Abstract
Objective
Hypertension predicts the development of diabetes. However, there are still lacking high-quality studies on the correlation between mean arterial pressure (MAP) and incident diabetes. We aimed to explore the relationship between MAP and diabetes in Chinese adults.
Design
This is a secondary retrospective cohort study and the data were downloaded from the ‘DATADRYAD’ database (www.Datadryad.org).
Participants
The study included 210 418 adults without diabetes at baseline between 2010 and 2016 across 32 sites and 11 cities in China.
Setting
The target-independent and dependent variables were MAP measured at baseline and diabetes occurred during follow-up. Cox proportional hazards regression was used to explore the relationship between MAP and diabetes.
Primary outcome measures
The outcome was incident diabetes, which was defined as fasting blood glucose ≥7.00 mmol/L and/or self-reported diabetes during follow-up. Patients were censored either at the time of the diagnosis or at the last visit, whichever comes first.
Results
3927 participants developed diabetes during a 5-year follow-up. After adjusting covariates, MAP positively correlated with diabetes (HR=1.008, 95% CI 1.005 to 1.011, p<0.001), and the absolute risk difference was 0.02%. E-value analysis and multiple imputations were used to explore the robustness of the results. The relationship between MAP and diabetes was also non-linear, and the inflection point of MAP was 100.333 mm Hg. Subgroup analysis revealed a stronger association between MAP and diabetes in people with age (≥30,<50 years old), fasting plasma glucose <6.1 mmol/L and drinking. Additionally, receiver operating characteristic (ROC) curves showed the predictive performance of MAP for diabetes was similar to systolic blood pressure (SBP) (area under the curve (AUC)=0.694 with MAP vs AUC=0.698 with SBP).
Conclusions
MAP is an independent predictor for a 5-year risk of incident diabetes among Chinese adults. The relationship between MAP and diabetes is also non-linear. When MAP is below 100.333 mm Hg, MAP is closely positively related to diabetes.
Keywords: general diabetes, general endocrinology, risk management, diabetes & endocrinology
Strengths and limitations of this study.
Our research sample was large and participants were from multiple centres, well representative of the Chinese population.
We expounded a non-linear relationship, and it is the first study to identify the inflection point of mean arterial pressure’s effect on diabetes.
The subgroup analysis helped us explore other potential risks in the association between mean arterial pressure and incident diabetes.
The researchers did not perform a 2-hour oral glucose tolerance test or measure glycosylated haemoglobin level, which may underestimate the incidence of diabetes.
Introduction
The worldwide incidence of diabetes mellitus has increased significantly. Diabetes has emerged as a major epidemic in China. According to an extensive, nationally representative survey of Chinese adults, the estimated overall prevalence of diabetes had risen to be 10.9% in 2013.1 Diabetes has become one of the important public health issues that causes disability and premature death. It is a debilitating disease with potentially various complications, which reduces the quality of life and causes serious socioeconomic effects. Thence, identifying risk factors of incident diabetes is critical to prevent diabetes.
It is well known that hypertension and diabetes frequently coexist. Some researchers revealed that hypertension was closely related to impaired glucose tolerance and diabetes mellitus.2–4 A Chinese study showed that high blood pressure was positively related to incident diabetes.5 Some studies explored that elevated systolic blood pressure (SBP) levels by 1 mm Hg were associated with a 0.6%–4.0% increased risk of type 2 diabetes mellitus (T2DM).6–11 Similar findings showed that a 1 mm Hg increase in diastolic blood pressure (DBP) levels increased the risk of new-onset diabetes by 5.2%.12 SBP is the maximum pressure exerted on the arterial wall caused by the contraction of the ventricle, and DBP is the lowest pressure in the artery measured during ventricular relaxation.13 However, mean arterial pressure (MAP) is the average blood pressure throughout a cardiac cycle. Besides, MAP is the steady flow of blood through the aorta and its arteries, and MAP reflects peripheral resistance and cardiac output.14 In addition, MAP is a composite blood pressure index that considers SBP, DBP and pulse pressure (PP). Therefore, MAP can reflect blood pressure status more comprehensively. However, there were only a few studies that have assessed the relationship between MAP and incident diabetes. A rural Chinese cohort study demonstrated that MAP was positively correlated with T2DM in Chinese women.15 Moreover, two studies in Cameroon and Iran revealed that MAP was as strong a predictor of diabetes as SBP and DBP.16 17 Considering their small sample size and ethnic differences, we conducted the study to explore the potential relationship between MAP and incident diabetes in a large cohort of Chinese adults across 32 sites and 11 cities.
Methods
Data source, participants
The data of all participants were downloaded for free from the ‘DATADRYAD’ database (www.Datadryad.org). The raw data were provided by Chen et al,18 and the participant records were fully anonymised before we accessed them. The original study enrolled 685 277 adult Chinese persons >20 years old with at least two visits between 2010 and 2016 across 32 sites and 11 cities in China. Variables were as follows: age, gender, body mass index (BMI), DBP, SBP, fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum creatinine (Scr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), smoking and drinking status, family history of diabetes, year of follow-up and censor of diabetes at follow-up. In our research, we added MAP and PP. Participants were excluded at baseline in the original study, as follows: (1) no available information on weight, height and gender, (2) extreme BMI values (<15 kg/m2 or >55 kg/m2), (3) visit intervals <2 years, (4) no available FPG value, (5) participants diagnosed with diabetes at baseline and participants with undefined diabetes status at follow-up. We further excluded participants with incomplete blood pressure (n=24). To reduce interference, we excluded those whose MAP was below means minus three SD or more than means plus three SD (n=1391).19 Finally, 2 10 418 subjects were included in the secondary analysis.
Study design
The original study documented the design of the study.18 All subjects were required to do a questionnaire about demographics and lifestyle when they visited the health check-up centre. Trained staff measured their height and weight. Weight was measured in light clothing without shoes to the nearest 0.1 kg. The height is accurate to 0.1 cm. BMI was equal to the weight divided by the square of height, which is accurate to 0.1 kg/m2. Fasting venous blood samples were collected after fasting at least 10 hours after each visit. An automatic analyser measured FPG, Scr, TC, TG, HDL-C, LDL-C, ALT and AST. The staff used a standard mercury sphygmomanometer to measure their blood pressure. MAP and PP were calculated as MAP=1/3 SBP +2/3 DBP and PP=SBP–DBP.20 The data were collected under standardised conditions and carried out by trained staff in accordance with uniform procedures. Laboratory methods were carefully standardised through strict internal and external quality control. The target independent and dependent variables were MAP measured at baseline and incident diabetes during follow-up, respectively. As a retrospective cohort study, it decreased the risk of selection bias and observation bias.
Diagnosis criteria
The definitions of diabetes were fasting blood glucose ≥7.00 mmol/L and/or self-reported diabetes during follow-up. Patients were censored either at the time of the diagnosis or at the last visit, whichever comes first.
Patient and public involvement
Given this was a secondary retrospective cohort study, no patient was involved in the study.
Statistical analysis
First, we dealt with the missing values of covariates. Missing continuous variables were mainly supplemented by means or median. Since the missing values of HDL-C, LDL-C and AST were about 50%, we converted them as categorical variables based on the tertiles. And the missing categorical variables in each covariate were considered as a group.
Next, we analysed the baseline characteristics of participants. All participants were arranged into four groups, including low MAP group (MAP <80 mm Hg), medium MAP group (80≤MAP<90 mm Hg), high MAP group (90≤MAP<100 mm Hg) and very high MAP group (MAP ≥100 mm Hg). Continuous variables were described as the means±SDs (normal distribution) or medians (quartiles) (skewed distribution), and categorical variables were described as frequency or percentages. We tested differences between means and proportions of the groups based on the one-way ANOVA (analysis of variance) test for normally distributed quantitative variables, the Kruskal-Wallis H test for skewed quantitative variables and the χ2 test for categorical variables.21 Third, we calculated the person-years of follow-up from the first visit to the time of the diagnosis of diabetes or at the last visit, whichever came first.15 Person-years incidence and cumulative incidence were applied to describe the incidence rate.22 Cox proportional hazard regression models were used to detect the predictive role of MAP on the risk of diabetes. The results from unadjusted, minimally adjusted analyses and fully adjusted analyses were presented simultaneously in our study. The strategy for selecting covariates to adjust was mainly based on clinical experience, literature reports and statistical methods. The principle of statistical methods is that we adjusted the covariances which changed the matched HR by more than 10%.23 In the minimally adjusted model, we adjusted for the demographic covariates, including age, gender, BMI, family history of diabetes, smoking and drinking status. In fully adjusted model, we adjusted for all demographics and biochemical covariates, including age, gender, BMI, family history of diabetes, smoking and drinking status, FPG, TC, TG, HDL-C, LDL-C, ALT, AST and Scr. Additionally, we calculated the absolute risk difference (ARD). To do sensitivity analysis, we treated MAP as a categorical variable to explore the relationship between MAP level and incident diabetes. We explored the potential for unmeasured confounding between MAP and diabetes by calculating E-values.24 To ensure the robustness of results, multiple imputations were also used to replace the missing values to reduce the bias caused by missing covariables.25 The results of multiple regression analysis in this study adopted the values of imputed data, in which the estimates from each imputation were combined according to Rubin’s rules.26 Given that MAP was a continuous variable, we also verify the non-linear correlation of MAP and incident diabetes by using a generalised additive model (GAM). If the relationship was non-linear, from the perspective of a smoothing plot, a two-stage linear regression model would calculate the threshold effect of the MAP on diabetes. If there was an evident relationship between MAP and diabetes, it would calculate the inflection point. Moreover, the Cox proportional hazard models were used to do subgroup analysis (age, gender, BMI, FPG, HDL-C, LDL-C, family history of diabetes, smoking and drinking status). According to the clinical cut point or binary, the continuous variables were converted to categorical variables. Each stratification has undergone a fully adjusted analysis, except for the stratification factor itself. The likelihood ratio test was used to examine the modifications and interactions of subgroups. The Kaplan-Meier method was used to compare survival estimates and cumulative event rates. And the log-rank test was conducted to compare the Kaplan-Meier HRs for adverse events. The impact of SBP, DBP, PP and MAP on incident diabetes was evaluated by the receiver operating characteristics (ROC) curve.
Statistical analyses were done by the statistical software package R (http://www.R-project.org, The R Foundation) and Empower-Stats (http://www.empowerstats.com, X&Y Solutions, Boston, MA). The tests were two-tailed, and p<0.05 was statistically significant.
Results
Our study included a total of 210 418 participants (54.7% men and 45.3% women). The mean age of all participants was 42.0±12.6 years old. During the 5-year follow-up period, 3927 participants developed diabetes. The mean SBP, DBP, PP and MAP were 118.7±15.8 mm Hg, 73.9±10.4 mm Hg, 44.8±11.6 mm Hg, 88.9±11.2 mm Hg, respectively. The mean FPG was 4.9±0.6 mmol/L. The number of participants with the missing value of TC, TG, HDL-C and LDL-C was 4854, 4887, 94 000 and 92874, respectively. Besides, the missing value of Scr, ALT and AST was 11173, 1782 and 122458, respectively. In addition, the missing value of smoking and drinking status was 150 497 and 150 497, respectively.
Baseline characteristics of participants
Table 1 illustrated basic clinical measurements, biochemical tests and other parameters of the participants. We divided participants into four groups, including low MAP group (MAP <80 mm Hg), medium MAP group (80≤MAP<90 mm Hg), high MAP group (90≤MAP<100 mm Hg) and very high MAP group (MAP ≥100 mm Hg). The results showed that in the very high MAP group, the subjects had higher age, BMI, SBP, DBP, PP, MAP, FPG, Scr, TC, TG, ALT, AST and more current smoker and drinker. Besides, fewer participants at high HDL-C level in the very high MAP group and more participants at high LDL-C level. In addition, the low MAP group (MAP <80 mm Hg) had a higher incidence of family history of diabetes.
Table 1.
The baseline characteristics of participants
| MAP group | Low MAP group (MAP <80 mm Hg) | Medium MAP group (80≤MAP<90 mm Hg) | High MAP group (90≤MAP<100 mm Hg) | Very high MAP group (MAP ≥100 mm Hg) | P value |
| Participants | 47 430 | 70 126 | 57 530 | 35 332 | |
| Age (years) | 38.30±9.74 | 39.96±11.33 | 43.17±13.21 | 49.20±14.12 | <0.001 |
| Gender | <0.001 | ||||
| Male | 14 926 (31.47%) | 37 150 (52.98%) | 38 008 (66.07%) | 25 017 (70.81%) | |
| Female | 32 504 (68.53%) | 32 976 (47.02%) | 19 522 (33.93%) | 10 315 (29.19%) | |
| BMI (Kg/m2) | 21.60±2.73 | 22.77±3.08 | 23.91±3.29 | 25.12±3.37 | <0.001 |
| SBP (mm Hg) | 100.88±7.28 | 113.80±7.63 | 125.70±8.29 | 141.09±11.76 | <0.001 |
| DBP (mm Hg) | 61.60±4.44 | 70.47±4.13 | 78.81±4.58 | 89.45±6.48 | <0.001 |
| PP (mm Hg) | 39.28±8.32 | 43.32±10.10 | 46.89±11.38 | 51.64±13.82 | <0.001 |
| MAP (mm Hg) | 74.69±3.93 | 84.92±2.85 | 94.44±2.85 | 106.66±5.62 | <0.001 |
| FPG (mmol/L) | 4.75±0.55 | 4.87±0.58 | 4.98±0.62 | 5.11±0.66 | <0.001 |
| Scr (umol/L) | 64.99±13.89 | 69.61±14.93 | 72.38±15.12 | 73.78±16.38 | <0.001 |
| TC (mmol/L) | 4.51±0.83 | 4.64±0.86 | 4.78±0.90 | 4.96±0.93 | <0.001 |
| TG (mmol/L) | 0.84 (0.61–1.17) | 1.01 (0.71–1.46) | 1.19 (0.83–1.77) | 1.41 (1.00–2.09) | <0.001 |
| HDL-C (mmol/L) | <0.001 | ||||
| Low | 6775 (14.28%) | 12 260 (17.48%) | 11 615 (20.19%) | 7884 (22.31%) | |
| Medium | 8399 (17.71%) | 12 574 (17.93%) | 10 317 (17.93%) | 6688 (18.93%) | |
| High | 10 777 (22.72%) | 13 261 (18.91%) | 9696 (16.85%) | 6172 (17.47%) | |
| Not recorded | 21 479 (45.29%) | 32 031 (45.68%) | 25 902 (45.02%) | 14 588 (41.29%) | |
| LDL-C (mmol/L) | <0.001 | ||||
| Low | 10 722 (22.61%) | 13 561 (19.34%) | 9421 (16.38%) | 5163 (14.61%) | |
| Medium | 8756 (18.46%) | 12 905 (18.40%) | 10 658 (18.53%) | 6854 (19.40%) | |
| High | 6559 (13.83%) | 11 912 (16.99%) | 11 938 (20.75%) | 9095 (25.74%) | |
| Not recorded | 21 393 (45.10%) | 31 748 (45.27%) | 25 513 (44.35%) | 14 220 (40.25%) | |
| ALT (U/L) | 14.20 (11.00–20.10) | 17.20 (12.40–25.90 | 20.00 (14.30–31.00) | 22.60 (16.00–34.00) | <0.001 |
| AST (U/L) | <0.001 | ||||
| Low | 9198 (19.39%) | 10 381 (14.80%) | 6586 (11.45%) | 3158 (8.94%) | |
| Medium | 6386 (13.46%) | 9877 (14.08%) | 8166 (14.19%) | 4794 (13.57%) | |
| High | 4126 (8.70%) | 8869 (12.65%) | 9409 (16.35%) | 7010 (19.84%) | |
| Not recorded | 27 720 (58.44%) | 40 999 (58.46%) | 33 369 (58.00%) | 20 370 (57.65%) | |
| Smoking status | <0.001 | ||||
| Current smoker | 1563 (3.30%) | 3856 (5.50%) | 3982 (6.92%) | 2576 (7.29%) | |
| Ever smoker | 339 (0.71%) | 839 (1.20%) | 861 (1.50%) | 507 (1.43%) | |
| Never smoker | 9831 (20.73%) | 15 600 (22.25%) | 13 006 (22.61%) | 6961 (19.70%) | |
| Not recorded | 35 697 (75.26%) | 49 831 (71.06%) | 39 681 (68.97%) | 25 288 (71.57%) | |
| Drinking status | <0.001 | ||||
| Current drinker | 126 (0.27%) | 345 (0.49%) | 467 (0.81%) | 391 (1.11%) | |
| Ever drinker | 1193 (2.52%) | 3040 (4.34%) | 3043 (5.29%) | 1635 (4.63%) | |
| Never drinker | 10 414 (21.96%) | 16 910 (24.11%) | 14 339 (24.92%) | 8018 (22.69%) | |
| Not recorded | 35 697 (75.26%) | 49 831 (71.06%) | 39 681 (68.97%) | 25 288 (71.57%) | |
| Family history of diabetes | <0.001 | ||||
| No | 46 324 (97.67%) | 68 599 (97.82%) | 56 411 (98.05%) | 34 753 (98.36%) | |
| Yes | 1106 (2.33%) | 1527 (2.18%) | 1119 (1.95%) | 579 (1.64%) |
Values are n (%) or mean±SD.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipid cholesterol; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride.
The incidence rate of incident diabetes
Table 2 revealed that 3927 participants developed diabetes during a 5-year follow-up. The total incidence rate of diabetes was 598.78 per 100 000 person-years. Specifically, the incidence rates of the four MAP groups were 190.02, 360.74, 698.40 and 1354.31 per 100 000 person-years, respectively. Compared with the low MAP group, participants with increased MAP levels had a higher cumulative incidence. The cumulative incidence of total incident diabetes and each of the MAP groups was 1.854% (1.797%–1.911%), 0.590% (0.521%–0.659%), 1.125% (1.047%–1.203%), 2.185% (2.065%–2.304%) and 4.209% (3.999%–4.418%), respectively.
Table 2.
Incidence rate of incident diabetes
| MAP (mm Hg) | Participants (n) | DM events (n) | Cumulative incidence (95% CI) | Per 100 000 person-year |
| Total | 210 418 | 3927 | 1.854% (1.797% to 1.911%) | 598.78 |
| MAP group | ||||
| Low MAP group (MAP <80 mm Hg) | 47 430 | 280 | 0.590% (0.521% to 0.659%) | 190.02 |
| Medium MAP group (80≤MAP<90 mm Hg) |
70 126 | 789 | 1.125% (1.047% to 1.203%) | 360.74 |
| High MAP group (90≤MAP<100 mm Hg) |
57 530 | 1257 | 2.185% (2.065% to 2.304%) | 698.40 |
| very high MAP group (MAP ≥100 mm Hg) | 35 332 | 1487 | 4.209% (3.999% to 4.418%) | 1354.31 |
DM, diabetes mellitus; MAP, mean arterial pressure.
Univariate analysis
Table 3 demonstrated a positive association between age, BMI, SBP, DBP, PP, MAP, FPG, Scr, TC, TG, LDL-C, ALT, AST, family history of diabetes, smoking, drinking and incident diabetes. In contrast, HDL-C negatively correlated with incident diabetes. Meanwhile, men had a higher risk of developing diabetes than women.
Table 3.
The results of univariate analysis
| Statistics | HR (95% CI) | P value | |
| Age (years) | 42.018±12.603 | 1.066 (1.063 to 1.068) | <0.001 |
| Gender | |||
| Male | 115 101 (54.701%) | 1.0 | |
| Female | 95 317 (45.299%) | 0.499 (0.465 to 0.535) | <0.001 |
| BMI (Kg/m2) | 23.214±3.330 | 1.236 (1.227 to 1.245) | <0.001 |
| SBP (mm Hg) | 118.722±15.827 | 1.043 (1.041 to 1.044) | <0.001 |
| DBP (mmHg) | 73.937±10.397 | 1.050 (1.047 to 1.053) | <0.001 |
| PP (mmHg) | 44.784±11.563 | 1.041 (1.038 to 1.043) | <0.001 |
| MAP (mmHg) | 88.866±11.218 | 1.059 (1.057 to 1.062) | <0.001 |
| FPG (mmol/L) | 4.913±0.610 | 10.821 (10.336 to 11.329) | <0.001 |
| Scr (umol/L) | 70.027±15.329 | 1.006 (1.005 to 1.007) | <0.001 |
| TC (mmol/L) | 4.704±0.889 | 1.429 (1.387 to 1.472) | <0.001 |
| TG (mmol/L) | 1.328±1.015 | 1.263 (1.251 to 1.275) | <0.001 |
| HDL-C (mmol/L) | |||
| Low | 38 534 (18.313%) | 1.0 | |
| Medium | 37 978 (18.049%) | 0.873 (0.795 to 0.959) | 0.005 |
| High | 39 906 (18.965%) | 0.781 (0.709 to 0.860) | <0.001 |
| Not recorded | 94 000 (44.673%) | 0.585 (0.539 to 0.634) | <0.001 |
| LDL-C (mmol/L) | |||
| Low | 38 867 (18.471%) | 1.0 | |
| Medium | 39 173 (18.617%) | 1.134 (1.023 to 1.258) | 0.017 |
| High | 39 504 (18.774%) | 1.672 (1.519 to 1.841) | <0.001 |
| Not recorded | 92 874 (44.138%) | 0.791 (0.720 to 0.868) | <0.001 |
| ALT (U/L) | 23.855±22.007 | 1.004 (1.004 to 1.005) | <0.001 |
| AST (U/L) | |||
| Low | 29 323 (13.936%) | 1.0 | |
| Medium | 29 223 (13.888%) | 1.424 (1.239 to 1.636) | <0.001 |
| High | 29 414 (13.979%) | 2.798 (2.467 to 3.173) | <0.001 |
| Not recorded | 122 458 (58.197%) | 1.368 (1.217 to 1.538) | <0.001 |
| Smoking status | |||
| Current smoker | 11 977 (5.692%) | 1.0 | |
| Ever smoker | 2546 (1.210%) | 0.813 (0.628 to 1.051) | 0.113 |
| Never smoker | 45 398 (21.575%) | 0.449 (0.395 to 0.511) | <0.001 |
| Not recorded | 150 497 (71.523%) | 0.596 (0.535 to 0.665) | <0.001 |
| Drinking status | |||
| Current drinker | 1329 (0.632%) | 1.0 | <0.001 |
| Ever drinker | 8911 (4.235%) | 0.484 (0.346 to 0.677) | |
| Never drinker | 49 681 (23.611%) | 0.482 (0.355 to 0.655) | <0.001 |
| Not recorded | 150 497 (71.523%) | 0.510 (0.377 to 0.689) | <0.001 |
| Family history of diabetes | |||
| No | 206 087 (97.942%) | 1.0 | |
| Yes | 4331 (2.058%) | 1.733 (1.477 to 2.033) | <0.001 |
Values are n (%) or mean±SD.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipid cholesterol; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride.
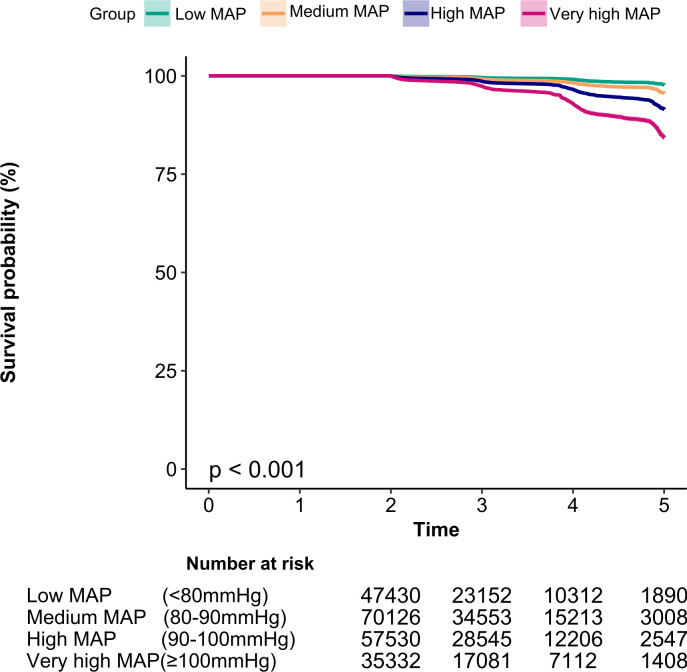
Figure 1 described the results of Kaplan-Meier curves of the cumulative hazards. The risk of developing diabetes was different between the four MAP groups (log-rank test, p<0.001). With the increase in MAP level, the cumulative risk of diabetes gradually increased. Thus, the very high MAP group faced the maximum risk of diabetes.
Figure 1.
Kaplan-Meier event-free survival curve of incident diabetes based on MAP groups (log-rank, p<0.001). MAP, mean arterial pressure.
The results of the relationship between MAP and incident diabetes
Table 4 showed the Cox proportional hazard regression model, which assessed the relationship between MAP and incident diabetes. We simultaneously presented the non-adjusted and two adjusted models. In non-adjusted model, MAP was positively correlated with diabetes (HR=1.059, 95% CI 1.057 to 1.062, p<0.001), and the ARD was 0.09%. In the minimally adjusted model (model I), we adjusted for the demographic covariates, including age, gender, BMI, SBP, DBP, family history of diabetes, smoking and drinking status, the results did not change significantly (HR: 1.018, 95% CI 1.015 to 1.021, p<0.001), and the ARD was 0.02%. In the fully adjusted model (model II), we adjusted for all demographics and biochemical covariates extracted from the raw data, including age, gender, BMI, FPG, TC, TG, HDL-C, LDL-C, ALT, AST, Scr, family history of diabetes, smoking and drinking status. We found that the relationship still exists (HR=1.008, 95% CI 1.005 to 1.011, p<0.001), and the ARD was 0.02%. The results showed that for every 1 mmHg increased in MAP, the risk of diabetes increased by 0.8%.
Table 4.
Relationship MAP and the incident diabetes in different models
| Variable | Crude model HR, 95% CI, P value |
ARD | Model I HR, 95% CI, P value |
ARD | Model II HR, 95% CI, P value |
ARD |
| MAP | 1.059 (1.057 to 1.062) <0.001 | 0.09% | 1.018 (1.015 to 1.021) <0.001 | 0.02% | 1.008 (1.005 to 1.011) <0.001 | 0.02% |
| MAP group | ||||||
| Low MAP group (MAP <80 mm Hg) | Ref. | Ref. | Ref. | |||
| Medium MAP group (80≤MAP<90 mm Hg) |
1.886 (1.646 to 2.162)<0.001 | 0.53% | 1.249 (1.089 to 1.433) 0.002 | 0.11% | 1.070 (0.932 to 1.228) 0.339 | 0.30% |
| High MAP group (90≤MAP<100 mm Hg) |
3.648 (3.204 to 4.152)<0.001 | 1.59% | 1.580 (1.383 to 1.805)<0.001 | 0.36% | 1.177 (1.029 to 1.345) 0.017 | 0.57% |
| Very high MAP group (MAP ≥100 mm Hg) | 7.219 (6.354 to 8.203)<0.001 | 3.62% | 1.896 (1.657 to 2.169)<0.001 | 0.54% | 1.265 (1.105 to 1.448) 0.001 | 0.72% |
Crude model: we did not adjust for other covariates.
Model I: we adjust for age, gender, BMI, family history of diabetes, smoking and drinking status.
Model II: we adjust for age, gender, BMI, FPG, TC, TG, HDL-C, LDL-C, ALT, AST, Scr, family history of diabetes, smoking and drinking status.
ALT, alanine aminotransferase; ARD, absolute risk difference; AST, aspartate aminotransferase; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratios; LDL-C, low-density lipid cholesterol; Ref, reference; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride.
Sensitivity analysis
We converted MAP into a categorical variable. Compared with the low MAP group in the full model, the risk of diabetes increased by 26.5%, and the absolute risk difference was 0.72% increased in the very high MAP group. In addition, as the level of MAP increases, the risk of diabetes increased accordingly (table 4). Besides, we generated an E-value to assess the sensitivity to unmeasured confounding. The E-value was 1.10. The E-value was greater than the RR of unmeasured confounders and incident diabetes, suggesting unmeasured or unknown confounders had little effect on the relationship between MAP and diabetes. After replacing the missing values through multiple imputations, the relationship between MAP and incident diabetes did not change (HR=1.008, 95% CI 1.005 to 1.011, p<0.001) (Supplementary file). The results showed that our findings were robust.
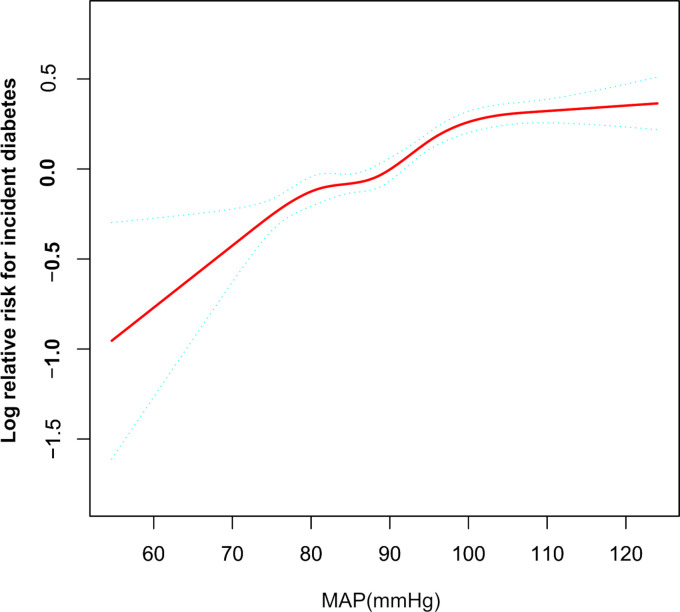
The analysis of the non-linear relationship
We established a GAM to verify the non-linearity in the association between MAP and incident diabetes (figure 2). The result showed a non-linear relationship between MAP and diabetes (after adjusting for age, gender, BMI, TC, TG, HDL-C, LDL-C, ALT, AST, Scr, family history of diabetes, smoking and drinking status). According to a two-piecewise linear regression model, we found that the inflection point of MAP was 100.333 mm Hg (log-likelihood ratio test p<0.001). When MAP was less than 100.333 mm Hg, MAP was positively related to diabetes (HR:1.022, 95% CI 1.017 to 1.027, p<0.001). In contrast, when MAP was more than 100.333 mm Hg, their relationship tended to be saturated (HR: 1.005, 95% CI 0.998 to 1.012, p=0.163) (table 5).
Figure 2.
The non-linear relationship between MAP and incident diabetes. A non-linear relationship between MAP and incident diabetes was probed after adjusting for age, gender, BMI, TC, TG, HDL-C, LDL-C, ALT, AST, Scr, family history of diabetes, smoking and drinking status. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratios; LDL-C, low-density lipid cholesterol; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride.
Table 5.
The result of two-piecewise linear regression model
| Incident diabetes (HR, 95% CI, P value) | |
| Fitting model by standard linear regression | 1.015 (1.012 to 1.018)<0.001 |
| Fitting model by two-piecewise linear regression | |
| Inflection point of MAP (mm Hg) | 100.333 |
| ≤100.333 | 1.022 (1.017 to 1.027)<0.001 |
| >100.333 | 1.005 (0.998 to 1.012) 0.163 |
| P for log likelihood ratio test | <0.001 |
We adjusted age, gender, BMI, TC, TG, HDL-C, LDL-C, ALT, AST, Scr, family history of diabetes, smoking and drinking status.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipid cholesterol; MAP, mean arterial pressure; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride.
The results of subgroup analysis
We performed a subgroup analysis to detect other potential risks of the relationship between MAP and incident diabetes. We treated age, gender, BMI, FPG, HDL-C, LDL-C, family history of diabetes, smoking and drinking status as the stratification variables to evaluate the trend of effect sizes in these variables. Table 6 showed that age, FPG and drinking could modify the relationship between MAP and diabetes (all p values for interaction <0.05). We found a stronger association in the population with age (≥30,<50 years old) (HR=1.021, 95% CI 1.011 to 1.030, p<0.001), FPG <6.1 mmol/L (HR=1.016, 95% CI 1.012 to 1.020, p<0.001) and drinking (current drinker: HR=1.029, 95% CI 0.998 to 1.060, p=0.063; ever drinker: HR=1.018, 95% CI 1.002 to 1.034, p=0.025).
Table 6.
Effect size of MAP on diabetes in prespecified and exploratory subgroups
| Characteristic | Participants | HR (95% CI) P value for interaction |
| Age (years) | <0.001 | |
| 20 to <30 | 28 597 | 1.000 (0.973 to 1.028) 0.985 |
| 30 to <40 | 82 782 | 1.021 (1.011 to 1.030)<0.001 |
| 40 to <50 | 45 093 | 1.012 (1.005 to 1.019) 0.001 |
| 50 to <60 | 29 609 | 1.006 (1.001 to 1.012) 0.020 |
| 60 to <70 | 17 271 | 1.001 (0.995 to 1.008) 0.658 |
| ≥70 | 7066 | 0.999 (0.991 to 1.007) 0.881 |
| Gender | 0.085 | |
| Male | 115101 | 1.005 (1.002 to 1.009) 0.003 |
| Female | 95 317 | 1.011 (1.006 to 1.016)<0.001 |
| BMI (kg/m2) | 0.943 | |
| <18.5 | 12 066 | 0.997 (0.958 to 1.038) 0.892 |
| ≥18.5,<24 | 116 485 | 1.008 (1.002 to 1.014) 0.005 |
| ≥24,<28 | 64 156 | 1.009 (1.004 to 1.013)<0.001 |
| ≥28 | 17 711 | 1.007 (1.001 to 1.014) 0.015 |
| FPG (mmol/L) | <0.001 | |
| <6.1 | 203 401 | 1.016 (1.012 to 1.020)<0.001 |
| ≥6.1 | 7017 | 0.999 (0.994 to 1.003) 0.603 |
| HDL-C (mmol/L) | 0.242 | |
| Low | 38 534 | 1.009 (1.003 to 1.015) 0.003 |
| Medium | 37 978 | 0.999 (0.992 to 1.005) 0.686 |
| High | 39 906 | 1.005 (0.998 to 1.012) 0.204 |
| Not recorded | 94 000 | 1.011 (1.006 to 1.017)<0.001 |
| LDL-C (mmol/L) | 0.523 | |
| Low | 38 867 | 1.009 (1.002 to 1.016) 0.016 |
| Medium | 39 173 | 1.006 (0.999 to 1.013) 0.082 |
| High | 39 504 | 1.002 (0.996 to 1.008) 0.505 |
| Not recorded | 92 874 | 1.012 (1.006 to 1.017)<0.001 |
| Smoking status | 0.188 | |
| Current smoker | 11 977 | 1.008 (0.998 to 1.018) 0.138 |
| Ever smoker | 2546 | 1.040 (1.014 to 1.066) 0.002 |
| Never smoker | 45 398 | 1.015 (1.007 to 1.023)<0.001 |
| Not recorded | 150 497 | 1.006 (1.003 to 1.010)<0.001 |
| Drinking status | 0.047 | |
| Current drinker | 1329 | 1.029 (0.998 to 1.060) 0.063 |
| Ever drinker | 8911 | 1.018 (1.002 to 1.034) 0.025 |
| Never drinker | 49 681 | 1.010 (1.003 to 1.017) 0.003 |
| Not recorded | 150 497 | 1.007 (1.003 to 1.010)<0.001 |
| Family history of diabetes | 0.109 | |
| No | 206 087 | 1.007 (1.004 to 1.010)<0.001 |
| Yes | 4331 | 1.020 (1.005 to 1.035) 0.010 |
Note 1: Above model adjusted for age, gender, BMI, FPG, TC, TG, HDL-C, LDL-C, ALT, AST, Scr, family history of diabetes, smoking and drinking status.
Note 2: In each case, the model is not adjusted for the stratification variable.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipid cholesterol; Scr, serum creatinine; TC, total cholesterol; TG, triglyceride.
Cut-off point of blood pressure for predicting incident diabetes
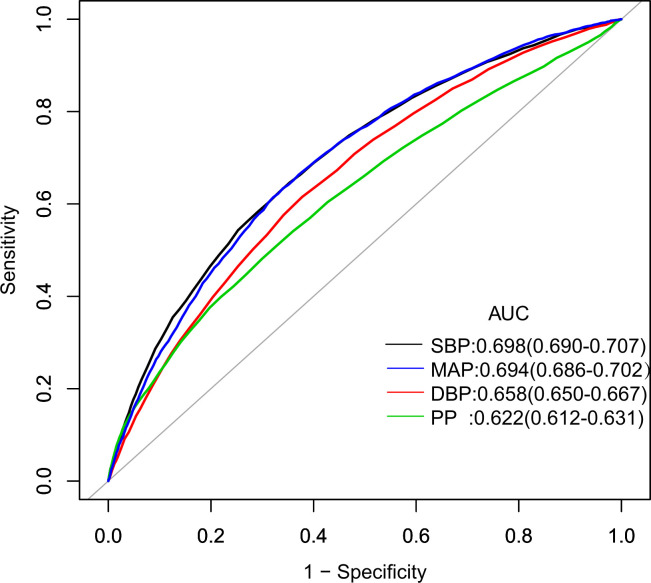
We analysed the performances and optimal value of cut-off point of various blood pressure indices for predicting incident diabetes, including SBP, DBP, PP and MAP. Our results supported that all four blood pressure indices were associated with the risk of diabetes. Areas under the ROC curves were 0.698 (95% CI 0.690 to 0.707) for SBP, 0.694 (95% CI 0.686 to 0.702) for MAP, 0.658 (95% CI 0.650 to 0.667) for DBP and 0.622 (95% CI 0.612 to 0.631) for PP, respectively. The optimal cut-off point of MAP was 92.833 mm Hg, sensitivity was 63.41%, and specificity was 65.93%. As an indicator reflecting the blood pressure status comprehensively, MAP was similar to SBP in predicting diabetes risk. Moreover, MAP was a better predictor of diabetes risk than DBP and PP (figure 3).
Figure 3.
Receiver operating characteristics (ROC) curves with incident diabetes. ROC curves with incident diabetes as the status variable. The areas under the receiver operating characteristics curve (AUCs) were SBP, DBP, MAP and PP. DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
Discussion
The present study showed that MAP was an independent predictor for a 5-year risk of incident diabetes after adjusting some covariates. Furthermore, taking MAP equal to the 100.333 mm Hg boundary, this relationship was different on both sides (left (HR:1.022, 95% CI 1.017 to 1.027, p<0.001)); right (HR: 1.005, 95% CI 0.998 to 1.012, p=0.163)). When MAP was below 100.333 mm Hg, the relationship between MAP and incident diabetes is significant. Subgroup analysis showed a stronger association in the population with age (≥30, <50 years old), FPG <6.1 mmol/L and drinking. Additionally, MAP and SBP have quite similar predictive performance for diabetes (area under the curve (AUC)=0.694 with MAP vs AUC=0.698 with SBP).
Previously, some studies have probed the potential relationship between MAP and incident diabetes. However, most studies were not conducted in the Chinese population.16 17 In an Iranian study with 701 participants,16 the researchers found that MAP played the same role in predicting the progression of diabetes as SBP and DBP. The AUC for diabetes was 0.589 for MAP, 0.582 for SBP and 0.658 for DBP. In comparison, the AUCs in our study were larger, 0.694 for MAP, 0.698 for SBP and 0.658 for DBP.16 The difference may be caused by our study’s larger sample size and longer follow-up years. Another study in Cameroon also reached similar conclusions: MAP, SBP and DBP were significantly correlated with diabetes.16 Contrary to the results of these studies, blood pressure could not predict diabetes risk in a case-referent study among 33 336 participants.27 A similar study in a Chinese population showed that SBP and DBP were not predictors of incident diabetes.28 We compared these studies mentioned above, and the inconsistent results may come from the following points: (1) the research population was different and the sample size significantly differed, (2) these findings did not expound the non-linear relationship, (3) these studies did not consider the effect of some important covariates on the relationship between MAP and diabetes. In short, our findings further confirmed that MAP was an independent predictor for diabetes risk in a large Chinese cohort.
A recent study based on 12 284 participants from rural areas in China’s Henan province showed that an increase in MAP could predict the risk of T2DM in women. In our study, the Cox proportional hazard regression model revealed that MAP was positively related to diabetes in women, consistent with that study. However, we found that this relationship also exists in men. The difference may be that our research sample was larger (210 418), and they were from multiple centres, more representative of the Chinese population. Besides, we adjusted different covariates. We adjusted age FPG, ALT, AST and Scr than their research, and they were linked to MAP and diabetes in previous studies.29–32 In their study, they found a non-linear association between MAP and T2DM. But they did not mention the inflection point. In contrast, we used a two-stage linear regression model to describe the non-linear relationship between MAP and diabetes. We found that the inflection point of MAP was 100.333 mm Hg. When MAP was below 100.333 mm Hg, an increase in MAP caused an increased risk of developing diabetes. When MAP was more than 100.333 mm Hg, their relationship tended to be saturated. Besides, we found that there was a stronger association in the population with age (≥30,<50 years old), FPG <6.1 mmol/L and drinking, which may be due to the fact that in the population with age (<30 or ≥50 years old), FPG ≥6.1 mmol/L and never drinking, the impact of other risk factors on the incident diabetes exceeds the effects of MAP on diabetes. It is worth mentioning that we analysed the performances and optimal value of the cut-off point of various blood pressure indices. The ROC curve showed that the predictive performance of MAP and SBP was similar in predicting diabetes risk, which was better than DBP and PP. Given that MAP is the average blood pressure throughout a cardiac cycle and a composite blood pressure index that reflects blood pressure status more comprehensively, the effect of MAP on the incident diabetes can better reflect the relationship between blood pressure and diabetes in the real world. Our findings illustrated that people with MAP<100.333 mm Hg could pay more attention to control MAP to prevent incident diabetes, especially controlling MAP below 92.833 mm Hg. Our findings may be helpful for future research to establish a diagnostic or predictive model of the risk of incident diabetes. And a detailed understanding of MAP as a potential risk factor for incident diabetes will help clinicians provide more personalised prevention and management protocols.
Hypertension and diabetes are often concurrent. Several previous studies showed that hypertension and diabetes have common mediators, including obesity, inflammation, oxidative stress, insulin resistance and endothelial dysfunction.33–35 So far, researchers have not discovered a direct causal nexus between hypertension and diabetes. Given MAP is a composite blood pressure indicator, elevated MAP is caused by elevated systolic and/or DBP. High blood pressure could lead to endothelial dysfunction, then reduced peripheral vascular flow, which affects insulin delivery and increases insulin resistance.36–38 It was assumed that oxidative stress induced by hypertension could affect the function of pancreatic β cells, which in turn reduces insulin secretion.39 Besides, glucose metabolism can be modified by oxidative stress-related cytokines, which may be indirectly related to diabetes.40 Meanwhile, some researches showed that patients with diabetes and hypertension have low-grade inflammatory reactions.41–43 Correspondingly, inflammatory markers such as adhesion molecules, cytokines and C reactive protein are increased in these patients.44 Another study found that about 50% of patients with essential hypertension appear to develop insulin resistance and have an increased risk of diabetes.45 Thus, insulin resistance could be one of the potential links between blood pressure and diabetes. To our knowledge, high MAP can cause arterial stiffness to progress.46 However, arterial stiffness could cause impaired microcirculation, and insulin cannot be delivered to target tissues, affecting glucose metabolism and leading to diabetes.47
There were some strengths in our study, as follows: (1) our research sample was large and participants were from multiple centres, more representative of the Chinese population, (2) our study quantitatively assessed the specific relationship between MAP and diabetes, (3) we expounded a non-linear relationship and it was the first study to identify the inflection point of MAP’s effect on diabetes, (4) we used rigorous statistical adjustments to reduce confounders' interference with the results, (5) we treated the MAP as a categorical variable, E-value analysis and multiple imputations to do a sensitivity analysis, (6) the subgroup analysis helped us explore other potential risks in the association between MAP and incident diabetes, (7) we used the ROC curves to compare the predictive performance of various blood pressure indices for the risk of diabetes.
There were still some potential limitations. First, the raw data were from the Chinese population; thus, it needs caution to translate and generalise our findings to other races. The generalisability of our findings might be limited. Besides, other related factors were not included in the data, such as glycosylated haemoglobin, medication history, socioeconomic factors, etc. Second, they did not perform a 2-hour oral glucose tolerance test. According to the 1999 WHO diagnostic criteria for diabetes, the definition of diabetes in our study may lead to miss some diabetic patients.48 However, the 2-hour oral glucose tolerance test was not feasible in such a large cohort. Third, there were some missing values in several variables. However, in order to control bias, we did not exclude missing values for covariates. We mainly supplemented the missing continuous variables with means or median, and others were converted as categorical variables. Besides, we added the multiple imputation method to do sensitivity analysis. Fourth, we only measured MAP and other parameters at baseline, and we did not focus on their changes during follow-up. Fifth, the potential for residual confounding exists in our study, as with all retrospective analysis. However, we adjusted for some confounding factors to the possible influences, and we used the E-value sensitivity analysis to quantify the potential implications of unmeasured confounders and found that unmeasured confounders were unlikely to explain the findings. Sixth, the follow-up duration of this study was 5 years. Once the follow-up time was longer, the relationship between MAP and diabetes may be more significant. Finally, the conclusions were based on retrospective observational design, so prospective studies were needed to further evaluate the relationship between MAP and diabetes.
Conclusion
MAP is an independent predictor for 5-year risk of incident diabetes among Chinese adults. The relationship between MAP and incident diabetes is also non-linear. MAP is positively correlated with incident diabetes when MAP is below 100.333 mm Hg. A detailed understanding of MAP as a potential risk factor for incident diabetes will help clinicians provide more personalised prevention and management protocols. This retrospective observational study provides association inference rather than establishing a causal relationship between MAP and diabetes. Therefore, our findings need to be interpreted cautiously and further validated by prospective research.
Supplementary Material
Footnotes
YW and HH contributed equally.
Contributors: YW and HH conceived and designed the research, drafted the manuscript. JC and RC did statistical analysis. XZ and HC took part in the discussion. DY revised the manuscript and acts as the guarantor. All authors read and approved the final manuscript.
Funding: This study was supported by the Discipline Construction Ability Enhancement Project of Shenzhen Municipal Health Commission (SZXJ2017031).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data sharing statementThe raw data can be downloaded from ‘DATADRYAD’ database (www.Datadryad.org). Dryad Digital Repository. https://datadryad.org/stash/dataset/doi:10.5061/dryad.ft8750v.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The original study followed guidelines outlined by the Helsinki Declaration and was approved by the Rich Healthcare Group Review Board, as did our secondary retrospective cohort study. The information was retrieved retrospectively and patient consent was not required.
References
- 1.Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515–23. 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim M-J, Lim N-K, Choi S-J, et al. Hypertension is an independent risk factor for type 2 diabetes: the Korean genome and epidemiology study. Hypertens Res 2015;38:783–9. 10.1038/hr.2015.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutter MK. Night-Time blood pressure: a role in the prediction and prevention of diabetes? Diabetologia 2016;59:234–6. 10.1007/s00125-015-3814-2 [DOI] [PubMed] [Google Scholar]
- 4.Wu YT, Song L, Liu XX, et al. Time-cumulated blood pressure exposure and incident impairment of glucose tolerance and diabetes mellitus. BMC Cardiovasc Disord 2017;17:106. 10.1186/s12872-017-0537-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun F, Tao Q, Zhan S. An accurate risk score for estimation 5-year risk of type 2 diabetes based on a health screening population in Taiwan. Diabetes Res Clin Pract 2009;85:228–34. 10.1016/j.diabres.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–75. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta AK, Dahlof B, Dobson J, et al. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial--Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care 2008;31:982–8. 10.2337/dc07-1768 [DOI] [PubMed] [Google Scholar]
- 8.Dudina A, Cooney MT, Bacquer DD, et al. Relationships between body mass index, cardiovascular mortality, and risk factors: a report from the SCORE Investigators. Eur J Cardiovasc Prev Rehabil 2011;18:731–42. 10.1177/1741826711412039 [DOI] [PubMed] [Google Scholar]
- 9.Sheikh MA, Lund E, Braaten T. The predictive effect of body mass index on type 2 diabetes in the Norwegian women and cancer study. Lipids Health Dis 2014;13:164. 10.1186/1476-511X-13-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emdin CA, Anderson SG, Woodward M, et al. Usual blood pressure and risk of new-onset diabetes: evidence from 4.1 million adults and a meta-analysis of prospective studies. J Am Coll Cardiol 2015;66:1552–62. 10.1016/j.jacc.2015.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aikens RC, Zhao W, Saleheen D, et al. Systolic blood pressure and risk of type 2 diabetes: a Mendelian randomization study. Diabetes 2017;66:543–50. 10.2337/db16-0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise J. High blood pressure is linked to increased risk of diabetes. BMJ 2015;351:h5167. 10.1136/bmj.h5167 [DOI] [PubMed] [Google Scholar]
- 13.Hsu C-H, Chang J-B, Liu I-C, et al. Mean arterial pressure is better at predicting future metabolic syndrome in the normotensive elderly: a prospective cohort study in Taiwan. Prev Med 2015;72:76–82. 10.1016/j.ypmed.2014.12.036 [DOI] [PubMed] [Google Scholar]
- 14.Safar ME. Pulse pressure in essential hypertension: clinical and therapeutical implications. J Hypertens 1989;7:769–76. 10.1097/00004872-198910000-00001 [DOI] [PubMed] [Google Scholar]
- 15.Guo C, Qin P, Li Q, et al. Association between mean arterial pressure and risk of type 2 diabetes mellitus: the rural Chinese cohort study. Prim Care Diabetes 2020;14:448–54. 10.1016/j.pcd.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 16.Janghorbani M, Amini M. Comparison of systolic and diastolic blood pressure with pulse pressure and mean arterial pressure for prediction of type 2 diabetes: the Isfahan diabetes prevention study. Endokrynol Pol 2011;62:324–30. [PubMed] [Google Scholar]
- 17.Mbanya VN, Mbanya J-C, Kufe C, et al. Effects of Single and Multiple Blood Pressure Measurement Strategies on the Prediction of Prevalent Screen-Detected Diabetes Mellitus: A Population-Based Survey. J Clin Hypertens 2016;18:864–70. 10.1111/jch.12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Zhang X-P, Yuan J, et al. Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study. BMJ Open 2018;8:e21768. 10.1136/bmjopen-2018-021768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Hu X, Zhang Q, et al. Non-high-density lipoprotein cholesterol: High-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: Results from a population-based cohort study. J Diabetes 2018;10:708–14. 10.1111/1753-0407.12650 [DOI] [PubMed] [Google Scholar]
- 20.Kodama S, Horikawa C, Fujihara K, et al. Meta-Analysis of the quantitative relation between pulse pressure and mean arterial pressure and cardiovascular risk in patients with diabetes mellitus. Am J Cardiol 2014;113:1058–65. 10.1016/j.amjcard.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Hu H, Chen M, et al. Association of triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis 2020;19:33. 10.1186/s12944-020-01213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin H, Chen Z, Zhang Y, et al. Triglyceride to high-density lipoprotein cholesterol ratio is associated with incident diabetes in men: a retrospective study of Chinese individuals. J Diabetes Investig 2020;11:192–8. 10.1111/jdi.13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12:1500–24. 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 24.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019;321:602–3. 10.1001/jama.2018.21554 [DOI] [PubMed] [Google Scholar]
- 25.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 26.Zou Y, Zhong L, Hu C, et al. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: a 5-year longitudinal cohort study. Lipids Health Dis 2021;20:28. 10.1186/s12944-021-01457-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norberg M, Stenlund H, Lindahl B, et al. Components of metabolic syndrome predicting diabetes: no role of inflammation or Dyslipidemia*. Obesity 2007;15:1875–85. 10.1038/oby.2007.222 [DOI] [PubMed] [Google Scholar]
- 28.Wang J-J, Qiao Q, Miettinen ME, et al. The metabolic syndrome defined by factor analysis and incident type 2 diabetes in a Chinese population with high postprandial glucose. Diabetes Care 2004;27:2429–37. 10.2337/diacare.27.10.2429 [DOI] [PubMed] [Google Scholar]
- 29.Balkau B, Soulimane S, Lange C, et al. Are the same clinical risk factors relevant for incident diabetes defined by treatment, fasting plasma glucose, and HbA1c? Diabetes Care 2011;34:957–9. 10.2337/dc10-1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao F, Huang X-L, Jiang X-P, et al. Independent effect of alanine transaminase on the incidence of type 2 diabetes mellitus, stratified by age and gender: a secondary analysis based on a large cohort study in China. Clin Chim Acta 2019;495:54–9. 10.1016/j.cca.2019.03.1636 [DOI] [PubMed] [Google Scholar]
- 31.Kunutsor SK, Abbasi A, Apekey TA. Aspartate aminotransferase - risk marker for type-2 diabetes mellitus or red herring? Front Endocrinol 2014;5:189. 10.3389/fendo.2014.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin P, Lou Y, Cao L, et al. Dose-Response associations between serum creatinine and type 2 diabetes mellitus risk: a Chinese cohort study and meta-analysis of cohort studies. J Diabetes 2020;12:594–604. 10.1111/1753-0407.13038 [DOI] [PubMed] [Google Scholar]
- 33.Reaven GM. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: similarities and differences. J Clin Hypertens 2011;13:238–43. 10.1111/j.1751-7176.2011.00439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung BMY, Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep 2012;14:160–6. 10.1007/s11883-012-0227-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnett DK. Analysis of large electronic health record databases supports blood Pressure-Incident diabetes association. J Am Coll Cardiol 2015;66:1563–5. 10.1016/j.jacc.2015.07.058 [DOI] [PubMed] [Google Scholar]
- 36.Julius S, Gudbrandsson T, Jamerson K, et al. The hemodynamic link between insulin resistance and hypertension. J Hypertens 1991;9:983–6. 10.1097/00004872-199111000-00001 [DOI] [PubMed] [Google Scholar]
- 37.Pinkney JH, Stehouwer CD, Coppack SW, et al. Endothelial dysfunction: cause of the insulin resistance syndrome. Diabetes 1997;46 Suppl 2:S9–13. 10.2337/diab.46.2.S9 [DOI] [PubMed] [Google Scholar]
- 38.Meigs JB, O'donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham offspring study. Diabetes 2006;55:530–7. 10.2337/diabetes.55.02.06.db05-1041 [DOI] [PubMed] [Google Scholar]
- 39.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 2004;24:816–23. 10.1161/01.ATV.0000122852.22604.78 [DOI] [PubMed] [Google Scholar]
- 40.Orban Z, Remaley AT, Sampson M, et al. The differential effect of food intake and beta-adrenergic stimulation on adipose-derived hormones and cytokines in man. J Clin Endocrinol Metab 1999;84:2126–33. 10.1210/jcem.84.6.5747 [DOI] [PubMed] [Google Scholar]
- 41.Lontchi-Yimagou E, Sobngwi E, Matsha TE, et al. Diabetes mellitus and inflammation. Curr Diab Rep 2013;13:435–44. 10.1007/s11892-013-0375-y [DOI] [PubMed] [Google Scholar]
- 42.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. 10.1038/nri2925 [DOI] [PubMed] [Google Scholar]
- 43.Dinh QN, Drummond GR, Sobey CG, et al. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014;2014:1–11. 10.1155/2014/406960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsounis D, Bouras G, Giannopoulos G, et al. Inflammation markers in essential hypertension. Med Chem 2014;10:672–81. 10.2174/1573406410666140318111328 [DOI] [PubMed] [Google Scholar]
- 45.Lima NKC, Abbasi F, Lamendola C, et al. Prevalence of insulin resistance and related risk factors for cardiovascular disease in patients with essential hypertension. Am J Hypertens 2009;22:106–11. 10.1038/ajh.2008.263 [DOI] [PubMed] [Google Scholar]
- 46.Demirci MS, Gungor O, Kircelli F, et al. Impact of mean arterial pressure on progression of arterial stiffness in peritoneal dialysis patients under strict volume control strategy. Clin Nephrol 2012;77:105–13. 10.5414/CN107223 [DOI] [PubMed] [Google Scholar]
- 47.Chirinos JA, Segers P, Gillebert TC, et al. Central pulse pressure and its hemodynamic determinants in middle-aged adults with impaired fasting glucose and diabetes: the Asklepios study. Diabetes Care 2013;36:2359–65. 10.2337/dc12-1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Data sharing statementThe raw data can be downloaded from ‘DATADRYAD’ database (www.Datadryad.org). Dryad Digital Repository. https://datadryad.org/stash/dataset/doi:10.5061/dryad.ft8750v.