Abstract
Necrotizing fasciitis is a rare soft tissue infection characterized by a rapidly spreading infection of the subcutaneous tissue. Early diagnosis is important as it requires immediate and complete debridement of infected tissues and antibiotic therapy. Necrotizing fasciitis usually involves the extremities, abdomen, and groin, but rarely involves the head and neck. Necrotizing fasciitis has an aggressive course; however, in rare cases, it can present in a subacute indolent form which can be misdiagnosed as other cutaneous diseases. Our case is a unique presentation of subacute necrotizing fasciitis of the posterior neck, which was initially diagnosed as a herpes zoster infection, in a patient with undiagnosed diabetes mellitus, which was complicated with diabetic ketoacidosis and sepsis.
Keywords: Necrotizing fasciitis, subacute necrotizing fasciitis, herpes zoster, diabetes mellitus
Introduction
Necrotizing fasciitis (NF) is a soft tissue infection characterized by a subtle but often rapid spread of inflammation and necrosis. It starts in the fascia, muscles, and subcutaneous fat, eventually leading to necrosis of the overlying skin. 1 The most common symptoms are swelling, pain, and erythema. 2 Thus, early diagnosis is difficult or delayed sometimes and diagnosed as cellulitis or abscess. The infection can be polymicrobial and caused by both aerobic and anaerobic organisms. Streptococcus species are the most common agents, followed by Staphylococcus aureus. 1 The most common sites of infection are extremities (28%), particularly the upper limbs, followed by the perineum (21%), trunk (18%), and head or neck (5%). 3
Generally, the infection starts from a skin break in 80% of cases, leading to rapid transit through muscle fascia without cutaneous signs of infection. Cutaneous signs appear later, with pain out of proportion to the infected area. Over the next 3–5 days, skin breakdown begins, accompanied by bullae and cutaneous gangrene. 4 This acute presentation usually requires urgent surgical intervention based on the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score. 5 However, NF can present in a subacute form but has not been reported frequently in the literature.6,7
In the United States, NF affects about four in a million individuals per year. 4 Diabetes mellitus is the most common co-morbidity associated with NF, with up to 45%–71% of patients having diabetes.2,3 Patients with a history of alcoholism, end-stage renal disease, liver cirrhosis, and other immunocompromised conditions are at high risk to suffer from NF. 4 Herein, we present a rare case of subacute NF in a 69-year-old female with undiagnosed diabetes mellitus (DM) that was initially diagnosed as a herpes zoster infection.
Case presentation
A 69-year-old female presented to the Emergency Department (ED) with a complaint of inability to move her neck due to pain in February of 2022. The patient had no known medical history, and her last medical evaluation was in 2017. On review of her medical records, the patient was seen in the clinic 2 weeks before her presentation for evaluation of a rash on her posterior neck which was first noticed approximately 2 weeks before clinic evaluation. The provider felt the rash may represent herpes zoster as it was painful, and vesicles were present; however, it did cross the midline. The patient was placed on valacyclovir and instructed to follow up in 3 days to ensure improvement as unlike herpes zoster the rash did cross the midline. Despite noting a progressive increase in pain to the posterior neck after initial evaluation she did not follow-up as instructed.
On presentation to the ED, she complained of fatigue, generalized weakness, blurred vision, and severe neck pain. Physical examination was significant for a heart rate of 135 BPM, blood pressure (BP) of 157/86 mm Hg, respiration rate (RR) of 23 BPM, SpO2 of 97% on room air, and temperature of 36.7 C. She appeared disheveled, alert, oriented, and without focal neurological deficits. She had dry mucous membranes and an ulcerated, necrotic area of the posterior neck (Figure 1(a)). The borders of the wound, surrounding skin, and soft tissues were extremely tender to touch.
Figure 1.
Progression stages of the necrotizing fasciitis wound. (a) ulcerated, necrotic area of the posterior neck on presentation; (b) computed tomography (CT) of the soft tissue of the neck showed marked inflammation at the posterior aspect of the neck with soft tissue gas collections consistent with necrotizing fasciitis; (c) after debridement for extensive necrotizing fasciitis of the head and neck; and (d) progressive healing of the wound.
Initial laboratory studies (Table 1) revealed a new diagnosis of DM presenting with diabetic ketoacidosis (DKA). Initial Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was 6 which shows intermediate risk for NF. Computed tomography (CT) of the soft tissue of the neck (Figure 1(b)) showed marked inflammation at the posterior aspect of the neck with soft tissue gas collections consistent with NF. The spinal cord appeared uninvolved.
Table 1.
Initial abnormal laboratory results.
| Test | Result | Reference range |
|---|---|---|
| Complete blood count | ||
| Hemoglobin | 12.4 | 13.2–16.6 g/dL |
| White blood count | 40.8 | 3.4–9.6 × 109/L |
| Platelets | 401 | 135–317 × 109/L |
| Comprehensive metabolic panel | ||
| Sodium | 123 | 135–145 mmol/L |
| Potassium | 4.6 | 3.6–5.2 mmol/L |
| Chloride | 83 | 98–107 mmol/L |
| Bicarbonate | 12 | 22–29 mmol/L |
| Anion gap | 28 | 7–15 |
| Glucose | 452 | 70–140 mg/dL |
| Alkaline phosphatase | 184 | 40–129 U/L |
| Albumin | 2.1 | 3.5–5.0 g/dL |
| Lactate | 2.9 | 0.5–2.2 mmol/L |
| Beta-hydroxybutyrate | 4.7 | <0.4 mmol/L |
| Venous blood gas | ||
| pH | 7.31 | 7.32–7.43 |
| pCO2 | 27 | 41–51 mm Hg |
| Base excess | −13 | mmol/L |
| HCO3 | 13 | mmol/L |
| Endocrine | ||
| Hemoglobin A1c | 11.6 | 4.2%–5.6% |
The patient was started on treatment for sepsis and DKA protocol. She was transferred to a higher level of care where she underwent debridement for extensive NF of the head and neck (Figure 1(c)). Blood and neck swab cultures were positive for S. aureus and Streptococcus agalactiae. She remained hospitalized for 2 weeks and needed surgical debridement four times. After her discharge, she continues to follow up with outpatient wound care, has had one outpatient surgery for application of Integra wound matrix, and is progressively healing (Figure 1(d)) (Figure 2).
Figure 2.
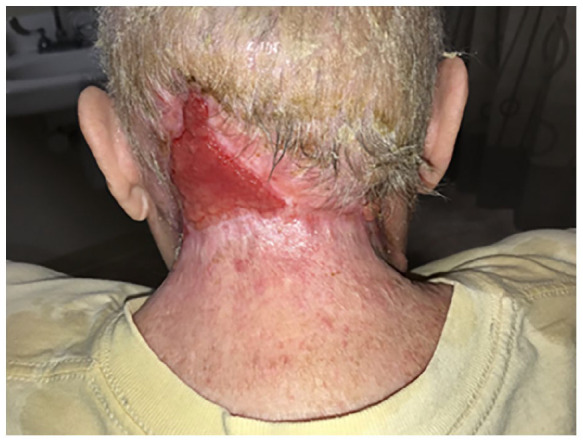
Healing necrotizing fasciitis wound.
Discussion
This case is a rare presentation of NF due to its location in the posterior neck and presentation as an indolent process over approximately a 1-month duration. The presence of bullae filled with serous fluid is an important diagnostic clue for NF and could be suggestive of this condition. 3 The patient’s blistering lesions likely represented a bullous bacterial infection rather than herpes zoster. In addition, patients with DM have higher rates of misdiagnosis and longer time to surgery. 8 However, our patient had an undiagnosed DM which could have misled the initial diagnosis.
This case’s blistering lesions likely represented a bullous bacterial infection, although there is the possibility that an initial rare bilateral herpes zoster infection could have seeded a subacute NF. Herpes zoster tends to be unilateral in its presentation and very rarely crosses the midline or involves widely separate dermatomes.9,10 There are case reports involving multidermatomal cervical herpes zoster. One case report described subacute NF in the setting of a confirmed disseminated cutaneous herpes zoster in an immunosuppressed patient on methotrexate occurring over a 3-week span from initial rash to the diagnosis of subacute NF. 11 There are also existing case reports of varicella infections complicated by NF, mainly in children; however, the timing of rash to diagnosis is generally less than 1 week, with one case after 9 days of the onset of the rash.12–15 A recent case report describes a similar infection in an adult female described as herpes zoster complicated by NF that can be argued also represents a subacute NF infection given the onset of rash to the diagnosis of NF of over 3 weeks. 16 This case did not obtain initial photographs of the rash or laboratory confirmation of herpes zoster on initial presentation. However, this patient had clinically declined despite 20 days of oral antibiotic and antiviral therapy.
The diagnostic criteria for subacute NF have been a subject of debate. Previous literature proposed different definitions, but there was never a consensus regarding a clear set of criteria.17–19 According to the latest proposed criteria, the diagnosis of subacute NF requires five clinical points: (1) an initial indolent course with no systemic abnormalities, (2) progressive cutaneous changes across the afflicted area with gradual tissue necrosis, (3) progression despite antimicrobial treatment, (4) rapid development of NF or systemic sepsis symptoms with sudden worsening, and (5) histological characteristics similar to NF. The first two points are a must for diagnosis. Our patient met all diagnostic criteria for subacute NF except meeting the histological characteristics of NF as she did not undergo a biopsy for diagnosis. 17
Conclusion
This case represents an important lesson for clinicians in reconsidering herpes zoster diagnosis in those cases in which the lesions cross the midline. The case is also a reminder of the importance of follow-up in such cases where the lesions in question do not follow a classic pattern, especially in diabetic patients where NF tends to present atypically. More research studies are needed to ascertain the pathophysiology of subacute NF and determine clear diagnostic criteria.
Acknowledgments
The authors have no acknowledgments to disclose.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for their anonymized information and clinical images to be published in this article.
ORCID iD: Anwar Khedr  https://orcid.org/0000-0002-2730-3031
https://orcid.org/0000-0002-2730-3031
References
- 1. Lancerotto L, Tocco I, Salmaso R, et al. Necrotizing fasciitis: classification, diagnosis, and management. J Trauma Acute Care Surg 2012; 72(3): 560–566. [DOI] [PubMed] [Google Scholar]
- 2. Goh T, Goh LG, Ang CH, et al. Early diagnosis of necrotizing fasciitis. Br J Surg 2014; 101: e119–e125. [DOI] [PubMed] [Google Scholar]
- 3. Wong CH, Chang HC, Pasupathy S, et al. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003; 85: 1454–1460. [PubMed] [Google Scholar]
- 4. Wallace HA, Perera TB. Necrotizing Fasciitis, https://www.ncbi.nlm.nih.gov/books/NBK430756/ (2021, accessed May 2022). [PubMed]
- 5. Wong CH, Khin LW, Heng KS, et al. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32(7): 1535–1541. [DOI] [PubMed] [Google Scholar]
- 6. Roberts M, Crasto D, Roy D. A case of subacute necrotizing fasciitis due to serratia marcescens. J Clin Aesthet Dermatol 2021; 14: 55–58. [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmad H, Haider J, Siddiqui SS, et al. Subacute peristomal necrotizing fasciitis detected during adjuvant chemotherapy for adenocarcinoma rectum: case report on a unique presentation and description of a simple surgical strategy for treatment. Cureus 2018; 10: e2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan JH, Koh BT, Hong CC, et al. A comparison of necrotising fasciitis in diabetics and non-diabetics: a review of 127 patients. Bone Joint J 2016; 98-B(11): 1563–1568. [DOI] [PubMed] [Google Scholar]
- 9. Dayan RR, Peleg R. Herpes zoster—typical and atypical presentations. Postgrad Med 2017; 129(6): 567–571. [DOI] [PubMed] [Google Scholar]
- 10. Shin JW, Kim DH, Whang KU, et al. A case of zoster duplex bilateralis. Ann Dermatol 2009; 21: 423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jarrett P, Ha T, Oliver F. Necrotizing fasciitis complicating disseminated cutaneous herpes zoster. Clin Exp Dermatol 1998; 23(2): 87–88. [DOI] [PubMed] [Google Scholar]
- 12. Shirley R, Mackey S, Meagher P. Necrotising fasciitis: a sequelae of varicella zoster infection. J Plast Reconstr Aesthet Surg 2011; 64(1): 123–127. [DOI] [PubMed] [Google Scholar]
- 13. Wilson GJ, Talkington DF, Gruber W, et al. Group A streptococcal necrotizing fasciitis following varicella in children: case reports and review. Clin Infect Dis 1995; 20(5): 1333–1338. [DOI] [PubMed] [Google Scholar]
- 14. Sewell GS, Hsu VP, Jones SR. Zoster gangrenosum: necrotizing fasciitis as a complication of herpes zoster. Am J Med 2000; 108: 520–521. [DOI] [PubMed] [Google Scholar]
- 15. Cozzupoli GM, Gui D, Cozza V, et al. Necrotizing fasciitis following herpes zoster ophthalmicus in an immunocompromised patient. Case Rep Ophthalmol Med 2019; 2019: 4534153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Can B, Gözel B. Necrotizing fasciitis after herpes zoster infection: a rare case with diagnostic difficulties. Cureus 2022; 14(5): e24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong CH, Wang YS. What is subacute necrotizing fasciitis? A proposed clinical diagnostic criteria. J Infect 2006; 52(6): 415–419. [DOI] [PubMed] [Google Scholar]
- 18. Barker FG, Leppard BJ, Seal DV. Streptococcal necrotising fasciitis: comparison between histological and clinical features. J Clin Pathol 1987; 40(3): 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jarrett P, Rademaker M, Duffill M. The clinical spectrum of necrotising fasciitis: a review of 15 cases. Aust N Z J Med 1997; 27(1): 29–34. [DOI] [PubMed] [Google Scholar]



