Abstract
Stem and progenitor cells located within stem cell niches maintain the renewal and regeneration of tissues and organs throughout the life of an adult organism. Stem cell niche component dysfunction might alter the activity of stem cells and ultimately lead to the development of difficult-to-treat chronic or acute disorders. Of note, some cases of idiopathic male infertility, a highly prevalent diagnosis with no specific treatment options, might be associated with a spermatogonial stem cell(SSC) niche disturbance. To overcome this disease entity, approaches aiming at launching the regeneration of an altered stem cell niche are worth considering. Particularly, mesenchymal stromal cells (MSCs) or their secretome might fulfill this task due to their promising contribution in recovering injured stem cell niches. However, the successful application of MSC-based treatment is limited by the uncovered mechanisms of action of MSCs and their secretome. Specific animal models should be developed or adapted to reveal the role of MSCs and their secretome in a stem cell niche recovery. In this review, in a bid to consider MSCs and their secretome as a therapeutic regenerative approach for idiopathic male infertility we focus on the rationale of SSC niche injury modeling.
Keywords: Regenerative medicine, Mesenchymal stem/stromal cells, Stem cell niche, Secretome, Male infertility, Tissue homeostasis
Graphical abstract
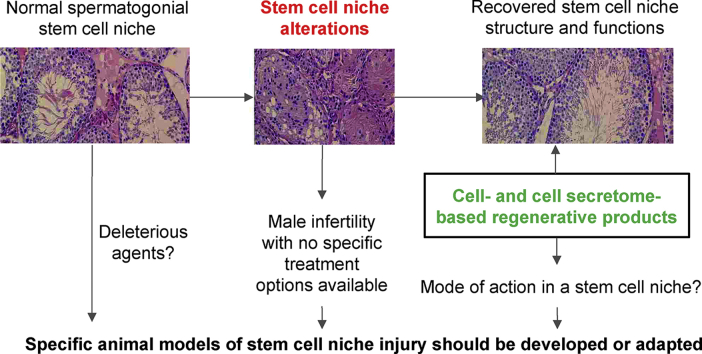
Throughout life, the cellular components of tissues and organs need timely renewal. To maintain this renewal, a pool of stem and progenitor cells is present in adult organisms [1]. Their activity of stem and progenitor cells is fine-tuned by a stem cell niche [2], a complex microenvironment of cells that interact via paracrine communication, metabolic, physical, and chemical cues. A dysregulation of stem cell niche components might alter the activity of stem cells and ultimately manifest as severe chronic or acute diseases [3,4]. Usually, given that the choice of therapeutic options is complicated by multiple mechanisms involved in niche dysfunction, these conditions are difficult to treat [5]. An example of such are some cases of idiopathic male infertility, which still lack effective therapeutic approaches despite its high worldwide prevalence [[6], [7], [8]].
Unlike some other niches, spermatogonial stem cell(SSC) niche cells are less capable of adopting new fates [9,10] that might provoke disease if any highly specialized component is altered. Therefore, to restore spermatogenesis, a complete restoration of the stem cell niche is required. Among regenerative medicine approaches, using of multipotent mesenchymal stromal cells (MSCs) might be an option. MSCs can replenish altered niche components through various mechanisms as well as mimic paracrine signals from adjacent niche cells to guarantee functional niche integrity during regeneration [11]. The effects of MSCs and their secretome have been investigated for the treatment of various disorders of tissue repair and regeneration, and several clinical trials have proven their efficacy [[12], [13], [14]]. However, their application to the restoration of stem cell niche disorders is yet far from being successful. One possible explanation could be the high variability of MSC therapeutic effects determined by the tissue source, donor parameters, approaches to manufacturing, route of delivery as well as the lack of clear characteristic criteria of MSC-based cell products [[15], [16], [17]]. The need to control the proportion of senescent/dysfunctional MSCs before injection [18] and maintain their viability after injection also complicates their effective use [19]. The risks of unwanted differentiation of injected cells, the formation of tumors and ischemic disorders due to the microemboli formation limit the launch of some studies [20]. Escaping these drawbacks could be achieved by using MSC secretome-derived products. However, despite the advantages of the MSC secretome over cell therapy, its application is also limited by the complexity of its standardization [21]. Notably, only a few number of studies aimed at exploring the pathogenic impact of a stem cell niche and the role of MSCs and their secretome in a stem cell niche recovery were carried out. Thus, to allow studying roles of MSCs and their secretome in SSC niche recovery, new animal models should be considered. Herein we focus on idiopathic male infertility modeling to expand the use of MSC-based products to address this unmet medical need.
Accumulating evidence points to the involvement of SSC niche components in the pathogenesis of idiopathic male infertility
It is estimated that infertility affects 8%–12% of couples worldwide, with the male factor being a primary or contributing cause in approximately 50% of couples. The increasing incidence of male infertility may be due to a multitude of factors, which can be stratified into congenital, acquired, and idiopathic factors. About 30%–50% of male infertility cases are idiopathic, with no discernible cause [22]. Men with a history of idiopathic infertility do not have obvious fertility problems, and physical examination and laboratory tests are normal. However, semen analysis detects sperm abnormalities that appear alone or in combination.
At present, only a number of possible causes and molecular mechanisms underlying idiopathic male infertility are known. In particular, genetic factors may be associated with male infertility. However, the majority of findings are so rare that additional independent cases are required to confirm the association of genetic factors with idiopathic male infertility as monogenic causes [23]. One can expect that next-generation sequencing (NGS)-based approaches might facilitate this task. Although NGS analysis of custom gene panels was able to identify genetic events possibly involved in idiopathic male infertility development [24,25], the majority of the cases remained unexplained. This suggests the possible involvement of other, non-genetic, factors in the pathogenesis of idiopathic male infertility.
Among the most studied epigenetic factors, global or gene-specific DNA methylation may be involved in the development of idiopathic male infertility, albeit the roles of histone modifications or chromatin protamination in spermatogenesis remain less evident [26]. Importantly, some signatures of differential DNA methylation regions may serve as predictors of response to particular treatments for idiopathic male infertility [27]. However, mechanisms leading to the alterations of DNA methylation profiles and their role in the development of male infertility still need elucidation. There have been extensive studies on other epigenetic factors such as miRNAs. However, similarly to previously discussed factors, additional data are needed to prove the presence of a relationship between aberrant miRNA expression and idiopathic male infertility [28].
Apparently, aberrant mRNA expression might be related to idiopathic male infertility. First, it has been shown that a bulk of the genes associated with idiopathic male infertility is related to reactive oxygen species (ROS). This could explain the significance of imbalance in ROS genes and their protein products present in seminal plasma as well as in the membrane components of damaged spermatozoa [29]. These findings are consistent with those of the overexpression of glutathione transferase genes in non-obstructive azoospermia(NOA) and oligospermia samples [30], where these genes were found to possibly detoxify ROS [31]. Second, aberrant expression can be observed not only in the cells of the spermatogenic epithelium, but also in somatic cells [32]. Accordingly, immature Sertoli cell fractions were revealed transcriptomes of single Sertoli cells obtained from patients with idiopathic male infertility. These groups exhibited enriched Gene Set Enrichment Analysis terms characteristic of infantile and pubertal Sertoli cells, were more proliferative in vitro, and had energy metabolism patterns typical to immature Sertoli cells. The ability of immature Sertoli cells to support germ cell colonies were worse, yet statistically insignificant, compared to normal adult Sertoli cells. Importantly, the functional immaturity of isolated Sertoli cells was curable by Wnt pathway inhibition that reveals the ability for therapeutic modulation of SSC niche properties [33]. Furthermore, some clinical studies showed that men with a history of idiopathic male infertility demonstrated significant signs of impaired Leydig cell function. That could be caused by disturbed paracrine communication between the seminiferous epithelium and Leydig cells or congenital dysfunction of both components [34]. Additionally, in a mouse model, the production of glial cell line-derived neurotrophic factor(GDNF) by peritubular myoid cells was demonstrated to be essential for spermatogonial development [35]. As testosterone induces GDNF expression, this might indicate the significance of the interplay between SSC niche components such as Leydig cells and peritubular myoid cells.
Taken together, many factors may be involved in the pathogenesis of idiopathic male infertility. Accumulating evidence points to the substantial role of SSC niche components in the pathogenesis of this disease.
MSCs and their secretome can recover injured stem cell niches
Multipotent stem and progenitor cells support the structure of tissues and their potency to renew and regenerate throughout the life. In order to do this, they require coherent regulation by the microenvironment, a stem cell niche that is made up of cells and cell–cell contacts, paracrine factors, metabolites, extracellular matrix (ECM), and physical and chemical cues. Given that a niche can regulate quiescence and differentiation of stem cells, the loss of individual components of a niche can affect its regenerative capacity [[36], [37], [38]]. Therefore, to be able to recover, a niche might exploit internal mechanisms preventing itself from degradation. Along with other niche components [39], MSCs might play a pivotal role in this process. Particularly, to replenish altered niche cellular components, MSCs might act as their precursors [40]. Nevertheless, at present, the importance of the paracrine effects of MSCs in restoring the cellular composition of tissue is generally accepted. Particularly, MSCs secrete cytokines and growth factors that protect tissue from damage by regulating the immune response, stimulating angiogenesis, and maintaining the viability of the microenvironment [11]. Paracrine secretion of MSCs also involves ECM components that may fulfill structural and signaling roles [41]. Extracellular vesicles secreted by MSCs can be important for tissue regeneration as well due to miRNA, mRNA, bioactive lipid, and protein transfer [42]. Moreover, MSC-derived apoptotic bodies may also be involved in tissue regeneration [43].
Cells that meet the minimal MSC characterization criteria have been found in many tissues of the body [16]. They are also present in many stem cell niches and can participate in the regeneration of injured stem cell niches as endogenous components [44,45]. This raises the question of the possibility of the effective use of MSCs isolated from a different tissue source in regenerative medicine [46]. Characterization and analysis of MSC secretome might shed light on this issue given that secretion of paracrine factors along with ECM components and extracellular vesicles by MSCs is accepted to be pivotal for tissue regeneration [47]. Indeed, the heterogeneity of MSCs is inherent among donors, cell populations from different tissues, or different phenotypes [48]. Furthermore, the secretome of MSCs demonstrates dissimilar functional properties [49] that might persist after transplantation to another tissue [50]. However, in some injury models, they overlap substantially and might be interchanged [51]. Furthermore, while administrating MSCs from a different tissue, the ability of the microenvironment to impose tissue-specific regenerative properties to transplanted cells might also limit heterogeneity and increase the efficacy of MSCs [41,52,53].
These hypotheses have been proven by distinct studies of MSCs or their secretome for the recovery of injured stem cell niches [[54], [55], [56]]. However, new models and indications are needed to make these efforts more effective and reproducible. In particular, SSC niche injury, as a model of idiopathic male infertility, can be used to prove the applicability of MSCs or MSCs secretome as an effective treatment for regeneration of an injured stem cell niche.
Application of MSCs or their secretome might be effective for the treatment of idiopathic male infertility
Indeed, a large number of studies are devoted to the use of MSCs as a “tool” for the restoration of spermatogenesis. In particular, it was demonstrated that the intratesticular injection of bone marrow MSCs promoted almost complete normalization of spermatogenesis in busulfan-treated hamsters [57]. Similar results have been shown in other animal models of busulfan-induced azoospermia [58]. For some models, it can be assumed that spermatogenesis might be restored due to paracrine stimulation provided by transplanted MSC to maintain the function of injured or dysregulated SSC niche components [59]. Moreover, the impact of paracrine factors secreted by MSCs on spermatogenesis restoration can be confirmed by the effective restoration of spermatogenesis in animal models of spermatogenesis failure after injection of human MSCs isolated from various tissues or their secretome [[60], [61], [62]].
Taken together, the application of MSCs or their secretome might be effective to resolve injury to spermatogenesis in various animal models. Therefore, the application of MSCs or their secretome for idiopathic male infertility treatment might be considered. To guarantee the best potency of MSC-based products, the model should reflect the possible role of the SSC niche in the pathogenesis of idiopathic male infertility.
Searching for a feasible toxicant to model SSC niche injury
Since idiopathic male infertility is a multifactorial disease, different SSC niche components should be damaged in a relevant idiopathic male infertility model. Additionally, the modeled injury to a niche should be, at least, partially reversible to allow therapeutic restoration of spermatogenesis. Among fully reversible models, one can name ischemic models [63]. However, in a reversible setting, spermatogenic epithelium is predominantly damaged conversely to somatic niche components. Apparently, as germ cells are more sensitive to ischemia than Sertoli cells and particularly Leydig cells, it seems impossible to damage SSC niche components while saving germ cell epithelium. Thus, testicular torsion for sufficient time periods to cause loss of all germinal elements in the testis still leaves the testis with considerable steroidogenic capacity [64]. Similarly, physically modeling SSC niche injury by testicular puncture might also be suboptimal as it can lead only to local disturbances [65]. Modeling of abdominal cryptorchidism seems to be a fairly optimal way to disrupt the SSC niche as it allows it to affect several components of the SSC niche. An additional advantage of this model is the reversibility of the modeled injuries [66]. However, to model abdominal cryptorchidism, animals must undergo two surgeries, which is less ethically acceptable and might be accompanied by an increased risk of infections.
Consequently, toxicant- and radiation-induced models might be more appropriate. Among the most common chemicals able to injure SSC niche, ethane-1,2-dimethanesulfonate or mono- (2-ethylhexyl) phthalate act primarily on Leydig cells or Sertoli cells, respectively [67,68]. Conversely, the methods of radiation exposure are dangerous mainly for the spermatogenic epithelium [69]. Therefore, other toxicants for modeling SSC niche injury can be considered [70]. To select an optimal chemical to damage the SSC niche and subsequent disorders of spermatogenesis, their modes of action along with reversibility of their effects within SSC niche were analyzed.
As a result of a literature search, doxorubicin might apparently be used to model idiopathic male infertility [Table 1]. Doxorubicin has the potential to cause cell death or cell growth arrest by inhibiting topoisomerase II and DNA intercalation, and the ability to turn into free radicals, initiating ROS production and inducing oxidative damage to cellular DNA and the mitochondria. Currently, it is believed that doxorubicin-induced cell death predominantly involves the function and dysfunction of the mitochondria and cell energy levels, which may provide a low selectivity for doxorubicin [71]. At least by stimulating the production of ROS, doxorubicin may damage somatic components of the SSC niche; however, not limited to this [72,73], an excess of which is characteristic of idiopathic male infertility [29]. Another advantage of doxorubicin is its relatively wide therapeutic interval and the ability to model dose-dependent effects [74]. Concurrently, the damaging effects of doxorubicin can be reversible [75]. In summary, doxorubicin may be the toxicant of choice for modeling idiopathic male infertility.
Table 1.
Impact of chemicals on an SSC niche injury.
| Chemical | SSC Toxicity |
Sertoli cell toxicity | Leydig cell toxicity | Peritubular macrophage toxicity | Peritubular myoid cell toxicity | Reversible/irreversible damage | References |
|---|---|---|---|---|---|---|---|
| Cisplatin | yes | no data | yes | no data | no data | Reversible | [85] |
| Doxorubicin | yes | yes | yes | no data | no data | Reversible | [81,86,87] |
| Cyclophosphamide | yes | – | – | no data | no data | Irreversible in combination (CHOP) | [81,88] |
| Chlorambucil | yes | – | yes | no data | no data | Reversible | [88,89] |
| Melphalan | yes | – | – | no data | no data | Reversible | [89,90] |
| Ifosfamide | yes | – | – | no data | no data | Reversible | [91] |
| Carmustine | yes | – | yes | no data | no data | Irreversible in combination (BEAM) | [92] |
| Busulfan | yes | yes | yes | no data | yes | Reversible | [59,93,94] |
| Cytarabine | yes | no data | – | no data | no data | no data | [80] |
| Vinblastine | yes | yes | no data | no data | no data | Reversible | [81,82] |
| Vincristine | yes | no data | yes | no data | no data | Irreversible in combination (BEACOPP) | [83] |
| Etoposide | yes | no data | no data | no data | no data | no data | [84] |
Abbreviations: CHOP: combination of cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate, and prednisone; BEAM: combination of carmustine, etoposide, cytarabine, and melphalan; BEACOPP: combination of bleomycin sulfate, etoposide phosphate, doxorubicin hydrochloride, cyclophosphamide, vincristine sulfate, procarbazine hydrochloride, and prednisone.
It is reasonable to select a control substance that will make a significant contribution to the restoration of spermatogenesis in order to use the doxorubicin-induced injury model. Given the mechanism of action of doxorubicin, one might consider antioxidants. First, although more reliable confirmation of the effects is required through large-scale placebo-controlled studies, the results of several clinical trials suggest that antioxidants (vitamin E, zinc sulfate) may increase the chances of conception in patients with idiopathic male infertility [76,77]. Further, according to published results [78,79] and our unpublished data, antioxidants might serve as control treatments in regenerative medicine therapeutics studies on idiopathic male infertility (Fig. S1, unpublished data).
Conclusions
Many injury models have demonstrated that regenerative medicine products, particularly MSC-based, can mediate the restoration of damaged tissue by recovering a stem cell niche. It is also suggested to further consider MSCs and their secretome in the treatment of male infertility disorders associated with a SSC niche injury. Still, a deeper study of their mode of action might be required to enhance potency. Therefore, relevant animal models of the SSC niche injury are needed to resolve this challenge. In this case, doxorubicin-induced injury might be promising due to its rather broad spectrum of toxicity and the reversibility of its damage. Importantly, features of this toxicant might be used in other stem cell niche injury models to study the potency of other novel drugs for regeneration.
Summary
In this review, the authors critically considered the promises and limitations of regenerative medicine to treating idiopathic male infertility focusing on the tight link of its pathogenesis with the SSC niche disturbance. The authors suggest that approaches aiming at launching the regeneration of an altered stem cell niche might be worth considering to overcome this disease entity.
In this regard, multipotent mesenchymal stromal cells (MSCs) could be proposed as a promising tool for the stimulation of the SSC niche restoration. However, the successful application of MSC-based treatment is limited by the uncovered mechanisms of action of MSCs and their secretome. Therefore, attention is paid to specific animal models that should be developed or adapted to reveal the role of MSCs and their secretome in a stem cell niche recovery. Hence, the authors conduct an in-depth comparative analysis of spermatogenesis disorder models in order to select an experimental exposure that damages the somatic components of the SSC niche. As a result, doxorubicin is suggested as a toxicant of choice for modeling the idiopathic male infertility, which is supported by our own experimental data.
Of note, many injury models have demonstrated that MSC-based products can mediate the restoration of damaged tissue by recovering a stem cell niche. In this case, use of doxorubicin-induced SSC niche injury might be successfully applied to other stem cell niche injury models to study the potency of other novel drugs for regeneration.
Funding
The study was carried out under the State Assignment of Lomonosov MSU and using the equipment provided within the Program of Development of Lomonosov MSU.
Ethics declarations
Animals were housed and used for experimental procedures in full compliance with Directive 2010/63/EU. The work was approved by local ethic committee (#90-G).
Consent for publication
All authors have agreed to publish this manuscript. All materials and images are original. No consent needs to declare.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
The authors gratefully acknowledge Dr. Malkov P.G. and Danilova N.V., Faculty of Medicine, Lomonosov Moscow State University, Russia, for their help in histological samples preparation. We also thank Dr. Ohobotov D.A. and Prof. Matskeplishvili S.T. for the valuable advice in the field of chemotherapy-induced damage of the reproductive system.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2022.01.015.
Appendix ASupplementary data
The following are the Supplementary data to this article:
Multimedia component 1
Multimedia component 2
References
- 1.Keyes B.E., Fuchs E. Stem cells: aging and transcriptional fingerprints. J Cell Biol. 2018;217:79–92. doi: 10.1083/jcb.201708099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryu B.Y., Orwig K.E., Oatley J.M., Avarbock M.R., Brinster R.L. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melo-Narváez M.C., Stegmayr J., Wagner D.E., Lehmann M. Lung regeneration: implications of the diseased niche and ageing. Eur Respir Rev. 2020;29:200222. doi: 10.1183/16000617.0222-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollini S., Smits A.M., Balbi C., Lazzarini E., Ameri P. Triggering endogenous cardiac repair and regeneration via extracellular vesicle-mediated communication. Front Physiol. 2018;9:1497. doi: 10.3389/fphys.2018.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez L.M., de Lucas B., Gálvez B.G. Unhealthy stem cells: when health conditions upset stem cell properties. Cell Physiol Biochem. 2018;46:1999–2016. doi: 10.1159/000489440. [DOI] [PubMed] [Google Scholar]
- 6.Mulhall J.P., Trost L.W., Brannigan R.E., Kurtz E.G., Redmon J.B., Chiles K.A., et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 7.Attia A.M., Abou-Setta A.M., Al-Inany H.G. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD005071.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smits R.M., Mackenzie-Proctor R., Yazdani A., Stankiewicz M.T., Jordan V., Showell M.G. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3 doi: 10.1002/14651858.CD007411.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rompolas P., Mesa K.R., Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Sousa e Melo F., de Sauvage F.J. Cellular plasticity in intestinal homeostasis and disease. Cell Stem Cell. 2019;24:54–64. doi: 10.1016/j.stem.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Sagaradze G.D., Basalova N.A., Efimenko A.Y., Tkachuk V.A. Mesenchymal stromal cells as critical contributors to tissue regeneration. Front Cell Dev Biol. 2020;8:576176. doi: 10.3389/fcell.2020.576176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y.Z., Gou M., Da L.C., Zhang W.Q., Xie H.Q. Mesenchymal stem cells for chronic wound healing: current status of preclinical and clinical studies. Tissue Eng B Rev. 2020;26:555–570. doi: 10.1089/ten.TEB.2019.0351. [DOI] [PubMed] [Google Scholar]
- 13.Yang S., Liu P., Jiang Y., Wang Z., Dai H., Wang C. Therapeutic applications of mesenchymal stem cells in idiopathic pulmonary fibrosis. Front Cell Dev Biol. 2021;9:639657. doi: 10.3389/fcell.2021.639657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y., Yu Y., Hu S., Chen Y., Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11:349. doi: 10.1038/s41419-020-2542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanelidis A.J., Premer C., Lopez J., Balkan W., Hare J.M. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction. Circ Res. 2017;120:1139–1150. doi: 10.1161/CIRCRESAHA.116.309819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanathan S., Shi Y., Galipeau J., Krampera M., Leblanc K., Martin I., et al. Mesenchymal stem versus stromal cells: international society for cell & gene therapy (ISCT®) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Sagaradze G.D., Nimiritsky P.P., Akopyan Z.A., Makarevich P.I., Efimenko A.Y. In: Biopharmaceuticals. Yeh M.K., Chen Y.C., editors. IntechOpen; London, UK: 2018. “Cell-Free therapeutics” from components secreted by mesenchymal stromal cells as a novel class of biopharmaceuticals; pp. 47–62. [Google Scholar]
- 18.Liu J., Ding Y., Liu Z., Liang X. Senescence in mesenchymal stem cells: functional alterations, molecular mechanisms, and rejuvenation strategies. Front Cell Dev Biol. 2020;8:258. doi: 10.3389/fcell.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolar J., Le Blanc K., Keating A., Blazar B.R. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cell. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang W.Z., Lin Y.H., Su L.J., Wu M.S., Jeng H.Y., Chang H.C., et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci. 2021;28:28. doi: 10.1186/s12929-021-00725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagaradze G., Grigorieva O., Nimiritsky P., Basalova N., Kalinina N., Akopyan Z., et al. Conditioned medium from human mesenchymal stromal cells: towards the clinical translation. Int J Mol Sci. 2019;20:1656. doi: 10.3390/ijms20071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal A., Baskaran S., Parekh N., Cho C.L., Henkel R., Vij S., et al. Male infertility. Lancet. 2021;397:319–333. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 23.Bracke A., Peeters K., Punjabi U., Hoogewijs D., Dewilde S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod Biomed Online. 2018;36:327–339. doi: 10.1016/j.rbmo.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Cannarella R., Precone V., Guerri G., Busetto G.M., Di Renzo G.C., Gerli S., et al. Clinical evaluation of a custom gene panel as a tool for precision male infertility diagnosis by next-generation sequencing. Life(Basel) 2020;10:242. doi: 10.3390/life10100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Precone V., Cannarella R., Paolacci S., Busetto G.M., Beccari T., Stuppia L., et al. Male infertility diagnosis: improvement of genetic analysis performance by the introduction of pre-diagnostic genes in a next-generation sequencing custom-made panel. Front Endocrinol (Lausanne) 2021;11:605237. doi: 10.3389/fendo.2020.605237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunes S., Arslan M.A., Hekim G.N.T., Asci R. The role of epigenetics in idiopathic male infertility. J Assist Reprod Genet. 2016;33:553–569. doi: 10.1007/s10815-016-0682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luján S., Caroppo E., Niederberger C., Arce J.C., Sadler-Riggleman I., Beck D., et al. Sperm DNA methylation epimutation biomarkers for male infertility and FSH therapeutic responsiveness. Sci Rep. 2019;9:16786. doi: 10.1038/s41598-019-52903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salas-Huetos A., James E.R., Aston K.I., Carrell D.T., Jenkins T.G., Yeste M. The role of miRNAs in male human reproduction: a systematic review. Andrology. 2020;8:7–26. doi: 10.1111/andr.12714. [DOI] [PubMed] [Google Scholar]
- 29.Kothandaraman N., Agarwal A., Abu-Elmagd M., Al-Qahtani M.H. Pathogenic landscape of idiopathic male infertility: new insight towards its regulatory networks. NPJ Genom Med. 2016;1:16023. doi: 10.1038/npjgenmed.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razavi S.M., Sabbaghian M., Jalili M., Divsalar A., Wolkenhauer O., Salehzadeh-Yazdi A. Comprehensive functional enrichment analysis of male infertility. Sci Rep. 2017;7:15778. doi: 10.1038/s41598-017-16005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llavanera M., Mateo-Otero Y., Bonet S., Barranco I., Fernández-Fuertes B., Yeste M. The triple role of glutathione S-transferases in mammalian male fertility. Cell Mol Life Sci. 2020;77:2331–2342. doi: 10.1007/s00018-019-03405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bansal S.K., Gupta N., Sankhwar S.N., Rajender S. Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L., Yao C., Xing X., Jing T., Li P., Zhu Z., et al. Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat Commun. 2020;11:5683. doi: 10.1038/s41467-020-19414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson A.M., Jørgensen N., Frydelund-Larsen L., Rajpert-De Meyts E., Skakkebæk N.E. Impaired Leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab. 2004;89:3161–3167. doi: 10.1210/jc.2003-031786. [DOI] [PubMed] [Google Scholar]
- 35.Chen L.Y., Willis W.D., Eddy E.M. Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc Natl Acad Sci U S A. 2016;113:1829–1834. doi: 10.1073/pnas.1517994113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukjanenko L., Jung M.J., Hegde N., Perruisseau-Carrier C., Migliavacca E., Rozo M., et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med. 2016;22:897–905. doi: 10.1038/nm.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segel M., Neumann B., Hill M.F.E., Weber I.P., Viscomi C., Zhao C., et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. 2019;573:130–134. doi: 10.1038/s41586-019-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eliazer S., Muncie J.M., Christensen J., Sun X., D'Urso R.S., Weaver V.M., et al. Wnt4 from the niche controls the mechano-properties and quiescent state of muscle stem cells. Cell Stem Cell. 2019;25:654–665. doi: 10.1016/j.stem.2019.08.007. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratnayake D., Nguyen P.D., Rossello F.J., Wimmer V.C., Tan J.L., Galvis L.A., et al. Macrophages provide a transient muscle stem cell niche via NAMPT secretion. Nature. 2021;591:281–287. doi: 10.1038/s41586-021-03199-7. [DOI] [PubMed] [Google Scholar]
- 40.Newton P.T., Li L., Zhou B., Schweingruber C., Hovorakova M., Xie M., et al. A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature. 2019;567:234–238. doi: 10.1038/s41586-019-0989-6. [DOI] [PubMed] [Google Scholar]
- 41.Novoseletskaya E., Grigorieva O., Nimiritsky P., Basalova N., Eremichev R., Milovskaya I., et al. Mesenchymal stromal cell-produced components of extracellular matrix potentiate multipotent stem cell response to differentiation stimuli. Front Cell Dev Biol. 2020;8:555378. doi: 10.3389/fcell.2020.555378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xunian Z., Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111:3100–3110. doi: 10.1111/cas.14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu Y., Sui B., Xiang L., Yan X., Wu D., Shi S., et al. Emerging understanding of apoptosis in mediating mesenchymal stem cell therapy. Cell Death Dis. 2021;12:596. doi: 10.1038/s41419-021-03883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wosczyna M.N., Konishi C.T., Perez Carbajal E.E., Wang T.T., Walsh R.A., Gan Q., et al. Mesenchymal stromal cells are required for regeneration and homeostatic maintenance of skeletal muscle. Cell Rep. 2019;27:2029–2035. doi: 10.1016/j.celrep.2019.04.074. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degirmenci B., Valenta T., Dimitrieva S., Hausmann G., Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature. 2018;558:449–453. doi: 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 46.Levy O., Kuai R., Siren E.M.J., Bhere D., Milton Y., Nissar N., et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y., Holmes C. Tissue regeneration capacity of extracellular vesicles isolated from bone marrow-derived and adipose-derived mesenchymal stromal/stem cells. Front Cell Dev Biol. 2021;9:648098. doi: 10.3389/fcell.2021.648098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zha K., Li X., Yang Z., Tian G., Sun Z., Sui X., et al. Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. Npj Regen Med. 2021;6:14. doi: 10.1038/s41536-021-00122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kehl D., Generali M., Mallone A., Heller M., Uldry A.-C., Cheng P., et al. Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential. Npj Regen Med. 2019;4:8. doi: 10.1038/s41536-019-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran T.T., Yamamoto Y., Gesta S., Kahn C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metabol. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin S., Lee J., Kwon Y., Park K.S., Jeong J.H., Choi S.J., et al. Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and wharton's jelly. Int J Mol Sci. 2021;22:845. doi: 10.3390/ijms22020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leuning D.G., Beijer N.R.M., du Fossé N.A., Vermeulen S., Lievers E., van Kooten C., et al. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci Rep. 2018;8:7716. doi: 10.1038/s41598-018-25700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayaz-Guner S., Alessio N., Acar M.B., Aprile D., Özcan S., Di Bernardo G., et al. A comparative study on normal and obese mice indicates that the secretome of mesenchymal stromal cells is influenced by tissue environment and physiopathological conditions. Cell Commun Signal. 2020;18:118. doi: 10.1186/s12964-020-00614-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salgado A.J., Pires A.O., Pinto L., Sousa N., Mateus-Pinheiro A., Teixeira F.G., et al. Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities. Front Cell Neurosci. 2015;9:249. doi: 10.3389/fncel.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam P.K., Wang K.K.W., Lo A.W.I., Tong C.S.W., Ching D.W.C., Wong K., et al. Interactome and reciprocal activation of pathways in topical mesenchymal stem cells and the recipient cerebral cortex following traumatic brain injury. Sci Rep. 2017;7:5017. doi: 10.1038/s41598-017-01772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J.W., Kim J.M., Choi M.E., Kim S.K., Kim Y.M., Choi J.S. Adipose-derived mesenchymal stem cells regenerate radioiodine-induced salivary gland damage in a murine model. Sci Rep. 2019;9:15752. doi: 10.1038/s41598-019-51775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamadon A., Mehrabani D., Rahmanifar F., Jahromi A.R., Panahi M., Zare S., et al. Induction of spermatogenesis by bone marrow-derived mesenchymal stem cells in busulfan-induced azoospermia in hamster. Int J Stem Cells. 2015;8:134–145. doi: 10.15283/ijsc.2015.8.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hajihoseini M., Vahdati A., Hosseini S.E., Mehrabani D., Tamadon A. Induction of spermatogenesis after stem cell therapy of azoospermic Guinea pigs. Vet Arh. 2017;87:333–350. [Google Scholar]
- 59.Anand S., Bhartiya D., Sriraman K., Mallick A. Underlying mechanisms that restore spermatogenesis on transplanting healthy niche cells in busulphan treated mouse testis. Stem Cell Rev Reports. 2016;12:682–697. doi: 10.1007/s12015-016-9685-1. [DOI] [PubMed] [Google Scholar]
- 60.Sagaradze G., Basalova N., Kirpatovsky V., Ohobotov D., Nimiritsky P., Grigorieva O., et al. A magic kick for regeneration: role of mesenchymal stromal cell secretome in spermatogonial stem cell niche recovery. Stem Cell Res Ther. 2019;10:342. doi: 10.1186/s13287-019-1479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian C., Meng Q., Lu J., Zhang L., Li H., Huang B. Human amnion mesenchymal stem cells restore spermatogenesis in mice with busulfan-induced testis toxicity by inhibiting apoptosis and oxidative stress. Stem Cell Res Ther. 2020;11:290. doi: 10.1186/s13287-020-01803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu J., Liu Z., Shu M., Zhang L., Xia W., Tang L., et al. Human placental mesenchymal stem cells ameliorate chemotherapy-induced damage in the testis by reducing apoptosis/oxidative stress and promoting autophagy. Stem Cell Res Ther. 2021;12:199. doi: 10.1186/s13287-021-02275-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stern J.A., Lui R.C., LaRegina M.C., Herbold D.R., Tolman K.C., Johnson F.E. Long-term outcome following testicular ischemia in the rat. J Androl. 1990;11:390–395. [PubMed] [Google Scholar]
- 64.Baker L.A., Turner T.T. Leydig cell function after experimental testicular torsion despite loss of spermatogenesis. J Androl. 1995;16:12–17. [PubMed] [Google Scholar]
- 65.Shufaro Y., Prus D., Laufer N., Simon A. Impact of repeated testicular fine needle aspirations (TEFNA) and testicular sperm extraction (TESE) on the microscopic morphology of the testis: an animal model. Hum Reprod. 2002;17:1795–1799. doi: 10.1093/humrep/17.7.1795. [DOI] [PubMed] [Google Scholar]
- 66.Sagaradze G.D., Basalova N.A., Kirpatovsky V.I., Ohobotov D.A., Grigorieva O.A., Balabanyan V.Y., et al. Application of rat cryptorchidism model for the evaluation of mesenchymal stromal cell secretome regenerative potential. Biomed Pharmacother. 2019;109:1428–1436. doi: 10.1016/j.biopha.2018.10.174. [DOI] [PubMed] [Google Scholar]
- 67.Kelce W.R., Zirkin B.R. Mechanism by which ethane dimethanesulfonate kills adult rat leydig cells: involvement of intracellular glutathione. Toxicol Appl Pharmacol. 1993;120:80–88. doi: 10.1006/taap.1993.1089. [DOI] [PubMed] [Google Scholar]
- 68.Lee J., Richburg J.H., Shipp E.B., Meistrich M.L., Boekelheide K. The fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology. 1999;140:852–858. doi: 10.1210/endo.140.2.6479. [DOI] [PubMed] [Google Scholar]
- 69.Richburg J.H., Nañez A., Williams L.R., Embree M.E., Boekelheide K. Sensitivity of testicular germ cells to toxicant-induced apoptosis in gld mice that express a nonfunctional form of fas ligand 1. Endocrinology. 2000;141:787–793. doi: 10.1210/endo.141.2.7325. [DOI] [PubMed] [Google Scholar]
- 70.Delessard M., Saulnier J., Rives A., Dumont L., Rondanino C., Rives N. Exposure to chemotherapy during childhood or adulthood and consequences on spermatogenesis and male fertility. Int J Mol Sci. 2020;21:1454. doi: 10.3390/ijms21041454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meredith A.M., Dass C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J Pharm Pharmacol. 2016;68:729–741. doi: 10.1111/jphp.12539. [DOI] [PubMed] [Google Scholar]
- 72.Tremblay A.R., Delbes G. In vitro study of doxorubicin-induced oxidative stress in spermatogonia and immature Sertoli cells. Toxicol Appl Pharmacol. 2018;348:32–42. doi: 10.1016/j.taap.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Akinjo O.O., Gant T.W., Marczylo E.L. Perturbation of microRNA signalling by doxorubicin in spermatogonial, Leydig and Sertoli cell lines in vitro. Toxicol Res (Camb) 2018;7:760–770. doi: 10.1039/c7tx00314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang C.C., Chen Y.T., Chen C.H., Chiang J.Y., Zhen Y.Y., Yip H.K. Assessment of doxorubicin-induced mouse testicular damage by the novel second-harmonic generation microscopy. Am J Transl Res. 2017;9:5275–5288. [PMC free article] [PubMed] [Google Scholar]
- 75.Poorvu P.D., Frazier A.L., Feraco A.M., Manley P.E., Ginsburg E.S., Laufer M.R., et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr. 2019;3:pkz008. doi: 10.1093/jncics/pkz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omu A.E., Dashti H., Al-Othman S. Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome. Eur J Obstet Gynecol Reprod Biol. 1998;79:179–184. doi: 10.1016/s0301-2115(97)00262-5. [DOI] [PubMed] [Google Scholar]
- 77.Showell M.G., Brown J., Yazdani A., Stankiewicz M.T., Hart R.J. In: Cochrane database syst. Rev. Showell M.G., editor. John Wiley & Sons; Chichester, UK: 2011. Antioxidants for male subfertility. [Google Scholar]
- 78.Badkoobeh P., Parivar K., Kalantar S.M., Hosseini S.D., Salabat A. Effect of nano-zinc oxide on doxorubicin- induced oxidative stress and sperm disorders in adult male Wistar rats. Iran J Reproductive Med. 2013;11:355–364. [PMC free article] [PubMed] [Google Scholar]
- 79.Olusoji M.J., Oyeyemi O.M., Asenuga E.R., Omobowale T.O., Ajayi O.L., Oyagbemi A.A. Protective effect of Gallic acid on doxorubicin-induced testicular and epididymal toxicity. Andrologia. 2017;49:e12635. doi: 10.1111/and.12635. [DOI] [PubMed] [Google Scholar]
- 80.Orth J.M., Gunsalus G.L., Lamperti A.A. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–794. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- 81.Bujan L., Walschaerts M., Brugnon F., Daudin M., Berthaut I., Auger J., et al. Impact of lymphoma treatments on spermatogenesis and sperm deoxyribonucleic acid: a multicenter prospective study from the CECOS network. Fertil Steril. 2014;102:667–674. doi: 10.1016/j.fertnstert.2014.06.008. e3. [DOI] [PubMed] [Google Scholar]
- 82.Russell L.D., Malone J.P., MacCurdy D. Effect of the microtubule disrupting agents, colchicine and vinblastine, on seminiferous tubule structure in the rat. Tissue Cell. 1981;13:349–367. doi: 10.1016/0040-8166(81)90010-0. [DOI] [PubMed] [Google Scholar]
- 83.Sieniawski M., Reineke T., Nogova L., Josting A., Pfistner B., Diehl V., et al. Fertility in male patients with advanced Hodgkin lymphoma treated with BEACOPP: a report of the German Hodgkin Study Group (GHSG) Blood. 2008;111:71–76. doi: 10.1182/blood-2007-02-073544. [DOI] [PubMed] [Google Scholar]
- 84.Marchetti F., Pearson F.S., Bishop J.B., Wyrobek A.J. Etoposide induces chromosomal abnormalities in mouse spermatocytes and stem cell spermatogonia. Hum Reprod. 2006;21:888–895. doi: 10.1093/humrep/dei416. [DOI] [PubMed] [Google Scholar]
- 85.Aydiner A., Aytekin Y., Topuz E. Effects of cisplatin on testicular tissue and the leydig cell-pituitary Axis. Oncology. 1997;54:74–78. doi: 10.1159/000227665. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi H., Tainaka H., Umezawa M., Takeda K., Tanaka H., Nishimune Y., et al. Evaluation of testicular toxicology of doxorubicin based on microarray analysis of testicular specific gene expression. J Toxicol Sci. 2011;36:559–567. doi: 10.2131/jts.36.559. [DOI] [PubMed] [Google Scholar]
- 87.Ward J.A., Wayne Bardin C., Knight M., Robinson J., Gunsalus G., Morris I.D. Delayed effects of doxorubicin on spermatogenesis and endocrine function in rats. Reprod Toxicol. 1988;2:117–126. doi: 10.1016/0890-6238(88)90007-x. [DOI] [PubMed] [Google Scholar]
- 88.Delic J.I., Stanley J.A., Harwood J.R. Testicular function in adult rats treated with the alkylating agent chlorambucil. Arch Androl. 1986;17:87–98. doi: 10.3109/01485018608986960. [DOI] [PubMed] [Google Scholar]
- 89.Generoso W.M., Witt K.L., Cain K.T., Hughes L., Cacheiro N.L., Lockhart A.M., et al. Dominant lethal and heritable translocation tests with chlorambucil and melphalan in male mice. Mutat Res Toxicol. 1995;345:167–180. doi: 10.1016/0165-1218(95)90052-7. [DOI] [PubMed] [Google Scholar]
- 90.Amory J.K., Hong S., Yu X., Muller C.H., Faustman E., Goldstein A. Melphalan, alone or conjugated to an FSH-β peptide, kills murine testicular cells in vitro and transiently suppresses murine spermatogenesis in vivo. Theriogenology. 2014;82:152–159. doi: 10.1016/j.theriogenology.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams D., Crofton P., Levitt G. Does ifosfamide affect gonadal function? Pediatr Blood Cancer. 2008;50:347–351. doi: 10.1002/pbc.21323. [DOI] [PubMed] [Google Scholar]
- 92.Chatterjee R., Mills W., Katz M., McGarrigle H.H., Goldstone A.H. Germ cell failure and Leydig cell insufficiency in post-pubertal males after autologous bone marrow transplantation with BEAM for lymphoma. Bone Marrow Transplant. 1994;13:519–522. [PubMed] [Google Scholar]
- 93.Chen X., Liang M., Wang D. Progress on the study of the mechanism of busulfan cytotoxicity. Cytotechnology. 2018;70:497–502. doi: 10.1007/s10616-018-0189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sasso-Cerri E., Oliveira B., de Santi F., Beltrame F.L., Caneguim B.H., Cerri P.S. The antineoplastic busulphan impairs peritubular and Leydig cells, and vitamin B12 stimulates spermatogonia proliferation and prevents busulphan-induced germ cell death. Biomed Pharmacother. 2017;95:1619–1630. doi: 10.1016/j.biopha.2017.08.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Multimedia component 2