Abstract
Introduction
Our previous pilot work suggests relational harm reduction strengthens relationships between people with HIV (PWH) who use drugs and their healthcare providers and improves HIV health outcomes. However, there is limited research examining ways that structural (eg, strategies like syringe service programmes) and/or relational (patient-provider relationship) harm reduction approaches in HIV clinical settings can mitigate experiences of stigma, affect patient-provider relationships and improve outcomes for PWH who use drugs. Our mixed methods, multisite, observational study aims to fill this knowledge gap and develop an intervention to operationalise harm reduction care for PWH who use drugs in HIV clinical settings.
Methods and analysis
Aim 1 will explore the relationship between healthcare providers’ stigmatising attitudes towards working with PWH who use drugs and providers’ acceptance and practice of structural and relational harm reduction through surveys (n=125) and interviews (n=20) with providers. Aim 2 will explore the interplay between patient-perceived harm reduction, intersectional stigma and clinical outcomes related to HIV, hepatitis C (if applicable) and substance use-related outcomes through surveys (n=500) and focus groups (k=6, total n=36) with PWH who use drugs. We will also psychometrically evaluate a 25-item scale we previously developed to assess relational harm reduction, the Patient Assessment of Provider Harm Reduction Scale. Aim 3 will use human-centred design approaches to develop and pretest an intervention to operationalise harm reduction care for PWH who use drugs in HIV clinical settings.
Ethics and dissemination
This study was approved via expedited review by the University of Pittsburgh Institutional Review Board (STUDY21090002). Study findings will be presented in peer-reviewed journals and public health conferences as well as shared with patient participants, community advisory boards and harm reduction organisations.
Trial registration number
Keywords: HIV & AIDS, PUBLIC HEALTH, Substance misuse
Strengths and limitations of this study.
We are the first, to our knowledge, to examine intersectional stigma in people with HIV who use drugs through the multiple lenses of HIV, substance use and race.
Our study will also be the first to examine harm reduction for people with HIV who use drugs from a relational perspective (ie, the patient–provider relationship) in additional to the traditional structural approach (eg, syringe service programmes, naloxone distribution).
We will survey multiple health provider types who interface with people with HIV who use drugs, including those traditionally not included in research (eg, front desk and administrative staff, pharmacists, dieticians, etc).
A primary limitation is that our study sites explicitly provide HIV primary services to PWH, and there may be less variability among provider attitudes and patient experiences than would be found outside of this specialist setting. However, extant literature suggests that HIV providers often feel unprepared to care for and carry negative attitudes towards patients who use drugs.
Background
There are significant HIV health disparities between people who use drugs and people who do not use drugs. Among all new HIV diagnoses in the USA in 2018, one in 10 were among people who inject drugs.1 High rates of HIV among people who inject drugs are particularly problematic given injection drug use increases risk for HIV transmission and acquisition and predicts poor retention in HIV primary care.2–5 Lack of retention in care is associated with poor clinical outcomes, such as unsuppressed viral load, which contributes to HIV incidence.6–9 People with HIV (PWH) who miss visits in their first year of HIV treatment have more than double the mortality risk of those retained in care.10 Moreover, HIV and hepatitis C (HCV) often co-occur, with an estimated 21% of PWH in the USA coinfected with HCV,11 and evidence that HIV viral load impacts severity of HCV infection.12 13
While social factors such as economic distress,14 trauma15 and comorbid mental health conditions16 all increase substance use rates and serve as barriers to care, there is strong evidence that experiences of stigma in healthcare settings by people who use drugs are common and contribute to poor healthcare outcomes.17–20 PWH who use drugs may experience stigma related to HIV status and substance use, while PWH of colour who use drugs may experience additional stigma through racial discrimination (eg, inequitable treatment based on race or ethnicity).21 Experiencing any kind of stigma in the healthcare setting is particularly deleterious. We previously found that experiencing HIV stigma in healthcare settings, but not in community settings, was associated with lack of viral suppression,20 while additional research illuminates the negative relationship between experienced HIV stigma in the healthcare setting and antiretroviral therapy (ART) adherence.22 Experiencing substance use stigma in healthcare settings is also damaging, with people who inject drugs reporting experiences of discrimination and derogatory language from their healthcare providers, contributing to decreased engagement in care.23
Our previous work suggests that harm reduction (HR) may strengthen the patient–provider relationship and mitigate the effects of stigma. HR refers to approaches aimed at reducing the negative consequences of health behaviours without necessarily eliminating the problematic health behaviours entirely.24–27 HR stands in opposition to the traditional medical model of addiction, in which any illicit drug use is labelled as abuse, and the moral model, which labels substance use as simply wrong.25 26 HR strategies such as syringe service programmes (SSP), naloxone distribution and medications for opioid use disorder effectively engage people who use drugs in care by providing services that are responsive to their needs without assuming abstinence as the ideal clinical outcome, while simultaneously working to reduce stigma in healthcare settings by honouring patient autonomy.26 28–33 Though HR is typically thought of as structural approaches (ie, policies or strategies like SSPs), HR also includes relational approaches to care, centred on improving the patient–provider relationship, which can be implemented by healthcare teams to improve outcomes for PWH who use drugs.27 34 35
We previously defined HR principles for healthcare settings to describe ways that clinicians can operationalise and provide relational HR care (ie, humanism, pragmatism, individualism, autonomy, incrementalism and accountability without termination).27 In our mixed methods study of an HIV clinic serving PWH who use drugs, we conducted patient surveys to test associations between perceptions of care related to HR (respect, user-friendly and unhurried care and clinic responsiveness) and self-reported ART adherence. After adjusting for race, age, ethnicity, gender identity, sexual orientation, homelessness and poverty status, the addition of the HR-related variables significantly predicted ART adherence.34 35
However, there is limited research examining ways that structural and relational HR in HIV clinical settings reduce experiences of stigma, affect patient–provider relationships and improve outcomes for PWH who use drugs. Given that integrated, coordinated HIV and substance use care is essential for optimising the health outcomes of PWH who use drugs,36 an intervention that draws on the principles of HR to address both HIV and substance use healthcare needs is essential. The knowledge gained from this study will enable us to develop an intervention to operationalise HR care in an HIV clinic setting and, ultimately, reduce health inequities for PWH who use drugs. The current manuscript provides a detailed overview of our study protocol.
Objectives
The study has three primary aims:
1. Explore the relationship between healthcare providers’ stigmatising attitudes towards working with PWH who use drugs and providers’ acceptance and practice of structural and relational HR to elucidate the context for intervention development. We will survey physicians, advanced practice providers, nurses, medical assistants, front-desk staff and social workers (n=125) and conduct qualitative interviews (n=40) at our study sites to develop a deeper understanding of providers’ attitudes towards working with PWH who use drugs as well as the ways that these attitudes are associated with the provision of structural and relational HR care. See online supplemental files 1,2 for copies of the survey and interview guide, respectively.
bmjopen-2022-067219supp001.pdf (185.1KB, pdf)
bmjopen-2022-067219supp002.pdf (112.4KB, pdf)
2. Explore the interplay between patient-perceived HR and stigma and clinical outcomes; specifically, the degree to which (a) relational HR moderates the effect of intersectional stigma experienced in healthcare settings (HIV-related and substance use-related stigma and racial discrimination) on patients’ perceptions of their relationship with providers, (b) structural HR moderates the relationship between the patient-provider relationship and clinical outcomes (ART adherence, retention in care, HIV and HCV viral suppression) and (c) patient-perceived HR care is directly associated with HIV clinical outcomes. We will survey PWH who use drugs (n=500) to assess their perceptions of providers’ relational HR care, experiences of intersectional stigma and perceived quality of relationships with their providers, and to explore other potential stigmatised identities and characteristics in patient focus groups (total n=36). We will also psychometrically evaluate our novel scale, the Patient Assessment of Provider Harm Reduction Scale (PAPHRS), to assess patients’ perceptions of the degree to which their providers deliver relational HR care. See online supplemental files 3,4 for copies of the survey and focus group guide, respectively.
bmjopen-2022-067219supp003.pdf (85.6KB, pdf)
bmjopen-2022-067219supp004.pdf (120.3KB, pdf)
Using human-centred design approaches,37 develop and pre-test an intervention to operationalise HR care for PWH who use drugs in HIV clinical settings. Using findings from aims 1 and 2, we will meet with community member and provider collaborators (n=20), including PWH who use drugs, HIV providers and HR experts, to review results and pinpoint the most valuable intervention approaches using human-centred design, ensuring that the intervention is responsive to end users’ needs.
Methods and analysis
Study design
The overarching aim of our observational study is to collect data that will inform development of an intervention to be tested in a subsequent clinical trial. We will use a sequential explanatory mixed-methods approach,38 following the surveys with semistructured interviews (aim 1) and focus groups (aim 2), in order to contextualise and gain in-depth understanding of survey findings. The study is funded from September 2021 through June 2026. Recruitment for the provider survey (aim 1) began in April 2022.
We will develop an intervention in aim 3, in which we will meet with community member and provider collaborators to review results from aims 1 and 2 and identify the most valuable intervention approaches using human-centred design and pretest this intervention by convening small groups or one-on-one meetings with providers in Pittsburgh and Birmingham (total n=12). These individuals will be different than those involved in intervention development. During these meetings, we will share the mockup design (the concept poster) of the intervention and explore preliminary feasibility, acceptability and appropriateness of our prototyped approach.
Setting
The University of Pittsburgh (Pitt) is the study coordinating centre. Study sites are two HIV clinics in Pittsburgh, Pennsylvania (PA) (Allegheny Health Network’s Positive Health Clinic (PHC), University of Pittsburgh Medical Centre’s HIV/AIDS Programme and one in Birmingham, Alabama (AL) (University of Alabama at Birmingham (UAB) 1917 Clinic). These are areas of the country that are disproportionately affected by both the HIV and opioid epidemics and have high HCV incidence rates. Additionally, while not a study site, the study involves close collaboration with a strong community partner, Birmingham AIDS Outreach (BAO), an AIDS service organisation providing social support services to more than 1000 PWH each year, most of whom receive HIV primary care at UAB’s 1917 Clinic. BAO will lead recruitment efforts and coordinate study activities in AL.
Participants
For both quantitative and qualitative portions of aim 1, providers are eligible if they have worked at one of the study sites for least 1 year; provide service or care to PWH or people who use drugs at high risk for HIV acquisition and are able to verbally consent, read and speak English. Providers may include any employee who directly interfaces with patients, including, but not limited to, physicians, nurses, social workers, pharmacists and front desk staff. Eligible providers may, but do not have to, participate in both the survey and interview components of Aim 1.
For both quantitative and qualitative portions of aim 2, patient participants must be ages 18 or older, have a confirmed HIV diagnosis, be able to verbally consent, read and speak English, have received HIV medical care from one of the study sites for at least 1 year and have lifetime or recent use (past 3 months) of illicit substances (excluding marijuana) or prescription drugs for non-medical reasons. As with aim 1, eligible participants may, but do not necessarily have to, complete both quantitative and qualitative portions.
Variables and data sources and measurement
Outcomes
There are five outcomes of interest in our study, all relating to the clinical health of PWH who use drugs. Four of these are collected as standards of care at our study sites and will be abstracted via patient electronic medical health record: HIV viral load (<200 copies/mL, virally suppressed39); HIV primary care appointment attendance (as measured by1 visits at least 90 days apart within 1 year=retained in HIV primary care40 and2 proportion of missed to scheduled visits (range 0–100%)41); HCV viral load, for those who have HCV and retention in opioid treatment care for those with opioid use disorder (proportion of kept to scheduled visits (range 0–100%)).
We will measure ART adherence via self-report through the validated Center for Adherence Support Evaluation (CASE) index.42 All study outcomes will be measured cross-sectionally, collecting all HIV primary care and opioid treatment care visits within a 12-month observation window and the HIV and HCV viral load data closest to the end of the observation window. Clinical data will be linked to survey data by study staff at the participating clinical sites. Analysis of these outcomes will enable us to explore: the relationship between patient-perceived HR care and clinical outcomes, relational HR as a potential moderator of the path between intersectional stigma and the patient-provider relationship and structural HR as a potential moderator of the path between intersectional stigma and the patient–provider relationship, in which stigma is explored as HIV-related and substance use-related stigma and racial discrimination).
Other variables
Table 1 includes a complete list of all data elements included in aims 1 through 2 of the study, including sources of data and methods of assessment, along with corresponding citations.
Table 1.
Aims 1 and 2 constructs and measurement tools
| Aim 1. Provider-reported | |
| Quantitative | |
| Provider attitudes | |
| Acceptance of HR |
|
| Structural HR |
|
| Structural HR |
|
| Qualitative | |
| Interviews |
|
| Aim 2. Provider-reported | |
| Qualitative | |
| Interviews |
|
| Aim 2. Patient-reported (PWH who use drugs) | |
| Qualitative | |
| Focus groups |
|
| Quantitative | |
| Experiences of stigma and discrimination in healthcare settings | |
| Patient–provider relationship | |
| Receipt of structural HR care |
|
| Receipt of relational HR care |
|
| Patient clinical outcomes (EHR data) |
|
| Qualitative | |
| Focus groups |
|
ART, antiretroviral therapy; HR, harm reduction; MOUD, medications for opioid use disorder; PAPHRS, Patient Assessment of Provider Harm Reduction Scale; PWH, people with HIV.
Bias
While participants may experience social desirability bias, the provider confidentiality and patient anonymity of the surveys is expected to mitigate this bias.
Statistical methods
Quantitative analysis and sample sizes
To analyse survey data from aim 1, we will stratify by site and use descriptive statistics and bivariate associations to explore how providers feel about HR care as well as to determine both organisational and individual practice of structural HR, since HR policy and structures might be in place at the organisational level, yet not practiced by individual providers. At an estimated sample size of n=125, we anticipate sufficient sample size at power=0.80. Recent simulation research on SEM factor analysis suggests appropriate sample sizes with moderate factor loading between n=90–120 across a range of solutions.43
In aim 2, we will construct a generalised SEM (gSEM) to assess associations between patient-reported (1) intersectional stigma (HIV-related and substance use-related stigma and racial discrimination) in healthcare settings and patient–provider relationships and (2) patient-provider relationships and clinical outcomes (ART adherence, retention in HIV and substance use care and suppression of HCV and HIV). This gSEM will be constructed using a mediation approach, wherein we will assess whether the patient–provider relationship mediates the relationship between intersectional stigma and clinical outcomes. Mediation will be examined by assessing total, direct and indirect effects. This approach will test the degree to which the relationship between intersectional stigma (HIV-related and substance use-related stigma and racial discrimination) in healthcare settings and clinical outcomes is explained by the qualities of the patient–provider relationship. With an estimated sample size of n=500 and expected reasonable ratio of sample size to number of parameter estimates as 5:1,44 we anticipate sufficient sample size with eight covariates (age, gender, sexual and gender minority status, income, race, ethnicity, substance use and study site).
We will also evaluate the novel relational HR instrument using both classical and modern psychometric techniques. Classical item analysis including item frequencies, item-total correlations, item frequency distributions and tests of monotonicity will be examined first. The underlying factor structure of PAPHRS items will be explored using factor analysis. The sample will be randomly split into two half samples, one for exploratory factor analysis (EFA) and the other for confirmatory factor analysis (CFA) using Mplus.
Our aim 2 sample size of 500 patients is based on longstanding practice for estimating sample size for SEMs with latent variables. Fritz and MacKinnon have posited that n=500 confers sufficient power (at 80%) to detect small mediation effects with a cross-sectional study.45 A sample size of 500 also confers sufficient power for the psychometric evaluation of PAPHRS. Suggested minimums of sample size for factor analysis include from 3 to 20 times the number of variables and absolute ranges from 100 to over 1000.46 The sample size of 500, which will be split into 250 for EFA and 250 for CFA, will give us 10 times the number of PAPHRS items, right in the middle of the suggested sample size range. Reise and Yu47 recommend that the unidimensional graded response model (GRM) be estimated with 500 cases. For convergent validity analyses, a sample of 200 participants is sufficient to provide power of 0.90 for correlations larger than 0.80 at alpha level of 0.05 with a two-tailed test. For comparisons between groups with expected differences, a sample size of 191 per group is needed to provide power of 0.90 for an effect size of 0.30 with alpha level of 0.05 and a two-tailed test.
Qualitative analysis
We will analyse interview and focus group data in NVivo V.1248 using thematic analysis.49 50 All five members of our qualitative team will participate in analysis and development of the coding framework by reading through transcripts, identifying major themes to contextualise the data and supplementing with field notes and corresponding analytic memos. We will code interviews and focus groups based on the initial coding framework, using processes of adjudication after each interview and iteratively modifying the codebook. This method of co-coding will continue until agreement on application of the codes is achieved. All interviews and focus groups will be coded, and at least 20% will be double-coded by two researchers and compared for consistency, in keeping with scholars’ recommendation to double-code between 10% and 25% of transcripts.51 To assess the extent to which the qualitative findings help explain the quantitative results, we will integrate quantitative and qualitative findings in a joint display to illustrate quantitative results with their corresponding qualitative themes.52 53
Recruitment
Provider recruitment
We will recruit providers by visiting sites’ staff meetings and via electronic messaging used by each study site for internal communications and will have a Research Coordinator at each of our sites to assist with these methods and serve as site-specific project champions. Surveys will be deployed via REDCap54 using confidential links. We will continually monitor response rates by provider type and site to ensure that each provider group is represented in the data. We will continue with monthly targeted electronic messages until our recruitment targets are met.
Patient recruitment
We will recruit 500 patients in total from our three study sites to complete a one-time survey on REDCap and 36 patients from our three study sites in total to participate in focus groups; patients may, but do not have to, participate in both data collection activities. We will use a multimodal recruitment plan, including word-of-mouth, flyers in provider waiting areas and patient rooms, messages sent through internal clinic systems for patients who receive electronic messages and in-person information during clinic visits. Recruitment messages will inform potential participants of eligibility requirements, the voluntary nature of participation, data to be collected including clinical records data, confidentiality of data and incentives.
Data collection
Data will be collected through a combination of surveys, focus groups or individual interviews, and electronic medical records, as previously described.
Data management and confidentiality
Since this study has minimal risks for participants, does not assign participants to study arms, does not perform an intervention, and is not a clinical trial, all data and safety monitoring will be conducted by the Project Director. Since this research does not qualify as a clinical trial, a Data and Safety Monitoring Plan is not required.
All study survey data will be collected electronically via REDCap using individual, confidential links and stored on Pitt servers. Participant identifiers will only be collected for purposes of linking survey data to medical records for subsequent analysis. This information, as well as consent forms, will be stored separately from the study materials. Electronic medical record data from each study site will be securely transferred to Pitt for analysis using Sharefile, a secure file sharing transfer service. The Pitt data team will immediately delete participant identifiers once assigning a study ID to each participant linking survey and clinical data. This clinical data, in addition to deidentified survey data abstracted from REDCap, will be stored on OneDrive.
For qualitative methods, identifiable data will be gathered to schedule interviews or focus groups, but these will not be linked to data for analysis. Because interviews and focus groups could potentially include identifiable data, these will be recorded on an audio recorder with 256-bit file encryption and device PIN locking to ensure data security. Once interviews are complete, any identifying information will be deleted from these files, and the audio tapes will be transferred to a Pitt desktop and subsequently submitted to a professional transcription service. No identifiable data will be transcribed, and once analysis is complete, the audio recording will be deleted.
Ethics and dissemination
Per NIH guidelines for multisite research, the study uses a single IRB, wherein the University of Pittsburgh serves as the IRB of record for UAB, BAO and PHC. The University of Pittsburgh Human Research Protection Office approved this study via expedited review on 1 November 2021.
Consent
For patient surveys associated with aim 2 (n=500), informed consent will be obtained electronically in REDCap. Consent will include the voluntary nature of participation, data to be collected including access to clinical records data, confidentiality of data and information about incentives. We have received a waiver to document consent for provider surveys (n=125) and interviews (n=40) associated with aim 1, and for patient focus groups associated with aim 2 (n=36). Provider survey consent will be obtained via a ‘click to consent’ function in REDCap, and, for patient and provider qualitative methods, verbal consent will be obtained by the research team immediately before data collection. Participants will be informed of the study aims and approach, voluntary nature of participation, right to exit the study with no penalty or risk of penalty, confidentiality of data and incentives. No human subjects’ data will be collected as part of aim 3, so consent for these methods will not be obtained. However, given the sensitive inclusion criteria for patients, expectations for confidentiality related to participation will occur at the start of each patient focus group or stakeholders meeting.
Dissemination plan
Study findings will be presented in peer-reviewed journals and public health conferences. Findings will also be shared with patient participants online or in in-person community forums held at study sites and with providers during regularly scheduled staff meetings. We will also share findings with the members of BAO’s and PHC’s community advisory boards, which is composed of researchers, community organisation representatives and PWH as well as a local HR organisation that provides services to people who use drugs.
Patient and public involvement
Aim 3 of this study will be devoted to designing a HR intervention via community collaborator meetings with PWH who use drugs, HIV providers and HR experts using human-centred design. Members of our community advisory boards will inform and direct dissemination of results.
Discussion
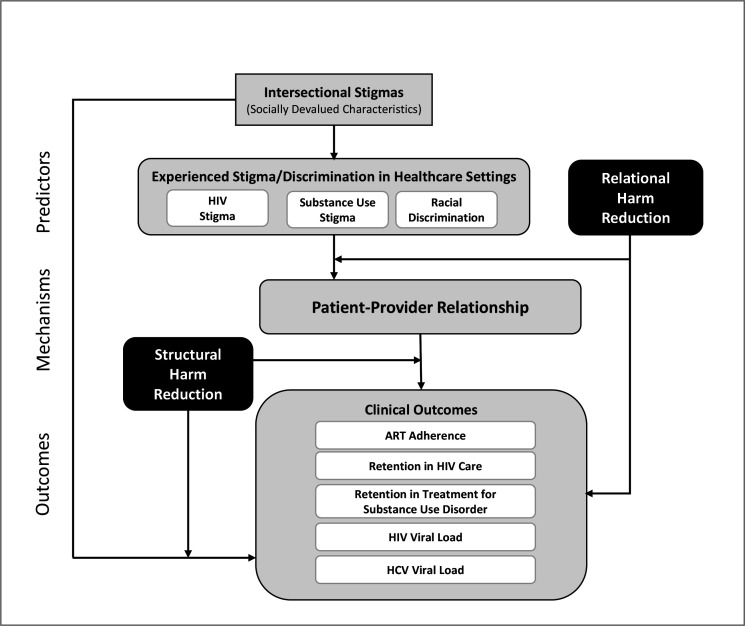
Ultimately this mixed methods observational study, taking place in two culturally distinct regions with similarly high HIV and HCV incidence rates, aims to discover whether HR approaches have the potential to improve HIV, HCV and substance use outcomes for PWH who use drugs. Given persistent racial health disparities, exploring racial discrimination experienced in healthcare settings is also critical. Our work builds on the Conceptual Framework for HIV-Related Stigma, Engagement in Care and Health Outcomes,55 which posits that multiple dimensions of stigma create different pathways to and effects on clinical outcomes for PWH. We are innovatively adapting this model (figure 1) to focus specifically on experienced HIV stigma in healthcare settings, to incorporate substance use stigma and racial discrimination in an exploration of intersectional stigma and to include our premise that the provision of HR can reduce and mitigate patients’ experiences of stigma in healthcare settings. We hypothesise that the effect of intersectional stigma on the patient–provider relationship is reduced in the presence of higher degrees of relational HR care, structural HR attenuates the effect of poor patient–provider relationships on clinical outcomes and higher degrees of HR care are associated with better clinical outcomes. Understanding the contributions of both structural and relational HR can help us determine which practices must be in place to improve patient outcomes.
Figure 1.
Modified conceptual framework. ART, antiretroviral therapy; HCV, hepatitis C virus.
A primary strength of our study is that we will collect data from a range of participants, including both patients and providers, and we will integrate both qualitative and quantitative methods to elicit rich data. Study results have the potential to contribute to changing standards of care for providers who work with PWH who use drugs and improve care for this population; therefore, it is paramount that both sets of stakeholders’ voices are included in all phases of the study. While many studies explore the effects of patient–provider relationships on clinical outcomes, our study is novel in that it includes the full range of treatment team members (e.g., receptionists, social workers, nurses, pharmacists) in our methods, rather than focusing on physicians alone. However, these strengths also add complexity to the protocol, as there are multiple stages of recruitment, data collection and analysis across two states and three HIV clinics.
Another potential challenge of this study, as with all research conducted during this time, is the ongoing challenges posed by the COVID-19 pandemic. For this reason, we have planned study activities, so that all phases of data collection may occur online as needed. Both principal investigators have experience conducting virtual interviews and focus groups, should this be necessary. Indeed, improving care for PWH who use drugs becomes even more critical as people with multiple vulnerabilities have increased risk for COVID-19, and rising rates of unemployment and poverty drive people further into survival economies, increasing risk for HIV and HCV.
Supplementary Material
Footnotes
Twitter: @emmakayphd
Contributors: MH and ESK developed the study and study protocol in collaboration with DB and JMT of the University of Alabama at Birmingham; RC, SC, JEE, MRF, SK and LY of the University of Pittsburgh; SF and VN of the Allegheny Health Network Center for Inclusion Health; and SK and BT, consultants to the study. The manuscript was written by ESK and MH with input and review from all authors. All authors read and approved the final manuscript.
Funding: Funding for this study was provided by the US National Institutes of Health, National Institute on Drug Abuse (1R01DA054832-01). The funder had no role in the design of the study, data collection, data analyses, interpretation of data, or preparation of this manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Centers for Disease Control and Prevention . Hiv and people who inject drugs, 2021. Available: https://www.cdc.gov/hiv/group/hiv-idu.html
- 2.SA-OX B, Wainberg ML, Newton-John TRO. Predictors of adult retention in HIV care: a systematic review. Electronic:1573–3254. 10.1007/s10461-016-1644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartzler B, Dombrowski JC, Williams JR, et al. Influence of substance use disorders on 2-year HIV care retention in the United States. AIDS Behav 2018;22:742–51. 10.1007/s10461-017-1826-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giordano TP, Hartman C, Gifford AL, et al. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials 2009;10:299–305. 10.1310/hct1005-299 [DOI] [PubMed] [Google Scholar]
- 5.Marks G, Patel U, Stirratt MJ, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications. J Acquir Immune Defic Syndr 2016;73:205–12. 10.1097/QAI.0000000000001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althoff KN, Rebeiro P, Brooks JT, et al. Disparities in the quality of HIV care when using us department of health and human services indicators. Clin Infect Dis 2014;58:1185–9. 10.1093/cid/ciu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano TP, Gifford AL, White AC, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis 2007;44:1493–9. 10.1086/516778 [DOI] [PubMed] [Google Scholar]
- 8.Horberg MA, Hurley LB, Silverberg MJ, et al. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS 2013;27:442–9. 10.1089/apc.2013.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park WB, Choe PG, Kim S-H, et al. One-Year adherence to clinic visits after highly active antiretroviral therapy: a predictor of clinical progress in HIV patients. J Intern Med 2007;261:268–75. 10.1111/j.1365-2796.2006.01762.x [DOI] [PubMed] [Google Scholar]
- 10.Mugavero MJ, Lin H-Y, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis 2009;48:248–56. 10.1086/595705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Office of AIDS Research . Hiv and opportunistic infections, coinfections, and conditions: HIV and hepatitis C: National Institutes of health, 2021. Available: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-and-hepatitis-c
- 12.Carvalho ARM, Pinto CMA, Baleanu D. HIV/HCV coinfection model: a fractional-order perspective for the effect of the HIV viral load. Adv Differ Equ 2018;2018:2. 10.1186/s13662-017-1456-z [DOI] [Google Scholar]
- 13.Salmon-Ceron D, Arends JE, Leoni C, et al. HIV/HCV coinfection: current challenges. In: Ozaras R, Salmon-Ceron D, eds. Viral hepatitis: chronic hepatitis. Springer International Publishing, 2019: 141–57. [Google Scholar]
- 14.Glei DA, Weinstein M, Drug WM. Drug and alcohol abuse: the role of economic insecurity. Am J Health Behav 2019;43:838–53. 10.5993/AJHB.43.4.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaysen D, Dillworth TM, Simpson T, et al. Domestic violence and alcohol use: trauma-related symptoms and motives for drinking. Addict Behav 2007;32:1272–83. 10.1016/j.addbeh.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degenhardt L, Bharat C, Glantz MD, et al. The epidemiology of drug use disorders cross-nationally: findings from the who's world mental health surveys. Int J Drug Policy 2019;71:103–12. 10.1016/j.drugpo.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Boekel LC, Brouwers EPM, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131:23–35. 10.1016/j.drugalcdep.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 18.Gilchrist G, Moskalewicz J, Slezakova S, et al. Staff regard towards working with substance users: a European multi-centre study. Addiction 2011;106:1114–25. 10.1111/j.1360-0443.2011.03407.x [DOI] [PubMed] [Google Scholar]
- 19.Brener L, Von Hippel W, Kippax S, et al. The role of physician and nurse attitudes in the health care of injecting drug users. Subst Use Misuse 2010;45:1007–18. 10.3109/10826081003659543 [DOI] [PubMed] [Google Scholar]
- 20.Kay ES, Rice WS, Crockett KB, et al. Experienced HIV-related stigma in health care and community settings: mediated associations with psychosocial and health outcomes. J Acquir Immune Defic Syndr 2018;77:257–63. 10.1097/QAI.0000000000001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pager D, Shepherd H. The sociology of discrimination: racial discrimination in employment, housing, credit, and consumer markets. Annu Rev Sociol 2008;34:181–209. 10.1146/annurev.soc.33.040406.131740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Algarin AB, Sheehan DM, Varas-Diaz N, et al. Health Care-Specific enacted HIV-related stigma's association with antiretroviral therapy adherence and viral suppression among people living with HIV in Florida. AIDS Patient Care STDS 2020;34:316–26. 10.1089/apc.2020.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muncan B, Walters SM, Ezell J, et al. "They look at us like junkies": influences of drug use stigma on the healthcare engagement of people who inject drugs in New York City. Harm Reduct J 2020;17:53. 10.1186/s12954-020-00399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Des Jarlais DC. Harm reduction--a framework for incorporating science into drug policy. Am J Public Health 1995;85:10–12. 10.2105/AJPH.85.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marlatt GA. Harm reduction: come as you are. Addict Behav 1996;21:779–88. 10.1016/0306-4603(96)00042-1 [DOI] [PubMed] [Google Scholar]
- 26.Marlatt GA, Larimer ME, Witkiewitz KA. Harm reduction. Second Edition. New York: Pragmatic Strategies for Managing High-Risk BehaviorsGuilford Publications, 2011. [Google Scholar]
- 27.Hawk M, Coulter RWS, Egan JE, et al. Harm reduction principles for healthcare settings. Harm Reduct J 2017;14:70. 10.1186/s12954-017-0196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeil R, Kerr T, Pauly B, et al. Advancing patient-centered care for structurally vulnerable drug-using populations: a qualitative study of the perspectives of people who use drugs regarding the potential integration of harm reduction interventions into hospitals. Addiction 2016;111:685–94. 10.1111/add.13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uyei J, Fiellin DA, Buchelli M, et al. Effects of naloxone distribution alone or in combination with addiction treatment with or without pre-exposure prophylaxis for HIV prevention in people who inject drugs: a cost-effectiveness modelling study. Lancet Public Health 2017;2:e133–40. 10.1016/S2468-2667(17)30006-3 [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Wood E, Wiebe L, et al. An external evaluation of a peer-run outreach-based syringe exchange in Vancouver, Canada. Int J Drug Policy 2010;21:418–21. 10.1016/j.drugpo.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krawczyk N, Buresh M, Gordon MS, et al. Expanding low-threshold buprenorphine to justice-involved individuals through mobile treatment: addressing a critical care gap. J Subst Abuse Treat 2019;103:1–8. 10.1016/j.jsat.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treloar C, Rance J, Yates K, et al. Trust and people who inject drugs: the perspectives of clients and staff of needle syringe programs. Int J Drug Policy 2016;27:138–45. 10.1016/j.drugpo.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 33.Sawangjit R, Khan TM, Chaiyakunapruk N. Effectiveness of pharmacy-based needle/syringe exchange programme for people who inject drugs: a systematic review and meta-analysis. Addiction 2017;112:236–47. 10.1111/add.13593 [DOI] [PubMed] [Google Scholar]
- 34.Hawk M, Friedman M, Coulter R. There’s More to HIV than a Pill”: Operationalizing and Measuring a Harm Reduction Approach to HIV Clinical Care. Poster Presentation at the 22nd International AIDS Conference. Amsterdam, Netherlands, 2018. [Google Scholar]
- 35.Hawk M, Coulter RWS, Egan JE, et al. Exploring the healthcare environment and associations with clinical outcomes of people living with HIV/AIDS. AIDS Patient Care STDS 2017;31:495–503. 10.1089/apc.2017.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haldane V, Cervero-Liceras F, Chuah FL, et al. Integrating HIV and substance use services: a systematic review. J Int AIDS Soc 2017;20:21585. 10.7448/IAS.20.1.21585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maguire M. Methods to support human-centred design. Int J Hum Comput Stud 2001;55:587–634. 10.1006/ijhc.2001.0503 [DOI] [Google Scholar]
- 38.Creswell JW, Plano Clark VL, Vicki L. Designing and conducting mixed methods research. In: Creswell JW, Clark P, Vicki L, eds. SAGE Publications, 2011. [Google Scholar]
- 39.Centers for Disease Control and Prevention . Understanding the HIV care continuum, 2018. Available: https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum.pdf
- 40.Institute of Medicine . Monitoring HIV care in the United States: indicators and data systems, 2012 [PubMed]
- 41.Pence BW, Bengtson AM, Boswell S. Who will show? predicting missed visits among patients in routine HIV primary care in the United States. AIDS Behav 2018:1–9. 10.1007/s10461-018-2215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannheimer SB, Mukherjee R, Hirschhorn LR, et al. The case adherence index: a novel method for measuring adherence to antiretroviral therapy. AIDS Care 2006;18:853–61. 10.1080/09540120500465160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf EJ, Harrington KM, Clark SL, et al. Sample size requirements for structural equation models: an evaluation of power, bias, and solution Propriety. Educ Psychol Meas 2013;76:913–34. 10.1177/0013164413495237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bentler PM, Chou C-P. Practical issues in structural modeling. Sociol Methods Res 1987;16:78–117. 10.1177/0049124187016001004 [DOI] [Google Scholar]
- 45.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci 2007;18:233–9. 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mundfrom DJ, Shaw DG, Ke TL. Minimum sample size recommendations for conducting factor analyses. Int J Test 2005;5:159–68. 10.1207/s15327574ijt0502_4 [DOI] [Google Scholar]
- 47.Reise SP, Yu J. Parameter recovery in the graded response model using MULTILOG. J Educ Meas 1990;27:133–44. 10.1111/j.1745-3984.1990.tb00738.x [DOI] [Google Scholar]
- 48.NVivo qualitative data analysis software . QSR international Pty LTD, version 12. QSR international, 2018
- 49.Braun V, Clarke V. Thematic analysis; 2012.
- 50.Clarke V, Braun V, Hayfield N. Thematic analysis. Qualitative psychology: A practical guide to research methods, 2015: 222–48. [Google Scholar]
- 51.O’Connor C, Joffe H. Intercoder reliability in qualitative research: debates and practical guidelines. International Journal of Qualitative Methods 2020;19. 10.1177/1609406919899220 [DOI] [Google Scholar]
- 52.Creswell JW, Clark VLP. Designing and conducting mixed methods research; 2007.
- 53.Clark VLP, Creswell JW. The mixed methods reader. Sage, 2008. [Google Scholar]
- 54.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turan B, Hatcher AM, Weiser SD, et al. Framing mechanisms linking HIV-related stigma, adherence to treatment, and health outcomes. Am J Public Health 2017;107:863–9. 10.2105/AJPH.2017.303744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watson H, Maclaren W, Kerr S. Staff attitudes towards working with drug users: development of the drug problems perceptions questionnaire. Addiction 2007;102:206–15. 10.1111/j.1360-0443.2006.01686.x [DOI] [PubMed] [Google Scholar]
- 57.Wagner AC, Hart TA, McShane KE, et al. Health care provider attitudes and beliefs about people living with HIV: initial validation of the health care provider HIV/AIDS stigma scale (HPASS). AIDS Behav 2014;18:2397–408. 10.1007/s10461-014-0834-8 [DOI] [PubMed] [Google Scholar]
- 58.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev 2000;57 Suppl 1:146–61. 10.1177/1077558700057001S07 [DOI] [PubMed] [Google Scholar]
- 59.Goddard P, Mallott MA, Grindle ME, eds. Reliability and validity of the Harm Reduction Acceptability Scale, 2003. [Google Scholar]
- 60.Goddard P. Changing attitudes towards harm reduction among treatment professionals: a report from the American Midwest. International Journal of Drug Policy 2003;14:257–60. 10.1016/S0955-3959(03)00075-6 [DOI] [Google Scholar]
- 61.Nyblade L, Jain A, Benkirane M, et al. A brief, standardized tool for measuring HIV-related stigma among health facility staff: results of field testing in China, Dominica, Egypt, Kenya, Puerto Rico and St. Christopher & Nevis. J Int AIDS Soc 2013;16:18718. 10.7448/IAS.16.3.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith LR, Earnshaw VA, Copenhaver MM, et al. Substance use stigma: reliability and validity of a theory-based scale for substance-using populations. Drug Alcohol Depend 2016;162:34–43. 10.1016/j.drugalcdep.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart AL, Nápoles-Springer AM, Gregorich SE, et al. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health Serv Res 2007;42:1235–56. 10.1111/j.1475-6773.2006.00637.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bodenlos JS, Grothe KB, Kendra K, et al. Attitudes toward HIV health care providers scale: development and validation. AIDS Patient Care STDS 2004;18:714–20. 10.1089/apc.2004.18.714 [DOI] [PubMed] [Google Scholar]
- 65.Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med 2006;21:661–5. 10.1111/j.1525-1497.2006.00399.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krier S, Bozich C, Pompa R. Pennsylvania HIV planning group intersectional stigma in healthcare settings survey 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067219supp001.pdf (185.1KB, pdf)
bmjopen-2022-067219supp002.pdf (112.4KB, pdf)
bmjopen-2022-067219supp003.pdf (85.6KB, pdf)
bmjopen-2022-067219supp004.pdf (120.3KB, pdf)