturation into MPO, while higher NO concentrations are inhibitory. RAW cells were induced from 0 to 24 h with IFN-γ and LPS, −/+ l-NAME. The generated cell supernatants were assayed for MPO expression and activity. Further we also transiently expressed the Flag-MPO β subunit in HEK cells and then treated with variable doses of NOC-18 for 18 h before cell harvest. (A, B) MPO protein expression −/+ l-NAME under indicated conditions. (C) MPO activity assays −/+ l-NAME under indicated conditions. D) Mean nitrite values estimated by the ozone-based chemiluminescence method (using the Sievers NO analyzer) from aliquots of the collected media (n = 3), under parallel conditions. (E) MPO expression and (F) activity as determined from transiently transfected HEK cells. All values depicted are mean n = 3, ±SD. *p < 0.05, by one-way ANOVA.
Low NO concentrations are protective against lung injury reperfusion on a Ex Vivo Lung Perfusion (EVLP) system prior to lung transplant: Among potential alternatives, the ex vivo lung perfusion (EVLP, Fig. 10A) serves as a novel platform that allows for accurate assessment of lung function and also aims to improve lung function of marginal lungs. This system (Fig. 10A) can be used to deliver NO via trachea tube (i.e. iNO Airway) or NO can be delivered mixed with the blood-base perfusate (iNO Membrane), in the EVLP circuit. Okamoto and colleagues earlier determined that blood-base perfusate mixed with NO (at 80 ppm) was protective against lung reperfusion injury of porcine (pig) lungs on the EVLP, and some of these lungs even became suitable for transplant (Table S3). We obtained these pig lung tissues which were either perfused with blood-base perfusate mixed with NO (M) or lungs which were given NO via the airways (A), along with control untreated lung tissues (C) from Dr. Okamoto. (Table S3). We first determined the level of lung sGC heterodimer by doing IPs in the generated tissue supernatants (Fig. 10B) and found that the sGC heterodimer was mostly retained on all lung samples that underwent blood perfusion with NO (M) including those which were given low NO via airways (A). The heterodimer retention was highest for samples 2 and 5 and sample 5 was also the lung which was suitable for transplant. Among the controls, lung sample 1 was also found to be transplant suitable and this also exhibited a strong sGC-α1β1 heterodimer before perfusion. The cGMP estimated from all these samples were in accordance with the respective strength of the lung sGC heterodimers (Fig. 10B and C). Doing biotin-switch assay on these very samples we found more SNO-Hbβ on the NO treated samples (Fig. 10 D). Taken together our data imply that low NO levels maintains sGC-α1β1heterodimer levels in injured lungs during perfusion on the EVLP and that the circulating blood perfusate displays an elevated SNO on Hbβ and thus may improve tissue oxygenation in the lungs [4,62].
4. Discussion
Based on our obtained results from these studies we construct a model depicting the impact of NO on hemeprotein maturation (Fig. 11). Low NO levels which corresponds to endogenously generated NO is essential for hemeprotein maturation of at least seven different hemeproteins. While low NO triggers heme-insertion into the sGCβ1 subunit thereby causing heterodimerization of sGC-α1β1 subunits, low NO can also cause heme-insertion into Mb or the Hb-αβ subunits by activation of the NO-sGC-cGMP pathway, following which the Hb heterodimerizes into Hb-αβ subunits. Low NO likewise also induces heme-insertion/maturation within all three NOSs (iNOS, eNOS and nNOS) and MPO. On the contrary high NO levels cause inhibition of heme-insertion in the NOSs and MPO. For sGC high NO causes breaking of the sGC heterodimer, thereby stalling the NO-sGC signal pathway and this in turn obstructs globin (Hb and Mb) heme-maturations. We also found that high NO levels can even reduce protein expression of globins such as Mb (Fig. 2F), when expressed in cell systems which lack sGC (COS-7) [33]. Here NO mediated proteosomal degradation mechanisms [59,60] maybe prevalent and overexpression of the NO receptor sGC, abrogated these effects (Fig. 2G), which suggests that sGC may also protect globin expression from NO. The good effect of low NO in causing sGC activation via sGC heterodimer buildup, can be effectively harnessed to revive or repair injured lungs on the EVLP. We find that both by perfusing blood mixed with low NO (M) or infusing low NO via the airways (A) can be protective against lung injury reperfusion on an EVLP and some of these NO treated lungs are also judged as suitable for transplant (Table S3). In all these instances of low NO based perfusions (via blood or airways), the basic molecular mechanism seems to be the resistance offered by low NO against breakup of the injured lung sGC heterodimer (Fig. 10, Fig. 11). However not all the porcine lungs treated with NO were suitable for transplant and those maybe due to either dysfunction in the NO-sGC-cGMP pathway further downstream beyond cGMP or other factors outside the NO-sGC axis. Nevertheless, it was remarkable that in all the cases the only lungs suitable for transplant were control 1 and NO treated sample 5 (Fig. 10B), both of which had the highest sGC heterodimer either to begin with (control 1) or after NO perfusion (sample 5).
Fig. 11.
Model depicting the impact of NO on hemeprotein maturation and its therapeutic role. While high NO levels inhibit hemeprotein maturation, low NO levels enables the heme-maturation of NOSs, sGC, Hb, Mb, and MPO. Among these hemeproteins the components of the NO-sGC-Globin axis, namely NOSs, sGC, Hb and Mb have their heme-maturation driven by low NO levels, and an active sGC is also needed for Hb, Mb and iNOS heme-maturation. NO at ppm levels also manifests itself as a therapy and is found to be protective against ischemia reperfusion lung injury on a ex vivo lung perfusion (EVLP) system by resisting the breakage of the lung sGC heterodimer.
The significance of our current study lies in the underappreciated dual role of NO. While low NO levels in cells might play a role in its proliferation by participating in signaling processes [63,64], high levels of NO can retard growth by acting as a toxic molecule and causing apoptosis [63,64]. Moreover a fine balance may exist in the maturation and activation of NOSs, sGC and the globins, all of which seem to be regulated by low NO levels. While it was previously determined that high NO can limit assembly of dimeric iNOS by possibly preventing heme insertion and decreasing heme availability [65], our current study explicitly shows that high levels of NO generated by iNOS inhibits its own heme-insertion and lowering the NO levels by using l-NAME is beneficial for iNOS heme-maturation (Fig. 7). Further we found by treating stable lines of eNOS and nNOS with variable doses from a NO donor, that low NO doses promotes while high NO levels directly inhibits eNOS/nNOS heme-insertion (Fig. 8). While it is known that NOS enzymes can self inhibit themselves by forming reversible inactive ferrous-NO or ferric-NO complexes (which can reactivate by dissociation of NO from the NOS heme) [4] and studies have actually shown that 70–90% of iNOS and nNOS are present in their inactive ferrous-NO forms. However such steady states might be existing at low NO levels, and are different from the state of NOSs at high NO levels, where they become inactive on account of being heme-deprived (Fig. 7, Fig. 8).
Our results obtained from low NO driven heme-insertions clearly demonstrate that sGC and the globins (Hb, Mb) undergo subunit heterodimerization (sGC-α1β1, Hb-αβ and a less defined Mb dimer) following heme-insertion, while excess NO disrupts heme-maturations (Fig. 1, Fig. 2, Fig. 3), which suggests that optimum low NO levels are absolutely essential to sustain these processes. In order to test the efficacy of the low NO driven heme-insertions in whole animal models, we tested the sGC or globin (Hb, Mb) heme-maturations in the lung, heart and blood tissues of NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) KO mice that we received from Prof. Tsutsui's lab. The lung sGC from the NOS double (n/eNOS−/−) and triple (n/i/eNOS−/−) KOs was significantly responsive to heme-independent sGC activator BAY-60 relative to WT (Fig. 4), suggesting that sGC was heme-free [24], signifying obstructed or incomplete sGC heme-maturations to be prevalent on account of lowering or loss of endogenous NO. This finding is correlative to the triple (n/i/eNOS−/−) KO mice which was recently shown to develop spontaneous pulmonary emphysema in juvenile mice [61]. sGC has been earlier implicated in COPD where its expression seems to be downregulated and may also develop heme-free sGC [66]. Our earlier studies on mouse models of allergic asthma as well as those in HASMCs derived from human asthma show that lung sGC is dysfunctional on account of being heme-free [41]. Analyzing the Hb in the blood tissue, we found that Hb was low in heme (Fig. 6), in the NOS triple (n/i/eNOS−/−) KOs and double (n/eNOS−/−) KO mice. Moreover we also found that Mb from the mouse hearts, displayed low Mb heme levels with a higher binding to hsp90, and the heme levels were lowest for the NOS triple (n/i/eNOS−/−) KO, followed by the double (n/eNOS−/−) KOs relative to WT (Fig. 5). Together these data imply that heme-maturation cascade in these knockouts is obstructed for the sGC and the globins. These findings may correlate to the low mortality of the NOS triple (n/i/eNOS−/−) KO, followed by the NOS double (n/eNOS−/−) KOs [45]. Importantly the NOS triple (n/i/eNOS−/−) KOs exhibit cardiovascular abnormalities, including hypertension, myocardial infractions, cardiac hypertrophy, bone marrow abnormalities, arteriosclerosis, diastolic heart failure etc., all of which may relate to obstructed heme-maturation of sGC and the globins (Hb and Mb) [61,67]. Moreover conditions of iron deficiency or anemia can cause myocardial infractions or make sGC heme-free as has been demonstrated in a recent study [68], and such conditions may also exist in the NOS triple (n/i/eNOS−/−) KO mice.
Endothelial cells as well as macrophages, components of hematopoietic microenvironment are potent NO producers, playing an active role in the modulation of human hematopoietic cell growth and differentiation [69]. Low levels of NO released by endothelial cells are critical for the maintenance of basal vascular tone. The synthesis of endothelial NO is increased in response to biochemical stimuli, including thrombin, adenosine diphosphate (ADP), and bradykinin; as well as mechanical stimuli, including shear stress and cyclic strain [69]. Studies have previously shown that inhibition of NO synthesis in the vasculature may lead to hypertension [70] or ischemic stroke [71], which are in part, through its effects on the vascular tone as well as is effect on the thrombotic potential [6]. The effects of low NO on sGC activation/maturation (Fig. 1) or on heme-maturation of the globins (Mb Hb) and the three NOSs (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8) may seem synchronous or even similar with the effect of the bioavailable NO [4], and these realizations are now made clearer by our current study. While Ikuta et al. showed for the first time that cGMP signaling induces human γ globin gene expression [72], later studies by Ikuta and colleagues showed that when mice were treated with NO to activate the NO-sGC pathway or in sGC overexpressing transgenic rats, it led to higher red blood cell count and total Hb levels [29], and at the same time reduced the leukocyte count. These implicit results clearly translates in our current study, which demonstrates that low NO levels can increase the heme-insertion on the newly synthesized globin (Hbα/β, Fig. 3), and this subsequently increases the Hb-αβ heterodimerization. The finding that this NO effect on the Hb heme-insertion, works via the NO-sGC-cGMP pathway and the fact that Hbβ (adult) induction requires sGC (Fig. 3F), proves that erythropoiesis may require low NO levels in-vivo or can be stimulated in-vitro by induction with low NO levels. The probable therapeutic applications of these findings suggest that in hematologic disorders such as in anemia, potential activators of the NO-sGC-cGMP pathway may stimulate erythropoiesis to supplement hemoglobin levels. However earlier reports described an inverse correlation between hemoglobin and NO metabolite levels in anemic conditions [73,74], which implied that NO is a contributing factor in anemia, without taking into account the dual role of NO on hemeprotein maturation, based on its effective concentration or time points of NO exposure. The collective implication of these findings however suggests that activation of NO-cGMP signaling may establish a hematologic adaptive response to rectify anemic conditions.
MPO plays a critical role in host defense and in inflammatory tissue injury, it is abundant in neutrophils, monocytes and in subpopulation of macrophages [54]. Our studies found that MPO’S catalytic activity can be modulated by NO generated from iNOS in RAW cells, implying that NO has a bimodal effect on MPO heme (Fig. 9A–D). While low NO levels (obtained from 0 to 10 μΜ concentrations of added NOC-18) progressively increased the MPO activity, high NO doses (obtained from >10 μΜ of added NOC-18, Fig. 9E and F) drastically inhibited its activity, probably by inhibiting is heme-maturation as we find for NOS enzymes (Fig. 7, Fig. 8). MPO is a heme peroxidase that is mainly expressed in neutrophils and is abundant in disease pathologies [[75], [76], [77]]. Multiple lines of evidence suggest a close nexus between MPO and cardiovascular diseases including coronary artery disease, congestive heart failure, arterial hypertension etc. [77]. It is abundantly present in human atherosclerotic lesions, where oxidative damage within the artery wall is implicated in the pathogenesis of atherosclerosis [78,79]. Since MPO catalyzes the formation of reactive oxidant species which contributes significantly towards development of cardiovascular diseases, therapeutic intervention to curb MPO activity is an ardent need. From this prospective our finding a key NO regulation of MPO activity becomes all the more significant, for better therapeutic intervention in specific cardiovascular disease pathologies.
While the actual difference between low and high doses of NOC-18 used in this study are not too severe taking into account the NO release rates which transition from less than 25 nM/min to greater than 50 nM/min (Fig. S1), to cause such drastic effects on heme-maturations in all these proteins. This suggests that NO-driven heme-insertion is modulated by fine changes in molecular mechanisms where such NO doses can become the tipping point. It becomes more remarkable considering the fact that these NO concentrations in vivo, in the steady state can actually be very low where such events can be further dependent upon time points of NO exposure which can enable or disable heme-maturations. Here S-nitrosylation (SNO) of hemeproteins can be a valid mechanism which can correlate with certain heme-proteins becoming heme-free on prolonged exposure as we see with sGC in mouse models of allergic asthma [41]. This finding suggests that increased SNO on sGC can even cause breaking of the sGC heterodimer, implying that NO exposure can even break protein interactions. Moreover initial SNO on sGC has also been suggested to enable its heme-maturation [55]. However further work is needed to establish such mechanisms for other hemeproteins which can cause initiation or inhibition of heme-insertion on NO exposure. The effect of NO for all the hemeproteins we studied that caused heme-maturation at low doses and inhibition at higher doses seemed similar in terms of concentration ranges from a single NO donor (NOC-18), which may also imply that the basic mechanism of heme-insertion is similar in all these hemeproteins. Low NO driven heme-insertion in the proteins that we found thus far appears to be a primary effect which first causes buildup of the sGC heterodimer, with subsequent activation to produce cGMP. This may transcriptionally induce synthesis of the globins (Hb or Mb) and may even enable iNOS heme-maturation (Fig. S4). A similar mechanism may even occur for the constitutive NOSs (eNOS and nNOS), which remains to be determined. However at this stage we cannot rule out additional mechanisms where low NO levels can itself directly act on the apo-globins or apo-NOSs to directly cause heme downloading using the GAPDH-Hsp90 heme transfer cascade [30]. This way low NO can act at many levels to cause prolonged heme-maturation in select proteins.
Application of low NO as a therapy: In order to validate the usefulness of low NO for therapeutic purposes we choose the EVLP system which aims to improve lung function of rejected lungs prior to lung transplant. Working with transplant surgeon Dr. T. Okamoto at the Cleveland Clinic we received porcine lungs that underwent EVLP with low NO mixed blood perfusions (via membrane M, Fig. 10) or were given NO via airways (A), where some of these lungs were found suitable for transplant after undergoing such EVLP. To investigate whether low NO was causative at least in part to improve lung functions on the EVLP we investigated the molecular mechanisms based on the effects of low NO on the lung sGC heterodimer that we earlier determined (Fig. 1). We found that low NO levels maintain sGC-α1β1 heterodimer levels in the injured lungs (ischemic reperfusion injury) during perfusion on the EVLP and that the circulating blood perfusate exhibits an elevated SNO on Hbβ and this may also contribute to improve tissue oxygenation in the lungs (Fig. 10A–E) [62]. These primary data from porcine lungs suggest that such application of low NO may also succeed for improving human lung function on the EVLP prior to lung transplant and these molecular mechanisms of low NO driven sGC heterodimer buildup and high SNO on Hbβ should also hold true for human lungs. Moreover our findings should encourage the use of sGC stimulators like BAY 41, following low NO treatment to produce increased cGMP or drugs which synergize with low NO to cause an elevated sGC heterodimer. Since sGC maturation in fetal airway smooth muscle cells (Human ASMCs), is essential for lung alveolar development in neonates [80], similar applications of low NO treatment aimed to improve fetal sGC maturation in neonates or low NO treatment of asthma HASMCs, where the lung sGC is dysfunctional [49] should also be pursued in future studies for better therapeutic intervention. Our present finding can thus amplify the therapeutic use of low NO levels and a will help widen the scope of research in these directions.
Author contributions
A. Ghosh, M. P. Sumi and B. Tupta designed the experiments. A. Ghosh, M. P. Sumi B. Tupta and K. Aulak performed all cell culture and biochemical studies. A. Ghosh, M. P. Sumi, S. C. Erzurum and D. J. Stuehr analyzed all the data. A. Ghosh wrote the manuscript. T. Okamoto provided pig blood and pig lung tissues on which he performed EVLP. M. Tsutsui and H. Shimokawa gave mice tissues including lungs and hearts from NOS double (n/eNOS−/−) and NOS triple (n/i/eNOS−/−) knock outs.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by National Institute of Health Grants R56HL139564, R01HL150049 (A.G.) and GM130624 (D.J.S.). The animal cases in the EVLP were supported by Mallinckrodt (T.O).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102478.
Abbreviations
- BAY-41
BAY 41–2272
- BAY-60
BAY 60–2770
- EVLP
Ex Vivo Lung Perfusion
- HASMC
Human airway smooth muscle cell
- Hb
Hemoglobin
- Mb
Myoglobin
- IFN-γ
Interferon gamma
- LPS
Lipopolysaccharides
- TNF-α
Tumor necrosis factor-alpha
- KYN
Kynurenine
- OVA
Ovalbumin
- SNO
S-Nitrosation.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Gantner B.N., LaFond K.M., Bonini M.G. Nitric oxide in cellular adaptation and disease. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101550. Epub 2020/05/22. PubMed PMID: 32438317; PMCID: PMC7235643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen K., Pittman R.N., Popel A.S. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxidants Redox Signal. 2008;10(7):1185–1198. doi: 10.1089/ars.2007.1959. Epub 2008/03/12. PubMed PMID: 18331202; PMCID: PMC2932548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. Epub 1980/11/27. PubMed PMID: 6253831. [DOI] [PubMed] [Google Scholar]
- 4.Jin R.C., Loscalzo J. Vascular nitric oxide: formation and function. Hematol. Res. Rev. 2010;2010(1):147–162. doi: 10.2147/JBM.S7000. Epub 2011/05/17. PubMed PMID: 21572574; PMCID: PMC3092409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer R.M., Ferrige A.G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–526. doi: 10.1038/327524a0. Epub 1987/06/11. PubMed PMID: 3495737. [DOI] [PubMed] [Google Scholar]
- 6.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88(8):756–762. doi: 10.1161/hh0801.089861. Epub 2001/04/28. PubMed PMID: 11325866. [DOI] [PubMed] [Google Scholar]
- 7.Radomski M.W., Palmer R.M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br. J. Pharmacol. 1987;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. Epub 1987/09/01. PubMed PMID: 3311265; PMCID: PMC1853617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samama C.M., Diaby M., Fellahi J.L., Mdhafar A., Eyraud D., Arock M., Guillosson J.J., Coriat P., Rouby J.J. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology. 1995;83(1):56–65. doi: 10.1097/00000542-199507000-00007. Epub 1995/07/01. PubMed PMID: 7605019. [DOI] [PubMed] [Google Scholar]
- 9.Simon D.I., Stamler J.S., Jaraki O., Keaney J.F., Osborne J.A., Francis S.A., Singel D.J., Loscalzo J. Antiplatelet properties of protein S-nitrosothiols derived from nitric oxide and endothelium-derived relaxing factor. Arterioscler. Thromb. 1993;13(6):791–799. doi: 10.1161/01.atv.13.6.791. Epub 1993/06/01. PubMed PMID: 8388713. [DOI] [PubMed] [Google Scholar]
- 10.Ahluwalia A., Foster P., Scotland R.S., McLean P.G., Mathur A., Perretti M., Moncada S., Hobbs A.J. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of P-selectin expression and leukocyte recruitment. Proc. Natl. Acad. Sci. U. S. A. 2004;101(5):1386–1391. doi: 10.1073/pnas.0304264101. Epub 2004/01/27. PubMed PMID: 14742866; PMCID: PMC337062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sneddon J.M., Vane J.R. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 1988;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. Epub 1988/04/01. PubMed PMID: 3258664; PMCID: PMC280087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napoli C., Paolisso G., Casamassimi A., Al-Omran M., Barbieri M., Sommese L., Infante T., Ignarro L.J. Effects of nitric oxide on cell proliferation: novel insights. J. Am. Coll. Cardiol. 2013;62(2):89–95. doi: 10.1016/j.jacc.2013.03.070. Epub 2013/05/15. PubMed PMID: 23665095. [DOI] [PubMed] [Google Scholar]
- 13.Wink D.A., Hines H.B., Cheng R.Y., Switzer C.H., Flores-Santana W., Vitek M.P., Ridnour L.A., Colton C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011;89(6):873–891. doi: 10.1189/jlb.1010550. Epub 2011/01/15. PubMed PMID: 21233414; PMCID: PMC3100761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh V.K., Mehrotra S., Narayan P., Pandey C.M., Agarwal S.S. Modulation of autoimmune diseases by nitric oxide. Immunol. Res. 2000;22(1):1–19. doi: 10.1385/IR:22:1:1. Epub 2000/08/17. PubMed PMID: 10945224. [DOI] [PubMed] [Google Scholar]
- 15.Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006;355(19):2003–2011. doi: 10.1056/NEJMsa063904. Epub 2006/11/10. PubMed PMID: 17093251. [DOI] [PubMed] [Google Scholar]
- 16.Stuehr D.J. Mammalian nitric oxide synthases. Biochim. Biophys. Acta. 1999;1411(2–3):217–230. doi: 10.1016/s0005-2728(99)00016-x. Epub 1999/05/13. PubMed PMID: 10320659. [DOI] [PubMed] [Google Scholar]
- 17.Stuehr D.J., Haque M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmacol. 2019;176(2):177–188. doi: 10.1111/bph.14533. Epub 2018/11/08. PubMed PMID: 30402946; PMCID: PMC6295403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marletta M.A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78(6):927–930. doi: 10.1016/0092-8674(94)90268-2. Epub 1994/09/23. PubMed PMID: 7522970. [DOI] [PubMed] [Google Scholar]
- 19.Kapil V., Khambata R.S., Jones D.A., Rathod K., Primus C., Massimo G., Fukuto J.M., Ahluwalia A. The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol. Rev. 2020;72(3):692–766. doi: 10.1124/pr.120.019240. Epub 2020/06/25. PubMed PMID: 32576603. [DOI] [PubMed] [Google Scholar]
- 20.Bryan N.S., Bian K., Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front. Biosci. 2009;14:1–18. doi: 10.2741/3228. Epub 2009/03/11. PubMed. PMID: 19273051. [DOI] [PubMed] [Google Scholar]
- 21.Derbyshire E.R., Marletta M.A. Biochemistry of soluble guanylate cyclase. Handb. Exp. Pharmacol. 2009;191:17–31. doi: 10.1007/978-3-540-68964-5_2. Epub 2008/12/18. PubMed PMID: 19089323. [DOI] [PubMed] [Google Scholar]
- 22.Derbyshire E.R., Marletta M.A. Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. Epub 2012/03/13. PubMed PMID: 22404633. [DOI] [PubMed] [Google Scholar]
- 23.Stasch J.P., Pacher P., Evgenov O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123(20):2263–2273. doi: 10.1161/CIRCULATIONAHA.110.981738. Epub 2011/05/25. PubMed PMID: 21606405; PMCID: PMC3103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stasch J.P., Schmidt P., Alonso-Alija C., Apeler H., Dembowsky K., Haerter M., Heil M., Minuth T., Perzborn E., Pleiss U., Schramm M., Schroeder W., Schroder H., Stahl E., Steinke W., Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br. J. Pharmacol. 2002;136(5):773–783. doi: 10.1038/sj.bjp.0704778. PubMed PMID: 12086987; PMCID: PMC1573403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandner P., Berger P., Zenzmaier C. The potential of sGC modulators for the treatment of age-related fibrosis: a mini-review. Gerontology. 2017;63(3):216–227. doi: 10.1159/000450946. Epub 2016/10/27. PubMed PMID: 27784018. [DOI] [PubMed] [Google Scholar]
- 26.Sandner P., Zimmer D.P., Milne G.T., Follmann M., Hobbs A., Stasch J.P. Soluble guanylate cyclase stimulators and activators. Handb. Exp. Pharmacol. 2019 doi: 10.1007/164_2018_197. Epub 2019/01/29. PubMed PMID: 30689085. [DOI] [PubMed] [Google Scholar]
- 27.Tsai E.J., Kass D.A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 2009;122(3):216–238. doi: 10.1016/j.pharmthera.2009.02.009. PubMed PMID: 19306895; PMCID: PMC2709600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moras M., Lefevre S.D., Ostuni M.A. From erythroblasts to mature red blood cells: organelle clearance in mammals. Front. Physiol. 2017;8:1076. doi: 10.3389/fphys.2017.01076. Epub 2018/01/10. PubMed PMID: 29311991; PMCID: PMC5742207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikuta T., Sellak H., Odo N., Adekile A.D., Gaensler K.M. Nitric oxide-cGMP signaling stimulates erythropoiesis through multiple lineage-specific transcription factors: clinical implications and a novel target for erythropoiesis. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0144561. Epub 2016/01/05. PubMed PMID: 26727002; PMCID: PMC4699757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tupta B., Stuehr E., Sumi M.P., Sweeny E.A., Smith B., Stuehr D.J., Ghosh A. GAPDH is involved in the heme-maturation of myoglobin and hemoglobin. Faseb. J. 2022;36(2) doi: 10.1096/fj.202101237RR. Epub 2022/01/01. PubMed PMID: 34972240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cokic V.P., Smith R.D., Beleslin-Cokic B.B., Njoroge J.M., Miller J.L., Gladwin M.T., Schechter A.N. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J. Clin. Invest. 2003;111(2):231–239. doi: 10.1172/JCI16672. Epub 2003/01/18. PubMed PMID: 12531879; PMCID: PMC151872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh A., Garee G., Sweeny E.A., Nakamura Y., Stuehr D.J. Hsp90 chaperones hemoglobin maturation in erythroid and nonerythroid cells. Proc. Natl. Acad. Sci. U. S. A. 2018;115(6):E1117–E1126. doi: 10.1073/pnas.1717993115. Epub 2018/01/24. PubMed PMID: 29358373; PMCID: PMC5819442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh A., Dai Y., Biswas P., Stuehr D.J. Myoglobin maturation is driven by the hsp90 chaperone machinery and by soluble guanylyl cyclase. Faseb. J. 2019;33(9):9885–9896. doi: 10.1096/fj.201802793RR. Epub 2019/06/07. PubMed PMID: 31170354; PMCID: PMC6704453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiabrando D., Marro S., Mercurio S., Giorgi C., Petrillo S., Vinchi F., Fiorito V., Fagoonee S., Camporeale A., Turco E., Merlo G.R., Silengo L., Altruda F., Pinton P., Tolosano E. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J. Clin. Invest. 2012;122(12):4569–4579. doi: 10.1172/JCI62422. PubMed PMID: 23187127; PMCID: PMC3533534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas D.D., Miranda K.M., Colton C.A., Citrin D., Espey M.G., Wink D.A. Heme proteins and nitric oxide (NO): the neglected, eloquent chemistry in NO redox signaling and regulation. Antioxidants Redox Signal. 2003;5(3):307–317. doi: 10.1089/152308603322110887. Epub 2003/07/26. PubMed PMID: 12880485. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh A., Stuehr D.J. Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc. Natl. Acad. Sci. U. S. A. 2012;109(32):12998–13003. doi: 10.1073/pnas.1205854109. PubMed PMID: 22837396; PMCID: PMC3420196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donadee C., Raat N.J., Kanias T., Tejero J., Lee J.S., Kelley E.E., Zhao X., Liu C., Reynolds H., Azarov I., Frizzell S., Meyer E.M., Donnenberg A.D., Qu L., Triulzi D., Kim-Shapiro D.B., Gladwin M.T. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. Epub 2011/07/13. PubMed PMID: 21747051; PMCID: PMC3891836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owusu B.Y., Stapley R., Patel R.P. Nitric oxide formation versus scavenging: the red blood cell balancing act. J. Physiol. 2012;590(20):4993–5000. doi: 10.1113/jphysiol.2012.234906. Epub 2012/06/13. PubMed PMID: 22687616; PMCID: PMC3497558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Premont R.T., Reynolds J.D., Zhang R., Stamler J.S. Role of nitric oxide carried by hemoglobin in cardiovascular physiology: developments on a three-gas respiratory cycle. Circ. Res. 2020;126(1):129–158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019/10/09. PubMed PMID: 31590598; PMCID: PMC7034631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh A., Stasch J.P., Papapetropoulos A., Stuehr D.J. Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J. Biol. Chem. 2014;289(22):15259–15271. doi: 10.1074/jbc.M114.559393. PubMed PMID: 24733395; PMCID: PMC4140884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh A., Koziol-White C.J., Asosingh K., Cheng G., Ruple L., Groneberg D., Friebe A., Comhair S.A., Stasch J.P., Panettieri R.A., Jr., Aronica M.A., Erzurum S.C., Stuehr D.J. Soluble guanylate cyclase as an alternative target for bronchodilator therapy in asthma. Proc. Natl. Acad. Sci. U. S. A. 2016;113(17):E2355–E2362. doi: 10.1073/pnas.1524398113. PubMed PMID: 27071111; PMCID: PMC4855555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai Y., Faul E.M., Ghosh A., Stuehr D.J. NO rapidly mobilizes cellular heme to trigger assembly of its own receptor. Proc. Natl. Acad. Sci. U. S. A. 2022;119(4) doi: 10.1073/pnas.2115774119. Epub 2022/01/21. PubMed PMID: 35046034; PMCID: PMC8795550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waheed S.M., Ghosh A., Chakravarti R., Biswas A., Haque M.M., Panda K., Stuehr D.J. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic. Biol. Med. 2010;48(11):1548–1558. doi: 10.1016/j.freeradbiomed.2010.02.038. PubMed PMID: 20211245; PMCID: PMC2866197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abu-Soud H.M., Hazen S.L. Nitric oxide modulates the catalytic activity of myeloperoxidase. J. Biol. Chem. 2000;275(8):5425–5430. doi: 10.1074/jbc.275.8.5425. Epub 2000/02/22. PubMed PMID: 10681518. [DOI] [PubMed] [Google Scholar]
- 45.Tsutsui M., Tanimoto A., Tamura M., Mukae H., Yanagihara N., Shimokawa H., Otsuji Y. Significance of nitric oxide synthases: lessons from triple nitric oxide synthases null mice. J. Pharmacol. Sci. 2015;127(1):42–52. doi: 10.1016/j.jphs.2014.10.002. Epub 2015/02/24. PubMed PMID: 25704017. [DOI] [PubMed] [Google Scholar]
- 46.Niikawa H., Okamoto T., Ayyat K.S., Itoda Y., Farver C.F., McCurry K.R. The protective effect of prone lung position on ischemia-reperfusion injury and lung function in an ex vivo porcine lung model. J. Thorac. Cardiovasc. Surg. 2019;157(1):425–433. doi: 10.1016/j.jtcvs.2018.08.101. Epub 2018/11/13. PubMed PMID: 30415898. [DOI] [PubMed] [Google Scholar]
- 47.Niikawa H., Okamoto T., Ayyat K.S., Itoda Y., Sakanoue I., Farver C.F., Yun J.J., McCurry K.R. Cellular ex vivo lung perfusion beyond 1 hour may improve marginal donor lung assessment. J. Surg. Res. 2020;250:88–96. doi: 10.1016/j.jss.2019.09.073. Epub 2020/02/07. PubMed PMID: 32028151. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto T., Wheeler D., Farver C.F., McCurry K.R. Transplant suitability of rejected human donor lungs with prolonged cold ischemia time in low-flow acellular and high-flow cellular ex vivo lung perfusion systems. Transplantation. 2019;103(9):1799–1808. doi: 10.1097/TP.0000000000002667. Epub 2019/02/26. PubMed PMID: 30801532. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh A., Koziol-White C.J., Jester W.F., Jr., Erzurum S.C., Asosingh K., Panettieri R.A., Jr., Stuehr D.J. An inherent dysfunction in soluble guanylyl cyclase is present in the airway of severe asthmatics and is associated with aberrant redox enzyme expression and compromised NO-cGMP signaling. Redox Biol. 2021;39 doi: 10.1016/j.redox.2020.101832. Epub 2020/12/29. PubMed PMID: 33360351; PMCID: PMC7772568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh A., Chawla-Sarkar M., Stuehr D.J. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. Faseb. J. 2011;25(6):2049–2060. doi: 10.1096/fj.10-180554. PubMed PMID: 21357526; PMCID: PMC3101027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hausladen A., Rafikov R., Angelo M., Singel D.J., Nudler E., Stamler J.S. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc. Natl. Acad. Sci. U. S. A. 2007;104(7):2157–2162. doi: 10.1073/pnas.0611191104. Epub 2007/02/09. PubMed PMID: 17287342; PMCID: PMC1892991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinder A.G., Rogers S.C., Khalatbari A., Ingram T.E., James P.E. The measurement of nitric oxide and its metabolites in biological samples by ozone-based chemiluminescence. Methods Mol. Biol. 2008;476:11–28. doi: 10.1007/978-1-59745-129-1_2. Epub 2009/01/23. PubMed PMID: 19157006. [DOI] [PubMed] [Google Scholar]
- 53.Baek K.J., Thiel B.A., Lucas S., Stuehr D.J. Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J. Biol. Chem. 1993;268(28):21120–21129. Epub 1993/10/05. PubMed PMID: 7691806. [PubMed] [Google Scholar]
- 54.Strzepa A., Pritchard K.A., Dittel B.N. Myeloperoxidase: a new player in autoimmunity. Cell. Immunol. 2017;317:1–8. doi: 10.1016/j.cellimm.2017.05.002. Epub 2017/05/18. PubMed PMID: 28511921; PMCID: PMC5665680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernhoff N.B., Derbyshire E.R., Underbakke E.S., Marletta M.A. Heme-assisted S-nitrosation desensitizes ferric soluble guanylate cyclase to nitric oxide. J. Biol. Chem. 2012;287(51):43053–43062. doi: 10.1074/jbc.M112.393892. Epub 2012/10/25. PubMed PMID: 23093402; PMCID: PMC3522300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaffrey S.R., Snyder S.H. The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE. 2001;2001(86):pl1. doi: 10.1126/stke.2001.86.pl1. PubMed PMID: 11752655. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto T., Wheeler D., Liu Q., Quintini C., Hata J.S., McCurry K.R. Variability in pressure of arterial oxygen to fractional inspired oxygen concentration ratio during cellular ex vivo lung perfusion: implication for decision making. Transplantation. 2015;99(12):2504–2513. doi: 10.1097/TP.0000000000000776. Epub 2015/12/03. PubMed PMID: 26627676. [DOI] [PubMed] [Google Scholar]
- 58.Nagao S., Osuka H., Yamada T., Uni T., Shomura Y., Imai K., Higuchi Y., Hirota S. Structural and oxygen binding properties of dimeric horse myoglobin. Dalton Trans. 2012;41(37):11378–11385. doi: 10.1039/c2dt30893b. Epub 2012/08/14. PubMed PMID: 22885714. [DOI] [PubMed] [Google Scholar]
- 59.Kim S., Ponka P. Effects of interferon-gamma and lipopolysaccharide on macrophage iron metabolism are mediated by nitric oxide-induced degradation of iron regulatory protein 2. J. Biol. Chem. 2000;275(9):6220–6226. doi: 10.1074/jbc.275.9.6220. Epub 2000/02/29. PubMed PMID: 10692416. [DOI] [PubMed] [Google Scholar]
- 60.Lee C.M., Wilderman P.R., Park J.W., Murphy T.J., Morgan E.T. Tyrosine nitration contributes to nitric oxide-stimulated degradation of CYP2B6. Mol. Pharmacol. 2020;98(3):267–279. doi: 10.1124/molpharm.120.000020. Epub 2020/08/21. PubMed PMID: 32817462; PMCID: PMC7469253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato K., Tsutsui M., Noguchi S., Iha Y., Naito K., Ogoshi T., Nishida C., Tahara M., Yamashita H., Wang K.Y., Toyohira Y., Yanagihara N., Masuzaki H., Shimokawa H., Tanimoto A., Yatera K. Spontaneous pulmonary emphysema in mice lacking all three nitric oxide synthase isoforms. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-01453-6. Epub 2021/11/13. PubMed PMID: 34764368; PMCID: PMC8586362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Premont R.T., Stamler J.S. Essential role of hemoglobin betaCys93 in cardiovascular physiology. Physiology. 2020;35(4):234–243. doi: 10.1152/physiol.00040.2019. Epub 2020/06/04. PubMed PMID: 32490751; PMCID: PMC7474257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukumura D., Kashiwagi S., Jain R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 2006;6(7):521–534. doi: 10.1038/nrc1910. Epub 2006/06/24. PubMed PMID: 16794635. [DOI] [PubMed] [Google Scholar]
- 64.Hickok J.R., Thomas D.D. Nitric oxide and cancer therapy: the emperor has NO clothes. Curr. Pharmaceut. Des. 2010;16(4):381–391. doi: 10.2174/138161210790232149. Epub 2010/03/20. PubMed PMID: 20236067; PMCID: PMC3782103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albakri Q.A., Stuehr D.J. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J. Biol. Chem. 1996;271(10):5414–5421. doi: 10.1074/jbc.271.10.5414. Epub 1996/03/08. PubMed PMID: 8621396. [DOI] [PubMed] [Google Scholar]
- 66.Glynos C., Dupont L.L., Vassilakopoulos T., Papapetropoulos A., Brouckaert P., Giannis A., Joos G.F., Bracke K.R., Brusselle G.G. The role of soluble guanylyl cyclase in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;188(7):789–799. doi: 10.1164/rccm.201210-1884OC. Epub 2013/07/12. PubMed PMID: 23841447. [DOI] [PubMed] [Google Scholar]
- 67.Nakata S., Tsutsui M., Shimokawa H., Suda O., Morishita T., Shibata K., Yatera Y., Sabanai K., Tanimoto A., Nagasaki M., Tasaki H., Sasaguri Y., Nakashima Y., Otsuji Y., Yanagihara N. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation. 2008;117(17):2211–2223. doi: 10.1161/CIRCULATIONAHA.107.742692. Epub 2008/04/17. PubMed PMID: 18413498. [DOI] [PubMed] [Google Scholar]
- 68.Inserte J., Barrabes J.A., Aluja D., Otaegui I., Baneras J., Castellote L., Sanchez A., Rodriguez-Palomares J.F., Pineda V., Miro-Casas E., Mila L., Lidon R.M., Sambola A., Valente F., Rafecas A., Ruiz-Meana M., Rodriguez-Sinovas A., Benito B., Buera I., Delgado-Tomas S., Beneitez D., Ferreira-Gonzalez I. Implications of iron deficiency in STEMI patients and in a murine model of myocardial infarction. JACC Basic Transl Sci. 2021;6(7):567–580. doi: 10.1016/j.jacbts.2021.05.004. Epub 2021/08/10. PubMed PMID: 34368505; PMCID: PMC8326269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cokic V.P., Schechter A.N. Effects of nitric oxide on red blood cell development and phenotype. Curr. Top. Dev. Biol. 2008;82:169–215. doi: 10.1016/S0070-2153(07)00007-5. Epub 2008/02/20. PubMed PMID: 18282521. [DOI] [PubMed] [Google Scholar]
- 70.Stamler J.S., Loh E., Roddy M.A., Currie K.E., Creager M.A. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89(5):2035–2040. doi: 10.1161/01.cir.89.5.2035. Epub 1994/05/01. PubMed PMID: 7514109. [DOI] [PubMed] [Google Scholar]
- 71.Howard T.D., Giles W.H., Xu J., Wozniak M.A., Malarcher A.M., Lange L.A., Macko R.F., Basehore M.J., Meyers D.A., Cole J.W., Kittner S.J. Promoter polymorphisms in the nitric oxide synthase 3 gene are associated with ischemic stroke susceptibility in young black women. Stroke. 2005;36(9):1848–1851. doi: 10.1161/01.STR.0000177978.97428.53. Epub 2005/08/16. PubMed PMID: 16100023; PMCID: PMC1494105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ikuta T., Ausenda S., Cappellini M.D. Mechanism for fetal globin gene expression: role of the soluble guanylate cyclase-cGMP-dependent protein kinase pathway. Proc. Natl. Acad. Sci. U. S. A. 2001;98(4):1847–1852. doi: 10.1073/pnas.98.4.1847. Epub 2001/02/15. PubMed PMID: 11172039; PMCID: PMC29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anstey N.M., Granger D.L., Hassanali M.Y., Mwaikambo E.D., Duffy P.E., Weinberg J.B. Nitric oxide, malaria, and anemia: inverse relationship between nitric oxide production and hemoglobin concentration in asymptomatic, malaria-exposed children. Am. J. Trop. Med. Hyg. 1999;61(2):249–252. doi: 10.4269/ajtmh.1999.61.249. Epub 1999/08/27. PubMed PMID: 10463675. [DOI] [PubMed] [Google Scholar]
- 74.Choi J.W., Pai S.H., Kim S.K., Ito M., Park C.S., Cha Y.N. Iron deficiency anemia increases nitric oxide production in healthy adolescents. Ann. Hematol. 2002;81(1):1–6. doi: 10.1007/s00277-001-0409-4. Epub 2002/01/25. PubMed PMID: 11807627. [DOI] [PubMed] [Google Scholar]
- 75.Ekmekci O.B., Donma O., Sardogan E., Yildirim N., Uysal O., Demirel H., Demir T. Iron, nitric oxide, and myeloperoxidase in asthmatic patients. Biochemistry (Mosc.) 2004;69(4):462–467. doi: 10.1023/b:biry.0000026205.89894.25. Epub 2004/06/02. PubMed PMID: 15170385. [DOI] [PubMed] [Google Scholar]
- 76.Monteseirin J., Bonilla I., Camacho J., Conde J., Sobrino F. Elevated secretion of myeloperoxidase by neutrophils from asthmatic patients: the effect of immunotherapy. J. Allergy Clin. Immunol. 2001;107(4):623–626. doi: 10.1067/mai.2001.113566. Epub 2001/04/11. PubMed PMID: 11295649. [DOI] [PubMed] [Google Scholar]
- 77.Ndrepepa G. Myeloperoxidase - a bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta. 2019;493:36–51. doi: 10.1016/j.cca.2019.02.022. Epub 2019/02/25. PubMed PMID: 30797769. [DOI] [PubMed] [Google Scholar]
- 78.Nicholls S.J., Hazen S.L. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005;25(6):1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. Epub 2005/03/26. PubMed PMID: 15790935. [DOI] [PubMed] [Google Scholar]
- 79.Podrez E.A., Abu-Soud H.M., Hazen S.L. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic. Biol. Med. 2000;28(12):1717–1725. doi: 10.1016/s0891-5849(00)00229-x. Epub 2000/08/18. PubMed PMID: 10946213. [DOI] [PubMed] [Google Scholar]
- 80.Britt R.D., Jr., Thompson M.A., Kuipers I., Stewart A., Vogel E.R., Thu J., Martin R.J., Pabelick C.M., Prakash Y.S. Soluble guanylate cyclase modulators blunt hyperoxia effects on calcium responses of developing human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309(6):L537–L542. doi: 10.1152/ajplung.00232.2015. Epub 2015/08/09. PubMed PMID: 26254425; PMCID: PMC4572415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.