Abstract
Introduction
In Portugal as in other countries, data on the epidemiology of asthma are mainly grounded in questionnaire studies. Additionally, the detailed characterisation of asthma in terms of disease severity, control and phenotypes remain scarce. Studies assessing the prevalence of asthma and its subgroups using accurate methods are needed. This study aims to determine the prevalence of asthma, difficult-to-treat asthma and severe asthma, and to evaluate sociodemographic and clinical characteristics of those patients, in mainland Portugal.
Methods and analysis
A population-based nationwide study with a multicentre stepwise approach will be conducted between 2021 and 2023 in 38 primary care centres of the Portuguese National Health Service. The stepwise approach will comprise four stages: Stage 0—telephone call invitation to adult subjects (≥18 years) randomly selected (n~15 000); stage 1—telephone screening interview assessing the participants’ respiratory symptoms (n~7500); stage 2—diagnostic visit, including physical examination, diagnostic tests (eg, spirometry, fraction of exhaled nitric oxide, blood eosinophil count) and patient-reported outcome measures for diagnostic confirmation of those identified with possible asthma at stage 1 (n~1800); stage 3—further evaluation of patients with asthma and of patients with difficult-to-treat asthma and severe asthma, after 3 months (n~460). At stage 3, data will be collected from a review of the patient’s electronic health records, a follow-up telephone call and the CARATm (Caracteristicas Auto-reportadas de Asma em Tecnologias Móveis) app database. The prevalence of asthma, difficult-to-treat asthma and severe asthma will be determined as the percentage of patients with asthma confirmed from the overall population (stage 1). For the analysis of factors associated with asthma, difficult-to-treat asthma and severe asthma, logistic regression models will be explored.
Ethics and dissemination
Ethical approvals for the study were obtained from the ethics committee of the local health unit of Matosinhos, Porto (38/CES/JAS), Alto Minho (38/2021/CES) and the regional health administration of Lisbon-Vale do Tejo (035/CES/INV/2021). Results will be published in peer-reviewed journals.
Trial registration number
Keywords: Asthma, EPIDEMIOLOGY, Chronic airways disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The design of this study is based on a stepwise approach, divided into four stages, that mimics the clinical practice diagnostic pathway for asthma.
This study allows a reliable diagnosis and characterisation of asthma patients through the use of questionnaires together with clinical examination that includes lung function assessment.
A mHealth application to engage patients on self-management of their disease and to improve completeness of the data collection is used.
The 3-month follow-up with lack of optimised management might be a source of error in the estimation of the prevalence of asthma subgroups.
Heterogeneity in data collection across the country regions may prove challenging and a possible source of bias.
Introduction
Asthma is a common chronic disease affecting all age groups. Worldwide, it is estimated that about 5%–10% of people have asthma,1 with a significant wide variation across regions and countries.2 To date, epidemiological data on prevalence have used heterogeneous methodologies due to difficulties in defining and diagnosing asthma, leading to highly variable estimates of asthma prevalence.3 Some studies used non-standardised questionnaires, others included participants based on the assessment of lung function or responsiveness to bronchodilators. In Portugal, the 2011 National Asthma Survey was based on telephone interviews and indicated a prevalence of 6.8%.4 Yet, previous studies have estimated a prevalence between 3.3% and 15%.5 Furthermore, similarly to the USA and Denmark Asthma Surveys, the 2005–2006 Portuguese Health Survey, suggested that there is an underdiagnosis of asthma, particularly in the male population and in the country’s southern regions.5 The Portuguese National Programme for Respiratory Diseases maintains strategic research objectives in epidemiological surveillance both in asthma prevalence and its underdiagnosis,6 which is in line with the recommendations from the Global Initiative for Asthma (GINA).7
Additionally, national data on the characterisation of patients with asthma, in terms of severity and control, remain lacking.8 Worldwide, it is estimated that up to 17% of patients have difficult-to-treat asthma and 3.7% have severe asthma,9 which are more likely to experience life-threatening exacerbations and consume additional healthcare resources. It is important to differentiate these severity groups, as difficult-to-treat asthma might be manageable in primary healthcare, eventually with concomitant support from a secondary care specialist, whereas severe asthma requires a specialised second or tertiary care approach.10 Knowledge on the distribution of severity and characteristics of patients in each group will better support clinical management of the disease and will inform personalised health policies for a future and smarter allocation of healthcare resources.
The primary aim of this study is to determine the prevalence of asthma, difficult-to-treat asthma and severe asthma in Portugal. The secondary aims are to evaluate the sociodemographic and clinical characteristics of patients with asthma.
Methods and analysis
Patient and public involvement
No patient involved
Study design
EPI-ASTHMA is a population-based, nationwide, cross-sectional, prevalence study. This study will be conducted between 2021 and 2023, and will involve 38 primary care centres (PCCs) of the Portuguese National Health Service (NHS), geographically distributed across all mainland Portugal Health Regions (North, Centre, Lisbon Metropolitan Area, Alentejo and Algarve). The study will be conducted using a stepwise approach parallel to a clinical practice diagnostic methodology. First, subjects will be invited for a screening telephone interview to report respiratory symptoms, those who fulfil the eligible criteria will be invited for a clinical assessment in a mobile outpatient clinic. A subgroup of participants with confirmed asthma diagnosis will have a follow-up after 3 months for characterisation of their asthma profile, symptoms patterns, clinical features and treatment patterns. EPI-ASTHMA was first piloted in one local health unit located in the North region from May to October 2021, where all study procedures were tested; after which, implementation is planned to follow the order of approval of the ethics committee from each local health unit and regional health administrations. Currently, the study has been concluded in four PCC in Northern Portugal.
This study protocol is described according to The Strengthening the Reporting of Observational Studies in Epidemiology Statement: guidelines for reporting observational studies statement.11
Study population and sample size
Sample size was estimated using Cochran’s formula12 :
| (1) |
Where n is the sample size, Z is the statistic corresponding to level of confidence, P is expected prevalence of severe asthma, and d is the desired level of precision (ie, the margin of error). Applying a Z=1.96 (ie, 95% CI), an estimated prevalence in the population of p=0.42% and a margin of error of 0.15% the estimated sample size is 7141. In order to obtain 7500 participants on screening, we consider a stepwise approach with a 50% drop-out on invitation. Therefore, around 15 000 adults will be randomly selected from the Portuguese NHS database to be contacted and invited to enrol in the study. In Portugal, the majority of the population (including Portuguese nationals and legal residents) is registered in a PCC, therefore, the NHS database includes almost the entire resident population in the local administrative entity (ie, Nomenclature of Territorial Units for Statistics–NUTS). The sample stratification was based on demographic stratification NUTS III, small regions for specific diagnoses as per EUROSTAT classification. For each NUTS III, the PCCs were selected according to the feasibility of implementing the study (in total 38 PCCs, 31 are part of the regional health administrations and 7 of local health units). The number of participants will be randomly selected according to the proportional allocation of each PCC. The distribution of an estimated stratified sample by NUTS III and the correspondent study stages is presented in table 1. The study will include male or female subjects with at least 18 years old, registered in the NHS database, who give voluntary consent. Subjects with any specific physical and/or cognitive disabilities that prevented them from cooperating with the study procedures (eg, lung function tests) and/or understanding/answering self-reported questionnaires will be excluded.
Table 1.
Distribution of the estimated stratified sample28
| NUTS III | Population | Primary care centres | Stage 0 (0.15%) |
Stage 1 (50%) |
Stage 2 (24%) |
Stage 3 (25%) |
| Alto Minho/Cávado | 637 305 | 2 | 975 | 488 | 118 | 30 |
| Ave | 414 763 | 2 | 635 | 317 | 77 | 19 |
| Área Metropolitana do Porto | 1 719 362 | 6 | 2631 | 1316 | 319 | 81 |
| Alto Tâmega/Douro/Terras de Trás-os-Montes | 389 151 | 2 | 596 | 298 | 72 | 18 |
| Tâmega e Sousa | 419 811 | 2 | 643 | 321 | 78 | 20 |
| Oeste | 357 868 | 2 | 548 | 274 | 66 | 17 |
| Região de Aveiro | 363 424 | 2 | 556 | 278 | 67 | 17 |
| Região de Coimbra | 438 228 | 2 | 671 | 335 | 81 | 21 |
| Região de Leiria | 287 040 | 1 | 439 | 220 | 53 | 13 |
| Viseu Dão Lafões/Beiras/Serra da Estrela/Beira Baixa | 555 628 | 2 | 850 | 425 | 103 | 26 |
| Médio Tejo/Lezíria do Tejo | 474 802 | 2 | 727 | 363 | 88 | 22 |
| Área Metropolitana de Lisboa | 2 827 514 | 9 | 4328 | 2164 | 525 | 133 |
| Alentejo Litoral/Baixo Alentejo/Alto Alentejo/ Alentejo Central | 475 674 | 2 | 728 | 364 | 88 | 22 |
| Algarve | 440 543 | 2 | 674 | 337 | 82 | 21 |
| Total | 9 801 113 | 38 | 15 000 | 7500 | 1819 | 460 |
NUTS, Nomenclature of Territorial Units for Statistics.
Study procedures
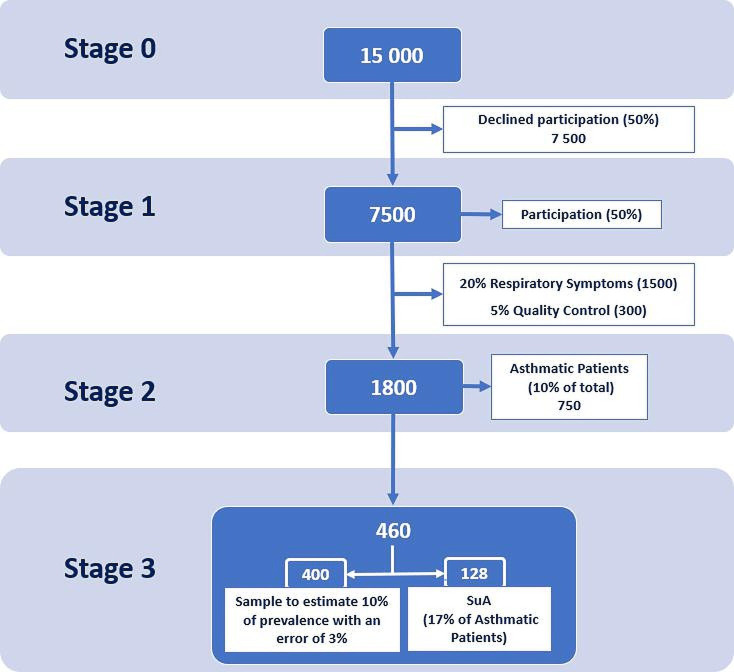
EPI-ASTHMA will use a stepwise approach, sequentially comprising the evaluation of symptoms patterns, clinical features and tests for diagnosis confirmation that includes the following stages (figure 1):
Figure 1.
Flow of participants through study’s stages. SuA, severe and difficult to treat asthma.
Stage 0—Study enrolment: Telephone call invitation to enrol participants.
Stage 1—Screening interview: Telephone call interview to assess respiratory symptoms.
Stage 2—Diagnostic visit: Clinical assessment, diagnostic confirmation and patient characterisation.
Stage 3—Subgroup asthma characterisation: follow-up of patients with asthma (subgroup) and of patients with difficult-to-treat asthma and severe asthma, after 3 months.
Stage 0: study enrolment
Around 15 000 randomised subjects 18 years or above will receive a telephone call invitation for enrolment in the study, to secure a sample size of 7500 participants for the next stage (assuming an acceptance of 50%).13 If needed, a second round of telephone calls will also be made to complete the number of patients in each region. Trained clinical secretaries will conduct this stage by following a semistructured guide during the telephone call. A screening log of potential eligible participants will be completed to document the main reasons for participants not entering the study. Subjects who fulfil the study eligibility criteria and give their oral consent will be contacted in stage 1.
Stage 1: screening interview
A computer-assisted telephone screening interview will be performed to 7500 participants. The interview will include validated patient-reported outcome measures for screening asthma14 and chronic obstructive pulmonary disease,15 dyspnoea16 and assessment of physical activity,17 18 among other questions formulated by the researchers (table 2). At this stage, participants will be considered eligible and invited to participate in stage 2 if they have, at least, one positive response in the Adult Asthma Score (A2 Score),14 that is, medical history of asthma or asthma medication intake or asthma symptoms. For quality control, 5% of not eligible participants (with a negative score) will also be invited to participate in stage 2. Interviews will be performed by a centralised team of trained and experienced interviewers. Each participant will be contacted for at least ten attempts during different occasions before being considered excluded. Prior to any question, participants will be asked to reinforce their oral consent to participate. A pilot study with 12 individuals was conducted to assess the clarity and feasibility of the screening interview before starting the data collection.19 The duration of each interview was no longer than 17 min.
Table 2.
Summary of the data collection per study’s stage
| Stage 1 | Stage 2 | Stage 3 | |
| Sociodemographic and anthropometric characteristics | × | ||
| Brief PA17 18 assessment | × | × | |
| Respiratory symptoms (wheeze, breathlessness) | × | × | × |
| CAPTURE15 | × | ||
| mMRC16 | × | ||
| Diagnosis of chronic respiratory disease | × | × | × |
| Comorbidities and allergies | × | × | × |
| Smoking habits and ETS | × | × | |
| A2 Score14 | × | ||
| Family history of asthma | × | × | |
| Age of asthma onset | × | ||
| Inhaler prescription/use | × | ||
| Mini-AQLQ29 30 | × | ||
| EQ-5D31 | × | ||
| Signs of asthma | × | ||
| CARAT21 | × | × | |
| Asthma control (according to GINA) | × | × | |
| Asthma pharmacological treatment | × | ×* | |
| Inhalation technique | × | ||
| Adherence to inhaled medication | × | ×* | |
| Other treatments | × | × | |
| No of exacerbations | × | ×* | |
| No of visits to emergency room | × | ×* | |
| No of hospital admissions and length of hospital stay | × | ×* | |
| No of unscheduled consultations | × | ×* | |
| No of consultations primary care team | × | ×* | |
| Referral for specialist care | × | ×* | |
| Standard measurements (blood pressure, height, weight) | × | ||
| Pre-BD and post-BD32 lung function | × | ||
| Pulmonary diagnostic tests (previously performed) | × | ||
| FeNO33 | × | ||
| Peripheral blood eosinophil and neutrophil counts | × | ||
| Use of health and fitness apps | × | × | |
| CARATm app use and opinion | × |
*In the previous 3 and 12 months.
A2 Score, A2 adult asthma score; CAPTURE, COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk; CARAT, Control of Allergic Rhinitis and Asthma Test; CARATm, Caracteristicas Auto-reportadas de Asma em Tecnologias Móveis; EQ-5D, European Quality of Life Five Dimension; ETS, Environmental tobacco smoke; FeNO, Fractional exhaled nitric oxide; Mini-AQLQ, Mini Asthma Quality of Life Questionnaire; mMRC, Modified British Medical Research Council; PA, Brief physical activity assessment; pre-BD and post-BD, Spirometry prebronchodilator and postbronchodilator.
Stage 2: diagnostic visit
The diagnostic visit will be conducted in a mobile outpatient clinic, located, whenever possible, in the close vicinity of each PCC. At this stage, participants will give a written informed consent before any study-related procedures. Stage 2 is expected to include 1800 participants, about 20% of participants from stage 1 who fulfil the eligibility criteria, plus 5% of participants for quality control. A detailed clinical assessment will be performed, including clinical history, physical examination, lung function tests, blood count and inflammatory biomarkers testing and patient-reported outcome measures (table 2). The clinical assessment will be carried out by a physician and the diagnostic tests by an experienced team to assure the quality and the harmonisation of procedures. All patients will be provided with a letter addressed to their primary care physician, containing the results of their clinical evaluation. Patients with asthma will also be invited to instal and use the CARATm app (‘Caracteristicas Auto-reportadas de Asma em Tecnologias Móveis’) in their daily life. CARATm is a Portuguese health mobile app developed to collect clinical data from patients with asthma, such as asthma control and medication adherence, which is also interoperable with the Portuguese Severe Asthma Registry (asmagrave.pt).
Stage 3: subgroup asthma characterisation
This stage is expected to include 460 participants: a randomised sample of patients with asthma, and all patients identified with difficult-to-treat or severe asthma. Patients with difficult-to-treat asthma and severe asthma will be defined according to GINA.20 This stage takes place 3 months after the diagnostic visit and consists of a review of patients’ electronic health records, a follow-up telephone call to the patient, and extraction of CARATm app data. The review of patients’ electronic health records by a physician of their PCC will ensure an asthma control assessment on two different time points, and will confirm the treatment profile for an adequate GINA severity classification7 (table 2).
Diagnosis criteria and definitions
According to GINA, uncontrolled asthma includes one or both of the following: poor symptom control and frequent exacerbations (≥2/year) requiring oral corticosteroids or serious exacerbations (≥1/year) requiring hospitalisation.20 In our study, poor symptom control will be based on a score less or equal than 24 in the Control of Allergic Rhinitis and Asthma Test.21 22 Data regarding exacerbations or serious exacerbations will be collected at stages 2 and 3.
Difficult-to-treat asthma is asthma that is uncontrolled despite treatment with medium-dose or high-dose inhaled corticosteroids with a second controller or with maintenance oral corticosteroids, or that requires high dose treatment to maintain symptom control and to reduce the risk of exacerbations.20 In our study, difficult-to-treat asthma will be defined as uncontrolled asthma, despite prescription of high intensity treatment (GINA step treatment 4–5). Severe asthma is a subset of difficult-to-treat asthma, that is, an uncontrolled asthma despite adherence with maximal optimised high-dose inhaled corticosteroid and a long-acting β2-agonist treatment and management of contributory factors, such as inhaler adherence and technique or asthma that worsens when high dose treatment is decreased. We will define severe asthma in the study as uncontrolled asthma despite prescription of high intensity treatment (GINA step treatment 4–5) and good treatment adherence (Visual Analogue Scale ≥50)23 and good inhaler technique (number of critical errors = (0–1)).24
Data storage, blinding and statistical analysis plan
Participants will be anonymised with a unique subject ID and their data will be anonymously stored in an appropriately secured server. In order to minimise diagnostic bias, researchers, data collectors and participants will be blinded to patient eligibility throughout data collection during stage 2. Over the stages, data will be collected for a specific electronic case report form by using blended data (primary and secondary data).
Statistical analysis will allow the characterisation of the study population and the estimation of each study’s endpoints. The prevalence of asthma, difficult-to-treat asthma and severe asthma with the respective 95% CIs will be calculated in relation to the entire study population (stage 1). Descriptive statistics such as (1) central tendency (eg, mode, median or mean); (2) localisation (eg, percentile); (3) dispersion (eg, IQR, SD) and (4) distribution (eg, skewness, kurtosis) will be used, depending on the type of each variable, to characterise the total sample or subgroups. Associations between two quantitative variables will be calculated using the Pearson’s correlation coefficient or Spearman correlation coefficient, in case of normality assumption are not verified. Association of two categorical variables will be determined using the χ2 test or Fisher’s exact test. Continuous variables between two groups will be compared with a t-test for independent samples or Mann-Whitney test, whereas for comparisons among three or more groups an one-way analysis of variance (ANOVA) or Kruskall-Wallis test will be used. Null-hypothesis statistical testing will also be applied in an exploratory manner to identify any relationship patterns among variables. Subgroup analysis per each asthma control level, GINA treatment step, short-acting beta agonists overuse among others will also be conducted. Measurements of effect size will be presented and confidence intervals at 95% CI will be estimated to account for the uncertainty of the sample estimates. Interim analysis will be conducted per region after all stages are completed to monitor the safety of study procedures and completeness of data collection. This analysis may encompass potential changes in logistical, monitoring and recruitment procedures to secure sample size.
For the analysis of factors associated with asthma, difficult-to-treat asthma and severe asthma, logistic regression models will be explored, taking into consideration these as dependent variables and sociodemographic, clinical, among other variables, as independent variables. The variables to be included in the multivariate model will be selected from the univariate analyses, when p value <0.100, those that do not meet these criteria, but are considered clinically relevant, will also be possible candidates. The final model will be analysed from two perspectives: (1) the calibration using the Hosmer-Lemeshow goodness of fit test and (2) the discrimination, using receiver operating characteristic (ROC) analysis. All results will be considered statistically significant when p<0.05.
Discussion
The EPI-ASTHMA study will address knowledge gaps in the epidemiology and characterisation of asthma in mainland Portugal, using a multicentre approach that combines different data collection methods, including a mHealth solution. Furthermore, it will provide a more complete picture of the prevalence of difficult-to-treat asthma and severe asthma in Portugal, and will allow a better understanding of these patients' characteristics, treatment profile and healthcare resource use. Further knowledge on the distribution of severity and characteristics of patients in each group will better support clinical management of the disease and inform personalised health policies for a smarter allocation of the needed healthcare resources. This ambitious study is aligned with the global need of epidemiological studies to monitor the trends in the prevalence of asthma, which is vital to ensure that health policies fit the populations’ needs.2 25 26
The study design is one of its strengths. The combination of standardised questionnaires with a standardised clinical evaluation, including lung function tests will allow a more accurate diagnosis and characterisation of asthma. However, few limitations and potential risks must be acknowledged. The definition of difficult-to-treat asthma and severe asthma will be based on the GINA treatment steps (4–5) and the assessment of asthma control during the study stages 2 and 3, which is only 3 months apart. An optimised management during the follow-up period was not included, which is also part of the GINA criteria to distinguish these asthma subgroups. This will probably be a source of error in the estimation of the prevalence of these subgroups, linked to the observational nature of this study. Thirty-eight PCCs will be conveniently selected, which can be seen as a source of bias. Yet, we considered the geographic areas and population distribution of each Portuguese region as well as their interest to participate and willingness to collect data and not only based on previous asthma research experience which will preserve the study real-world nature. Clinical secretaries (stage 0) and physicians (stages 2 and 3) will collaborate across different country’s regions, which may introduce some heterogeneity in data collection. Nevertheless, both clinical secretaries and physicians will be previously trained and continuously motivated by the management team, which will be available to clarify doubts on a 24/7 basis. To deal with possible risks, the study’s steering committee will supervise the execution and will take academic responsibility for the study. Another limitation is the need for this multicentre study to be approved by the ethics committees responsible for each regional health administration/local health unit. Although all applications will be similar in their content, they will differ in a number of aspects to comply with each institution’s formal requirements, resulting in additional complexity and may hinder the compliance with the study timeline. To mitigate these risks, an experienced contracted research organisation that knows the specificities of each administration/health unit is supporting us through all the process.
EPI-ASTHMA will be managed combining traditional management methodologies with an ‘agile’ approach27 using the SCRUM framework on Jira software. This combination is expected to fulfil the need to make the study adaptable and to anticipate future needs that are important and can be forgotten due to the dimension of the study.
Conclusion
EPI-ASTHMA will be the first population-based study in Portugal to determine the prevalence of asthma, difficult-to-treat asthma and severe asthma and to better understand the patient’s disease characteristics, treatment patterns and use of healthcare resources. The knowledge generated by this nationwide robust study has great potential to inform health policies and to improve the clinical outcomes of patients with asthma in Portugal.
Ethics and dissemination
The study will follow the tenants of the Declaration of Helsinki and the Oviedo Convention. Ethical approvals and data privacy clearance for the study were obtained by the Ethics Committee and Data Protection Officer of the local health unit of Matosinhos, Porto (ULSM; 38/CES/JAS, March 12th, 2021), Alto Minho (ULSAM; 38/2021/CES, 17 June 2021) and the Regional Health Administration of Lisbon and Vale do Tejo (ARSLVT; 035/CES/INV/2021; 3 December 2021) and is under revision in the remain regional ethical committees for health administration/local health units. All participants will receive both verbal and written information explaining the purpose of the study and they will have to provide verbal and written informed consent. Results will be presented at both national and international scientific meetings and published in peer-reviewed journals. Seasonal newsletters will be provided to the funders of the study as well as all the involved participants.
Supplementary Material
Footnotes
Contributors: Jc-d-S, JAF and FB conceptualised the study. CJá wrote the statistical analysis plan and PT conducted the sample size calculation. CJá and CJo wrote the first draft of the manuscript. CJá, DB, CJo, FL, JS, LA, MJB, MP, PT, FB, JAF and JC-d-S contributed to and refined the manuscript for scientific content. All authors read and approved the final version of the manuscript.
Funding: Funding for this study was provided by AstraZeneca. N/A for the award/grant number.
Disclaimer: The funding body had no role in the conducting or reporting of the study.
Competing interests: JC-d-S reports Advisory Board from Boheringer Ingelheim, personal fees and Advisory Board from GSK, grants, personal fees and Advisory Board from AstraZeneca, personal fees and Advisory Board from Bial, non-financial support from Mundipharma, personal fees from Sanofi, Advisory Board from Novartis, outside the submitted work. JAF declares grants from or research agreements with AstraZeneca, Mundipharma, Sanofi Regeneron and Novartis. Personal fees for lectures and attending advisory boards from AstraZeneca, GSK, Mundipharma, Novartis, Sanofi Regeneron and TEVA. MP and FB are employees of AstraZeneca, Produtos Farmacêuticos. The remaining authors declare no conflicts of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343–73. 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 2.Asher MI, García-Marcos L, Pearce NE, et al. Trends in worldwide asthma prevalence. Eur Respir J 2020;56. doi: 10.1183/13993003.02094-2020. [Epub ahead of print: 24 12 2020]. [DOI] [PubMed] [Google Scholar]
- 3.Sá-Sousa A, Jacinto T, Azevedo LF, et al. Operational definitions of asthma in recent epidemiological studies are inconsistent. Clin Transl Allergy 2014;4:24. 10.1186/2045-7022-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sa-Sousa A, Morais-Almeida M, Azevedo LF, et al. Prevalence of asthma in Portugal - The Portuguese National Asthma Survey. Clin Transl Allergy 2012;2:15. 10.1186/2045-7022-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Sousa JC, Santo ME, Colaço T, et al. Asthma in an urban population in Portugal: a prevalence study. BMC Public Health 2011;11:347. 10.1186/1471-2458-11-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Saúde D-G. Programa Nacional para as Doenças Respiratórias 2017. Lisboa: Direção-Geral da Saúde, 2017. [Google Scholar]
- 7.Global Initiative for Asthma . Global strategy for asthma management and prevention, 2021. [Google Scholar]
- 8.Sá-Sousa A, Amaral R, Morais-Almeida M, et al. Asthma control in the Portuguese national asthma survey. Rev Port Pneumol 2015;21:209–13. 10.1016/j.rppnen.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 9.Narasimhan K. Difficult to treat and severe asthma: management strategies. Am Fam Physician 2021;103:286–90. [PubMed] [Google Scholar]
- 10.Ryan D, Murphy A, Ställberg B, et al. 'SIMPLES': a structured primary care approach to adults with difficult asthma. Prim Care Respir J 2013;22:365–73. 10.4104/pcrj.2013.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007;18:800–4. 10.1097/EDE.0b013e3181577654 [DOI] [PubMed] [Google Scholar]
- 12.Kasiulevičius V, apoka V, Filipavičiūtė R. Sample size calculation in epidemiological studies. Gerontologija 2006;7:225–31. [Google Scholar]
- 13.Lopes C, Torres D, Oliveira A. Inquérito Alimentar Nacional E de Atividade Física IAN-AF 2015-2016: relatório de resultados, 2017. Available: https://repositorioaberto.up.pt/bitstream/10216/111073/2/257104.pdf
- 14.Sá-Sousa A, Pereira AM, Almeida R, et al. Adult asthma Scores-Development and validation of multivariable scores to identify asthma in surveys. J Allergy Clin Immunol Pract 2019;7:183–90. 10.1016/j.jaip.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 15.Martinez FJ, Mannino D, Leidy NK, et al. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017;195:748–56. 10.1164/rccm.201603-0622OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Standardized Questionaries on respiratory symptoms. BMJ 1960;2:1665. 10.1136/bmj.2.5213.166513688719 [DOI] [Google Scholar]
- 17.Cruz J, Jácome C, Oliveira A, et al. Construct validity of the brief physical activity assessment tool for clinical use in COPD. Clin Respir J 2021;15:530–9. 10.1111/crj.13333 [DOI] [PubMed] [Google Scholar]
- 18.Marshall AL, Smith BJ, Bauman AE, et al. Reliability and validity of a brief physical activity assessment for use by family doctors. Br J Sports Med 2005;39:294–7. 10.1136/bjsm.2004.013771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Teijlingen E, Hundley V. The importance of pilot studies. Nurs Stand 2002;16:33–6. 10.7748/ns2002.06.16.40.33.c3214 [DOI] [PubMed] [Google Scholar]
- 20.Global Initiative for Asthma . A new Pocket Guide, “Diagnosis and Management of Difficult-to-treat and Severe Asthma in adolescent and adult patients. 2021.
- 21.Fonseca JA, Nogueira-Silva L, Morais-Almeida M, et al. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy 2010;65:1042–8. 10.1111/j.1398-9995.2009.02310.x [DOI] [PubMed] [Google Scholar]
- 22.Fonseca JA, Nogueira-Silva L, Morais-Almeida M, et al. Control of allergic rhinitis and asthma test (CARAT) can be used to assess individual patients over time. Clin Transl Allergy 2012;2:16. 10.1186/2045-7022-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohta K, Jean Bousquet P, Akiyama K, et al. Visual analog scale as a predictor of GINA-defined asthma control. The SACRA study in Japan. J Asthma 2013;50:514–21. 10.3109/02770903.2013.786726 [DOI] [PubMed] [Google Scholar]
- 24.Bosnic-Anticevich SZ, Cvetkovski B, Azzi EA, et al. Identifying critical errors: addressing inhaler technique in the context of asthma management. Pulm Ther 2018;4:1–12. 10.1007/s41030-018-0051-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bousquet JW. Global surveillance, prevention and control of chronic respiratory diseases : a comprehensive approach 2007.
- 26.Global Asthma Network . The global asthma report 2018. Auckland, New Zealand, 2018. [Google Scholar]
- 27.Pavlović KB, Berić I, Berezljev L. Agile transformation in clinical research. European Project Management Journal 2018;8:65–70. 10.18485/epmj.2018.8.1.8 [DOI] [Google Scholar]
- 28.Instituto Nacional de Estatística . Censos 2011. XV Recenseamento Geral da População : V Recenseamento Geral da Habitação. Resultados definitivos : Portugal. Lisboa : INE, 2012. IV Recenseamento Geral de Habitação. Lisboa, Portugal: Instituto Nacional de … 2012.
- 29.Ferreira PL, Mendonça C, Newparth N. Quality of life in asthma: cultural adaptation of Juniper’s AQLQ to Portuguese 1998;7:590. [Google Scholar]
- 30.Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J 1999;14:32–8. 10.1034/j.1399-3003.1999.14a08.x [DOI] [PubMed] [Google Scholar]
- 31.Ferreira PL, Ferreira LN, Pereira LN. Contributos para a Validação dA Versão Portuguesa do EQ-5D. 26. Med Port: Acta, 2013: 664. [PubMed] [Google Scholar]
- 32.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic Society and European respiratory Society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dweik RA, Boggs PB, Erzurum SC, et al. An official ats clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602–15. 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.