Abstract
We present the unique case of a gastropericardial fistula with a rare, delayed presentation in a man in his 70s. Relevant surgeries include Watchman Left Atrial Appendage Closure device placement 1 year prior to arrival and gastric bypass surgery 20 years prior to arrival. The patient presented to the emergency department with weakness, diarrhoea and left knee pain. He was admitted for cellulitis of the left lower extremity, prosthetic septic arthritis of the left knee and group G streptococcus bacteraemia. His hospital course was complicated by acute chest pain and dyspnoea. Imaging revealed pneumopericardium. Oesophagogastroduodenoscopy visualisation confirmed the diagnosis of gastropericardial fistula. The patient could not be transferred to a tertiary centre for definitive management because of the effect of the COVID-19 pandemic on tertiary hospital volumes. After pericardial drainage and administration of antimicrobials without improvement, the patient was discharged to hospice care at his request and died 1 day after discharge.
Keywords: Pericardial disease, Stomach and duodenum, Infectious diseases, Adult intensive care, Pneumomediastinum
Background
A gastropericardial fistula is an abnormal communication between the stomach and the pericardial space. Associated risk factors include: previous bariatric, gastric and oesophageal surgeries; cancer of the stomach or oesophagus; and hiatal hernias.1 Most cases reviewed in the literature presented with chest pain or epigastric pain as the primary complaint,1–3 whereas our patient presented atypically with red-flag symptoms not arising until day 7. Significant complications have been reported in the literature, including, but not limited to, tamponade physiology, septic shock, pericarditis and death. Therefore, prompt recognition and treatment are paramount.2 We present the case of a rare delayed presentation of a polymicrobial gastropericardial fistula in a critically ill patient.
Case presentation
A man in his 70s presented to the emergency department (ED) with the chief complaint of weakness, diarrhoea and left knee pain. His medical history included paroxysmal atrial fibrillation after Watchman Left Atrial Appendage Closure device 1 year prior to arrival, chronic obstructive pulmonary disease, coronary artery disease, type II diabetes mellitus, remote peptic ulcer disease and peripheral artery disease. Relevant surgical history included gastric bypass 20 years prior to arrival, oesophagogastroduodenoscopy (OGD) 2 years prior to arrival and left knee arthroplasty. The patient did have an ED visit 1 week prior to his hospitalisation for management of atrial fibrillation with rapid ventricular response; however, he was safely discharged home after his heart rate was controlled. There was no documented physiological decline, weight loss, dysphagia or dyspepsia on review of his medical records over the prior months.
Initially, the patient was admitted with a diagnosis of sepsis secondary to cellulitis of the left lower extremity, group G streptococcus (GGS) septic arthritis of the left knee, GGS bacteraemia and acute kidney injury. The patient also had ulceration of the left great toe with MRI findings indicative of osteomyelitis. He was started on ceftriaxone and taken to the operating room by the orthopaedic surgery team for resection arthroplasty and removal of implants on day 1. On day 5, he underwent amputation of the left great toe. The patient’s acute kidney injury resolved, subsequent blood cultures were negative for persistent bacteraemia and the patient was set to be discharged to acute rehabilitation with plans to complete 42 total days of ceftriaxone via a peripherally inserted central catheter.
However, on day 6, the patient developed dyspnoea, hypoxia and chest discomfort. A repeat chest X-ray did not reveal an obvious abnormality. ECG findings showed atrial fibrillation at 110 beats per minute with an intraventricular conduction delay and non-specific ST changes (figure 1), similar to his ECG on admission. A review of an echocardiogram (ECHO) performed on day 2 showed a normal left ventricular ejection fraction and was otherwise unchanged from an ECHO performed 6 months prior to this admission.
Figure 1.
ECG: no diffuse ST-segment changes contrary to other cases reported in the literature.
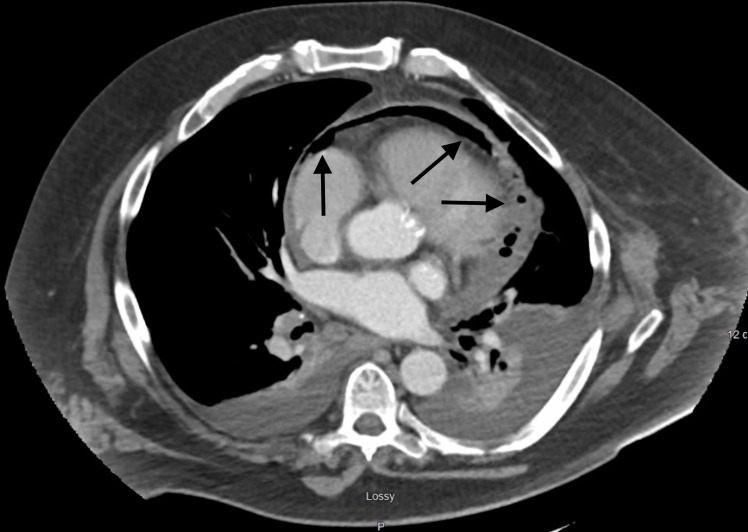
On day 7, the patient described increasing chest discomfort and palpitations when lying supine. He developed a significant leucocytosis (14.4–28.4) on day 8 and complained of worsening cough with ‘gurgling breathing’. Blood cultures were repeated. On day 9, the patient’s leucocytosis had increased from 28.4 to 37.8, and blood cultures showed no growth. The patient continued to require 2 L/min of oxygen via nasal cannula. A CT scan of the chest, abdomen and pelvis with intravenous contrast was ordered. Findings were notable for emphysematous pericarditis (figure 2) with both gas and fluid in the pericardial sac and bilateral pleural effusions. There were no pleural effusions noted on his previous ECHO. Additional findings of left clavicular head osteomyelitis and suspected acute cholecystitis were also noted.
Figure 2.
CT of the chest with intravenous contrast—emphysematous pericarditis—both fluid and gas in the pericardial sac with moderate posterior pericardial effusion. Bilateral pleural effusions with bilateral compressive atelectasis and consolidations are also seen.
The cardiothoracic surgery team was consulted, and a pericardial window was performed on day 10. On induction of anaesthesia, before the pericardial window procedure, the patient developed ventricular fibrillation. Multiple defibrillations were performed with return of spontaneous circulation after 10 min of advanced cardiac life support. The aetiology of the patient’s cardiac arrest was thought to be due to tamponade physiology; 200 mL of cloudy pericardial fluid was removed from the posterior pericardial sac, and 24-French Blake drains were placed. Pericardial fluid cultures grew Candida albicans, Candida glabrata, Enterococcus faecalis and methicillin-resistant Staphylococcus aureus. The infectious disease specialist was consulted, and the patient was started on vancomycin, micafungin and ampicillin in addition to the ceftriaxone for his GGS prosthetic knee infection. The patient remained hypotensive in the postarrest and postprocedure setting and was started on norepinephrine and vasopressin for his septic shock. However, due to atrial fibrillation with a rapid ventricular response, the patient’s vasopressors were subsequently changed to phenylephrine. He was successfully extubated after the pericardial window procedure and maintained on bilevel positive airway pressure (BiPAP).
The patient remained in the intensive care unit through day 17 until weaned off his vasopressor requirement and BiPAP. Due to his polymicrobial sepsis, a limited immunodeficiency workup performed was negative. Symptomatic anaemia developed on day 15 was refractory to multiple transfusions over the next few days. A haemolytic evaluation was negative, and the patient had no apparent source of blood loss. Given the patient’s history of bariatric surgery and remote anastomotic ulcer, gastroenterology was consulted for endoscopic evaluation. They recommended conservative treatment with transfusions and proton pump inhibitor therapy in light of the patient’s multiple severe comorbidities. On day 19, the patient had recurrent desaturations and was placed on BiPAP. Chest X-ray showed a new right-sided pleural effusion; however, he was unable to tolerate positioning for an attempt at right-sided thoracentesis.
Over the next 5 days, the patient maintained high output from his chest drains, and a barium oesophagram was performed on day 24. This study showed a suspected communication between the oesophagus and mediastinum (figure 3). The aetiology of this fistula was unclear. OGD 2 years prior showed no documented ulcerations, the patient had no recent oesophageal instrumentation and his most recent invasive procedure was his Watchman device placement 1 year prior to arrival. Discussion between gastroenterology and cardiothoracic surgery concluded that, due to significant comorbidities, the patient would be unlikely to survive an open repair and would need transfer to a tertiary care centre for oesophageal stenting. However, due to the ongoing COVID-19 pandemic, bed availability was scarce. Later the same day, the patient was reintubated for deteriorating respiratory status. He was started on total parenteral nutrition (TPN) and vasopressors.
Figure 3.
Oesophagram—extravasation of contrast is visualised from the distal oesophagus into the mediastinum.
His antibiotics were changed to vancomycin, micafungin and ampicillin-sulbactam. Due to his recurrent anaemia and melanic stools, an OGD was performed and revealed a large gastric perforation with gastropericardial fistula without signs of bleeding (figure 4). The oesophagus had extensive inflammatory changes, and there was concomitant ulceration of the gastrojejunal anastomosis. Unfortunately, no biopsies were taken. At this time, it was determined that this lesion would not be amenable to a stent and would need resection or flap repair.
Figure 4.
Oesophagogastroduodenoscopy—gastric pouch with perforation and visualisation of cardiac tissue.
He was again extubated on day 31, with the capacity to discuss goals of care. There was still no bed availability at tertiary centres to pursue surgical intervention. The discussion of attempted intervention at our institution was readdressed with the surgical team. The patient was informed that he would likely need indefinite TPN due to the extensiveness of the surgery. During the shared decision-making process, the patient expressed his wishes not to pursue surgical intervention in favour of pursuing hospice. The patient was then discharged to an inpatient hospice centre for comfort care, where he died 1 day later.
Differential diagnosis
There were multiple differential diagnoses for our patient’s condition, the most likely being occult ulceration in the setting of his historic gastric bypass surgery. The patient had a known gastrojejunal anastomotic ulceration, although his communication with the pericardium is present at a different location. The patient’s most recent OGD was 2 years prior to his hospital stay. Therefore, it is possible that the patient developed a new area of ulceration in the interim. Another possible aetiology was stress ulceration related to hospitalisation and critical illness. The patient may have had an existing small ulceration that expanded in part due to his proinflammatory state at presentation. Lastly, iatrogenic injury from perforation of the left atrium from Watchman device placement was also considered as this was the patient’s most recent instrumentation. The fact that the patient was asymptomatic between his procedure and hospitalisation makes this a less probable primary cause. Unfortunately, no biopsies were taken on OGD; therefore, we were unable to tell if the fistula was malignant in nature.
Treatment
Due to multiorganism bacteraemia, broad-spectrum antibiotics were required, including vancomycin, ampicillin-sulbactam, micafungin and ceftriaxone. The patient would have continued these antibiotics until 6 weeks postsurgical repair. The definitive treatment for our patient would have included surgical repair of his gastric defect. Initially, the lesion was thought to be amenable to stenting. However, OGD showed this not to be the case. Management would have included gastro-oesophageal resection versus direct repair with flap. Due to the extensiveness of the surgery, high morbidity and mortality risk, and the risk of requiring TPN indefinitely, the patient decided to withdraw treatment and pursue hospice care.
Outcome and follow-up
The patient elected to be discharged to hospice and declined further intervention after extensive discussions about care goals and his prognosis. He was at peace with his decision to proceed with hospice after weighing this decision throughout his illness and before each procedure; however, he had a great deal of stress regarding the financial situation he would leave for his wife and family. The patient died within 24 hours of discontinuing supportive treatments.
Discussion
While rare, gastropericardial fistulae have been previously reported in the literature. Most, if not all, patients with gastropericardial fistulae present with a chief complaint of chest or epigastric pain or discomfort.1–3 However, patients can also present with life-threatening complications such as pleural effusions, cardiac tamponade, septic shock and cardiogenic shock.1–3 These fistulae appear to be associated with a history of oesophageal or gastric surgery, hiatal hernias, active cancer of the oesophagus or stomach, or peptic ulcer disease.1–3 One study reported a case associated with longstanding shoulder pain as the presenting symptom.4 One of the most common associations between cases involves patients with a prior history of gastric and oesophageal surgery.1 However, gastropericardial fistulae do not appear immediately after the surgery in most cases, with one case being reported 27 years after bariatric surgery.5 It is currently unclear why cases attributed to surgical aetiologies of gastropericardial fistulae present at varied time points. Other unique cases have been reported, such as perforation and fistula formation associated with lye ingestion.6
Patients can present with ECG changes similar to pericarditis (diffuse ST changes) and pyopneumocardium subsequently culturing gastrointestinal flora.1 7 8 Our patient’s case is unique in that he presented initially with complaints of weakness and left lower extremity pain. His initial and subsequent ECGs were negative for diffuse ST-segment changes, and no documentation of chest pain or chest discomfort was made until day 6. Unfortunately, it is unclear why our patient had a delayed presentation. There was no instrumentation to his gastro-oesophageal junction while in the hospital prior to developing his symptoms.
Most cases of gastropericardial fistulae require CT imaging for diagnosis.9 In cases where CT imaging is equivocal, OGD is recommended for definitive diagnosis as it has high sensitivity given the nature of the procedure.9 Plain radiographs are not specific for gastropericardial fistulae but may reveal pneumopericardium and pericardial thickening.9 For our patient, the CT scan of his chest showed pyopneumopericardium, and the oesophagram suggested an oesophageal-pericardial fistula. The diagnosis of gastropericardial fistula was not made until OGD was performed with direct visualisation.
In conclusion, patients with gastropericardial fistulae can face devastating complications if diagnosis and treatment are delayed. Surgical advances since the year 2000 have decreased the reported mortality from 69% to 11%.1 Unfortunately, there is no single symptom or sign that appears to be pathognomonic for these cases as most present with chest pain and ECG changes. Gastropericardial fistula should be considered in patients presenting with chest pain and pericarditis-type ECG changes, particularly if there is an identified pericardial effusion in patients with a known history of gastric or oesophageal surgery. Pericardial effusions were commonly reported in the literature, so bedside echocardiography by an experienced physician may aid in diagnosis. However, CT imaging with OGD follow-up is recommended for definitive diagnosis.
Learning points.
The constellation of sepsis, chest pain with ECG changes similar to pericarditis and pericardial effusion on bedside echocardiography in the emergency department consider gastropericardial fistula on the differential diagnosis.
Oesophagogastroduodenoscopy with direct cardiac visualisation is the best modality for confirmatory diagnosis. CT and fluoroscopy may aid in the diagnosis but are not confirmatory tests. Chest X-ray findings of pneumopericardium are non-specific and insensitive.
Gastropericardial fistula should be considered as part of the differential diagnosis in patients with polymicrobial pyopneumopericardium without alternate explanation.
Acknowledgments
We would like to thank Tracy Koehler PhD for her assistance in reviewing our manuscript.
Footnotes
Contributors: AP, MF and NM for writing and editing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Azzu V. Gastropericardial fistula: getting to the heart of the matter. BMC Gastroenterol 2016;16:96. 10.1186/s12876-016-0510-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni B, Chen Y-L, Lin P-C, et al. Gastropericardial fistula induced acute purulent pericarditis. Am J Emerg Med 2021;46:801.e1–801.e3. 10.1016/j.ajem.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 3.Rathur A, Al-Mohamad H, Steinhoff J, et al. Chest pain from pneumopericardium with gastropericardial fistula. Case Rep Cardiol 2021;2021:1–3. 10.1155/2021/5143608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sailing N, Falensteen AM, Larsen LG. Non-traumatic perforation of gastric ulcer in a hiatal hernia to the pericardium. Acta Med Scand 2009;213:225–6. 10.1111/j.0954-6820.1983.tb03722.x [DOI] [PubMed] [Google Scholar]
- 5.Martin R, Reuhland B, Carlson L, et al. Gastropericardial fistula presenting 27 years after bariatric surgery. CPC-EM 2017;1:435–6. 10.5811/cpcem.2017.6.34372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Susarla S, Khouzam RN, Lowell D, et al. Gastropericardial fistula presenting 22 years after lye ingestion. Can J Cardiol 2005;21:371–2. [PubMed] [Google Scholar]
- 7.Lee J, Ramkumar S, Ha P, et al. Pyopneumopericarditis from a gastropericardial fistula: a case report. Euro Heart J - Case Rep 2021;5. 10.1093/ehjcr/ytab408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim WJ, Choi EJ, Oh Y-W, et al. Gastropericardial fistula-induced pyopneumopericardium after esophagectomy with esophagogastrectomy. Ann Thorac Surg 2011;91:e10–11. 10.1016/j.athoracsur.2010.09.082 [DOI] [PubMed] [Google Scholar]
- 9.Davidson JP, Connelly TM, Libove E, et al. Gastropericardial fistula: radiologic findings and literature review. J Surg Res 2016;203:174–82. 10.1016/j.jss.2016.03.015 [DOI] [PubMed] [Google Scholar]