Abstract
Background
The abnormal expression of long non-coding RNA (lncRNA) Opa-interacting protein 5 antisense RNA 1 (OIP5-AS1) has been observed in many human cancers and the underlying mechanisms have been well studied. However, the function of OIP5-AS1 in acute kidney injury (AKI) remains unclear.
Methods
To explore the role of OIP5-AS1 in the progression of AKI, the cisplatin-induced AKI mouse and cell model were established. To confirm the potential protective effect of OIP5-AS1 during cisplatin-induced AKI, rescue experiments were performed. Targetscan was used to predict the potential targets of miR-144-5p. To further determine whether the effect of miR-144-5p during cisplatin-induced AKI was mediated by PMK2, the recuse experiments using PMK2 overexpressing vector was applied.
Results
OIP5-AS1 was significantly downregulated both in cisplatin-induced AKI mice and human renal tubular cell line HK-2 cells. Moreover, overexpression of OIP5-AS1 efficiently promoted cell growth and reduced cisplatin-induced apoptosis of HK-2 cells. Furthermore, OIP5-AS1 was identified as a sponge of miR-144-5p, and upregulation of miR-144-5p could significantly reverse overexpression of OIP5-AS1-induced protective effect on the damage of cisplatin to HK-2 cells. In addition, pyruvate kinase M2 (PKM2) was found to be a direct target of miR-144-5p, and overexpression of PKM2 efficiently reversed the effect of miR-144-5p mimics on the damage in cisplatin-stimulated HK-2 cells.
Conclusions
OIP5-AS1 reduced the apoptosis of cisplatin-stimulated renal epithelial cells by targeting the miR-144-5p/PKM2 axis, which extended the regulatory network of lncRNAs in cisplatin-induced AKI and also provided a novel therapeutic target for AKI treatment.
Keywords: OIP5-AS1, miR-144-5p, PKM2, AKI, Cell viability, Apoptosis
At a glance commentary
Scientific background on the subject
Various lncRNAs have been reported to be involved in the development and progression of acute kidney injury (AKI). However, the biological role and underlying mechanisms of lncRNA OIP5-AS1 in AKI remain unknown.
What this study adds to the field
This study demonstrated that OIP5-AS1 reduced the apoptosis of cisplatin-induced renal epithelial cells by targeting the miR-144-5p/PKM2 axis. These findings imporved our understanding of the pathogenesis of cisplatin-induced AKI and provided a potential therapeutic target for AKI treatment.
Acute kidney injury (AKI) is not a single disease but a loose collection of multiple syndromes such as sepsis, cardiorenal syndrome, and urinary tract obstruction [1]. AKI occurs in approximately 20% of hospitalized patients and more common in critically ill patients [2]. AKI causes about 2 million deaths every year, and increasing evidence indicated that the incidence of AKI in intensive care is more than 50% of patients [3]. The pathophysiological process of AKI includes acute tubular epithelial cell damage, excessive inflammation and acute vascular dysfunction [4]. During AKI, the loss of kidney function can be evaluated via monitoring the changes of renal function-related markers, such as serum creatinine (SCr) and blood urea nitrogen (BUN) levels [5,6]. Although clinical treatments are available, the development of more efficient therapies for AKI is still urgent. Better understanding of the underlying molecular mechanisms in the progression of AKI would contribute to the identification of potential therapeutic targets.
Long chain non-coding RNAs (lncRNAs) are a class of non-coding RNAs with approximately 200 nucleotides in length and lack protein coding function, but can regulate a series of biological processes in human eukaryotic cells [7]. LncRNA OIP5-AS1 (Opa-interacting protein 5 antisense RNA 1), has been found to be abnormally expressed and closely associated with a variety of progressions in various human cancers. For instance, OIP5-AS1 promotes the resistance of doxorubicin through targeting the miR-137-3p/PTN axis in osteosarcoma [8]. Overexpression of OIP5-AS1 can inhibit cell viability, colony formation, invasion and migration, while induce cell cycle arrest at G1 phase and apoptosis of multiple myeloma cells in vitro, and can also suppress tumorigenesis in vivo [9]. OIP5-AS1 promotes cell proliferation, migration and induce angiogenesis through regulating the miR-3163/VEGFA axis in hepatocellular carcinoma [10]. Overexpression of OIP5-AS1 can aggravate the progression through targeting the miR-153-3p/ZBTB2 axis in gastric cancer cells [11]. In pancreatic cancer, OIP5-AS1 promotes cell growth by sponging miR-342-3p via the AKT/ERK pathway [12]. In addition, the expression of OIP5-AS1 is also closely related to the prognosis of cancer patients, in which upregulation of OIP5-AS1 predicts a poor prognosis of patients with thyroid cancer due to its regulation of cell proliferation and migration [13]. These evidences confirmed the important functions of OIP5-AS1 in human cancers. Interestingly, a recent study reported that OIP5-AS1 could induce epithelial-to-mesenchymal transition (EMT) and renal fibrosis by binding to miR-30c-5p in diabetic nephropathy [14]. Meanwhile, EMT played essential roles in the pathogenesis of renal fibrosis [15], and the mechanism of chronic kidney disease (CKD) post-AKI is characterized by progressive renal fibrosis [16]. Hence, we thought that OIP5-AS1 might play a potential role in AKI, and paid our attentions on it.
Materials and methods
The mouse model of cisplatin-induced AKI
A total of 20 BALB/c mice (SPF, male, 1–8 weeks old and approximately 20 g) were purchased from the Institute of Medical Laboratory Animals, Chinese Academy of Medical Sciences. Mice were kept under the following conditions: normal grade, free access to food and drink. The cisplatin-induced AKI mice model was established as previously described [17]. Briefly, mice were intraperitoneally injected with 20 mg/kg cisplatin and kept for different time periods, while mice in the vehicle group were injected with equal amount of saline simultaneously. All experiment procedures were performed following the Animal Experimental Guide of the First Affiliated Hospital of Zhengzhou University and approved by the First Affiliated Hospital of Zhengzhou University.
The measurement of renal function and tissue morphology
The renal function of mice was evaluated by the serum creatinine and BUN level of mice using the respective detection assay kit (built, Nanjing, China). For the observation of renal tissue morphology, the renal tissues were removed from mice and fixed with 10% formaldehyde aqueous solution, then paraffin-embedded, sliced and strained by hematoxylin-eosin (HE). Subsequently, the pathological changes were observed using a light microscope. In addition, the score of renal tissue damage was evaluated according to the percentage of damaged tubules as previously reported [18]. 0 indicates no obvious damage.1 indicates the damage was less than 25%. 2 indicates the damage was 25–50%. 3 indicates the damage was 50–75%. 4 indicates the damage was more than 75%.
Cell culture and treatment
Human renal tubular cell line HK-2 was obtained from the American Type Culture Collection (ATCC, USA). Cells were cultured in DMEM medium containing 10% fetal bovine serum at 37 °C with 5% CO2. To construct the AKI cell model, different concentration of cisplatin (10−9, 10−8, 10−7 and 10−6 M) was added into cultured HK-2 cells for 24 h as previously reported [19].
Cell transfection
Cell transfection was performed using the RNAi Max and Lipofectamine 3000 with Plus Reagent. The cDNA sequence of OIP5-AS1 was amplified and cloned into the expression vector pcDNA3.1 (ABM, Canada) to construct the OIP5-AS1 overexpressing plasmid. MiR-144-5p mimics and miR-NC were obtained from RiboBio (Guangzhou, China). For overexpression of PMK2, the cDNA sequence of PMK2 was also amplified and cloned into pcDNA3.1 vector. The empty pcDNA3.1 vector was used as the negative control.
qPCRs
Total RNAs were extracted from cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA). PrimeScript Reverse Transcriptase (TaKaRa) was used for reverse transcription. Then qRT-PCR assay was performed with the platform LightCycler 480 (Roche, Basel, Switzerland). The relative expression of targets was calculated using the 2−ΔΔCT method with GAPDH and U6 as the internal reference. The primer sequences were as follows: miR-144-5p forward: 5′-TGCCGTTTGGCCATTGTAAAAC-3′, reverse: 5′-CAAGTCCAGGGTCCGAGGT-3′; OIP5-AS1 forward: 5′ CAAGAATCCCAGGGCTGATA 3′, reverse: 5′ GATGTTGGGAAGCATCTGGT 3′'; GAPDH forward: 5′-CCACCCAGAAGACTGTGGAT-3′, reverse: 5′-TTCAGCTCAGGGATGACCTT-3′; U6 forward: 5′-CTCGCTTCGGCAGCACA-3′, reverse: 5′-AACGCTTCACGAAATTGCGT-3′.
Western blot
Total proteins were isolated from cultured cells using RIPA lysis buffer (Beyotime, Nantong, China). Protein concentrations were determined using the BCA Protein Assay Kit. Then approximately equal amount of protein samples were separated by 12% SDS-PAGE and transferred onto PVDF membranes. After blocking in 3% skim milk, the membranes were incubated with primary antibodies at 1:500 against PMK2, C-caspase 3, C-caspase 9, Bax and Bcl-2 at 4 °C overnight. After blocking, the membranes were submitted to horseradish peroxidase-conjugated secondary antibody for 1 h. β-actin (1:2000) was used as the internal reference. All antibodies were obtained from Abcam, Cambridge, MA. Signals were detected using the EasyBlot ECL Kit (Sangon Biotech, Shanghai, China), and the grey-scale value of target proteins was evaluated using the ChemiDoc XRS System.
Luciferase reporter assay
StarBase (http://starbase.sysu.edu.cn/, v2.0) [20] and Targetscan database (http://www.targetscan.org/vert_72/, v7.2) [21] were used to predict the potential targets of OIP5-AS1 and miR-144-5p, respectively. The fragment of OIP5-AS1(GACATTC) and PMK2 (CTATAGTA) wild type (WT) for miR-144-5p binding site were cloned into the firefly luciferase gene in the pMIR Basic vector (OBiO Biology, Shanghai).
In addition, the OIP5-AS1 (GATCGTC) and PMK2 mutant plasmids (CTACTATA) were designed in the putative miR-144-5p binding sites. Then the luciferase reporter plasmids were co-transfected with miR-144-5p mimics or miR-NC into HK-2 cells using the Lipofectamine 3000 kit. After transfection for 48 h, cells were lysed, and the relative luciferase activity was detected using a dual luciferase assay system.
RNA pull-down assay
RNA pull down assay was performed as previously described [22]. The probe of WT and MUT miR-144-5p was biotinylated (Sangon, Shanghai, China) and transfected into HK-2 cells (WT miR-144-5p: 5′-GGAUAUCAUCAUAUACUGUAAG-3′; MUT miR-144-5p: 5′-CCTUAUCAUCAUAUAGACATTC-3′). After 48 h, cells were collected and lysed, then incubated with Dynabeads M−280 streptavidin (Invitrogen, Carlsbad, CA, USA) for 10 min. Finally, the enriched RNA was analyzed by qRT-PCR.
Apoptosis analysis
Cell apoptosis was evaluated by flow cytometry after treatment using the Annexin V: FITC Apoptosis Detection Kit II (BD Biosciences, San Jose, CA) following the manufacturer's instructions. Briefly, after transfection for 48 h, cells were centrifuged, and re-suspended with binding buffer. Then 5 μL of Annexin V-FITC and 10 μL of propidium iodide (PI) were added. Subsequently, apoptosis rate was evaluated by Flow cytometry (BD Biosciences).
CCK-8 assay
To measure cell viability, approximately 5 × 103 HK-2 cells were seeded into 96-well plates. Then 100 μL of CCK8 solution (Dojindo Molecular Technologies, Japan) was added to each well at 24, 48, 72, and 96 h. Finally, the optical density (OD) at 450 nm was detected using a microplate reader (Bio Tek Instruments, USA).
Statistical analysis
All experiments were repeated at least 3 times. Data were presented as the mean ± standard deviation (SD). Difference between two groups was determined by t test, and difference among multiple groups was determined by one-way ANOVA using the SPSS 19.0 software. p < 0.05 was considered as the significant threshold.
Results
OIP5-AS1 was significantly downregulated in cisplatin-induced AKI model both in vitro and in vivo
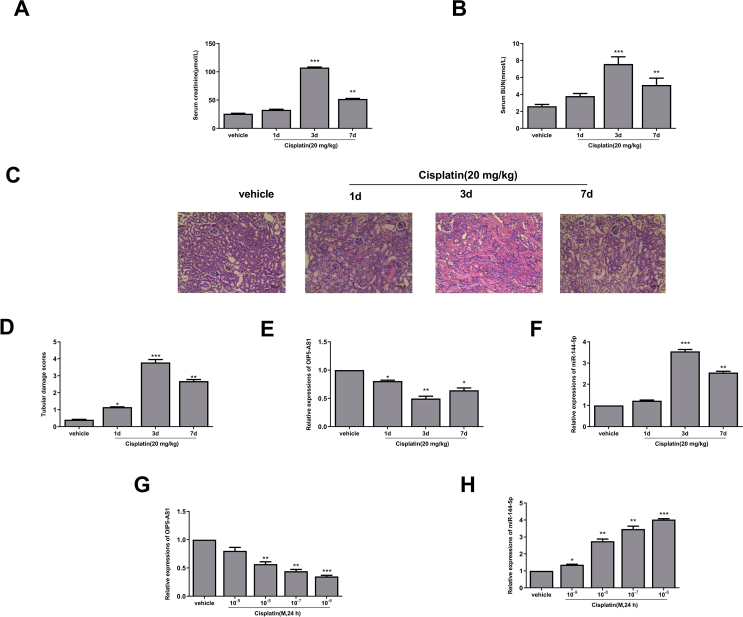
To explore the role of OIP5-AS1 in the progression of AKI, the cisplatin-induced AKI mice model was firstly established. The results indicated that the expression levels of renal function-related makers such as serum creatinine (p < 0.001, p < 0.01) [Fig. 1A] and serum BUN (p < 0.001, p < 0.01) [Fig. 1B] were significantly increased in cisplatin-induced AKI mice compared with that in vehicle group, and their expression levels were highest on the third day. Meanwhile, HE staining results in the renal tissues showed that the morphology of renal tissues in the vehicle group was almost normal, while tubular epithelial cells were edematous and necrotic, and exhibited obvious inflammatory cell infiltration in the cisplatin-induced AKI mice [Fig. 1C]. The injury scores of renal tissues were also evaluated, and the results showed that renal tissue injury scores were significantly increased after treatment with cisplatin at day 1 (p < 0.05), day 3 (p < 0.01) and day 7 (p < 0.001) compared with the vehicle group [Fig. 1D]. These data suggested that the cisplatin-induced AKI mice model was successfully established and could be used for the subsequent experiments. Moreover, the expression of OIP5-AS1 in the renal tissues of cisplatin-induced mice was significantly downregulated at day 1 (p < 0.05), day 3 (p < 0.01) and day 7 (p < 0.05) compared with that in the vehicle group [Fig. 1E]. Interestingly, the expression of miR-144-5p was oppositely upregulated at day 3 (p < 0.001) and day 7 (p < 0.01) compared with that in the vehicle group [Fig. 1F]. To confirm this, HK-2 cells were treated with different concentrations of cisplatin for 24 h, and a dose-dependent decrease of the expression levels of OIP5-AS1 (p < 0.01, p < 0.001) [Fig. 1G] and a dose-dependent increase of the expression levels of miR-144-5p (p < 0.05, p < 0.01, p < 0.001) [Fig. 1H] were observed in the cisplatin treatment group compared with that in the vehicle group. These results suggested that OIP5-AS1 and miR-144-5p play important roles during the progression of cisplatin-induced AKI.
Fig. 1.
Altered expression of OIP5-AS1 and miR-144-5p in cisplatin-induced AKI model. The mice were induced with or without cisplatin for 1 d, 3 d and 7 d. (A–C) The serum level of creatinine (A) and BUN (B). (C) The morphology of renal tissues was evaluated by HE staining assay. Scale bar = 50 μm. (D) The tubular injury score. (E and F) The mRNA level of OIP5-AS1 (E) and miR-144-5p (F) were evaluated by qRT-PCR. (G and H) The HK-2 cells were treated with or without different concentrations of cisplatin for 24 h. The mRNA expression level of OIP5-AS1 (G) and miR-144-5p (H) was evaluated by qRT-PCR. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Overexpression of OIP5-AS1 promoted cell viability and suppressed apoptosis of cisplatin-induced HK-2 cells
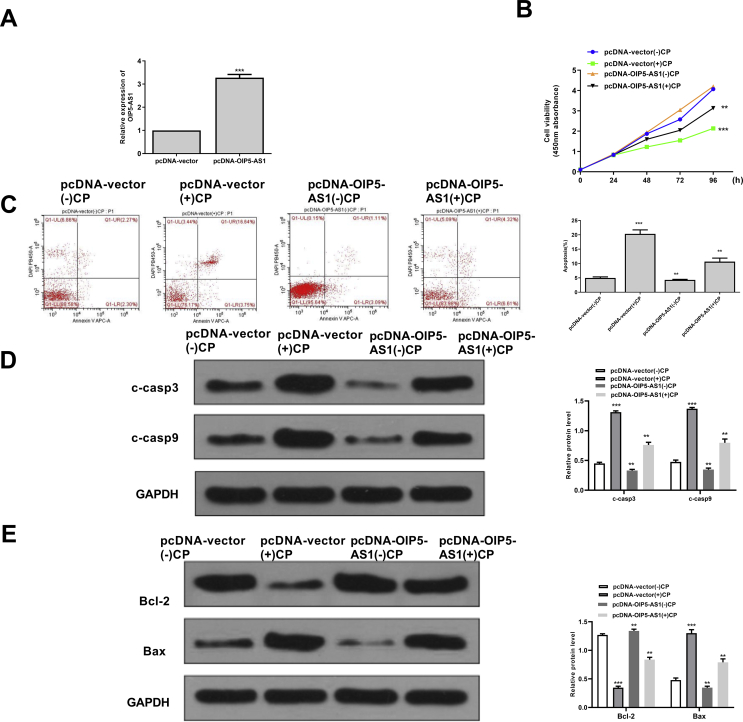
To investigate the specific function of OIP5-AS1, the overexpressing vector pc-DNA-OIP5-AS1 was constructed and transfected into HK-2 cells. The transfection efficiency was evaluated by qRT-PCR assay and the results showed that pc-DNA-OIP5-AS1 significantly increased the expression levels of OIP5-AS1 compared with negative control (pc-DNA-vector) (p < 0.001) [Fig. 2A]. Cisplatin obviously reduced cell viability of pc-DNA-vector transfected HK-2 cells (p < 0.05), while overexpression of OIP5-AS1 markedly increased cell viability of cisplatin-stimulated HK-2 cells (p < 0.05) [Fig. 2B]. In addition, cisplatin significantly induced the apoptosis of pc-DNA-vector transfected HK-2 cells (p < 0.05), while overexpression of OIP5-AS1 markedly suppressed the apoptosis of cisplatin-stimulated HK-2 cells (p < 0.05) [Fig. 2C]. Furthermore, the expression levels of apoptosis-related proteins were evaluated and the results showed that the expression levels of C-caspase 3 and C-caspase 9 were all significantly increased in cisplatin-stimulated HK-2 cells transfected with pc-DNA-vector (p < 0.001), while overexpression of OIP5-AS1 markedly decreased the expression levels of C-caspase 3 and C-caspase 9 in the cisplatin-stimulated HK-2 cells (p < 0.01) [Fig. 2D]. Meanwhile, cisplatin significantly increased the expression levels of Bax and decreased the expression levels of Bcl-2 in HK-2 cells transfected with pc-DNA-vector (p < 0.001), while overexpression of OIP5-AS1 obviously reversed the effects of cisplatin on the expression of Bax and Bcl-2 in HK-2 cells (p < 0.01) [Fig. 2E]. These results confirmed a potential inhibitory effect of OIP5-AS1 on cisplatin-induced acute kidney injury.
Fig. 2.
The effect of overexpression of OIP5-AS1 on the cell viability and apoptosis of cisplatin-stimulated HK-2 cells. (A) The transfection efficiency of pc-DNA-OIP5-AS1 and pcDNA-vector was evaluated by qRT-PCR. (B) HK-2 cells were transfected with pc-DNA-OIP5-AS1 and pcDNA-vector, then induced with cisplatin for 24, 48, 72 and 96 h. Cell viability was evaluated by CCK-8 kit. (C–E) The HK-2 cells were transfected with pc-DNA-OIP5-AS1 and pcDNA-vector, then induced with or with cisplatin for 24 h. (C) The apoptosis rate was evaluated by flow cytometry. (D) The protein expression levels of apoptosis-related makers C-caspase 3 and C-caspase 9 were detected by Western blot. (E) The protein expression levels of Bax and Bcl-2 were evaluated by Western blot. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. pcDNA-vector (−) CP group.
LncRNA OIP5-AS1 acts as a sponge of miR-144-5p
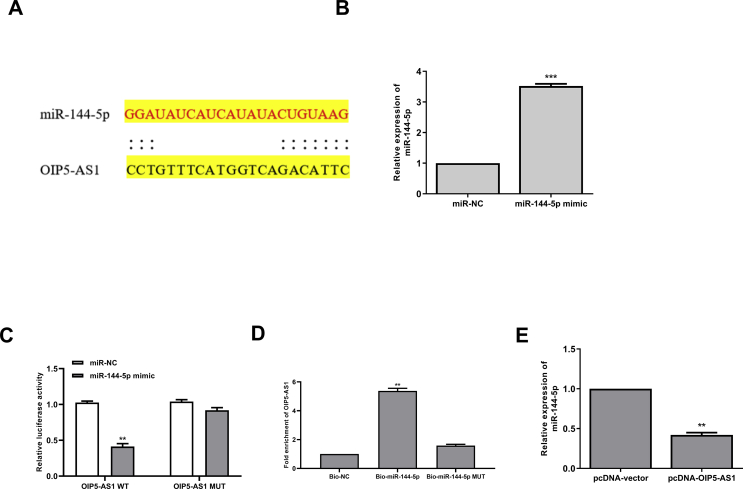
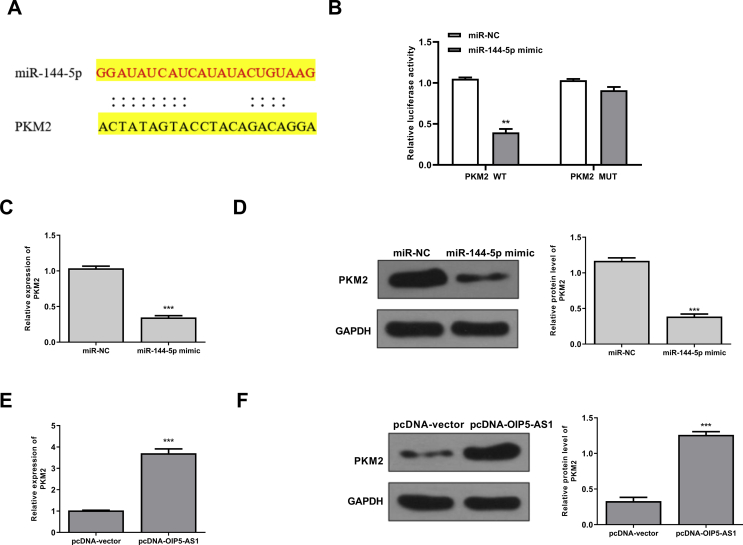
To explore the underlying molecular mechanisms of OIP5-AS1 in cisplatin-induced AKI, the potential targets of OIP5-AS1 were predicted using Starbase online database, and the results showed that miR-144-5p might be a target of OIP5-AS1 [Fig. 3A]. Then HK-2 cells were transfected with miR-144-5p mimics or miR-NC, and qRT-PCR assay showed that overexpression of miR-144-5p could significantly increase the expression levels of miR-144-5p compared with miR-NC (p < 0.001) [Fig. 3B]. To determine the relationship between OIP5-AS1 and miR-144-5p, the fragments of OIP5-AS1 containing wild type(WT) or mutant (MUT) putative binding sites against miR-144-5p were cloned into luciferase reporter plasmid and luciferase reporter assay was performed. The results showed that overexpression of miR-144-5p markedly decreased the relative luciferase activity of WT OIP5-AS1 compared with miR-NC (p < 0.01), while showed no obvious change of MUT OIP5-AS1 [Fig. 3C]. Furthermore, RNA pull-down assay was performed using biotinylated miR-144-5p and the results showed that OIP5-AS1 was significantly enriched by biotinylated miR-144-5p (p < 0.01), but exhibited no change by biotinylated MUT miR-144-5p [Fig. 3D]. In addition, the expression levels of miR-144-5p were markedly decreased in pcDNA-OIP5-AS1 transfected HK-2 cells compared with that in pcDNA-vector transfected cells (p < 0.01) [Fig. 3E]. These results indicated that OIP5-AS1 might function though targeting miR-144-5p during cisplatin-induced AKI.
Fig. 3.
OIP5-AS1 acts as a sponge of miR-144-5p. (A) The putative binding site between OIP5-AS1 and miR-144-5p was predicted by Starbase database. (B) HK-2 cells were transfected with miR-144-5p mimics or miR-NC, and the mRNA level of miR-144-5p was evaluated by qRT-PCR. (C) The relative luciferase activity of WT or MUT OIP5-AS1 was detected by the dual luciferase assay system. (D) The enrichment fold of OIP5-AS1 was evaluated by RNA pull down assay. (E) HK-2 cells were transfected with pc-DNA-OIP5-AS1 or pcDNA-vector, the mRNA expression of miR-144-5p was evaluated by qRT-PCR. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Upregulation of miR-144-5p obviously reversed the effects of overexpression of OIP5-AS1 on cell viability and apoptosis in cisplatin-stimulated HK-2 cells
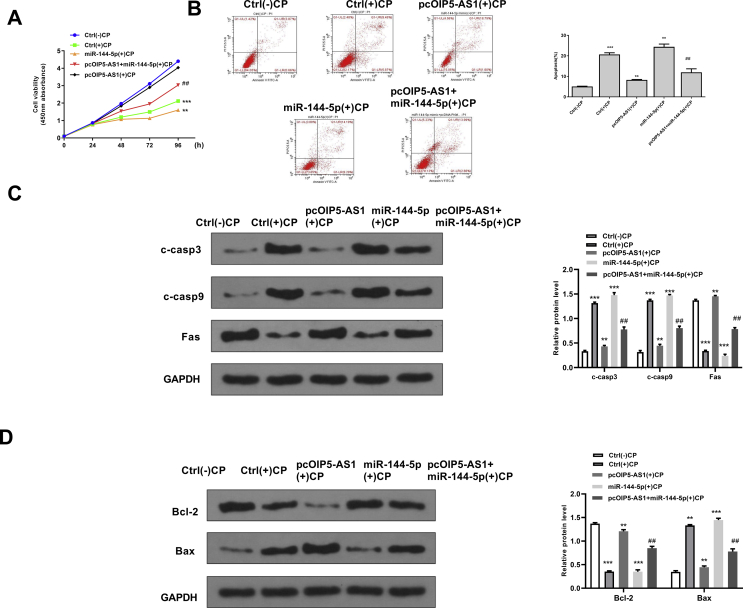
To confirm the potential protective effect of OIP5-AS1 during cisplatin-induced acute kidney injury, the rescue assays were performed. Cisplatin treatment significantly inhibited cell viability of HK-2 cells (p < 0.001), and overexpression of OIP5-AS1 markedly attenuated the inhibitory effect of cisplatin on cell viability, while co-transfection with pcDNA-OIP5-AS1 and miR144-5p mimics partially reversed the effect of overexpression of OIP5-AS1 on cell viability in cisplatin-stimulated HK-2 cells (p < 0.01) [Fig. 4A]. For apoptosis, cisplatin treatment significantly induced cell apoptosis of HK-2 cells (p < 0.001), and overexpression of OIP5-AS1 inhibited cisplatin-induced apoptosis (p < 0.01), while co-transfection with pcDNA-OIP5-AS1 and miR144-5p mimics partially reversed the effect of overexpression of OIP5-AS1 on the cell apoptosis in cisplatin-stimulated HK-2 cells (p < 0.01) [Fig. 4B]. Meanwhile, the expression of apoptosis-related proteins was detected and the results showed that overexpression of OIP5-AS1 significantly decreased the protein expression levels of C-caspase 3, C-caspase 9, Bax and increased the expression levels of Bcl-2 in cisplatin-stimulated HK-2 cells (p < 0.05), while co-transfection with pcDNA-OIP5-AS1 and miR144-5p mimics obviously and partially reversed the effects of overexpression of OIP5-AS1 on the expression of these apoptosis-related makers (p < 0.05) [Fig. 4C and D]. These results suggested that upregulation of miR-144-5p could obviously reverse the effects of overexpression of OIP5-AS1 on cell viability and apoptosis in cisplatin-stimulated HK-2 cells.
Fig. 4.
MiR-144-5p mimics significantly reversed the effects of overexpression of OIP5-AS1 on cell viability and apoptosis in cisplatin-stimulated HK-2 cells. HK-2 cells were transfected with pcDNA-OIP5-AS1, or ac-transfected with pcDNA-OIP5-AS1 and miR-144-5p mimics, then cells were treated with or without cisplatin. (A) Cell viability for different times was evaluated by CCK-8 kit. (B) The apoptosis rate for 24 h was evaluated by flow cytometry. (C) The protein expression of C-caspase 3 and C-caspase 9 for 24 h was detected by Western blot. (D) The protein expression levels of Bax and Bcl-2 for 24 h were detected by Western blot. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. control group without cisplatin. ##p < 0.01 vs. pcDNA-OIP5-AS1 group with cisplatin.
PMK2 was a target of miR-144-5p
Next, Targetscan was used to predict the potential targets of miR-144-5p, and the results showed that there was a potential binding site between miR-144-5p and 3′-UTR of PMK2 mRNA [Fig. 5A]. To validate this, we performed the luciferase reporter assay using either WT or MUT 3′-UTR of PMK2 mRNA lacking the miR-144-5p binding site in HK-2 cells. When miR-144-5p was overexpressed, the relative luciferase activity was significantly reduced in HK-2 cells transfected with the luciferase reporter vector containing WT 3′-UTR of PMK2 (p < 0.01), but exhibited no obvious change with that containing the MUT 3′-UTR of PMK2 [Fig. 5B]. Meanwhile, miR-144-5p mimics decreased the expression levels of PMK2 at both mRNA (p < 0.001) [Fig. 5C] and protein levels (p < 0.001) [Fig. 5D] compared with miR-NC group in HK-2 cells. In addition, overexpression of OIP5-AS1 increased the expression levels of PMK2 at both mRNA (p < 0.001) [Fig. 5E] and protein levels (p < 0.001) [Fig. 5F] compared with negative group (pcDNA-vector) in HK-2 cells. These results indicated that PMK2 was a direct target of miR-144-5p, and regulated by OIP5-AS1 and miR-144-5p.
Fig. 5.
PMK2 was a target of miR-144-5p. (A) The putative binding site between miR-144-5p and PMK2 mRNA was predicted by Targetscan. (B) The relative luciferase activity of WT or MUT 3′-UTR of PMK2 was detected by the dual luciferase assay system. (C and D) HK-2 cells were transfected with miR-144-5p mimics or miR-NC, then the mRNA level (C) and protein level (D) of PMK2 were evaluated. (F) HK-2 cells were transfected with pcDNA-OIP5-AS1 or pcDNA-vector, then the mRNA level (E) and protein level (F) of PMK2 were evaluated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
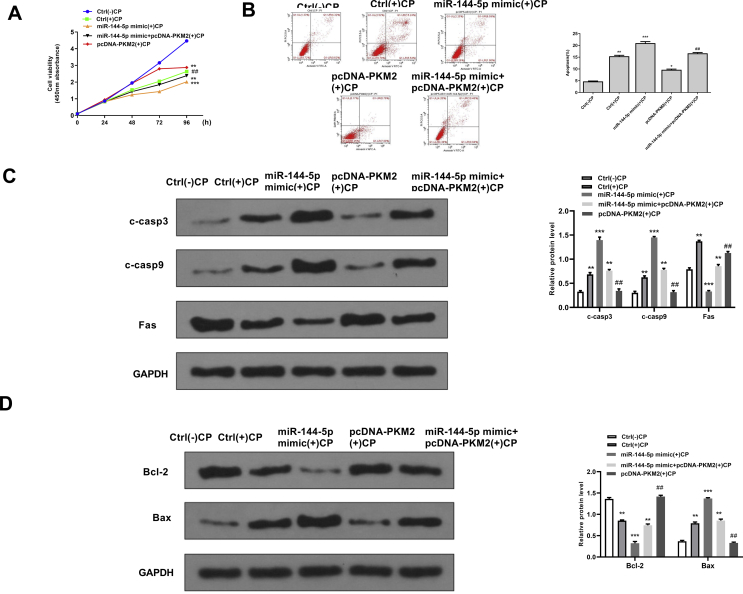
Overexpression of PMK2 significantly reversed the effects of miR-144-5p mimics on cell viability and apoptosis in cisplatin-stimulated HK-2 cells
To further explore whether the effects of miR-144-5p during cisplatin-induced AKI were mediated by PMK2, the recuse assay using PMK2 overexpressing vector was performed. For cell viability, miR-144-5p mimics significantly decreased the cell viability of cisplatin-stimulated HK-2 cells (p < 0.05), while co-transfection with miR-144-5p mimics and pcDNA-PMK2 obviously reversed the effect of overexpression of miR-144-5p on cell viability in cisplatin-stimulated HK-2 cells (p < 0.01) [Fig. 6A]. For apoptosis, miR-144-5p mimics significantly induced apoptosis of cisplatin-stimulated HK-2 cells (p < 0.01), while co-transfection with miR-144-5p mimics and pcDNA-PMK2 obviously reversed the effect of overexpression of miR-144-5p on cell apoptosis in cisplatin-stimulated HK-2 cells (p < 0.01) [Fig. 6B]. Meanwhile, the expression levels of apoptosis-related proteins were evaluated by Western blot, and the results showed that miR-144-5p mimics significantly increased the expression levels of C-caspase 3, C-caspase 9, Bax and decreased the expression levels of Bcl-2 (p < 0.01), while co-transfection with miR-144-5p mimics and pcDNA-PMK2 obviously reversed the effects of miR-144-5p mimics on the expression of these apoptosis-related makers (p < 0.01) [Fig. 6C and D]. These data suggested that the effects of miR-144-5p during cisplatin-induced AKI were partially mediated by PMK2.
Fig. 6.
Overexpression of PMK2 significantly reversed the effects of miR-144-5p mimics on cell viability and apoptosis in cisplatin-stimulated HK-2 cells. HK-2 cells were transfected with miR-144-5p mimics, or co-transfected with miR-144-5p mimics and pcDNA-PMK2, then induced with or without cisplatin. (A) The cell viability for different times was evaluated by CCK-8 kit. (B) The apoptosis rate for 24 h was evaluated by flow cytometry. (C) The protein expression of C-caspase 3 and C-caspase 9 for 24 h was detected by Western blot. (D) The protein expression levels of Bax and Bcl-2 for 24 h were detected by Western blot. ∗∗p < 0.01, ∗∗∗p < 0.001 vs. control group without cisplatin. ##p < 0.01 vs. miR-144-5p mimics group with cisplatin.
Discussion
The kidneys are the primary targets for toxic effects of various chemical agents, so drug-induced AKI is common in clinical medicine [23]. Cisplatin, a commonly used chemotherapeutic agent, has been demonstrated to have a major limitation due to its severe nephrotoxicity [24]. After a single dose of cisplatin (50–100 mg/m2), approximately one-third of the patients develop nephrotoxicity [25,26]. Therefore, cisplatin has been frequently used to induce AKI model to investigate the molecular mechanisms in AKI progression [27]. Here, the cisplatin-induced AKI mice model was established. We found that the expression levels of renal function-related makers including serum creatinine and serum BUN were significantly increased in cisplatin-induced AKI mice, and injury scores of renal tissues were significantly increased after treatment with cisplatin.
In the last decades, various lncRNAs have been identified to be closely associated with the progression of AKI. For instance, HOTAIR is significantly downregulated in kidney tissues in sepsis rats and overexpression of HOTAIR efficiently attenuates renal injury through inhibiting the apoptosis of kidney tissues by targeting the miR-34a/Bcl-2 signaling pathway [28]. CRNDE can reduce sepsis-induced renal injury by inhibiting the activation of the TLR3/NF-κB signaling pathway [29]. Overexpression of NEAT1 can aggravate the LPS-induced renal injury through targeting miR-204 and activating the NF-κB signaling pathway [30]. The expression of LINC00520 was markedly upregulated and overexpression of LINC00520 can aggravate ischemia/reperfusion induced acute kidney injury by targeting the miR-27b-3p/OSMR axis [31]. Downregulation of TUG1 contributes to the progression of sepsis-associated acute kidney injury through targeting the miR-142-3p/sirtuin 1 axis and regulating the NF-κB signaling pathway [32]. PVT1 has been identified to promote LPS-induced septic acute kidney injury through modulating the TNFα and JNK/NF-κB pathways in human renal epithelial HK-2 cells [33]. In addition, several lncRNAs including PRINS, PlncRNA-1, XIST and CCAT1 have been demonstrated to play essential roles during the progression of AKI [[34], [35], [36], [37]]. These reports confirmed the crucial roles of lncRNAs during AKI development. Although previous studies indicated that OIP5-AS1 might play potential role in AKI [14,15], further details remain unclear. In this study, we found that OIP5-AS1 was significantly downregulated in both the renal tissues of cisplatin-induced AKI mice model and cisplatin-induced HK-2 cell model. Moreover, overexpression of OIP5-AS1 could efficiently attenuate cisplatin-induced renal injury through promoting cell viability and inhibiting apoptosis of HK-2 cells. These data demonstrated that OIP5-AS1 participated in AKI through regulating renal epithelial cell apoptosis, suggesting that OIP5-AS1 might be a potential therapeutic target for AKI.
It has been reported that lncRNAs always function as competing endogenous RNAs (ceRNAs) of microRNAs (miRNAs) in human diseases [38]. In this study, luciferase reporter assay and RNA pull-down assays were performed to confirm the binding relationship between OIP5-AS1 and miR-144-5p. Previous studies have demonstrated that miR-144-5p plays important roles in various human diseases including lung squamous cell carcinoma, bladder cancer and sickle cell disease [[39], [40], [41]]. MiR-144-5p limits the formation of experimental abdominal aortic aneurysm by inhibiting M1 macrophage-mediated inflammation through downregulating TLR2 and OLR1 [42]. MiR-144-5p inhibits cell proliferation, invasion and migration of colorectal cancer cells through targeting CDKL1 [43]. Inhibition of miR-144-5p can enhance the radio-sensitivity of non-small cell lung cancer cells through directly targeting ATF2 [44]. In addition, abnormal expression of miR-144-5p was also observed in a series of other human cancers including bladder cancer, oral cavity squamous cell carcinoma, esophageal cancer, and lung adenocarcinoma [[45], [46], [47], [48]]. Here, we also found that the expression of miR-144-5p was markedly upregulated in both the renal tissues of cisplatin-induced AKI mice model and cisplatin-induced HK-2 cell model. Meanwhile, overexpression of miR-144-5p obviously reversed the effects of overexpression of OIP5-AS1 on cell viability and apoptosis in cisplatin-stimulated HK-2 cells. Specifically, overexpression of OIP5-AS1 significantly decreased the expression levels of C-caspase 3, C-caspase 9, Bax and increased the expression levels of Bcl-2, while additional miR-144-5p partially reversed the effects of overexpression of OIP5-AS1. Our findings demonstrated that the function of OIP5-AS1 during AKI was partially mediated by miR-144-5p.
PKM2 (pyruvate kinase M2 isoform) has been identified to play an anti-apoptotic role in the progression of many human cancers. PKM2 is a rate-limiting terminal glycolytic enzyme and can catalyze the last step of glycolysis [49]. Increasing evidence has demonstrated that PKM2 acts as an anti-apoptotic factor against the AKT signaling pathway, which controls the cell survival and apoptosis [50]. It was reported that PKM2 could efficiently attenuate heat stress-induced damage in mouse kidneys through regulating the AKT and HIF-1α signaling pathway [51]. Knockdown of PKM2 can induce autophagic cell death in prostate cancer cells through affecting the AKT/mTOR signaling pathway [52]. PKM2 serves as the target of miRNAs to participate in the progression of human cancers. For example, miR-338-3p can suppress the growth, metastasis and glycolysis of glioma cells via downregulating PKM2 [53]. MiR-374b re-sensitizes hepatocellular carcinoma cells to sorafenib therapy through antagonizing PKM2-mediated glycolysis pathway [54]. PKM2, targeted by lncRNA FEZF1-AS1, can promote tumor proliferation and metastasis in colorectal cancer [55]. In addition, it was demonstrated that mitochondrial PKM2 could regulate oxidative stress-induced apoptosis through stabilizing Bcl2 [56]. In this study, PKM2 was predicted to be a direct target of miR-144-5p. Furthermore, the relative luciferase activity was significantly reduced in HK-2 cells transfected with the luciferase gene containing WT 3′-UTR of PMK2, but exhibited no obvious change with that containing the MUT 3′-UTR of PMK2. And overexpression of miR-144-5p obviously decreased the expression levels of PKM2, while overexpression of OIP5-AS1 increased the expression levels of PKM2. Moreover, overexpression of PMK2 obviously reversed the effects of miR-144-5p mimics on cell viability and apoptosis in cisplatin-stimulated HK-2 cells. All these data suggested that OIP5-AS1 affected the progression of AKI through regulating the miR-144-5p/PKM2 axis. Although our in vitro experiments indicated that OIP5-AS1 significantly reduced cisplatin-induced renal epithelial cell apoptosis through targeting the miR-144-5p/PKM2 axis, in vivo experiments should also be performed to determine the roles of the OIP5-AS1/miR-144-5p axis in AKI.
Conclusion
In summary, we first demonstrated that OIP5-AS1 was significantly downregulated in cisplatin-induced AKI both in vitro and in vivo. Specifically, OIP5-AS1 efficiently reduced the apoptosis of renal epithelial cells in cisplatin-induced AKI through targeting the miR-144-5p/PKM2 axis, suggesting that OIP5-AS1 might be a potential therapeutic target for AKI.
Compliance with ethical standards
Research involving animals
All experiment protocols were performed according to the First Affiliated Hospital of Zhengzhou University Animal Experimental Guide and approved by the First Affiliated Hospital of Zhengzhou University.
Informed consent
Not applicable.
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2021.07.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ronco C., Bellomo R., Kellum J.A. Acute kidney injury. Lancet. 2019;394:1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 2.Farrar A. Acute kidney injury. Nurs Clin North Am. 2018;53:499–510. doi: 10.1016/j.cnur.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Moore P.K., Hsu R.K., Liu K.D. Management of acute kidney injury: core curriculum 2018. Am J Kidney Dis. 2018;72:136–148. doi: 10.1053/j.ajkd.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Li Y.F., Jing Y., Hao J., Frankfort N.C., Zhou X., Shen B., et al. MicroRNA-21 in the pathogenesis of acute kidney injury. Protein Cell. 2013;4:813–819. doi: 10.1007/s13238-013-3085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singbartl K., Kellum J.A. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819–825. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 7.Jarroux J., Morillon A., Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 8.Sun X., Tian C., Zhang H., Han K., Zhou M., Gan Z., et al. Long noncoding RNA OIP5-AS1 mediates resistance to doxorubicin by regulating miR-137-3p/PTN axis in osteosarcoma. Biomed Pharmacother. 2020;128:110201. doi: 10.1016/j.biopha.2020.110201. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Wang H., Ruan J., Zheng W., Yang Z., Pan W. Long non-coding RNA OIP5-AS1 suppresses multiple myeloma progression by sponging miR-27a-3p to activate TSC1 expression. Cancer Cell Int. 2020;20:155. doi: 10.1186/s12935-020-01234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C., Yang Q., Pan S., Lin X., Xu G., Luo Y., et al. LncRNA OIP5-AS1 promotes cell proliferation and migration and induces angiogenesis via regulating miR-3163/VEGFA in hepatocellular carcinoma. Cancer Biol Ther. 2020;21:604–614. doi: 10.1080/15384047.2020.1738908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhi X.H., Jiang K., Ma Y.Y., Zhou L.Q. OIP5-AS1 promotes the progression of gastric cancer cells via the miR-153-3p/ZBTB2 axis. Eur Rev Med Pharmacol Sci. 2020;24:2428–2441. doi: 10.26355/eurrev_202003_20510. [DOI] [PubMed] [Google Scholar]
- 12.Meng X., Ma J., Wang B., Wu X., Liu Z. Long non-coding RNA OIP5-AS1 promotes pancreatic cancer cell growth through sponging miR-342-3p via AKT/ERK signaling pathway. J Physiol Biochem. 2020;76:301–315. doi: 10.1007/s13105-020-00734-4. [DOI] [PubMed] [Google Scholar]
- 13.Li Q., Chen W., Luo R., Zhang Z., Song M., Chen W., et al. Upregulation of OIP5-AS1 predicts poor prognosis and contributes to thyroid cancer cell proliferation and migration. Mol Ther Nucleic Acids. 2020;20:279–291. doi: 10.1016/j.omtn.2019.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Fu J.X., Sun G.Q., Wang H.L., Jiang H.X. LncRNA OIP5-AS1 induces epithelial-to-mesenchymal transition and renal fibrosis in diabetic nephropathy via binding to miR-30c-5p. J Biol Regul Homeost Agents. 2020;34:961–968. doi: 10.23812/20-199-A-68. [DOI] [PubMed] [Google Scholar]
- 15.Grande M.T., Sánchez-Laorden B., López-Blau C., De Frutos C.A., Boutet A., Arévalo M., et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 16.Li C., Shen Y., Huang L., Liu C., Wang J. Senolytic therapy ameliorates renal fibrosis postacute kidney injury by alleviating renal senescence. FASEB J. 2021;35 doi: 10.1096/fj.202001855RR. [DOI] [PubMed] [Google Scholar]
- 17.Sharp C.N., Siskind L.J. Developing better mouse models to study cisplatin-induced kidney injury. Am J Physiol Renal Physiol. 2017;313:F835–F841. doi: 10.1152/ajprenal.00285.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang H.R., Gandolfo M.T., Ko G.J., Racusen L., Rabb H. The effect of murine anti-thymocyte globulin on experimental kidney warm ischemia-reperfusion injury in mice. Transpl Immunol. 2009;22:44–54. doi: 10.1016/j.trim.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Qin W., Xie W., Yang X., Xia N., Yang K. Inhibiting microRNA-449 attenuates cisplatin-induced injury in NRK-52E cells possibly via regulating the SIRT1/P53/BAX pathway. Med Sci Monit. 2016;22:818–823. doi: 10.12659/MSM.897187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams B.D., Anastasiadou E., Esteller M., He L., Slack F.J. The inescapable influence of noncoding RNAs in cancer. Cancer Res. 2015;75:5206–5210. doi: 10.1158/0008-5472.CAN-15-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4 doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Ren L., Zhao Q., Lu G., Ren M., Lu X., et al. TRPC1 exacerbate metastasis in gastric cancer via ciRS-7/miR-135a-5p/TRPC1 axis. Biochem Biophys Res Commun. 2020;529:85–90. doi: 10.1016/j.bbrc.2020.05.181. [DOI] [PubMed] [Google Scholar]
- 23.Ozkok A., Edelstein C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res Int. 2014;2014:967826. doi: 10.1155/2014/967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han X., Chesney R.W. TauT protects against cisplatin-induced acute kidney injury (AKI) established in a TauT transgenic mice model. Adv Exp Med Biol. 2009;643:113–122. doi: 10.1007/978-0-387-75681-3_12. [DOI] [PubMed] [Google Scholar]
- 25.Lebwohl D., Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34:1522–1534. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 26.Shiraishi F., Curtis L.M., Truong L., Poss K., Visner G.A., Madsen K., et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000;278:F726–F736. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- 27.Holditch S.J., Brown C.N., Lombardi A.M., Nguyen K.N., Edelstein C.L. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci. 2019;20:3011. doi: 10.3390/ijms20123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Z.J., Zhang M.Y., Fan Z.W., Sun W.L., Tang Y. Influence of lncRNA HOTAIR on acute kidney injury in sepsis rats through regulating miR-34a/Bcl-2 pathway. Eur Rev Med Pharmacol Sci. 2019;23:3512–3519. doi: 10.26355/eurrev_201904_17717. [DOI] [PubMed] [Google Scholar]
- 29.Sun B.Q., Sui Y.D., Huang H., Zou X.B., Chen S.C., Yu Z.K. Effect of lncRNA CRNDE on sepsis-related kidney injury through the TLR3/NF-κB pathway. Eur Rev Med Pharmacol Sci. 2019;23:10489–10497. doi: 10.26355/eurrev_201912_19688. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Qiu J., Chen B., Lin Y., Chen Y., Xie G., et al. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-κB pathway. Int Immunopharmacol. 2018;59:252–260. doi: 10.1016/j.intimp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Tian X., Ji Y., Liang Y., Zhang J., Guan L., Wang C. LINC00520 targeting miR-27b-3p regulates OSMR expression level to promote acute kidney injury development through the PI3K/AKT signaling pathway. J Cell Physiol. 2019;234:14221–14233. doi: 10.1002/jcp.28118. [DOI] [PubMed] [Google Scholar]
- 32.Liu X., Hong C., Wu S, Song S, Yang Z, Cao L, et al. Downregulation of lncRNA TUG1 contributes to the development of sepsis-associated acute kidney injury via regulating miR-142-3p/sirtuin 1 axis and modulating NF-κB pathway. J Cell Biochem. 2019;120:11331–11341. doi: 10.1002/jcb.28409. [DOI] [PubMed] [Google Scholar]
- 33.Huang W., Lan X., Li X., Wang D., Sun Y., Wang Q., et al. Long non-coding RNA PVT1 promote LPS-induced septic acute kidney injury by regulating TNFα and JNK/NF-κB pathways in HK-2 cells. Int Immunopharmacol. 2017;47:134–140. doi: 10.1016/j.intimp.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Yu T.M., Palanisamy K., Sun K.T., Day Y.J., Shu K.H., Wang I.K., et al. RANTES mediates kidney ischemia reperfusion injury through a possible role of HIF-1α and LncRNA PRINS. Sci Rep. 2016;6:18424. doi: 10.1038/srep18424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu D., Zhou K., Liu J., Zheng P., Li P., Cheng W., et al. Long non-coding RNA PlncRNA-1 regulates cell proliferation, apoptosis, and autophagy in septic acute kidney injury by regulating BCL2. Int J Clin Exp Pathol. 2018;11:314–323. [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Q., Wang L. LncRNA XIST serves as a ceRNA to regulate the expression of ASF1A, BRWD1M, and PFKFB2 in kidney transplant acute kidney injury via sponging hsa-miR-212-3p and hsa-miR-122-5p. Cell Cycle. 2020;19:290–299. doi: 10.1080/15384101.2019.1707454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu S., Dong L., Jing X., Gen-Yang C., Zhan-Zheng Z. Abnormal lncRNA CCAT1/microRNA-155/SIRT1 axis promoted inflammatory response and apoptosis of tubular epithelial cells in LPS caused acute kidney injury. Mitochondrion. 2020;53:76–90. doi: 10.1016/j.mito.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida A., Seki N., Mizuno K., Misono S., Yamada Y., Kikkawa N, et al. Involvement of dual-strand of the miR-144 duplex and their targets in the pathogenesis of lung squamous cell carcinoma. Cancer Sci. 2019;110:420–432. doi: 10.1111/cas.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushita R., Seki N., Chiyomaru T., Inoguchi S., Ishihara T., Goto Y., et al. Tumour-suppressive microRNA-144-5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br J Cancer. 2015;113:282–289. doi: 10.1038/bjc.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li B., Zhu X., Ward C.M., Starlard-Davenport A., Takezaki M., Berry A., et al. MIR-144-mediated NRF2 gene silencing inhibits fetal hemoglobin expression in sickle cell disease. Exp Hematol. 2019;70:85–96. doi: 10.1016/j.exphem.2018.11.002. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi X., Ma W., Li Y., Wang H., Pan S., Tian Y., et al. MiR-144-5p limits experimental abdominal aortic aneurysm formation by mitigating M1 macrophage-associated inflammation: suppression of TLR2 and OLR1. J Mol Cell Cardiol. 2020;143:1–14. doi: 10.1016/j.yjmcc.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Pei J., Xiao W., Zhu D., Ji X., Shi L., Deng X. WITHDRAWN: LncRNA DSCAM-AS1 promotes proliferation, migration and invasion of colorectal cancer cells via modulating miR-144-5p/CDKL1. Life Sci. 2019:117050. doi: 10.1016/j.lfs.2019.117050. [DOI] [PubMed] [Google Scholar]
- 44.Song L., Peng L., Hua S, Li X, Ma L, Jie J, et al. miR-144-5p enhances the radiosensitivity of non-small-cell lung cancer cells via targeting ATF2. BioMed Res Int. 2018;2018:5109497. doi: 10.1155/2018/5109497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugawara S., Yamada Y., Arai T., Okato A., Idichi T., Kato M., et al. Dual strands of the miR-223 duplex (miR-223-5p and miR-223-3p) inhibit cancer cell aggressiveness: targeted genes are involved in bladder cancer pathogenesis. J Hum Genet. 2018;63:657–668. doi: 10.1038/s10038-018-0437-8. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen N.J., Jensen D.H., Lelkaitis G., Kiss K., Charabi B.W., Ullum H., et al. MicroRNA-based classifiers for diagnosis of oral cavity squamous cell carcinoma in tissue and plasma. Oral Oncol. 2018;83:46–52. doi: 10.1016/j.oraloncology.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Gao Z., Zhang P., Xie M., Gao H., Yin L., Liu R. miR-144/451 cluster plays an oncogenic role in esophageal cancer by inhibiting cell invasion. Cancer Cell Int. 2018;18:184. doi: 10.1186/s12935-018-0679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu N., Yong S., Kim H.K., Choi Y.L., Jung Y., Kim D., et al. Identification of tumor suppressor miRNAs by integrative miRNA and mRNA sequencing of matched tumor-normal samples in lung adenocarcinoma. Mol Oncol. 2019;13:1356–1368. doi: 10.1002/1878-0261.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Q., Chen X., Ma J., Peng H., Wang F., Zha X., et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabriele F., Martinelli C., Comincini S. Prostate cancer cells at a therapeutic gunpoint of the autophagy process. J Cancer Metastasis Treat. 2018;4:17. [Google Scholar]
- 51.Chen B., Yang B., Zhu J., Wu J., Sha J., Sun J., et al. Hsp90 relieves heat stress-induced damage in mouse kidneys: involvement of antiapoptotic PKM2-AKT and autophagic HIF-1α signaling. Int J Mol Sci. 2020;21:1646. doi: 10.3390/ijms21051646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dey P., Kundu A., Sachan R., Park J.H., Ahn M.Y., Yoon K., et al. PKM2 knockdown induces autophagic cell death via AKT/mTOR pathway in human prostate cancer cells. Cell Physiol Biochem. 2019;52:1535–1552. doi: 10.33594/000000107. [DOI] [PubMed] [Google Scholar]
- 53.Liu X., Zhu Q., Guo Y., Xiao Z., Hu L., Xu Q., et al. LncRNA LINC00689 promotes the growth, metastasis and glycolysis of glioma cells by targeting miR-338-3p/PKM2 axis. Biomed Pharmacother. 2019;117:109069. doi: 10.1016/j.biopha.2019.109069. [DOI] [PubMed] [Google Scholar]
- 54.Zhang M., Zhang H., Hong H., Zhang Z. MiR-374b re-sensitizes hepatocellular carcinoma cells to sorafenib therapy by antagonizing PKM2-mediated glycolysis pathway. Am J Cancer Res. 2019;9:765–778. [PMC free article] [PubMed] [Google Scholar]
- 55.Bian Z., Zhang J., Li M., Feng Y., Wang X., Zhang J., et al. LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin Cancer Res. 2018;24:4808–4819. doi: 10.1158/1078-0432.CCR-17-2967. [DOI] [PubMed] [Google Scholar]
- 56.Liang J., Cao R., Wang X., Zhang Y., Wang P., Gao H., et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2017;27:329–351. doi: 10.1038/cr.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.