Abstract
Background:
Platelet-rich plasma (PRP) has been established as safe and effective for knee osteoarthritis (OA). Another orthobiologic therapy, microfragmented adipose tissue (MFAT), has gained attention because of its heterogeneous cell population (including mesenchymal stem cells). However, prospective comparative data on MFAT are lacking. Because of the safety, efficacy, and simplicity of PRP, new therapeutics such as MFAT should be compared directly with PRP.
Purpose:
To compare patient-reported outcomes of a single injection of PRP versus MFAT for knee OA.
Study Design:
Randomized controlled trial; Level of evidence, 2.
Methods:
A total of 58 patients with symptomatic knee OA (Kellgren-Lawrence grades 1-4) were randomized to receive a single injection of either leukocyte-rich PRP or MFAT under ultrasound guidance. PRP was created by processing 156 mL of whole blood. MFAT was created by harvesting 30 mL of adipose tissue via standard lipoaspiration. Scores for the Knee injury and Osteoarthritis Outcome Score (KOOS) subscales and visual analog scale for pain with Activities of Daily Living (VAS-ADL) were recorded at baseline and at 1, 3, and 6 months after the injection. The primary outcome was the KOOS–Pain subscore at 6 months after the injection.
Results:
The PRP group (n = 30) had a mean volume of 5.12 ± 1.12 mL injected. This consisted of a mean platelet count of 2673.72 ± 1139.04 × 103/µL and mean leukocyte count of 25.36 ± 13.27 × 103/µL (67.81% lymphocytes, 18.66% monocytes, and 12.33% neutrophils). The MFAT group (n = 28) had a mean volume of 7.92 ± 3.87 mL injected. The mean total nucleated cell count was 3.56 ± 4.62 million/mL. In both groups, KOOS subscale and VAS-ADL scores improved from baseline, and there was no significant difference between the PRP and MFAT groups in the final KOOS–Pain subscore (80.38 ± 16.07 vs 81.61 ± 16.37, respectively; P = .67) or any other outcome score.
Conclusion:
A single injection of either PRP or MFAT resulted in a clinically meaningful improvement for patients with knee OA at 6 months, with no difference between treatment groups.
Registration:
NCT04351087 (ClinicalTrials.gov identifier).
Keywords: knee osteoarthritis, platelet-rich plasma, microfragmented adipose tissue, mesenchymal stem cell
Platelet-rich plasma (PRP) is a solution of densely concentrated platelets that can be used to treat osteoarthritis (OA). 14 It is created by harvesting autologous whole blood and centrifuging it to concentrate the platelets. This solution is rich in anti-inflammatory and anabolic proteins and has been shown to induce chondroprotection, leading to its use for the treatment of degenerative conditions such as OA. 3,29,30,36,37 For knee OA, several randomized controlled trials and meta-analyses have demonstrated the safety and efficacy of PRP. 5,12,31,34,42
Although the efficacy of PRP for the treatment of knee OA has been demonstrated, other autologous therapeutics that have been developed to treat OA have not undergone the same degree of clinical study. One of these options is microfragmented adipose tissue (MFAT). It is created by performing lipoaspiration at the point of care to obtain autologous adipose tissue. Adipose tissue is then rinsed with saline to remove blood and oil and passed through a filter to make it easier to inject. 15 MFAT is composed of a heterogeneous cell population including fibroblasts, macrophages, adipocytes, and mesenchymal stem cells. 19 Animal models have demonstrated that MFAT promotes cartilage repair. 41 Early human studies have demonstrated that MFAT injections for knee OA result in improved patient-reported outcomes (PROs). 22,28,39
Dallo et al 17 found that a single injection of MFAT resulted in superior 6-month outcomes compared with a series of 3 PRP + viscosupplement injections. While this was the first prospective randomized trial comparing these interventions, several factors in the PRP + viscosupplement group complicated the study’s conclusions. First, the device used produced low-concentration, leukocyte-poor PRP. This concentration has been reported as not superior to placebo for knee OA. 6 Second, while PRP + viscosupplement therapy holds promise, its clinical efficacy is still debatable. 4 To date, no study has compared the clinical outcomes of single injections of MFAT versus PRP alone for knee OA.
We conducted a prospective randomized trial comparing a single dose of PRP versus a single dose of MFAT for the treatment of knee OA. This article presents early results (6 months after the injection). We hypothesized that both treatment methods would result in significantly improved PROs at 6-month follow-up compared with baseline.
Methods
The study protocol was approved by our institutional review board and is registered at clinicaltrials.gov. The study design was a prospective, randomized comparative study of PRP versus MFAT for knee OA. From June 2020 through July 2021, patients seen in the sports medicine clinic for knee OA were screened and recruited for enrollment.
Patient Selection
Patients were screened according to the inclusion and exclusion criteria presented in Table 1. Patients who met the criteria for enrollment and provided written consent were randomized using a 1:1 allocation ratio according to a computer-generated block randomization scheme. Patients were randomized to receive a single injection of either PRP or MFAT. Because of differences in harvest between the 2 groups, none of the patients, investigators, or research staff was blinded.
Table 1.
Inclusion and Exclusion Criteria a
| Inclusion Criteria |
|---|
|
| Exclusion Criteria |
|
a KOOS, Knee injury and Osteoarthritis Outcome Score; NSAID, nonsteroidal anti-inflammatory drug; OA, osteoarthritis; PRP, platelet-rich plasma.
Outcome Measures
Demographic data collected from all patients included age, sex, body mass index, and Kellgren-Lawrence grade of OA in the index knee. The PRO measures administered included the following: (1) Knee injury and Osteoarthritis Outcome Score (KOOS) subscales (Pain, Symptoms, Activities of Daily Living [ADL], Sport and Recreation [Sport/Rec], and Quality of Life [QoL]), (2) visual analog scale for pain with Activities of Daily Living (VAS-ADL; 100-point scale), and (3) Tegner activity scale. PRO scores were recorded at baseline and at 1, 3, and 6 months after the injection. In addition, patients were monitored for adverse events over the same intervals (with an additional 2-week wound check for the MFAT group).
The primary outcome was the KOOS–Pain subscore at 6 months after the injection. The responder rate was also reported using the Patient Acceptable Symptom State (PASS) for each KOOS subscale. 8
Procedural Details
After randomization, all patients were instructed to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin 7 days before the procedure, avoid exercise the day before and the day of the procedure, and arrive for the procedure well hydrated. 24 –26 Patients then underwent either the PRP or the MFAT procedure according to their allocation.
PRP Procedure
For the PRP procedure, 156 mL of whole blood was harvested by venipuncture from the antecubital fossa and mixed with 24 mL of Anticoagulant Citrate Dextrose Solution, Solution A (Citra Labs). This solution was concentrated using double-spin centrifugation (Angel cPRP system using the 2% hematocrit setting; Arthrex). 3 For both whole blood and the final PRP, 0.5 mL was analyzed immediately for complete blood counts using a hemoanalyzer (XN-350; Sysmex). The aliquot of PRP that underwent cell counts was then frozen at –80°C. At the conclusion of the study, all PRP samples underwent growth factor analysis by enzyme-linked immunosorbent assay.
After the aliquot of 0.5 mL was taken for analysis, the remaining PRP (up to a maximum volume of 8 mL) was injected using an ultrasound-guided superolateral approach with a 25-gauge, 1.5-inch needle. All injections were performed by the same physician (M.B.), who has extensive experience with interventional ultrasound.
MFAT Procedure
For the MFAT procedure, adipose tissue was aspirated from subcutaneous tissue of the abdomen or flank (depending on the ease of harvest determined by the patient’s body habitus). Under sterile precautions, the aspiration site was injected with 10 mL of 1% lidocaine. A small incision was made with a No. 11 scalpel blade, and 120 mL of Klein solution was injected into the adipose layer bilaterally. The solution was allowed to sit for 15 minutes to achieve proper anesthesia and vasoconstriction. Then, 30 mL of adipose tissue was aspirated (15 mL from each side) using a 13-gauge cannula. The lipoaspirate was rinsed with sterile saline and resized via mechanical agitation using the Lipogems system. 15 Once all adipose tissue had been processed according to manufacturer guidelines and in accordance with minimal manipulation, up to 8 mL was transferred into a syringe for the injection. The knee injection was carried out using the same approach described for the PRP group, except that a 21-gauge needle was used because of higher viscosity. Any remaining MFAT that was not injected was sent for a total nucleated cell count.
Postinjection Care
For pain control, patients were permitted to ice and use acetaminophen and NSAIDs as needed. Patients were allowed to bear weight as tolerated. They were instructed to avoid high-impact and sporting activity for 1 week and then were allowed to resume activities as tolerated. Patients allocated to the PRP group were scheduled for 1-month follow-up, whereas the MFAT group was scheduled for an additional 2-week wound check. The remaining follow-up visits were performed at the same intervals (1, 3, and 6 months).
Cellular Analysis
The PRP group underwent routine complete blood count analysis using the hemoanalyzer as described above. At the conclusion of the study, all frozen PRP aliquots were analyzed for platelet-derived growth factor, insulin-like growth factor–1, vascular endothelial growth factor, and transforming growth factor–β. The concentrations were determined in duplicate aliquots of all samples and standards using enzyme-linked immunosorbent assay kits specific for each growth factor (R&D Systems) according to manufacturer recommendations.
MFAT Processing for Total Nucleated Cell Count
MFAT samples were processed for stromal vascular fraction (SVF) isolation to ensure accurate quantification of the total nucleated cell count, as previously described. 1,7 The samples were washed twice with phosphate-buffered saline for the removal of residual blood via centrifugation at 250g for 3 minutes. The liquid phases were removed between washes. The MFAT samples were digested in collagenase A (1 mg/mL) solution under gentle agitation for 1 hour at 37°C. The collagenase digest was neutralized by the addition of an equal volume of Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum and centrifuging at 800g for 5 minutes to separate the SVF pellet from the adipocytes. The SVF was washed twice with phosphate-buffered saline and centrifuged at 800g for 5 minutes after each wash. The cell suspension was filtered through a 40-µm cell strainer. The supernatant was discarded, and the cell pellet termed the SVF was resuspended in 5 mL of DMEM containing 10% fetal bovine serum. Cell viability was determined via trypan blue dye exclusion. The total nucleated cell count was calculated by counting an aliquot of the resulting suspension using a hemocytometer and an inverted light microscope.
Statistical Analysis
Continuous variables were summarized as means with standard deviations, and categorical variables were reported as frequencies and percentages. The KOOS subscale and VAS-ADL scores were summarized by treatment group (PRP vs MFAT) at baseline and follow-up time points, and scores were compared between the treatment groups with unpaired t tests at the 6-month time point. Repeated-measures analysis of variance was performed to detect the effect of treatment and/or time on the VAS-ADL and KOOS subscale scores.
Using the established PASS for each KOOS subscale (Pain, 73.6; Symptoms, 71.2; ADL, 84.5; Sport/Rec, 47.5; and QoL, 47.0), 8 the proportion of patients in each treatment group who reached the PASS were summarized and compared using the Fisher exact test. Additionally, the mean volume of whole blood and PRP injected; cell counts for platelets, red blood cells, white blood cells, and leukocytes; and percentage differential of each cell line were summarized to include means, standard deviations, and ranges. MFAT volume, SVF, and percentage viability were summarized to include means, standard deviations, and ranges.
An a priori power analysis was undertaken based on the minimal clinically important difference (MCID) for the KOOS–Pain of 9 points between baseline and final follow-up. 8 With an alpha error of .05 and an anticipated SD of 15 points for the KOOS–Pain subscore, a total of 88 patients (44 per treatment group) would be required to detect a 9-point difference between treatment groups with 80% power. The initial study design was to enroll 110 patients (55 per group) to account for up to 20% loss to follow-up. Owing to a change in regulatory requirements during the study, enrollment was halted after 71 patients. A repeated power calculation demonstrated that the study achieved 56% power to detect a between-group difference in excess of the 9-point MCID for the KOOS–Pain.
Results
Patient Demographics
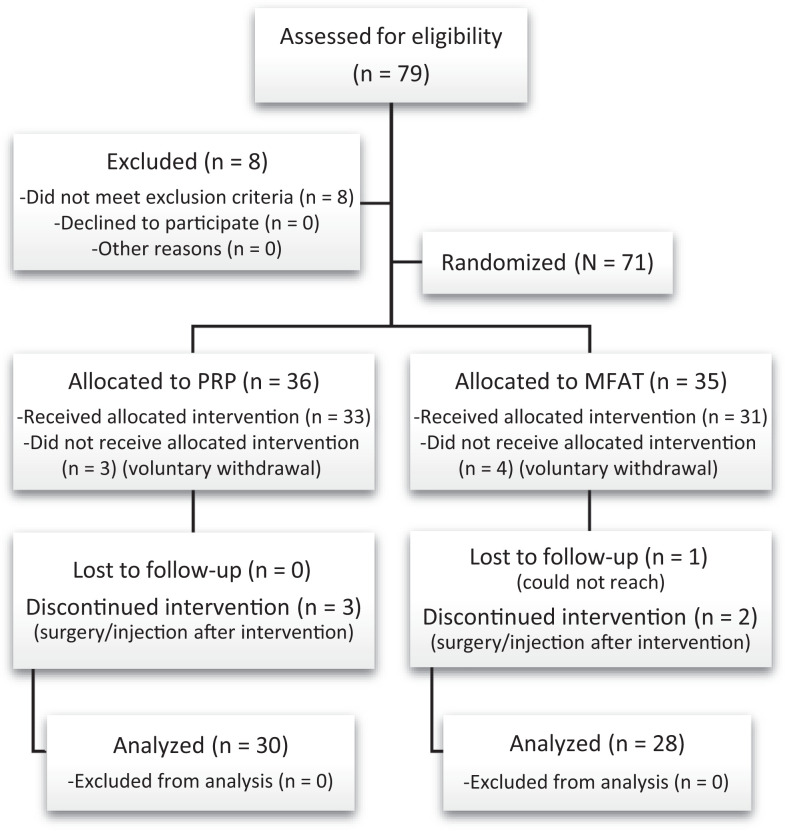
A total of 79 patients were assessed for eligibility. After consent and screening, 71 patients were randomized (36 to PRP and 35 to MFAT). At the primary endpoint of 6 months, 58 patients (82%) had completed 6-month follow-up (30 in the PRP group and 28 in the MFAT group) and were analyzed in the final data set (Figure 1).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. MFAT, microfragmented adipose tissue; PRP, platelet-rich plasma.
Baseline patient demographics are presented in Table 2. There was no significant difference between the groups regarding age, body mass index, or race. However, more men were randomized to the PRP group and more women to the MFAT group.
Table 2.
Patient Demographics a
| PRP (n = 30) | MFAT (n = 28) | P Value | |
|---|---|---|---|
| Age, y | 51.9 ± 2.4 | 56.1 ± 1.7 | .17 |
| Body mass index, kg/m2 | 31.0 ± 0.8 | 31.0 ± 0.9 | .90 |
| Sex, n (%) | .01 | ||
| Male | 20 (66.7) | 8 (28.6) | |
| Female | 10 (33.3) | 20 (71.4) | |
| Race, n (%) | .99 | ||
| White | 24 (80.0) | 24 (85.7) | |
| Black | 4 (13.3) | 3 (10.7) | |
| Other | 2 (6.7) | 1 (3.6) | |
| Kellgren-Lawrence grade, n | .16 | ||
| 1 | 6 | 2 | |
| 2 | 8 | 5 | |
| 3 | 12 | 11 | |
| 4 | 4 | 10 |
a Data are reported as mean ± SD unless otherwise indicated. Boldface P values indicate a statistically significant difference between groups (P < .05). MFAT, microfragmented adipose tissue; PRP, platelet-rich plasma.
The cellular composition of each group is presented in Table 3. The mean volume of PRP injected was 5.12 ± 1.12 mL. The mean platelet count in PRP was 2673.72 ± 1139.04 × 103/μL. PRP was leukocyte rich (white blood cells: 25.36 ± 13.27 × 103/µL for PRP vs 5.43 ± 1.49 × 103/µL for whole blood). The leukocyte differential consisted of 67.81% lymphocytes, 18.66% monocytes, and 12.33% neutrophils. The mean volume of MFAT injected was 7.92 ± 3.87 mL, with a mean total nucleated cell count of 3.56 ± 4.62 million/mL and 97.96% cell viability.
Table 3.
Composition of Cellular Products a
| PRP: Cellular Composition (Versus Whole Blood) | |
|---|---|
| Injection volume, mL | PRP: 5.12 ± 1.12 |
| Platelet, 103/μL | PRP: 2673.72 ± 1139.04 Whole blood: 177.56 ± 43.74 |
| WBC, 103/μL | PRP: 25.36 ± 13.27 Whole blood: 5.43 ± 1.49 |
| RBC, 106/μL | PRP: 0.37 ± 0.73 Whole blood: 3.66 ± 0.75 |
| Neutrophil, % | PRP: 12.33 ± 13.37 Whole blood: 57.29 ± 9.67 |
| Lymphocyte, % | PRP: 67.81 ± 12.45 Whole blood: 30.75 ± 8.34 |
| Monocyte, % | PRP: 18.66 ± 6.11 Whole blood: 8.75 ± 1.65 |
| PRP: Growth Factor Composition | |
| TGF-β, ng/dL | 126.07 ± 7.17 |
| VEGF, ng/dL | 1037.52 ± 774.08 |
| IGF-1, ng/dL | 47.60 ± 20.88 |
| PDGF, ng/dL | 31.22 ± 10.39 |
| MFAT: Cellular Composition | |
| Volume, mL | 7.92 ± 3.87 |
| Total nucleated cell count, million/mL | 3.56 ± 4.62 |
| Viability, % | 97.96 ± 1.33 |
a Data are reported as mean ± SD. IGF-1, insulin-like growth factor–1; MFAT, microfragmented adipose tissue; PDGF, platelet-derived growth factor; PRP, platelet-rich plasma; RBC, red blood cell; TGF-β, transforming growth factor–β; VEGF, vascular endothelial growth factor; WBC, white blood cell.
Outcome Scores
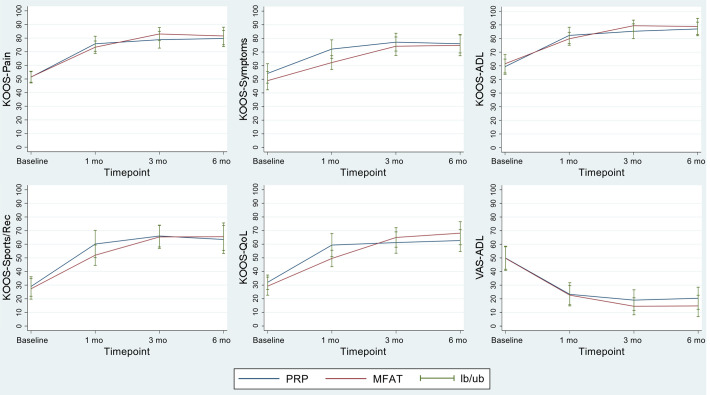
For the primary outcome (KOOS–Pain), no difference was noted between the PRP and MFAT groups at 6 months (80.38 ± 16.07 vs 81.61 ± 16.37, respectively; P = .67). Both groups experienced statistically and clinically significant improvements at 6 months on all KOOS subscales and the VAS-ADL, with no significant differences between groups noted (Figure 2). The only statistically significant difference between the groups was observed at 1-month follow-up in the KOOS–Symptoms subscore. For both treatment groups, the MCID for the KOOS–Pain was exceeded at 1-month follow-up and maintained for 6 months (Table 4).
Figure 2.
Outcomes at baseline and follow-up times for the Knee injury and Osteoarthritis Outcomes Score (KOOS) subscales and visual analog scale (VAS) for pain with Activities of Daily Living (ADL). lb/ub, lower bound/upper bound; MFAT, microfragmented adipose tissue; PRP, platelet-rich plasma; QoL, Quality of Life; Sport/Rec, Sport and Recreation.
Table 4.
Patient-Reported Outcome Scores a
| Baseline | 1 mo | 3 mo | 6 mo | |
|---|---|---|---|---|
| KOOS–Pain | ||||
| PRP | 50.90 ± 11.64 | 75.72 ± 14.54 | 77.32 ± 17.99 | 80.38 ± 16.07 |
| MFAT | 51.48 ± 9.78 | 73.80 ± 11.48 | 81.48 ± 15.24 | 81.61 ± 16.37 |
| KOOS–Symptoms | ||||
| PRP | 53.92 ± 18.86 | 72.24 ± 17.85 b | 76.19 ± 17.78 | 76.38 ± 17.87 |
| MFAT | 49.64 ± 17.38 | 63.10 ± 13.01 b | 74.05 ± 17.49 | 75.37 ± 19.45 |
| KOOS-ADL | ||||
| PRP | 58.63 ± 15.20 | 82.45 ± 15.79 | 83.63 ± 16.86 | 87.38 ± 13.27 |
| MFAT | 62.11 ± 16.99 | 80.54 ± 12.12 | 88.19 ± 12.52 | 88.85 ± 15.05 |
| KOOS–Sport/Rec | ||||
| PRP | 28.23 ± 19.35 | 60.16 ± 26.31 | 64.17 ± 23.05 | 64.52 ± 27.67 |
| MFAT | 28.50 ± 19.57 | 53.83 ± 19.99 | 63.67 ± 22.05 | 65.86 ± 25.60 |
| KOOS-QoL | ||||
| PRP | 31.45 ± 14.39 | 58.47 ± 22.80 | 59.17 ± 23.14 | 63.71 ± 21.97 |
| MFAT | 30.21 ± 16.98 | 50.63 ± 15.51 | 65.00 ± 18.76 | 68.10 ± 21.41 |
| VAS-ADL | ||||
| PRP | 50.10 ± 21.73 | 22.97 ± 22.43 | 20.57 ± 21.29 | 19.74 ± 21.65 |
| MFAT | 48.97 ± 22.46 | 21.57 ± 18.02 | 16.00 ± 18.83 | 14.59 ± 19.80 |
| Tegner | ||||
| PRP | 3.06 ± 1.55 | 4.06 ± 1.73 | 3.93 ± 1.14 | 4.00 ± 1.59 |
| MFAT | 3.00 ± 2.05 | 3.73 ± 1.39 | 4.23 ± 1.38 | 4.34 ± 1.47 |
a Data are reported as mean ± SD. ADL, Activities of Daily Living; KOOS, Knee injury and Osteoarthritis Outcome Score; MFAT, microfragmented adipose tissue; PRP, platelet-rich plasma; QoL, Quality of Life; Sport/Rec, Sport and Recreation; VAS, visual analog scale.
b Statistically significant difference (P = .03).
The 6-month responder rates according to PASS criteria for the KOOS subscales are presented in Table 5. There was no statistical difference between the PRP and MFAT groups in the number of patients crossing the PASS threshold for any subscale.
Table 5.
Patients Who Met the PASS for the KOOS at 6 Months a
| PRP (n = 30) | MFAT (n = 28) | P Value | |
|---|---|---|---|
| KOOS–Pain | 20 (66.7) | 20 (71.4) | .78 |
| KOOS–Symptoms | 21 (70.0) | 20 (71.4) | .99 |
| KOOS-ADL | 19 (63.3) | 21 (75.0) | .40 |
| KOOS–Sport/Rec | 21 (70.0) | 23 (82.1) | .36 |
| KOOS-QoL | 24 (80.0) | 24 (85.7) | .73 |
a Data are reported as n (%). ADL, Activities of Daily Living; KOOS, Knee injury and Osteoarthritis Outcome Score; MFAT, microfragmented adipose tissue; PASS, Patient Acceptable Symptom State; PRP, platelet-rich plasma; QoL, Quality of Life; Sport/Rec, Sport and Recreation.
Discussion
The most important finding of this study was that both PRP and MFAT resulted in clinically and statistically significant improvements on all outcome measures for knee OA at 6 months after the injection, with no difference between treatment methods. Patients in both groups reported similar improvements in pain, mechanical symptoms, functional ability, and quality of life. These improvements were observed within 1 month and were stable to the 6-month primary endpoint. Both treatment methods resulted in a mean KOOS–Pain subscore improvement of approximately 30 points, which far exceeded the MCID of 9 points. The responder rates were also similar between groups, ranging between 67% and 71%, for the KOOS–Pain; interestingly, higher responder rates (80%-86%) were noted for the KOOS-QoL. The study findings are important because the growing availability of biologic interventions is outpacing the ability to properly study them. This is the first randomized study to compare PRP to MFAT, and the results can aid physicians and patients in understanding and selecting treatment options.
Numerous randomized trials and meta-analyses support the use of PRP for knee OA compared with placebo, steroid, and viscosupplement. # These data have established PRP as the gold standard of orthobiologic interventions. 14 Therefore, from a pragmatic clinical perspective, new orthobiologics should be compared with PRP as a benchmark to compare efficacy and determine if the increased cost and invasiveness are justified. The distinct cell populations in MFAT, including mesenchymal stem cells, may pique the interest of patients and clinicians. Additionally, the processing methods used to create MFAT are technically more complex than PRP, which could lead to the assumption that this technique is more advanced. However, this present study demonstrated that PRP is equally effective compared with MFAT at 6 months. This is important because the PRP procedure is simpler to perform, does not require additional training (including lipoaspiration), is less expensive, and is easier to repeat as needed.
The only other study to compare PRP with another orthobiologic was performed by Anz et al. 2 They randomized 90 patients, who were not blinded to the treatment allocation, to receive either leukocyte-rich PRP or bone marrow aspirate concentrate (BMAC) for knee OA. The rationale for using BMAC is similar to PRP; it contains a large quantity of anabolic and anti-inflammatory proteins that may be useful in treating OA. However, the study by Anz et al found no difference between the groups at any time point for 1 year. When we consider the breadth of comparative PRP studies, not only has PRP demonstrated superiority to placebo and standard-of-care treatment options (steroid and viscosupplement), but the work of Anz et al and the present study also demonstrate that PRP results in similar outcomes to more invasive treatment options such as BMAC and MFAT. There may be clinical scenarios in which individual patients could see benefits from BMAC and MFAT, but for the general patient population, PRP should be the orthobiologic treatment of choice.
Before our current study, the only other randomized study using MFAT for knee OA was performed by Dallo et al. 17 There were 50 patients randomized, in an unblinded fashion, to receive a single MFAT injection or a series of 3 PRP + viscosupplement injections. At 6 months, there was a statistical difference in the KOOS–Symptoms subscore, favoring MFAT. However, this difference did not cross the established MCID threshold. At 1 year, there was no difference between the groups for the VAS pain or KOOS. While both Dallo et al and the present study used the same MFAT preparation system, the PRP arm was significantly different. In our study, we used a single injection of PRP (alone) with a higher platelet dose. This reduces the number of injections required, eliminates the need for combining PRP with viscosupplement, and achieves similar outcomes compared with MFAT.
Limitations
This study has several limitations that warrant a discussion. The most important is that this study did not meet the power determined a priori to be sufficient to detect a difference between groups. This occurred because of evolving regulatory perspectives within our institution. Initially, the investigators and our institutional review board agreed that because these products were autologous, minimally manipulated, and used in a same-day procedure, no additional regulation was required. However, this viewpoint changed over time, and it was concluded that these interventions represented a greater than nonsignificant risk and therefore required oversight by the US Food and Drug Administration (FDA). Because an investigational device exemption application cannot be filed in the middle of a trial, study enrollment was stopped. It should be noted that there were no adverse safety events that led to stoppage of the trial. This change was only because of institutional perspective. While this outcome was unfortunate, it demonstrates a dynamic that is likely not limited to this trial and this institution. The regulatory landscape in orthobiologics is often unclear and confusing. Given the evolution of regulatory guidance, it may be best to seek the perspective (and risk determination) of the FDA through the agency’s Q-submission program before initiating a similar study in the future.
Additionally, 6-month outcomes are relatively short term. However, approved standard-of-care injections such as steroid and viscosupplement can be repeated at this same interval, and trials studying biologics such as amniotic suspension use the same timeline. 20 Therefore, it is important to know the clinical outcomes of PRP and MFAT at those same time points. The unblinded design is another limitation. The need for blood draw and lipoaspiration makes patient blinding challenging, if not impossible. The same was noted in the work by Anz et al 2 and Dallo et al. 17 While this is a limitation, the greater placebo effect of increasingly more invasive procedures is well-documented. Therefore, one might expect MFAT to outperform PRP in an unblinded study, but that did not occur in our work or in that of Anz et al. This may point to the comparative effectiveness of PRP.
Our use of leukocyte-rich PRP could be a point of critique. Earlier work has suggested that leukocyte-poor PRP should be the standard for OA. 33 However, this is largely based on in vitro work demonstrating a negative effect of neutrophils and red blood cells on cartilage. 32,36 Comparative human studies have not demonstrated a deleterious effect of leukocytes. 21,27 Most recently, a double-blind randomized trial by Di Martino et al 18 demonstrated no difference in adverse events or patient outcomes between leukocyte-poor and leukocyte-rich PRP. Therefore, there is no comparative human study demonstrating that leukocytes influence outcomes for knee OA.
The inclusion of end-stage knee OA is also worth discussing. It is established that PRP is most effective for earlier stages of OA. 10 While we counsel our patients on this point, our sports medicine clinic treats younger patients with more advanced OA. These patients are often not ready or not ideal candidates for joint replacement; therefore, they often seek alternative treatment methods to delay the time to arthroplasty. It was therefore decided to include these patients in the trial. Other notable randomized controlled trials on PRP have included this population as well. 13 Additionally, symptom severity does not correlate with radiographic staging of OA, so there is no basis to exclude a patient purely based on radiographic severity. 35
This study was also limited by the lack of a placebo control group. This would be ideal because of the known placebo effect in OA trials. 43 However, because there are several treatment options with proven efficacy and superiority over placebo, it is becoming increasingly impractical and undesirable to use a placebo arm. 31,34,42 Because PRP is superior to placebo, that served as the control arm, against which we compared the new intervention, MFAT. The final limitation was that there was a significant difference in treatment allocation by sex. The randomization scheme was reviewed and found to be appropriate. Therefore, this difference represents a truly random occurrence. A regression analysis was performed and demonstrated that biological sex did not contribute to different performance within each treatment group. Therefore, despite the unequal allocation of men and women to each group, this did not skew the clinical outcomes.
Conclusion
A single injection of PRP or MFAT resulted in a clinically meaningful improvement for patients with knee OA at 6 months, with no difference between treatment groups.
Footnotes
Final revision submitted June 6, 2022; accepted June 14, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding for this study was provided through a research grant from the Lisa Dean Moseley Foundation. M.B. has received research funding from Arthrex and the Moseley Foundation and consulting and nonconsulting fees from Arthrex. C.K. has received grant support from DJO, education payments from CDC Medical, consulting fees from Arthrex and Zimmer Biomet, and nonconsulting fees from Arthrex and Smith & Nephew. S.D. has received grant support from Arthrex, education payments from Arthrex, and hospitality payments from Smith & Nephew and Stryker. D.F. has received education payments from CDC Medical; consulting fees from Bioventus, Ceterix, DePuy/Medical Device Business Services, Linvatec, Smith & Nephew, Vericel, and Zimmer Biomet; nonconsulting fees from Karl Storz Endoscopy and Smith & Nephew; and honoraria from Vericel. R.M. has received grant support from DJO and education payments from CDC Medical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from The Ohio State University (No. 2019H0448).
References
- 1. Agostini F, Rossi FM, Aldinucci D, et al. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res Ther. 2018;9(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anz AW, Hubbard R, Rendos NK, Everts PA, Andrews JR, Hackel JG. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 1 year: a prospective, randomized trial. Orthop J Sports Med. 2020;8(2):2325967119900958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baria M, Vasileff WK, Miller M, Borchers J, Flanigan DC, Durgam SS. Cellular components and growth factor content of platelet-rich plasma with a customizable commercial system. Am J Sports Med. 2019;47(5):1216–1222. [DOI] [PubMed] [Google Scholar]
- 4. Baria MR, Vasileff WK, Borchers J, et al. Treating knee osteoarthritis with platelet-rich plasma and hyaluronic acid combination therapy: a systematic review. Am J Sports Med. 2022;50(1):273–281. [DOI] [PubMed] [Google Scholar]
- 5. Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49(1):249–260. [DOI] [PubMed] [Google Scholar]
- 6. Bennell KL, Paterson KL, Metcalf BR, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021;326(20):2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bianchi F, Maioli M, Leonardi E, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063–2077. [DOI] [PubMed] [Google Scholar]
- 8. Boffa A, Andriolo L, Franceschini M, et al. Minimal clinically important difference and patient acceptable symptom state in patients with knee osteoarthritis treated with PRP injection. Orthop J Sports Med. 2021;9(10):23259671211026242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822–2827. [DOI] [PubMed] [Google Scholar]
- 10. Chahla J, Cole BJ. Editorial commentary. Platelet-rich plasma for knee osteoarthritis: a “novel” and effective symptomatic approach. Arthroscopy. 2019;35(1):118–120. [DOI] [PubMed] [Google Scholar]
- 11. Chen Z, Wang C, You D, Zhao S, Zhu Z, Xu M. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Medicine (Baltimore). 2020;99(11):e19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cole B, Fortier L, Karas V, et al. HA vs PRP: double-blind RCT comparing clinical outcomes and intra-articular biology for treatment of knee arthritis. Arthroscopy. 2015;31(6):e22–e23. [Google Scholar]
- 13. Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45(2):339–346. [DOI] [PubMed] [Google Scholar]
- 14. Cook CS, Smith PA. Clinical update: why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. 2018;11(4):583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coughlin RP, Oldweiler A, Mickelson DT, Moorman CT, 3rd. Adipose-derived stem cell transplant technique for degenerative joint disease. Arthrosc Tech. 2017;6(5):e1761–e1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659–670.e651. [DOI] [PubMed] [Google Scholar]
- 17. Dallo I, Szwedowski D, Mobasheri A, Irlandini E, Gobbi A. A prospective study comparing leukocyte-poor platelet-rich plasma combined with hyaluronic acid and autologous microfragmented adipose tissue in patients with early knee osteoarthritis. Stem Cells Dev. 2021;30(13):651–659. [DOI] [PubMed] [Google Scholar]
- 18. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50(3):609–617. [DOI] [PubMed] [Google Scholar]
- 19. Di Matteo B, El Araby MM, D’Angelo A, et al. Adipose-derived stem cell treatments and formulations. Clin Sports Med. 2019;38(1):61–78. [DOI] [PubMed] [Google Scholar]
- 20. Farr J, Gomoll AH, Yanke AB, Strauss EJ, Mowry KC. A randomized controlled single-blind study demonstrating superiority of amniotic suspension allograft injection over hyaluronic acid and saline control for modification of knee osteoarthritis symptoms. J Knee Surg. 2019;32(11):1143–1154. [DOI] [PubMed] [Google Scholar]
- 21. Filardo G, Kon E, Pereira Ruiz MT, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082–2091. [DOI] [PubMed] [Google Scholar]
- 22. Gobbi A, Dallo I, Rogers C, et al. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: a multi-centric, international study. Int Orthop. 2021;45(5):1179–1188. [DOI] [PubMed] [Google Scholar]
- 23. Gormeli G, Gormeli CA, Ataoglu B, Colak C, Aslanturk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958–965. [DOI] [PubMed] [Google Scholar]
- 24. Hamilton B, Tol JL, Knez W, Chalabi H. Exercise and the platelet activator calcium chloride both influence the growth factor content of platelet-rich plasma (PRP): overlooked biochemical factors that could influence PRP treatment. Br J Sports Med. 2015;49(14):957–960. [DOI] [PubMed] [Google Scholar]
- 25. Jayaram P, Yeh P, Patel SJ, et al. Effects of aspirin on growth factor release from freshly isolated leukocyte-rich platelet-rich plasma in healthy men: a prospective fixed-sequence controlled laboratory study. Am J Sports Med. 2019;47(5):1223–1229. [DOI] [PubMed] [Google Scholar]
- 26. Mannava S, Whitney KE, Kennedy MI, et al. The influence of naproxen on biological factors in leukocyte-rich platelet-rich plasma: a prospective comparative study. Arthroscopy. 2019;35(1):201–210. [DOI] [PubMed] [Google Scholar]
- 27. Mariani E, Canella V, Cattini L, et al. Leukocyte-rich platelet-rich plasma injections do not up-modulate intra-articular pro-inflammatory cytokines in the osteoarthritic knee. PLoS One. 2016;11(6):e0156137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mautner K, Bowers R, Easley K, Fausel Z, Robinson R. Functional outcomes following microfragmented adipose tissue versus bone marrow aspirate concentrate injections for symptomatic knee osteoarthritis. Stem Cells Transl Med. 2019;8(11):1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moussa M, Lajeunesse D, Hilal G, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352(1):146–156. [DOI] [PubMed] [Google Scholar]
- 30. Oudelaar BW, Peerbooms JC, Huis In’t Veld R, Vochteloo AJH. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med. 2019;47(2):479–487. [DOI] [PubMed] [Google Scholar]
- 31. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. [DOI] [PubMed] [Google Scholar]
- 32. Potpally N, Rodeo S, So P, Mautner K, Baria M, Malanga GA. A review of current management of knee hemarthrosis in the non-hemophilic population. Cartilage. 2021;13(1)(suppl):116S–121S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792–800. [DOI] [PubMed] [Google Scholar]
- 34. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884–891. [DOI] [PubMed] [Google Scholar]
- 35. Son KM, Hong JI, Kim DH, Jang DG, Crema MD, Kim HA. Absence of pain in subjects with advanced radiographic knee osteoarthritis. BMC Musculoskelet Disord. 2020;21(1):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135–2140. [DOI] [PubMed] [Google Scholar]
- 37. Sundman EA, Cole BJ, Karas V, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42(1):35–41. [DOI] [PubMed] [Google Scholar]
- 38. Tang JZ, Nie MJ, Zhao JZ, Zhang GC, Zhang Q, Wang B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res. 2020;15(1):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Genechten W, Vuylsteke K, Martinez PR, Swinnen L, Sas K, Verdonk P. Autologous micro-fragmented adipose tissue (MFAT) to treat symptomatic knee osteoarthritis: early outcomes of a consecutive case series. J Clin Med. 2021;10(11):2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu Q, Luo X, Xiong Y, et al. Platelet-rich plasma versus hyaluronic acid in knee osteoarthritis: a meta-analysis with the consistent ratio of injection. J Orthop Surg. 2020;28(1):2309499019887660. [DOI] [PubMed] [Google Scholar]
- 41. Xu T, Yu X, Yang Q, Liu X, Fang J, Dai X. Autologous micro-fragmented adipose tissue as stem cell-based natural scaffold for cartilage defect repair. Cell Transplant. 2019;28(12):1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu Z, Luo J, Huang X, Wang B, Zhang J, Zhou A. Efficacy of platelet-rich plasma in pain and self-report function in knee osteoarthritis: a best-evidence synthesis. Am J Phys Med Rehabil. 2017;96(11):793–800. [DOI] [PubMed] [Google Scholar]
- 43. Zhang W. The powerful placebo effect in osteoarthritis. Clin Exp Rheumatol. 2019;37(5)(suppl 120):118–123. [PubMed] [Google Scholar]