Abstract
The lives of thousands premature babies have been saved along the last thirty years thanks to the establishment and consolidation of pulmonary surfactant replacement therapies (SRT). It took some time to close the gap between the identification of the biophysical and molecular causes of the high mortality associated with respiratory distress syndrome in very premature babies and the development of a proper therapy. Closing the gap required the elucidation of some key questions defining the structure–function relationships in surfactant as well as the particular role of the different molecular components assembled into the surfactant system. On the other hand, the application of SRT as part of treatments targeting other devastating respiratory pathologies, in babies and adults, is depending on further extensive research still required before enough amounts of good humanized clinical surfactants will be available. This review summarizes our current concepts on the compositional and structural determinants defining pulmonary surfactant activity, the principles behind the development of efficient natural animal-derived or recombinant or synthetic therapeutic surfactants, as well as a the most promising lines of research that are already opening new perspectives in the application of tailored surfactant therapies to treat important yet unresolved respiratory pathologies.
Keywords: Surfactant replacement therapy, Air–liquid interface, Respiratory distress syndrome, ARDS, Lipid–protein interactions, DPPC
Pulmonary surfactant replacement therapies (SRT) are now consolidated as routine practices complementing advanced critical care procedures able to rescue lives of very premature babies [[1], [2], [3], [4]]. The continuous improvement in these clinical procedures has been accompanied of an intense research that has revealed key determinants of the molecular and biophysical mechanisms behind pulmonary surfactant function [5,6]. However, and in spite of the huge advance in this basic knowledge, the clinical surfactant preparations most used today in the neonatology services are still similar to the surfactant extracts from animal sources that were already used thirty years ago. This scenario may be about to change, with the development and presumably imminent translation to the clinics of entirely synthetic surfactant preparations that combine defined phospholipid species and synthetic peptides designed to mimic important functional determinants in the key surfactant proteins. Also, with the production of recombinant human surfactant proteins in amounts amenable to be the basis of a new generation of clinical surfactants. Another line of advancement in the context of SRT has to do with the tremendous improvement of the critical care facilities currently applied to rescue premature babies. The use of much less invasive and highly protective procedures such as the application of Continuous Positive Airway Pressure (CPAP), has reduced substantially the prophylactic use of SRT, which is mostly reserved for babies failing CPAP-only care [7]. This change in care recommendations makes crucial both the development of predictive tests to determine the babies that may still need SRT as early as possible [8], and the optimization of less invasive ways to administer therapeutic surfactant [9,10]. On the other hand, extensive basic research from biophysics and molecular biology approaches, also applied to a variety of animal models, has provided numerous evidences indicating that the pulmonary surfactant system results dramatically altered as a consequence of lung injury and inflammation associated to severe respiratory diseases. In spite of that, the incorporation of SRT into the arsenal of therapeutic strategies to treat or ameliorate devastating acute or chronic pathologies such as acute respiratory distress syndrome (ARDS) of several origins in adults and babies [11], including the one associated with COVID-19, chronic obstructive pulmonary disease (COPD), fibrosis, and many others, is still far from reality. In part because the investigation of the molecular mechanisms behind pulmonary surfactant impairment as a consequence of lung injury has been still unable to bring ways to produce better clinical surfactants with enhanced resistance to inactivation in enough amounts to treat both pediatric and adult patients at reasonable costs. This review offers a perspective on the basic concepts learnt about the principles defining a good clinical surfactant and on how the development of future enhanced surfactants could open new opportunities in respiratory medicine.
Pulmonary surfactant: the scenario to replicate or restore
The main activity of the pulmonary surfactant complexes, as assembled and secreted by type II pneumocytes into the alveolar spaces, is to form phospholipid-based films at the air–liquid interface, competent to reduce dramatically the surface tension of the thin layer of liquid coating the respiratory epithelium at the distal airways. These films reduce surface tension from around 70 mN/m in the case of an aqueous solution at 37 °C to values in the order of 20–25 mN/m, which are apparently reduced even further to less than 5 mN/m at the end of expiration. Such extremely low tension values are critical at the time when the lungs reduce their volume to a minimum and the delicate walls of alveoli are at risk of collapse.
A simple interfacial monolayer in which the phospholipid molecules organize orientated with their polar headgroups exposed to water and the hydrophobic acyl chains pointing to air is enough to reduce surface tension, as it can be easily tested in classical physical experiments in Langmuir surface balances [12]. If the phospholipids have a proper acyl chain composition, i.e. if they are rich in fully saturated fatty acids with no double bonds, the monolayer can even reach extremely low surface tensions, close to 0 mN/m, at least when they are compressed in vitro. However, surfactant films at the airways typically present a complex three-dimensional multilayered structure, connected with a rich network of membranes into the subphase [[13], [14], [15]] [see cartoon in Fig. 1]. It is thought that these membrane networks are important to work as a continuous supply of surface active molecules, to replace those that are being continuously lost as a consequence of oxidation and/or detachment during breathing dynamics [16]. However, different biophysical studies have proposed that such complex multilayered structure of the surface films could be also important to ensure enough mechanical stability along the demanding breathing mechanics [17,18].
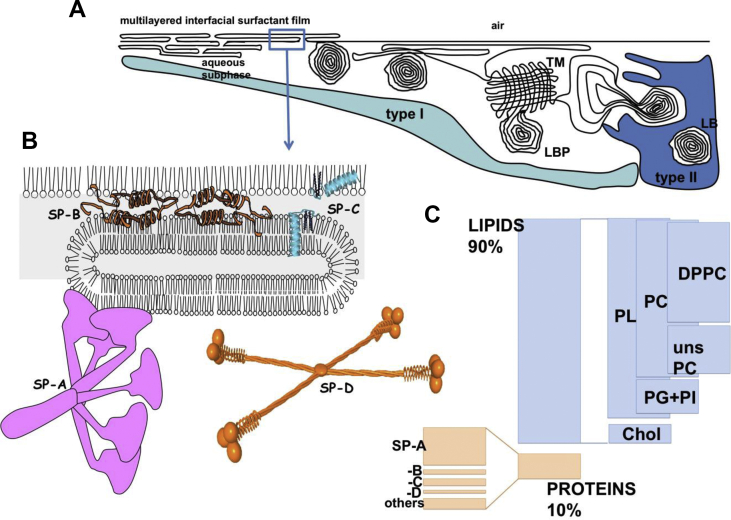
Fig. 1.
Biogenesis and composition of pulmonary surfactant interfacial films. A) Pulmonary surfactant is assembled and stored by type 2 pneumocytes into special organelles, the Lamellar Bodies (LB). Once the content of LB is secreted by pneumocytes into the alveolar spaces, part of them remain as compact lamellar body-like particles (LBP), while others are converted into tubular myelin (TM), an ordered array of membranes, whose function is still under debate. Ultimately, LBPs and TM transfer surface-active molecules into the air–liquid interface, to form a multilayered surface film competent to stabilize alveoli at the end of expiration. B) Surfactant includes 4 surfactant-associated proteins: the hydrophilic SP-A and SP-D and the hydrophobic, and deeply integrated into lipid, proteins SP-B and SP-C. C) Compositional proportions of different proteins and lipids in a typical pulmonary surfactant.
Lipids and proteins in surfactant are assembled and stored by type II pneumocytes into special organelles, the lamellar bodies (LBs), where they adopt a morphology consisting in densely packed membranes, organized in parallel or concentric geometries, with minimal interbilayer distances [19,20]. These structures are highly dehydrated, as a consequence of extreme packing generated at expenses of the hydrolysis of ATP by the lipid pump ABCA3, which uses the energy liberated by ATP to translocate the phospholipids that are assembled as multi-bilayers inside the lumen of LBs [[21], [22], [23]]. Upon the proper stimulus, and once their content is liberated into the alveolar spaces, LBs convert into extracellular LB-like particles (LBPs) that seem to preserve a compact state until reaching the interface, where they rapid and cooperatively unpack to transfer massively the surfactant material into the surface film [24,25].
The whole complexes of pulmonary surfactant can be extracted and purified from the lungs by making a bronchoalveolar lavage (BAL) (as explained for instance in Ref. [26]). If BAL is obtained from animal lungs, the whole material coating both distal and upper airways can be collected together. If made by bronchoscopy in patients, the material extracted will correspond to that present in the limited region washed. Once the cells coming with the lavage are discarded, the material pelleted by centrifugation constitutes the pulmonary surfactant complexes, whose typical composition is represented in Fig. 1. They are composed of approx. 90% by mass of lipids, including around 80% phospholipids and in the order of 10% neutral lipids, mainly cholesterol, and ∼10% of associated proteins, including the specific surfactant proteins (SP-) A, B, C and D [16,23,27]. Proteins SP-A (∼5–6% of total surfactant weight) and SP-D (associated with surfactant membranes in a minor (<1%) and highly variable amount) have hydrophilic character and belong to the collectin (Ca2+-dependent collagenous lectins) family. They play essential roles as part of innate defense mechanisms [[27], [28], [29]]. SP-A and SP-D bind to different motifs at the surface of microorganisms, allergens and environmental particles and facilitate their recognition and clearance by macrophages, the main immune cells in charge of keeping the large respiratory surface clean and sterile.
It is an interesting and intriguing problem how the biophysical activity of pulmonary surfactant to stabilize the mammalian lung has evolved to also integrate elements involved in the innate defense, with no clear evidence of what emerged first in the evolution of air-breathing animals [30]. In this sense, we still do not know whether all the structures of surfactant are related with the biophysical stabilization of the lung while being compatible with the presence of defense molecules, or whether some of the structures assembled by surfactant in the airways are rather involved in preventing the colonization of the respiratory epithelium by potential pathogenic entities. For instance, active surfactant is known to form a peculiar ordered array of membranes at the alveolar spaces, the so-called tubular myelin (TM), with the necessary participation of proteins SP-A and SP-B [31,32]. It was originally thought that TM could constitute a reservoir of surface active material, but it could actually be part of a network of structures designed to optimize protection against pathogens. Both proteins, SP-A [33,34] and SP-B [35], have revealed direct and indirect antibiotic activities, which could support this hypothesis.
Natural clinical surfactants: establishing the surfactant replacement therapy (SRT) paradigm
It was as early as 1959 when the lack of an operative surface active material was established by Mary Avery as the probable cause of the Hyaline Membrane Disease associated to neonatal Respiratory Distress Syndrome and the high mortality of premature babies [36]. Many attempts to supplement those babies with an exogenous surfactant failed since then until trials such as that from Fujiwara et al. were finally successful, later in the 80's of the last century [37]. The experiments with materials obtained from animal lungs had revealed that an extract in organic solvents prepared from BAL surfactant was enough to reproduce most of the surface tension reducing properties of the whole surfactant, at least in vitro [38,39]. Those extractive procedures with solvents such as chloroform/methanol mixtures are typically used by biochemists to obtain the lipid components of biological samples [40], and it was therefore assumed that the lipid moiety of surfactant was the main responsible for their surface tension reduction capabilities. Numerous biophysical studies thus established the role played by the peculiar lipid composition of pulmonary surfactant, including its unusually high proportion of disaturated phospholipids such as dipalmitoylphosphatidylcholine (DPPC), the most abundant phospholipid species in surfactant and the most active in terms of reducing surface tension [41]. Equally unusual compared with the phospholipid composition of other animal tissues resulted the presence in surfactant of phosphatidylglycerol (PG) a phospholipid whose presence was early established as an indicator of lung maturity [42,43].
However, any attempt to rescue preterm newborns with lipid mixtures mimicking the lipid composition of surfactant failed. It was proposed that the particular structure in which surfactant lipids are assembled by the pneumocytes in vivo, as the highly packed and dehydrated membranes of LBs, provides somehow a driving force for the lipids to spread and transfer into the interface, a key process to form the surface-active films that stabilize the lungs [44]. Several approaches inspired by this hypothesis, such as delivering dry surfactant lipid mixtures, also failed [45,46]. The pioneer successful experiments by Fujiwara directly supplemented preterm newborns with aqueous suspensions derived from organic extracts of surfactant from animal lungs [37], suggesting that those extracts contained something else that was strictly required and that was not present into the purely lipidic mixtures.
The missing piece was found around those years of the earliest successful SRT. The organic extracts prepared from natural surfactant purified by BAL contained minor but crucial amounts of two small proteins, later called SP-B and SP-C, which provide the surface active lipids with the ability to adsorb and spread very rapid and efficiently into the air–liquid interface [47,48]. Without these proteins, lipids have no tendency to move out from the bilayered structures in which they spontaneously assemble once exposed to aqueous environments, into the interface [5,6,23]. SP-B and SP-C had not been detected before because i) they are present in really low proportions in surfactant, in the order of 1% with respect to lipid mass, ii) they are extremely hydrophobic, which makes them to be co-extracted with lipids in organic solvents, a property of very few proteins or peptides, iii) because of this high hydrophobicity, these proteins are deeply embedded into the lipids in the surfactant complexes [Fig. 1], whose presence mask their detection, and iv) they have a rather unusual amino acid composition with very low proportion of the residues that typically react with protein-sensitive reagents. The presence of these hydrophobic surfactant-associated proteins caused the all-or-none difference between the biophysical behavior, and the ultimate physiological performance, of purely lipidic mixtures and those of the organic extracts from natural surfactants.
Once established that a lipid/protein extract obtained from a natural surfactant could be reconstituted to a suspension with the ability to efficiently form surface-active films, and thus serve in facilitating opening and stabilization of preterm lungs, the golden age of surfactant therapy was opened [[1], [2], [3], [4]]. The different natural clinical surfactants still in use were variations in the way to prepare a extract of the most hydrophobic components of surfactant using organic solvents applied to different materials of animal origen [49], and were able to reproduce much of the complex multilayered structure of surfactant suspensions and films [13]. As summarized in Table 1, all the materials that ultimately worked had in common the presence of enough amounts of the hydrophobic surfactant proteins, although varied in their relative amount, as well as in the qualitative and quantitative lipid composition, which depended strongly on the starting material (BAL or whole minced lungs) and on the way the organic extract was processed and purified to become a standardized product.
Table 1.
Composition of natural clinical pulmonary surfactant formulations.
| Surfactant | Source (animal) | Source (material) | % PL | %DPPC | Sup. | % Chol | Proteins |
|---|---|---|---|---|---|---|---|
| Native surfactant | Porcine | Lavage | 80–90 | 36–54 | – | 5 | SP-B+++ SP-C+++ |
| Alveofact (Beractant) | Bovine | Lavage | 88 | 39 | – | 3–4 | SP-B++ SP-C++ |
| BLES | Bovine | Lavage | 95 | 42 | – | 4 | SP-B++ SP-C++ |
| Curosurf (Poractant) | Porcine | Minced tissue | 99 | 35–56 | – | 0.3–1.6 | SP-B++ SP-C++ |
| Infasurf (Calfactant) | Bovine | Lavage | 90–94 | 40 | – | 5 | SP-B++ SP-C++ |
| Surfacen | Porcine | Lavage | 91–95 | 45 | – | 5 | SP-B++ SP-C++ |
| Survanta (Bovactant) | Bovine | Minced tissue | 84 | 75 | DPPC, PA, TGs | NR | SP-B+ SP-C++ |
Abbreviations: Chol: cholesterol; DPPC: dipalmitoyl phosphatidylcholine; PA: palmitic acid; PL: phospholipids; TGs: triglycerides.
Sup: supplemented with; NR: not reported.
Proteins: relative amounts in reference to full proportion in native surfactant (+++ 100%; ++ 40-70%; + 10-30%).
Data taken from Ref. [49] and manufacturers.
The key lipid and protein ingredients as learned from natural surfactants
Extensive research during the last decades has produced a model on the basic biophysical and molecular principles on how surfactant works to stabilize the airways, and what the role is of its most important components [5,6]. These studies required the development of key in vitro setups competent to mimic the behavior of air–liquid interfaces subjected to breathing-like physiologically meaningful conditions. The readers are referred to recent reviews describing in detail these approaches and how they allow assessing structure–function relationships in pulmonary surfactant preparations [12,50,51]. According to this research, the most important components in surfactant can be summarized as follows [illustrated in Fig. 2].
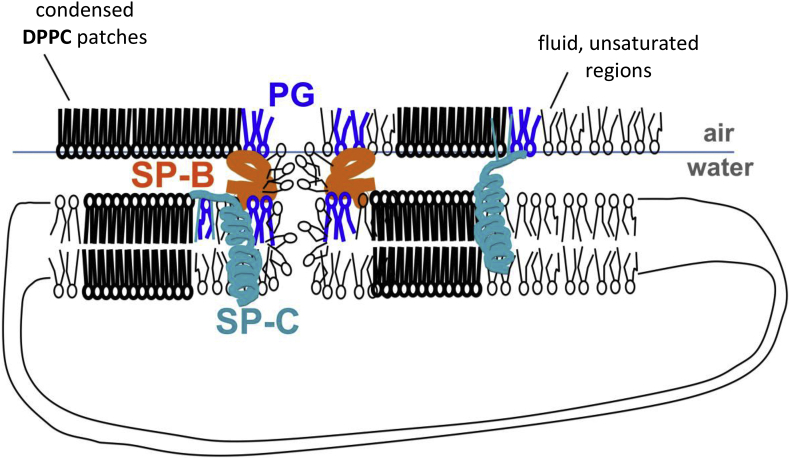
Fig. 2.
Key components of a clinical pulmonary surfactant. The ability of DPPC to pack into highly compact condensed films at the air–liquid interface is the key feature allowing pulmonary surfactant to reduce surface tension to very low values. The coexistence of segregated fluid regions, enriched in unsaturated phospholipids, is important to provide a dynamic character, critical for adsorption and re-spreading of surfactant bilayers along the interface. Hydrophobic surfactant proteins SP-B and SP-C partition into the disordered regions with preferential interaction with the boundaries between ordered and disordered phases. Both proteins require the interaction with the anionic phospholipids PG and PI. SP-B is the key protein to promote transfer of surface-active phospholipids between bilayers and monolayers and to provide maximal mechanical stability at the highest pressures of the films through the establishment of bilayer–monolayer and bilayer–bilayer contacts. SP-C is able to form complexes with SP-B, and participates in monolayer–bilayer transitions from the most fluid domains.
DPPC and saturated phospholipid species
As stated above, DPPC is the most abundant phospholipid species in lung surfactant from most mammals (typically in the order of 40–50% with respect to total surfactant mass) and the one able to produce by itself films with the lowest surface tension. The saturated acyl chains of DPPC can adopt a fully straight conformation, with all the C–C bonds in trans configuration, and this allows the molecule to rich maximal packing, occupying the minimal area per molecule under extreme compression. Unsaturated acyl chains, with the typical cis double bonds of the natural fatty acids, have kinks in their tails that prevent maximal packing, and as a consequence, cannot be in principle compressed to so extreme low tensions. It has been considered for long time that for DPPC-enriched films to reach full condensation upon compression, the operating temperature needs to be lower than the melting temperature of DPPC (41 °C). This temperature corresponds to the transition temperature at which DPPC (bi)layers go from a condensed, ordered state (the so-called gel phase), into a fluid disordered, highly mobile state (the liquid-crystalline phase). Consequently, it is assumed that evolution has selected DPPC as the basis for surfactant in homeothermic mammals because at a physiological temperature of 37 °C, DPPC films can still be compressed to the maximally packed condensed state required to achieve extremely low surface tensions. However, packing/condensation does not depend only on temperature, but also on hydration, which defines the so-called lyotropic phases (as temperature defines the thermotropic phases mentioned above) of lipids [5]. It has been recently demonstrated that the highly dehydrated state reached during the assembly of LBs, allows freshly secreted unused surfactant to maintain a ordered state up to temperatures well above 41 °C [52], which could be important for a good surfactant to sustain breathing even under challenging physiological situations such as feverish episodes. Apart from DPPC, other minor amounts of disaturated phospholipid species complement surfactant and possibly modulate their behavior under slightly different contexts [53,54], according to adaptive processes that still need to be elucidated.
Phosphatidylglycerol (PG) and polyhydroxylated anionic phospholipids
The phospholipid phosphatidylglycerol (PG) is a typical component of the plasma membrane of bacteria such as Escherichia coli, and of the membranes of plant cells, but very unusual in animal cell membranes. However, PG, and also phosphatidylinositol (PI), are the most abundant anionic phospholipid species in lung surfactant [42,43,55]. Typically, PI antecedes metabolically the appearance of PG, as it is a precursor in the pathway to its biosynthesis, but PI also constitutes a significant fraction of mature surfactant and in some animal species PI is even more abundant than PG [55]. The fraction of both (PG + PI) polyhydroxylated (their headgroup contains glycerol or inositol groups, rich in –OH) anionic phospholipids in surfactant accounts for about 10–15% of its mass. They appear at the end of gestation when the lungs mature and are being prepared for air respiration [42,43,55,56]. Extensive studies revealed that PG and PI but not other anionic phospholipids abundant in other tissues, establish selective interactions with the cationic hydrophobic proteins SP-B and SP-C [57,58]. Today it is considered that PG and PI act as a sort of cofactors required for proteins SP-B and SP-C to adopt the proper conformation that allows them to catalyze the efficient transfer of surface active phospholipids into the respiratory surface.
Equilibrated proportion of saturated/unsaturated phospholipid species
The analysis of the structure of pulmonary surfactant membranes [59,60] and interfacial films [61,62] early revealed a conspicuous remarkable feature: the coexistence until close to physiological temperatures of two different phases, one ordered and the other disordered, both with fluid character. This coexistence depends on the presence in surfactant of similar proportions of saturated (mainly DPPC) and unsaturated phospholipid species. At temperatures below the phase transition temperature of the saturated phospholipids, but above of that of the unsaturated species, surfactant layers segregate domains with different order/packing, and presumably composition. It has been interpreted that evolution has somehow selected such a particular compositional combination for lung surfactant to optimize two simultaneous, albeit somehow contradictory, properties. The presence of enough ordered phase is essential for surfactant films to rich maximal packing and produce minimal surface tension with maximal stability. Still, the simultaneous presence of disordered regions into the membranes and films provides to the system a dynamic enough behavior, crucial for rapid adsorption into the interface during expansion at inspiration and for rapid spreading along the interface once the surface expands again after each breathing cycle [59]. These dynamic properties emerging from the most disordered regions of surfactant layers need to be modulated by the hydrophobic proteins, which partitions preferentially into such disordered domains, even accumulating at the boundaries between ordered and disordered phases [63,64]. An equilibrated proportion of saturated and unsaturated phospholipid species, and therefore of ordered/stable and disordered/dynamic phases, in surfactant, is therefore critical for surfactant to behave as a sort of material “alloy”, exhibiting simultaneously physical properties of the two intermixed phases. The phase coexistence extends in surfactant layers along the length scale, from the micro- down to nano-meter sized domains [65,66]. Interestingly, surfactant from heterothermic mammals leaving and breathing at different body temperatures (such as for instance hibernating species) may exhibit important changes in the relative proportions of saturated/unsaturated phospholipids [[67], [68], [69]]. Phospholipid-promoted adaptive changes in surfactant composition and structure seem to be also linked to a proper modulation of the proportion of cholesterol. The conclusion is therefore that different lipid mixtures can still support optimal activity as a function of particular physiological contexts.
Cholesterol
In spite of what is stated above, the presence of cholesterol as a necessary component of a good pulmonary surfactant is still rather controversial. Cholesterol is the main neutral lipid in surfactant, in very variable proportions from 2 to 10% by mass, depending on species, diet, and as said, physiological situations [42,70]. It is clear that cholesterol is a part of natural surfactant, and that it modulates the physical properties of surfactant membranes and films [60,71,72]. In general terms, cholesterol increases the fluidity of the most ordered phases, which gain in mobility and dynamics in the presence of moderate amounts of cholesterol. It has been described that particularly in some animals, like heterothermic mammals able to modulate their physiological temperature depending on seasonal or circadian rhythms or on diet conditions, the proportion of cholesterol is an important feature that finely tunes surfactant properties [[67], [68], [69]]. This suggests that the highly variable amounts of cholesterol in surfactant from different individuals and species may be a consequence of cholesterol playing such a modulating role. It has been widely reported that an exacerbated amount of cholesterol in a deteriorated and at least partially inactivated surfactant is a hallmark of pathological contexts such as ARDS associated to lung injury [[73], [74], [75], [76]]. What is difficult to establish is whether the high cholesterol proportion is a cause or a consequence of a poorly performing surfactant system and the corresponding breathing demands. In the meantime, cholesterol is typically removed, or simply not considered, to produce most of the currently used clinical surfactants [49]. However, some surfactants such as calfactant (Infasurf), produced as extracts from whole surfactant from lavage of calf lungs, still contain the natural proportions of cholesterol and work well in SRT of premature babies [[77], [78], [79]]. Recent work has proposed that a minimal amount of cholesterol could be also important in surfactant to satisfy selected protein-lipid interactions with the hydrophobic proteins [80].
Hydrophobic surfactant proteins SP-B and SP-C
Numerous evidences have confirmed that air-breathing supported life is incompatible with total absence of surfactant proteins SP-B and SP-C. This is behind the lethality of the congenital lack of the expression of the SP-B gene, either in humans or in animal models, which at the end results in a lack of not only protein SP-B but also mature forms of protein SP-C [[81], [82], [83], [84], [85]]. This is also behind the inefficiency of purely lipidic protein-free clinical surfactants to sustain lung function. Numerous studies in vitro have revealed that a minimal proportion of these extremely hydrophobic surfactant proteins is absolutely required for surfactant lipids to adsorb rapid enough into the air–liquid interface and produce an almost immediate reduction in surface tension [6,23]. Interestingly, when tested in vitro, both SP-B and SP-C seem to exhibit redundant properties at least with respect to the promotion of rapid initial adsorption of surfactant into clean interfaces. Then during compression, the proteins, particularly SP-B, are crucial to facilitate reaching the lowest surface tension (<5 mN/m) during compression upon a very limited area reduction [86]. It is estimated that the change in surface area at the alveoli, between the inspiration and expiration moieties of the breathing cycles, is less than 20%. In the presence of SP-B, surface films with a proper lipid composition can reach the lowest tension values with so limited area reduction, which is not the case in the absence of the protein. Current models have proposed that this activity of SP-B could depend on the establishment of protein-promoted bilayer–bilayer and bilayer–monolayer contacts providing the surface film with a surplus of stability at the highest compression rates that avoids collapse of the most dynamic regions of the surfactant films, where SP-B typically partitions [see Fig. 2] [17,80,87,88]. SP-C is a 35-residue polypeptide that promotes bilayer–monolayer and monolayer–bilayer transitions, and has been related with the depuration of the surface film from the least active molecules [[89], [90], [91]]. It has been proposed that once the surface film is formed, the most dynamic components, possibly important to promote adsorption, need to be depurated for the film to become enriched in the most compression-driven packable elements, which become thus competent to reach and sustain the minimal tensions. SP-C could facilitate these compression-driven transitions while maintaining the excluded material closely apposed to the surface. Eventually, upon detachment of the excluded material from the surface film, SP-C could be in charge of targeting this spent surfactant for recycling or degradation [92]. Recent work has reported the participation of both proteins, SP-B and SP-C, in common supramolecular complexes, and their mutual regulation of some properties such as their oligomerization state or their ability to promote fusion or fragmentation of membranes [93].
An operative pulmonary surfactant, at least under homeostatic physiological circumstances, should fulfill these basic compositional principles with respect to DPPC, saturated/unsaturated proportion, anionic phospholipids, cholesterol and hydrophobic surfactant proteins. A deviation from these constraints, as a consequence of pathological contexts, could originate a surfactant with defective performance, either in terms of reduction in surface tension and stabilization of the lung at low volume, or in terms of recycling and long-term maintenance, contributing even further to pathogenesis [94]. A clinical surfactant that cannot fulfill these constraints, i.e. because of the lack of enough proportions of operative proteins, could require a different compositional optimization, behind the standards defined by natural surfactant. For instance, several clinical surfactants under development have been optimized upon addition of a variable proportion of palmitic acid (PA) [[95], [96], [97], [98]]. PA, with its saturated acyl chain, provides additional stability to surfactant films compressed to high packing states. Clinical surfactants lacking enough amounts of an operative SP-B protein typically require a higher proportion of DPPC, a certain proportion of PA, or both, to reach and maintain low enough surface tensions upon compression. The price to pay is that these surfactant preparations are much less dynamic and their suspensions exhibit more viscous behaviors than natural surfactants. This may become a problem at the time of delivering a highly concentrated bolus of therapeutic surfactant into the airways of a baby.
Making recombinant or synthetic humanized surfactant protein analogs
It is clear that the inclusion of enough amounts of operative versions of the hydrophobic surfactant proteins is the bottleneck in the production of the required amounts of clinical surfactants to treat not only premature babies but also patients of other respiratory pathologies. Clinical surfactants from natural origin are expensive to produce, very limited in amount, and difficult to standardize. Furthermore, the use of materials of animal origin has potential non-negligible intrinsic risks of the presence of pathogenic entities difficult to assess and prevent. Production of native-like proteins by expression of the human genes in appropriate recombinant systems is clearly the future to pursue. However, the high hydrophobicity of SP-B and SP-C and the complexity of their native assemblies, generated in vivo upon co-expression of very complex and large precursors, makes this task a really difficult challenge. Variable amounts of recombinant human SP-C (rhSP-C) are starting to be available already [99,100], and some first recombinant surfactants based in this rhSP-C have been already tested in clinical trials [101]. However, in spite of the successful production of the precursor of human SP-B in different expression systems [102,103], the production of recombinant hSP-B has been still an insuperable challenge.
As an alternative, several simplified versions of proteins SP-B and SP-C have been rationally designed and synthetized [104]. The production in large amounts of synthetic peptides is not cheap, but can be done under very controlled and reproducible conditions of quality. The proteins and their analogs are only needed in very small proportions and can be reasonably produced synthetically considering the surfactant amounts required to treat neonates. A more complex problem would be the production of the entirely synthetic surfactants that would be required to treat adult patients. In the meantime, different synthetic surfactant preparations are entering into clinical trials and providing very important information on the potential of SRT based on surfactant protein analogs [105,106]. The smaller hydrophobic protein, SP-C, has been produced synthetically in several laboratories, with some sequence variations to reduce self-aggregation tendencies and avoid problems with oxidation. For instance, the synthetic surfactant CHF5633, which has shown to be effective in preterm babies, contains a synthetic version of human SP-C in which valine residues and the single methionine in the wild type protein have been substituted by leucines, and the cysteines by serines [3,107]. The production of synthetic versions of SP-B, a larger protein of 79 amino acids, with three intramolecular and one intermolecular disulphide bridges, is much more difficult and different laboratories have designed simplified polypeptides mimicking just partial motifs of the wild type protein. The readers are referred to several reviews that summarize the different attempts to make synthetic functional analogs of SP-B [104,108,109].
In general terms, these synthetic protein analogs mimic only part of the activities of the native proteins, but can offer a reasonable ability to form interfacial films able to reduce surface tension in a stable manner. Synthetic preparations based strictly in synthetic analogs of SP-B, reproducing the structure and biophysical capabilities of the protein only to a limited extent, typically require a modified phospholipid composition, with higher proportions of DPPC and often significant amounts of PA. Remarkably, preparations combining analogs of the two proteins such as CHF5633, seem to work reasonably well with a much simpler composition that resembles much more the actual composition of native surfactant. Experiments carried out both in vitro and in vivo confirmed that the preparation containing both SP-B and SP-C analogs worked better than preparations containing just one of the analogs [110,111]. Although the synthetic peptides only mimic part of the structural motifs of the proteins, and is difficult to believe that these simplified versions could reproduce the supramolecular complexes apparently formed by SP-B and SP-C, it is possible that some of the SP-B/SP-C interactions could still be recapitulated, providing at least part of the biophysical features inherent to native lipid/protein surfactant complexes.
Combining the ingredients is not enough: building the proper structure
Once the key ingredients to formulate an operative clinical surfactant have been identified, it is important to remark that they need to be properly assembled for the lipid/protein or lipid/peptide complexes to exhibit optimal activity. In vivo, lipids and proteins are assembled together into highly compact, partly dehydrated, complexes that acquire the ability to transfer material into the air–liquid interface in a highly cooperative manner. Such highly efficient cooperative transfer is likely behind the formation of complex multilayered interfacial films with maximal stability to not only produce low surface tensions but to main those low tensions along many recurrent compression–expansion cycles. Numerous experiments in vitro have confirmed the importance of a high enough density/concentration of surfactant complexes in the subphase in order to form surface films with optimal performance. This has likely to do with such cooperative very rapid transfer of massive amounts of surface active molecules into the interface. Freshly secreted native complexes in the form of LBPs likely preserve local cooperative transfer capabilities once each LBP package touches the air–liquid interface [24]. Complexes reconstituted in vitro are presumably only partly recapitulating the native structure, and can only produce massive interfacial transfer if applied at high enough concentrations onto the respiratory surface. This is likely the reason why clinical surfactants are designed to be applied at very high concentrations, which in many cases is limited by the physical nature of the lipid/protein or lipid/peptide suspensions. Poractant alpha, one of the natural clinical surfactants more widely applied at the neonatal intensive care units (NICUs), is prepared at a nominal concentration of 80 mg of phospholipid per mL, the same concentration at which the synthetic surfactant CHF5633 has been tested in clinical trials [105]. Other surfactants, natural or synthetic, are delivered at lower concentrations because there are limitations in the maximum concentrations achievable depending on the composition and their subsequent viscosity. It is important to take into account that once a dosage of clinical surfactant is applied into the upper airways, it spreads and dilutes into the total volume of fluid coating the entire airways, with the subsequent reduction in concentration/density and the possible loss of cooperativity. The problem could be worse if, as a consequence of the respiratory failure and the lung injury, some edema has already occurred and additional fluid has leaked into the alveolar spaces [94]. This, apart from other problems (see below the reference to inactivation contexts), presumably originates a further reduction in the operative concentration of surfactant and a loss of efficiency. Similar loss of cooperativity could be expected if surfactant is administered not as a intratracheal concentrated bolus but as a certain volume of diluted suspensions nebulized during a period of time. Nebulization have been tested as a less-invasive alternative to deliver clinical surfactant, with only limited efficacy [9]. New approaches need to be designed to deliver surfactant in non-invasive ways without losing the characteristic cooperative behavior of freshly secreted natural surfactants. A promising alternative that is being recently explored is the administration of dry therapeutic surfactant powders, resulting from the elimination of solvent, i.e. by spry-drying, from lipid–protein surfactant mixtures [112]. Aerosolization of dry powder surfactant may preserve a rapid and cooperative adsorption following the efficient delivery of surfactant by a less-invasive procedure. The basic concept is still under development, including the development of optimal compositions affecting both biophysical behavior and excipients to facilitate the stability of dry lipid-based powders under appropriate storage and delivery conditions.
An interesting strategy explored in the literature to produce clinical surfactants with high efficacy is their transient or permanent exposure to highly concentrated hydrophilic polymers such as dextran, polyethylenglycol or hyaluronic acid (HA). It has been demonstrated that exposure to molecularly crowded environments such as those created by concentrated water-soluble polymers increase the activity of surfactants at diluted concentrations or in the presence of inhibitory compounds, at list as assessed in vitro [51,[113], [114], [115]]. It somehow recapitulate the effect of the presence in surfactant of the hydrophilic protein SP-A, which increases the surface activity of surfactant improving its cooperative adsorption to form surface-active interfacial films [[116], [117], [118]]. Physico-chemical studies have revealed that the exposure of surfactant to such crowded media induce their condensation into a sort of pre-activated state with much higher packing and conspicuous dehydration of the complexes, and that this induces somehow a re-organization of the distribution of lipids and proteins into more surface active states [113,114]. Administration of surfactant/polymer combinations does not work that well in vivo, possibly as a consequence of osmotic forces created by the highly concentrated polymers into the airways. However, some polymers like HA seem to induce a irreversible pre-activation of surfactant that is apparently retained even after removal of the polymer [113]. This illustrates that novel more efficient future clinical surfactants could be produced by mimicking some of the processes occurring during assembly and maturation of surfactant into the LBs of the pneumocytes but using alternative procedures accessible at the industrial productive platforms.
Tailoring surfactants for specific applications
Although the continuous improvement of perinatal care at the modern NICUs has reduced considerably the number of babies still requiring SRT [7], this technology keeps saving the lives of uncountable very premature babies whose lungs had still no time to mature and be ready for air breathing. The lungs of many of these babies mature properly once they can be opened thanks to the introduction of the exogenous surfactant. In these babies, the exogenous surfactant has only to play a transient role to minimize lung injury as a consequence of respiration in the absence of an operative surfactant. In a relatively short period of time, the exogenous surfactant is substituted by the endogenously produced material, once the lung matures and produces its proper surfactant. The consequence is that many of these babies only require one, at worse two, doses of clinical surfactant to initiate their air-breathing life. To what extent the clinical surfactant used in these SRT needs to be as close as possible to the optimal native material is questionable. It is plausible that this “starting” surfactant only needs to be good enough as a “biophysical initiator”, meaning that it is at least competent to form an interfacial surface active film in a rapid and efficient manner, stable for a sufficiently long period of time to allow the production and arrival of the endogenous material. This may be the reason why many different surfactant preparations, produced by different methods from natural sources, or assembled from the combination of a variety of lipids and proteins/peptides, seem apparently to work.
A completely different scenario is what a clinical surfactant likely encounters once delivered into an injured and inflamed lung like it is the case in babies suffering of meconium aspiration syndrome (MAS), or in other cases of ARDS as a consequence of lung injury and inflammation by multiple potential causes [12]. Endogenous surfactant may be then inactivated as a consequence of the liberation of enzymes (lipases, proteases) and oxidative radicals, and/or the presence into edematous airways of leaked serum and different inflammatory mediators. If this is the case, the injury of the alveolar epithelium may require tissue reparation before it is ready to produce new endogenous surfactant. Under such constraints, exogenous surfactant needs to be able to restore surfactant function even in the presence of a highly inactivating context, during a long enough period of time. The surfactant-promoted biophysical facilitation of breathing mechanics can thus prevent further inflammatory damage and contribute to progressively restore a proper lung performance. So far, SRT trials in cases of ARDS and lung injury have shown very limited success, possibly because of the limited efficacy of the available clinical surfactants, particularly under the very demanding circumstances associated with lung injury. The investigation of the biophysical and molecular mechanisms of surfactant inactivation has been very active during the last few years, including the crucial development of in vitro models to test and assess in qualitative and quantitative detail surfactant performance under physiologically meaningful inactivating scenarios (revised in Refs. [12,51,94,119]). However, it is difficult to predict to what extent the behavior of clinical surfactants under controlled in vitro conditions could model the complex scenarios generated by injured lungs in vivo. The in vitro models have at least allowed determining that there is room for improvement in the development of clinical surfactants with enhanced resistance to inactivation. The synthetic surfactant CHF5633, for instance, has shown some resistance to inactivation by both serum or meconium [94], and it certainly showed improved performance in vivo with respect to natural surfactants such as poractant-alpha when tested in animal models with lung injury [120]. To what extent such inactivation resistance resides in a particular lipid composition or in the concerted action of SP-B and SP-C analogs is something that requires further investigation. Interestingly, it seems that a synthetic surfactant such as CHF5633 might somehow exhibit much slower turnover in vivo than a natural preparation like poractant-alpha [121]. This may facilitate protection of the respiratory spaces during the long periods of time required to restore the endogenous production of the patient's surfactant. In any case, it opens a new line of research that could pursue tailoring different surfactants for specific clinical applications, for instance when longer residence times, or resistance to particular inhibitory contributions, may be required. Other alternatives may include the use in surfactant formulations of non-hydrolyzable analogs of surface-active phospholipids [122,123], which could exhibit higher protection against the action of inflammatory phospholipases typically leaked in injured lungs [124]. In a similar line, it has been proposed that supplementation of clinical surfactant with inhibitors of inflammatory phospholipases such as varespladib may aid to treat ARDS [125].
A particular case of recent interest has been the potential use of SRT in the treatment of COVID-19 patients [126]. Preliminary results upon application of SRT in some COVID-19 patients were encouraging [127,128] and several clinical trials are open at the time of writing this review, whose results should be delivered in brief allowing to assess the potential utility of surfactant replacement to improve the outcome of patients with severe COVID-19-associated ARDS. It has been reported that the SARS-Cov-2 virus has some tropism against type II cells, whose destruction is a probable contribution to the severity of the worst cases of COVID-19. In this sense, COVID-19 could be more similar to pathologies associated with a intrinsic lack of surfactant, such as neonatal RDS, than to secondary ARDS evolved from multifactorial lung injury and inflammation [129].
An additional avenue for improvement of materials amenable for SRT includes the incorporation of the hydrophilic surfactant proteins SP-A or SP-D. These proteins not only complement surfactant from the point of view of the protection against pathogens, but exhibit conspicuous anti-inflammatory properties [29,130,131]. Recombinant versions of human collectins SP-A and SP-D have been already produced [132,133], making feasible the production of more complete humanized surfactant preparations, with enhanced properties in the framework of certain pathologies such as bronchopulmonary dysplasia [134].
Another particular case that may require tailored clinical surfactants is their use as promising drug-delivery agents. Recent research has analyzed in detail the ability of pulmonary surfactant to facilitate spreading and diffusion of therapeutic molecules, including poorly soluble drugs or particles carrying siRNAs, using the respiratory air–liquid interface as a shuttle [[135], [136], [137]]. Different biophysical approaches using specific setups have revealed how surfactant can transport different molecules to long distances, once delivered at the air–liquid interface, which facilitates an efficient distribution all the way from the upper to the distal airways [[138], [139], [140]]. This can improve substantially the paradigm of the delivery of inhaled drugs at extents difficult to predict, but that could be potentially extensible to any therapeutic agent, including peptides and proteins such as antibodies or enzymes, gene therapy vectors or inhaled vaccines. It is conceivable that the compositional and structural determinants governing the ability of surfactant to vehiculize therapeutic agents via the air–liquid interface will be different to those defining full surface tension reduction capabilities. It is also important to consider that an optimized delivery into the distal airways will be likely connected with a fruitful transepithelial delivery into the systemic circulation, opening new avenues for the systemic delivery of drugs such as peptidic hormones or antibodies, through non-invasive pathways that are not available nowadays. Future research should be able to optimize novel surfactant preparations engineered for inhalative delivery, with particular features tailored for well-defined therapeutic entities.
Conclusions: the basic surfactant recipe
A basic recipe to produce a good clinical surfactant should consider a basic composition containing a) sufficient proportions of DPPC, b) an equilibrated combination of saturated and unsaturated phospholipids, c) enough proportion, in the order of 10–15%, of anionic polyhydroxylated phospholipids, particularly PG, and d) sufficient content of SP-B and SP-C proteins or their analogs. Clinical surfactants should be also produced as a highly concentrated suspensions able to form surface active interfacial films with high cooperativity, and/or be subjected to some treatments that could optimize their structure towards a highly packed/dehydrated state with higher activity and enhanced resistance to inactivation. Finally, this basic recipe should admit variations in composition and production methodologies as a function of the final therapeutic target, whether intended for non pathologic but immature lungs, injured lungs or healthy operative lungs requiring delivery of inhalative treatments.
The future of the wide but tailored application of SRT to patients of different respiratory pathologies is highly promising but is currently going through the bottleneck of the production of massive amounts of humanized surfactant proteins as a basis for the production of therapeutic surfactants of new generation. Their combination with selective drugs or bioactive elements (DNA, RNA, antibodies, enzymes and inhibitors, etc), together with the development of efficient non-invasive delivery strategies will pave the way towards a future world of personalized surfactant therapies.
Conflicts of interest
The author has received research grants from Chiesi Farmaceutici SpA, and Airway Therapeutics Inc. He served as lecturer for Chiesi Farmaceutici SpA. He has also been member of advisory boards for Chiesi Farmaceutici SpA and Airway Therapeutics. These companies produce clinical surfactants and/or surfactant proteins or related products, but have not participated in the preparation, review, or approval of the manuscript or in the decision to submit it for publication.
Acknowledgments
Research in the laboratory of the author is currently funded by grants from the Spanish Ministry of Science and Innovation (RTI2018-094564-B-I00) and the Regional Government of Madrid (P2018/NMT-4389).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Hentschel R., Bohlin K., van Kaam A., Fuchs H., Danhaive O. Surfactant replacement therapy: from biological basis to current clinical practice. Pediatr Res. 2020;88:176–183. doi: 10.1038/s41390-020-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niemarkt H.J., Hutten M.C., Kramer B.W. Surfactant for respiratory distress syndrome: new ideas on a familiar drug with innovative applications. Neonatology. 2017;111:408–414. doi: 10.1159/000458466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curstedt T., Halliday H.L., Speer C.P. A unique story in neonatal research: the development of a porcine surfactant. Neonatology. 2015;107:321–329. doi: 10.1159/000381117. [DOI] [PubMed] [Google Scholar]
- 4.Speer C.P., Sweet D.G., Halliday H.L. Surfactant therapy: past, present and future. Early Hum Dev. 2013;89(Suppl 1):S22–S24. doi: 10.1016/S0378-3782(13)70008-2. [DOI] [PubMed] [Google Scholar]
- 5.Castillo-Sanchez J.C., Cruz A., Perez-Gil J. Structural hallmarks of lung surfactant: lipid-protein interactions, membrane structure and future challenges. Arch Biochem Biophys. 2021;703:108850. doi: 10.1016/j.abb.2021.108850. [DOI] [PubMed] [Google Scholar]
- 6.Canadas O., Olmeda B., Alonso A., Perez-Gil J. Lipid-protein and protein-protein interactions in the pulmonary surfactant system and their role in lung homeostasis. Int J Mol Sci. 2020;21:3708. doi: 10.3390/ijms21103708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweet D.G., Carnielli V., Greisen G., Hallman M., Ozek E., Te Pas A., et al. European consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology. 2019;115:432–450. doi: 10.1159/000499361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autilio C., Echaide M., Benachi A., Marfaing-Koka A., Capoluongo E.D., Perez-Gil J., et al. A noninvasive surfactant adsorption test predicting the need for surfactant therapy in preterm infants treated with continuous positive airway pressure. J Pediatr. 2017;182:66–73.e1. doi: 10.1016/j.jpeds.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 9.Hartel C., Glaser K., Speer C.P. The miracles of surfactant: less invasive surfactant administration, nebulization, and carrier of topical drugs. Neonatology. 2021;118:225–234. doi: 10.1159/000516106. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds P., Bustani P., Darby C., Fernandez Alvarez J.R., Fox G., Jones S., et al. Less-invasive surfactant administration for neonatal respiratory distress syndrome: a consensus guideline. Neonatology. 2021;118:586–592. doi: 10.1159/000518396. [DOI] [PubMed] [Google Scholar]
- 11.De Luca D., Cogo P., Kneyber M.C., Biban P., Semple M.G., Perez-Gil J., et al. Surfactant therapies for pediatric and neonatal ARDS: ESPNIC expert consensus opinion for future research steps. Crit Care. 2021;25:75. doi: 10.1186/s13054-021-03489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Autilio C., Perez-Gil J. Understanding the principle biophysics concepts of pulmonary surfactant in health and disease. Arch Dis Child Fetal Neonatal Ed. 2019;104:F443–F451. doi: 10.1136/archdischild-2018-315413. [DOI] [PubMed] [Google Scholar]
- 13.Oseliero Filho P.L., Gerbelli B.B., Fornasier F., Chaves Filho A.B., Yoshinaga M.Y., Miyamoto S., et al. Structure and thermotropic behavior of bovine- and porcine-derived exogenous lung surfactants. Langmuir. 2020;36:14514–14529. doi: 10.1021/acs.langmuir.0c02224. [DOI] [PubMed] [Google Scholar]
- 14.Schurch S., Qanbar R., Bachofen H., Possmayer F. The surface-associated surfactant reservoir in the alveolar lining. Biol Neonate. 1995;67(Suppl 1):61–76. doi: 10.1159/000244207. [DOI] [PubMed] [Google Scholar]
- 15.Xu L., Yang Y., Zuo Y.Y. Atomic force microscopy imaging of adsorbed pulmonary surfactant films. Biophys J. 2020;119:756–766. doi: 10.1016/j.bpj.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Gil J., Weaver T.E. Pulmonary surfactant pathophysiology: current models and open questions. Physiology. 2010;25:132–141. doi: 10.1152/physiol.00006.2010. [DOI] [PubMed] [Google Scholar]
- 17.Bernardino de la Serna J., Vargas R., Picardi V., Cruz A., Arranz R., Valpuesta J.M., et al. Segregated ordered lipid phases and protein-promoted membrane cohesivity are required for pulmonary surfactant films to stabilize and protect the respiratory surface. Faraday Discuss. 2013;161:535–548. doi: 10.1039/c2fd20096a. discussion 63-89. [DOI] [PubMed] [Google Scholar]
- 18.Parra E., Perez-Gil J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem Phys Lipids. 2015;185:153–175. doi: 10.1016/j.chemphyslip.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Ochs M. The closer we look the more we see? Quantitative microscopic analysis of the pulmonary surfactant system. Cell Physiol Biochem. 2010;25:27–40. doi: 10.1159/000272061. [DOI] [PubMed] [Google Scholar]
- 20.Vanhecke D., Herrmann G., Graber W., Hillmann-Marti T., Muhlfeld C., Studer D., et al. Lamellar body ultrastructure revisited: high-pressure freezing and cryo-electron microscopy of vitreous sections. Histochem Cell Biol. 2010;134:319–326. doi: 10.1007/s00418-010-0736-4. [DOI] [PubMed] [Google Scholar]
- 21.Ban N., Matsumura Y., Sakai H., Takanezawa Y., Sasaki M., Arai H., et al. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282:9628–9634. doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- 22.Cheong N., Zhang H., Madesh M., Zhao M., Yu K., Dodia C., et al. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282:23811–23817. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim Biophys Acta. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Haller T., Dietl P., Stockner H., Frick M., Mair N., Tinhofer I., et al. Tracing surfactant transformation from cellular release to insertion into an air-liquid interface. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1009–L1015. doi: 10.1152/ajplung.00342.2003. [DOI] [PubMed] [Google Scholar]
- 25.Hobi N., Giolai M., Olmeda B., Miklavc P., Felder E., Walther P., et al. A small key unlocks a heavy door: the essential function of the small hydrophobic proteins SP-B and SP-C to trigger adsorption of pulmonary surfactant lamellar bodies. Biochim Biophys Acta. 2016;1863:2124–2134. doi: 10.1016/j.bbamcr.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Taeusch H.W., Bernardino de la Serna J., Perez-Gil J., Alonso C., Zasadzinski J.A. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: experimental. Biophys J. 2005;89:1769–1779. doi: 10.1529/biophysj.105.062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Álvarez B., Alonso A., Perez-Gil J. In eLS, John Wiley & Sons, Ltd (Ed.); 2019. Structure and function of pulmonary surfactant proteins. [Google Scholar]
- 28.Casals C., Campanero-Rhodes M.A., Garcia-Fojeda B., Solis D. The role of collectins and galectins in lung innate immune defense. Front Immunol. 2018;9:1998. doi: 10.3389/fimmu.2018.01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson A., Madsen J., Clark H.W. SP-A and SP-D: dual functioning immune molecules with antiviral and immunomodulatory properties. Front Immunol. 2021;11:622598. doi: 10.3389/fimmu.2020.622598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orgeig S., Morrison J.L., Daniels C.B. Evolution, development, and function of the pulmonary surfactant system in normal and perturbed environments. Compr Physiol. 2015;6:363–422. doi: 10.1002/cphy.c150003. [DOI] [PubMed] [Google Scholar]
- 31.Brasch F., Johnen G., Winn-Brasch A., Guttentag S.H., Schmiedl A., Kapp N., et al. Surfactant protein B in type II pneumocytes and intra-alveolar surfactant forms of human lungs. Am J Respir Cell Mol Biol. 2004;30:449–458. doi: 10.1165/rcmb.2003-0262OC. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y., Fujita Y., Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis. 1989;140:75–81. doi: 10.1164/ajrccm/140.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Coya J.M., Akinbi H.T., Saenz A., Yang L., Weaver T.E., Casals C. Natural anti-infective pulmonary proteins: in vivo cooperative action of surfactant protein SP-A and the lung antimicrobial peptide SP-BN. J Immunol. 2015;195:1628–1636. doi: 10.4049/jimmunol.1500778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H., Kuzmenko A., Wan S., Schaffer L., Weiss A., Fisher J.H., et al. Surfactant proteins A and D inhibit the growth of gram-negative bacteria by increasing membrane permeability. J Clin Investig. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan M.A., Akinbi H.T., Serrano A.G., Perez-Gil J., Wu H., McCormack F.X., et al. Antimicrobial activity of native and synthetic surfactant protein B peptides. J Immunol. 2006;176:416–425. doi: 10.4049/jimmunol.176.1.416. [DOI] [PubMed] [Google Scholar]
- 36.Avery M.E., Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara T., Maeta H., Chida S., Morita T., Watabe Y., Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 38.Hildebran J.N., Goerke J., Clements J.A. Pulmonary surface film stability and composition. J Appl Physiol Respir Environ Exerc Physiol. 1979;47:604–611. doi: 10.1152/jappl.1979.47.3.604. [DOI] [PubMed] [Google Scholar]
- 39.King R.J., Clements J.A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972;223:715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- 40.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 41.Fleming B.D., Keough K.M. Surface respreading after collapse of monolayers containing major lipids of pulmonary surfactant. Chem Phys Lipids. 1988;49:81–86. doi: 10.1016/0009-3084(88)90067-9. [DOI] [PubMed] [Google Scholar]
- 42.Bernhard W. Lung surfactant: function and composition in the context of development and respiratory physiology. Ann Anat. 2016;208:146–150. doi: 10.1016/j.aanat.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Hallman M., Kulovich M., Kirkpatrick E., Sugarman R.G., Gluck L. Phosphatidylinositol and phosphatidylglycerol in amniotic fluid: indices of lung maturity. Am J Obstet Gynecol. 1976;125:613–617. doi: 10.1016/0002-9378(76)90782-1. [DOI] [PubMed] [Google Scholar]
- 44.Bangham A.D., Morley C.J., Phillips M.C. The physical properties of an effective lung surfactant. Biochim Biophys Acta. 1979;573:552–556. doi: 10.1016/0005-2760(79)90229-7. [DOI] [PubMed] [Google Scholar]
- 45.Morley C.J., Bangham A.D., Miller N., Davis J.A. Dry artificial lung surfactant and its effect on very premature babies. Lancet. 1981;1:64–68. doi: 10.1016/s0140-6736(81)90002-7. [DOI] [PubMed] [Google Scholar]
- 46.Morley C.J., Banhham A.D., Johnson P., Thorburn G.D., Jenkin G. Physical and physiological properties of dry lung surfactant. Nature. 1978;271:162–163. doi: 10.1038/271162a0. [DOI] [PubMed] [Google Scholar]
- 47.Curstedt T., Johansson J., Barros-Soderling J., Robertson B., Nilsson G., Westberg M., et al. Low-molecular-mass surfactant protein type 1. The primary structure of a hydrophobic 8-kDa polypeptide with eight half-cystine residues. Eur J Biochem. 1988;172:521–525. doi: 10.1111/j.1432-1033.1988.tb13918.x. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi A., Fujiwara T. Proteolipid in bovine lung surfactant: its role in surfactant function. Biochem Biophys Res Commun. 1986;135:527–532. doi: 10.1016/0006-291x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- 49.Blanco O., Perez-Gil J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat Respiratory Distress Syndrome: role of the different components in an efficient pulmonary surfactant. Eur J Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 50.Possmayer F., Hall S.B., Haller T., Petersen N.O., Zuo Y.Y., Bernardino de la Serna J., et al. Recent advances in alveolar biology: some new looks at the alveolar interface. Respir Physiol Neurobiol. 2010;173(Suppl):S55–S64. doi: 10.1016/j.resp.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Zuo Y.Y., Veldhuizen R.A., Neumann A.W., Petersen N.O., Possmayer F. Current perspectives in pulmonary surfactant – inhibition, enhancement and evaluation. Biochim Biophys Acta. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 52.Cerrada A., Haller T., Cruz A., Perez-Gil J. Pneumocytes assemble lung surfactant as highly packed/dehydrated states with optimal surface activity. Biophys J. 2015;109:2295–2306. doi: 10.1016/j.bpj.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schipke J., Jutte D., Brandenberger C., Autilio C., Perez-Gil J., Bernhard W., et al. Dietary carbohydrates and fat induce distinct surfactant alterations in mice. Am J Respir Cell Mol Biol. 2021;64:379–390. doi: 10.1165/rcmb.2020-0335OC. [DOI] [PubMed] [Google Scholar]
- 54.Pynn C.J., Picardi M.V., Nicholson T., Wistuba D., Poets C.F., Schleicher E., et al. Myristate is selectively incorporated into surfactant and decreases dipalmitoylphosphatidylcholine without functional impairment. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1306–R1316. doi: 10.1152/ajpregu.00380.2010. [DOI] [PubMed] [Google Scholar]
- 55.Markin C.J., Hall S.B. The anionic phospholipids of bovine pulmonary surfactant. Lipids. 2021;56:49–57. doi: 10.1002/lipd.12273. [DOI] [PubMed] [Google Scholar]
- 56.Chapman J.F. Amniotic fluid tests for fetal lung maturation – the good, the bad, and the promising. Clin Lab Sci. 1994;7:95–99. [PubMed] [Google Scholar]
- 57.Cabre E.J., Loura L.M., Fedorov A., Perez-Gil J., Prieto M. Topology and lipid selectivity of pulmonary surfactant protein SP-B in membranes: answers from fluorescence. Biochim Biophys Acta. 2012;1818:1717–1725. doi: 10.1016/j.bbamem.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Gil J., Casals C., Marsh D. Interactions of hydrophobic lung surfactant proteins SP-B and SP-C with dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylglycerol bilayers studied by electron spin resonance spectroscopy. Biochemistry. 1995;34:3964–3971. doi: 10.1021/bi00012a014. [DOI] [PubMed] [Google Scholar]
- 59.Bernardino de la Serna J., Oradd G., Bagatolli L.A., Simonsen A.C., Marsh D., Lindblom G., et al. Segregated phases in pulmonary surfactant membranes do not show coexistence of lipid populations with differentiated dynamic properties. Biophys J. 2009;97:1381–1389. doi: 10.1016/j.bpj.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernardino de la Serna J., Perez-Gil J., Simonsen A.C., Bagatolli L.A. Cholesterol rules: direct observation of the coexistence of two fluid phases in native pulmonary surfactant membranes at physiological temperatures. J Biol Chem. 2004;279:40715–40722. doi: 10.1074/jbc.M404648200. [DOI] [PubMed] [Google Scholar]
- 61.Nag K., Perez-Gil J., Ruano M.L., Worthman L.A., Stewart J., Casals C., et al. Phase transitions in films of lung surfactant at the air-water interface. Biophys J. 1998;74:2983–2995. doi: 10.1016/S0006-3495(98)78005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Discher B.M., Maloney K.M., Schief W.R., Jr., Grainger D.W., Vogel V., Hall S.B. Lateral phase separation in interfacial films of pulmonary surfactant. Biophys J. 1996;71:2583–2590. doi: 10.1016/S0006-3495(96)79450-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wustneck R., Perez-Gil J., Wustneck N., Cruz A., Fainerman V.B., Pison U. Interfacial properties of pulmonary surfactant layers. Adv Colloid Interface Sci. 2005;117:33–58. doi: 10.1016/j.cis.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Nag K., Taneva S.G., Perez-Gil J., Cruz A., Keough K.M. Combinations of fluorescently labeled pulmonary surfactant proteins SP-B and SP-C in phospholipid films. Biophys J. 1997;72:2638–2650. doi: 10.1016/S0006-3495(97)78907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanco O., Cruz A., Ospina O.L., Lopez-Rodriguez E., Vazquez L., Perez-Gil J. Interfacial behavior and structural properties of a clinical lung surfactant from porcine source. Biochim Biophys Acta. 2012;1818:2756–2766. doi: 10.1016/j.bbamem.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 66.Cruz A., Vazquez L., Velez M., Perez-Gil J. Effect of pulmonary surfactant protein SP-B on the micro- and nanostructure of phospholipid films. Biophys J. 2004;86:308–320. doi: 10.1016/S0006-3495(04)74106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suri L.N., Cruz A., Veldhuizen R.A., Staples J.F., Possmayer F., Orgeig S., et al. Adaptations to hibernation in lung surfactant composition of 13-lined ground squirrels influence surfactant lipid phase segregation properties. Biochim Biophys Acta. 2013;1828:1707–1714. doi: 10.1016/j.bbamem.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Suri L.N., McCaig L., Picardi M.V., Ospina O.L., Veldhuizen R.A., Staples J.F., et al. Adaptation to low body temperature influences pulmonary surfactant composition thereby increasing fluidity while maintaining appropriately ordered membrane structure and surface activity. Biochim Biophys Acta. 2012;1818:1581–1589. doi: 10.1016/j.bbamem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 69.Lang C.J., Postle A.D., Orgeig S., Possmayer F., Bernhard W., Panda A.K., et al. Dipalmitoylphosphatidylcholine is not the major surfactant phospholipid species in all mammals. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1426–R1439. doi: 10.1152/ajpregu.00496.2004. [DOI] [PubMed] [Google Scholar]
- 70.Orgeig S., Daniels C.B. The roles of cholesterol in pulmonary surfactant: insights from comparative and evolutionary studies. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:75–89. doi: 10.1016/s1095-6433(01)00307-5. [DOI] [PubMed] [Google Scholar]
- 71.Discher B.M., Maloney K.M., Grainger D.W., Hall S.B. Effect of neutral lipids on coexisting phases in monolayers of pulmonary surfactant. Biophys Chem. 2002;101–102:333–345. doi: 10.1016/s0301-4622(02)00191-6. [DOI] [PubMed] [Google Scholar]
- 72.Discher B.M., Maloney K.M., Grainger D.W., Sousa C.A., Hall S.B. Neutral lipids induce critical behavior in interfacial monolayers of pulmonary surfactant. Biochemistry. 1999;38:374–383. doi: 10.1021/bi981386h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gunasekara L., Al-Saiedy M., Green F., Pratt R., Bjornson C., Yang A., et al. Pulmonary surfactant dysfunction in pediatric cystic fibrosis: mechanisms and reversal with a lipid-sequestering drug. J Cyst Fibros. 2017;16:565–572. doi: 10.1016/j.jcf.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 74.Vockeroth D., Gunasekara L., Amrein M., Possmayer F., Lewis J.F., Veldhuizen R.A. Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298:L117–L125. doi: 10.1152/ajplung.00218.2009. [DOI] [PubMed] [Google Scholar]
- 75.Markart P., Ruppert C., Wygrecka M., Colaris T., Dahal B., Walmrath D., et al. Patients with ARDS show improvement but not normalisation of alveolar surface activity with surfactant treatment: putative role of neutral lipids. Thorax. 2007;62:588–594. doi: 10.1136/thx.2006.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gunasekara L., Schurch S., Schoel W.M., Nag K., Leonenko Z., Haufs M., et al. Pulmonary surfactant function is abolished by an elevated proportion of cholesterol. Biochim Biophys Acta. 2005;1737:27–35. doi: 10.1016/j.bbalip.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Jeon G.W., Oh M., Sin J.B. Efficacy of surfactant-TA, calfactant and poractant alfa for preterm infants with respiratory distress syndrome: a retrospective study. Yonsei Med J. 2015;56:433–439. doi: 10.3349/ymj.2015.56.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramanathan R. Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J Perinatol. 2009;29(Suppl 2):S38–S43. doi: 10.1038/jp.2009.31. [DOI] [PubMed] [Google Scholar]
- 79.Logan J.W., Moya F.R. Animal-derived surfactants for the treatment and prevention of neonatal respiratory distress syndrome: summary of clinical trials. Ther Clin Risk Manag. 2009;5:251–260. doi: 10.2147/tcrm.s4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liekkinen J., Enkavi G., Javanainen M., Olmeda B., Perez-Gil J., Vattulainen I. Pulmonary surfactant lipid reorganization induced by the adsorption of the oligomeric surfactant protein B complex. J Mol Biol. 2020;432:3251–3268. doi: 10.1016/j.jmb.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 81.Ruhl N., Lopez-Rodriguez E., Albert K., Smith B.J., Weaver T.E., Ochs M., et al. Surfactant protein B deficiency induced high surface tension: relationship between alveolar micromechanics, alveolar fluid properties and alveolar epithelial cell injury. Int J Mol Sci. 2019;20:4243. doi: 10.3390/ijms20174243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nogee L.M. Alterations in SP-B and SP-C expression in neonatal lung disease. Annu Rev Physiol. 2004;66:601–623. doi: 10.1146/annurev.physiol.66.032102.134711. [DOI] [PubMed] [Google Scholar]
- 83.Melton K.R., Nesslein L.L., Ikegami M., Tichelaar J.W., Clark J.C., Whitsett J.A., et al. SP-B deficiency causes respiratory failure in adult mice. Am J Physiol Lung Cell Mol Physiol. 2003;285:L543–L549. doi: 10.1152/ajplung.00011.2003. [DOI] [PubMed] [Google Scholar]
- 84.Clark J.C., Wert S.E., Bachurski C.J., Stahlman M.T., Stripp B.R., Weaver T.E., et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nogee L.M., Garnier G., Dietz H.C., Singer L., Murphy A.M., deMello D.E., et al. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Investig. 1994;93:1860–1863. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schurch D., Ospina O.L., Cruz A., Perez-Gil J. Combined and independent action of proteins SP-B and SP-C in the surface behavior and mechanical stability of pulmonary surfactant films. Biophys J. 2010;99:3290–3299. doi: 10.1016/j.bpj.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cabre E.J., Malmstrom J., Sutherland D., Perez-Gil J., Otzen D.E. Surfactant protein SP-B strongly modifies surface collapse of phospholipid vesicles: insights from a quartz crystal microbalance with dissipation. Biophys J. 2009;97:768–776. doi: 10.1016/j.bpj.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krol S., Ross M., Sieber M., Kunneke S., Galla H.J., Janshoff A. Formation of three-dimensional protein-lipid aggregates in monolayer films induced by surfactant protein B. Biophys J. 2000;79:904–918. doi: 10.1016/S0006-3495(00)76346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plasencia I., Baumgart F., Andreu D., Marsh D., Perez-Gil J. Effect of acylation on the interaction of the N-Terminal segment of pulmonary surfactant protein SP-C with phospholipid membranes. Biochim Biophys Acta. 2008;1778:1274–1282. doi: 10.1016/j.bbamem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez-Horta A., Andreu D., Morrow M.R., Perez-Gil J. Effects of palmitoylation on dynamics and phospholipid-bilayer-perturbing properties of the N-terminal segment of pulmonary surfactant protein SP-C as shown by 2H-NMR. Biophys J. 2008;95:2308–2317. doi: 10.1529/biophysj.108.132845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Na Nakorn P., Meyer M.C., Flach C.R., Mendelsohn R., Galla H.J. Surfactant protein C and lung function: new insights into the role of alpha-helical length and palmitoylation. Eur Biophys J. 2007;36:477–489. doi: 10.1007/s00249-006-0102-6. [DOI] [PubMed] [Google Scholar]
- 92.Barriga A., Moran-Lalangui M., Castillo-Sanchez J.C., Mingarro I., Perez-Gil J., Garcia-Alvarez B. Role of pulmonary surfactant protein Sp-C dimerization on membrane fragmentation: an emergent mechanism involved in lung defense and homeostasis. Biochim Biophys Acta Biomembr. 2021;1863:183572. doi: 10.1016/j.bbamem.2021.183572. [DOI] [PubMed] [Google Scholar]
- 93.Cabre E.J., Martinez-Calle M., Prieto M., Fedorov A., Olmeda B., Loura L.M.S., et al. Homo- and hetero-oligomerization of hydrophobic pulmonary surfactant proteins SP-B and SP-C in surfactant phospholipid membranes. J Biol Chem. 2018;293:9399–9411. doi: 10.1074/jbc.RA117.000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Echaide M., Autilio C., Arroyo R., Perez-Gil J. Restoring pulmonary surfactant membranes and films at the respiratory surface. Biochim Biophys Acta Biomembr. 2017;1859:1725–1739. doi: 10.1016/j.bbamem.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 95.Bruni R., Taeusch H.W., Waring A.J. Surfactant protein B: lipid interactions of synthetic peptides representing the amino-terminal amphipathic domain. Proc Natl Acad Sci U S A. 1991;88:7451–7455. doi: 10.1073/pnas.88.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cochrane C.G., Revak S.D., Merritt T.A., Heldt G.P., Hallman M., Cunningham M.D., et al. The efficacy and safety of KL4-surfactant in preterm infants with respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153:404–410. doi: 10.1164/ajrccm.153.1.8542150. [DOI] [PubMed] [Google Scholar]
- 97.Notter R.H., Wang Z., Egan E.A., Holm B.A. Component-specific surface and physiological activity in bovine-derived lung surfactants. Chem Phys Lipids. 2002;114:21–34. doi: 10.1016/s0009-3084(01)00197-9. [DOI] [PubMed] [Google Scholar]
- 98.Spragg R.G., Smith R.M., Harris K., Lewis J., Hafner D., Germann P. Effect of recombinant SP-C surfactant in a porcine lavage model of acute lung injury. J Appl Physiol. 2000;88:674–681. doi: 10.1152/jappl.2000.88.2.674. [DOI] [PubMed] [Google Scholar]
- 99.Hafner D., Germann P.G., Hauschke D. Effects of rSP-C surfactant on oxygenation and histology in a rat-lung-lavage model of acute lung injury. Am J Respir Crit Care Med. 1998;158:270–278. doi: 10.1164/ajrccm.158.1.9712061. [DOI] [PubMed] [Google Scholar]
- 100.Lukovic D., Plasencia I., Taberner F.J., Salgado J., Calvete J.J., Perez-Gil J., et al. Production and characterisation of recombinant forms of human pulmonary surfactant protein C (SP-C): structure and surface activity. Biochim Biophys Acta. 2006;1758:509–518. doi: 10.1016/j.bbamem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 101.Spragg R.G., Taut F.J., Lewis J.F., Schenk P., Ruppert C., Dean N., et al. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med. 2011;183:1055–1061. doi: 10.1164/rccm.201009-1424OC. [DOI] [PubMed] [Google Scholar]
- 102.Banares-Hidalgo A., Perez-Gil J., Estrada P. Conformational stability of the NH2-terminal propeptide of the precursor of pulmonary surfactant protein SP-B. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sever N., Milicic G., Bodnar N.O., Wu X., Rapoport T.A. Mechanism of lamellar body formation by lung surfactant protein B. Mol Cell. 2021;81:49–66.e8. doi: 10.1016/j.molcel.2020.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mingarro I., Lukovic D., Vilar M., Perez-Gil J. Synthetic pulmonary surfactant preparations: new developments and future trends. Curr Med Chem. 2008;15:393–403. doi: 10.2174/092986708783497364. [DOI] [PubMed] [Google Scholar]
- 105.Ramanathan R., Biniwale M., Sekar K., Hanna N., Golombek S., Bhatia J., et al. Synthetic surfactant CHF5633 compared with poractant alfa in the treatment of neonatal respiratory distress syndrome: a multicenter, double-blind, randomized, controlled clinical trial. J Pediatr. 2020;225:90–96.e1. doi: 10.1016/j.jpeds.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 106.Sinha S.K., Lacaze-Masmonteil T., Valls i Soler A., Wiswell T.E., Gadzinowski J., Hajdu J., et al. A multicenter, randomized, controlled trial of lucinactant versus poractant alfa among very premature infants at high risk for respiratory distress syndrome. Pediatrics. 2005;115:1030–1038. doi: 10.1542/peds.2004-2231. [DOI] [PubMed] [Google Scholar]
- 107.Mikolka P., Curstedt T., Feinstein R., Larsson A., Grendar M., Rising A., et al. Impact of synthetic surfactant CHF5633 with SP-B and SP-C analogues on lung function and inflammation in rabbit model of acute respiratory distress syndrome. Physiol Rep. 2021;9 doi: 10.14814/phy2.14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johansson J., Curstedt T. Synthetic surfactants with SP-B and SP-C analogues to enable worldwide treatment of neonatal respiratory distress syndrome and other lung diseases. J Intern Med. 2019;285:165–186. doi: 10.1111/joim.12845. [DOI] [PubMed] [Google Scholar]
- 109.Walther F.J., Gordon L.M., Waring A.J. Design of surfactant protein B peptide mimics based on the Saposin fold for synthetic lung surfactants. Biomed Hub. 2016;1:1–21. doi: 10.1159/000451076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Almlen A., Stichtenoth G., Linderholm B., Haegerstrand-Bjorkman M., Robertson B., Johansson J., et al. Surfactant proteins B and C are both necessary for alveolar stability at end expiration in premature rabbits with respiratory distress syndrome. J Appl Physiol. 2008;104:1101–1108. doi: 10.1152/japplphysiol.00865.2007. [DOI] [PubMed] [Google Scholar]
- 111.Almlen A., Walther F.J., Waring A.J., Robertson B., Johansson J., Curstedt T. Synthetic surfactant based on analogues of SP-B and SP-C is superior to single-peptide surfactants in ventilated premature rabbits. Neonatology. 2010;98:91–99. doi: 10.1159/000276980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walther F.J., Chan H., Smith J.R., Tauber M., Waring A.J. Aerosol, chemical and physical properties of dry powder synthetic lung surfactant for noninvasive treatment of neonatal respiratory distress syndrome. Sci Rep. 2021;11:16439. doi: 10.1038/s41598-021-95999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lopez-Rodriguez E., Cruz A., Richter R.P., Taeusch H.W., Perez-Gil J. Transient exposure of pulmonary surfactant to hyaluronan promotes structural and compositional transformations into a highly active state. J Biol Chem. 2013;288:29872–29881. doi: 10.1074/jbc.M113.493957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopez-Rodriguez E., Ospina O.L., Echaide M., Taeusch H.W., Perez-Gil J. Exposure to polymers reverses inhibition of pulmonary surfactant by serum, meconium, or cholesterol in the captive bubble surfactometer. Biophys J. 2012;103:1451–1459. doi: 10.1016/j.bpj.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taeusch H.W., Dybbro E., Lu K.W. Pulmonary surfactant adsorption is increased by hyaluronan or polyethylene glycol. Colloids Surf B Biointerfaces. 2008;62:243–249. doi: 10.1016/j.colsurfb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Lopez-Rodriguez E., Pascual A., Arroyo R., Floros J., Perez-Gil J. Human pulmonary surfactant protein SP-A1 provides maximal efficiency of lung interfacial films. Biophys J. 2016;111:524–536. doi: 10.1016/j.bpj.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodriguez Capote K., McCormack F.X., Possmayer F. Pulmonary surfactant protein-A (SP-A) restores the surface properties of surfactant after oxidation by a mechanism that requires the Cys6 interchain disulfide bond and the phospholipid binding domain. J Biol Chem. 2003;278:20461–20474. doi: 10.1074/jbc.M212697200. [DOI] [PubMed] [Google Scholar]
- 118.Saenz A., Canadas O., Bagatolli L.A., Sanchez-Barbero F., Johnson M.E., Casals C. Effect of surfactant protein A on the physical properties and surface activity of KL4-surfactant. Biophys J. 2007;92:482–492. doi: 10.1529/biophysj.106.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez-Rodriguez E., Perez-Gil J. Structure-function relationships in pulmonary surfactant membranes: from biophysics to therapy. Biochim Biophys Acta. 2014;1838:1568–1585. doi: 10.1016/j.bbamem.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 120.Seehase M., Collins J.J., Kuypers E., Jellema R.K., Ophelders D.R., Ospina O.L., et al. New surfactant with SP-B and C analogs gives survival benefit after inactivation in preterm lambs. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Madsen J., Panchal M.H., Mackay R.A., Echaide M., Koster G., Aquino G., et al. Metabolism of a synthetic compared with a natural therapeutic pulmonary surfactant in adult mice. J Lipid Res. 2018;59:1880–1892. doi: 10.1194/jlr.M085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Notter R.H., Schwan A.L., Wang Z., Waring A.J. Novel phospholipase-resistant lipid/peptide synthetic lung surfactants. Mini Rev Med Chem. 2007;7:932–944. doi: 10.2174/138955707781662627. [DOI] [PubMed] [Google Scholar]
- 123.Schwan A.L., Singh S.P., Davy J.A., Waring A.J., Gordon L.M., Walther F.J., et al. Synthesis and activity of a novel diether phosphonoglycerol in phospholipase-resistant synthetic lipid:peptide lung surfactants. MedChemComm. 2011;2:1167–1173. doi: 10.1039/C1MD00206F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Luca D., Shankar-Aguilera S., Autilio C., Raschetti R., Vedovelli L., Fitting C., et al. Surfactant-secreted phospholipase A2 interplay and respiratory outcome in preterm neonates. Am J Physiol Lung Cell Mol Physiol. 2020;319:L95–L104. doi: 10.1152/ajplung.00462.2019. [DOI] [PubMed] [Google Scholar]
- 125.De Luca D., Vendittelli F., Trias J., Fraser H., Minucci A., Gentile L., et al. Surfactant and varespladib co-administration in stimulated rat alveolar macrophages culture. Curr Pharm Biotechnol. 2013;14:445–448. doi: 10.2174/1389201011314040010. [DOI] [PubMed] [Google Scholar]
- 126.Veldhuizen R.A.W., Zuo Y.Y., Petersen N.O., Lewis J.F., Possmayer F. The COVID-19 pandemic: a target for surfactant therapy? Expert Rev Respir Med. 2021;15:597–608. doi: 10.1080/17476348.2021.1865809. [DOI] [PubMed] [Google Scholar]
- 127.Avdeev S.N., Trushenko N.V., Chikina S.Y., Tsareva N.A., Merzhoeva Z.M., Yaroshetskiy A.I., et al. Beneficial effects of inhaled surfactant in patients with COVID-19-associated acute respiratory distress syndrome. Respir Med. 2021;185:106489. doi: 10.1016/j.rmed.2021.106489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heching M., Lev S., Shitenberg D., Dicker D., Kramer M.R. Surfactant for the treatment of ARDS in a patient with COVID-19. Chest. 2021;160:e9–e12. doi: 10.1016/j.chest.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bhatt R.M., Clark H.W., Girardis M., Busani S. Exogenous pulmonary surfactant in COVID-19 ARDS. The similarities to neonatal RDS suggest a new scenario for an ‘old’ strategy. BMJ Open Respir Res. 2021;8 doi: 10.1136/bmjresp-2020-000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arroyo R., Echaide M., Moreno-Herrero F., Perez-Gil J., Kingma P.S. Functional characterization of the different oligomeric forms of human surfactant protein SP-D. Biochim Biophys Acta Proteins Proteom. 2020;1868:140436. doi: 10.1016/j.bbapap.2020.140436. [DOI] [PubMed] [Google Scholar]
- 131.Arroyo R., Khan M.A., Echaide M., Perez-Gil J., Palaniyar N. SP-D attenuates LPS-induced formation of human neutrophil extracellular traps (NETs), protecting pulmonary surfactant inactivation by NETs. Commun Biol. 2019;2:470. doi: 10.1038/s42003-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arroyo R., Martin-Gonzalez A., Echaide M., Jain A., Brondyk W.H., Rosenbaum J., et al. Supramolecular assembly of human pulmonary surfactant protein SP-D. J Mol Biol. 2018;430:1495–1509. doi: 10.1016/j.jmb.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 133.Watson A., Sorensen G.L., Holmskov U., Whitwell H.J., Madsen J., Clark H. Generation of novel trimeric fragments of human SP-A and SP-D after recombinant soluble expression in E. coli. Immunobiology. 2020;225:151953. doi: 10.1016/j.imbio.2020.151953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arroyo R., Kingma P.S. Surfactant protein D and bronchopulmonary dysplasia: a new way to approach an old problem. Respir Res. 2021;22:141. doi: 10.1186/s12931-021-01738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guagliardo R., Perez-Gil J., De Smedt S., Raemdonck K. Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins. J Control Release Off J Control Release Soc. 2018;291:116–126. doi: 10.1016/j.jconrel.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 136.Hidalgo A., Cruz A., Perez-Gil J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur J Pharm Biopharm. 2015;95:117–127. doi: 10.1016/j.ejpb.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 137.Hidalgo A., Cruz A., Perez-Gil J. Pulmonary surfactant and nanocarriers: toxicity versus combined nanomedical applications. Biochim Biophys Acta Biomembr. 2017;1859:1740–1748. doi: 10.1016/j.bbamem.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 138.Baer B., Souza L.M.P., Pimentel A.S., Veldhuizen R.A.W. New insights into exogenous surfactant as a carrier of pulmonary therapeutics. Biochem Pharmacol. 2019;164:64–73. doi: 10.1016/j.bcp.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 139.Hidalgo A., Garcia-Mouton C., Autilio C., Carravilla P., Orellana G., Islam M.N., et al. Pulmonary surfactant and drug delivery: vehiculization, release and targeting of surfactant/tacrolimus formulations. J Control Release. 2021;329:205–222. doi: 10.1016/j.jconrel.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hidalgo A., Salomone F., Fresno N., Orellana G., Cruz A., Perez-Gil J. Efficient interfacially driven vehiculization of corticosteroids by pulmonary surfactant. Langmuir. 2017;33:7929–7939. doi: 10.1021/acs.langmuir.7b01177. [DOI] [PubMed] [Google Scholar]