Abstract
Objectives
To analyse whether reported fatigue, one of the most challenging manifestations of systemic lupus erythematosus (SLE), may bias the assessment of disease activity in SLE according to the Physician Global Assessment (PGA).
Methods
Patients from the Lupus BioBank of the upper Rhein database, a cross-sectional multicentre collection of detailed clinical and biological data from patients with SLE, were included. Patients had to fulfil the 1997 American College of Rheumatology criteria for SLE and the PGA (0–3 scale) at the time of inclusion had to be available. Fatigue was assessed according to the Fatigue Scale for Motor and Cognitive Functions. Univariate and multivariate regression models were built to determine which variables were associated with the PGA.
Results
A total of 350 patients (89% female; median age: 42 years, IQR: 34–52) were included. The median Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) score was 4 (IQR: 2–6). Of these 350 patients, 257 (73%) reported significant fatigue. The PGA (p=0.004) but not the SELENA-SLEDAI (p=0.43) was significantly associated with fatigue. Both fatigue and SELENA-SLEDAI were independently associated with the PGA in two different multivariate models.
Conclusion
Fatigue is independently associated with disease activity assessed using the PGA but not the SLEDAI. These findings highlight the fact that the PGA should capture only objectively active disease manifestations in order to improve its reliability.
Keywords: Systemic Lupus Erythematosus, Disease Activity, Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Fatigue is reported by two-thirds of patients with systemic lupus erythematosus (SLE) and remains one of the most challenging manifestations.
WHAT THIS STUDY ADDS
Fatigue is independently associated with disease activity assessed using the Physician Global Assessment (PGA) but not the Systemic Lupus Erythematosus Disease Activity Index.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In SLE, the PGA should not incorporate subjective manifestations such as fatigue.
Introduction
Systemic lupus erythematosus (SLE) is a complex chronic systemic disease characterised by proteiform clinical manifestations. Because of a course subject to unpredictable flares, disease activity assessment is particularly challenging in SLE, even for experienced physicians. Those flares can lead to severe organ damage. Distinguishing between disease activity and damage is crucial in SLE. Immunosuppressive treatment escalation is indicated in case of active disease while this would be ineffective (if not harmful) in case of damage. Moreover, persistent disease activity can be considered an important predictor of both organ damage and mortality in SLE1; therefore, the attainment of remission or at least of a low disease activity state is currently the main therapeutic objective in active SLE.2 3 Importantly, the current definitions of remission and low disease activity in SLE incorporate the Physician Global Assessment (PGA), which is the only score explicitly recommended in the last EULAR/American College of Rheumatology (ACR) recommendations for routine monitoring of SLE.4 In a recent systematic review by our group,5 the PGA was found to be a valid, responsive and feasible instrument. However, the PGA has high variability due to a lack of standardisation in its rating.6 Our group is currently driving the PGA International Standardisation Consensus in SLE study, which aims to standardise the PGA rating in SLE. Meanwhile, it remains currently unknown whether purely subjective (the so-called ‘type 2’7) manifestations such as fatigue should be incorporated in the PGA. Fatigue remains one of the most challenging manifestations reported by patients with SLE.8–10 Fatigue is highly multifactorial and can be related to disease activity, organ damage, psychobehavioural elements or totally independent of the disease itself.11–13 Because of this, it is crucial to determine whether fatigue could be a significant confounder of PGA ratings. The aim of the present study was to analyse whether fatigue may influence the assessment of the PGA by an international panel of experienced clinicians. Understanding the potential contribution of fatigue to PGA ratings will prove crucial to improve the standardisation of the PGA.
Methods
Study population
The Lupus BioBank of the upper Rhein (LBBR) database is a cross-sectional collection of detailed clinical and biological data from patients suffering from SLE, linked to a biobank. The LBBR was funded by the European Union through an INTERREG IV programme that involves a German and French network of 15 clinical departments localised in the Upper Rhein Valley, under the leadership of two centres (Department of Rheumatology and Clinical Immunology, Freiburg, Germany; and Clinical Immunology Department, Strasbourg University Hospital, Strasbourg, France). A total of 1073 patients with SLE have been included in the LBBR in January 2018. At the time of inclusion, phenotype and disease activity assessments (according to the Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) score) were performed by senior physicians specialised in the care of patients with SLE. The PGA was evaluated on a visual analogue scale of 3 cm length anchored by 0 and 3 with inner markers of 1 and 2.
To be included in the current study, patients from the LBBR database had to fulfil ≥4 of the 1997 ACR criteria for SLE and the PGA at the time of inclusion had to be available. Patients gave informed consent before inclusion. The LBBR database was approved by the national data protection commission, Commission Nationale Informatique et Liberté.
Definitions for fatigue, anxiety and depression
All definitions have been used previously in the FATILUP studies by our research group.8 14 Fatigue was assessed according to the Fatigue Scale for Motor and Cognitive Functions (FSMC), a self-administered questionnaire composed of 20 items which evaluate motor and cognitive fatigue separately and provides an aggregated score for both. Cut-off values for mild, moderate and severe cognitive and motor fatigue were defined according to the definitions validated by Penner et al.15 In accordance with those definitions, we defined significant fatigue as patients with a global FSMC score ≥42 (which corresponds to mild, moderate or severe fatigue) and severe fatigue as those with a score ≥63. In the LBBR database, fatigue ratings were collected as categorical data according to these validated thresholds and not as continuous numerical values. Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS), a self-rating questionnaire that contains two subscales measuring symptoms of depression and anxiety during the previous week. The HADS includes seven statements on each disorder, and each response is rated on a 4-point rating scale (0–3), with a higher score depicting a worse condition (for each subscale the total score is at most 21). A score of ≥11 is considered a clinically significant disorder for each subscale. In the LBBR cohort, anxiety and depression ratings were also collected as categorical data according to the validated thresholds and not as continuous numerical values.
Statistical analysis
Data are presented as median (25th–75th percentile IQR) or counts and percentages. Comparisons between independent groups were made using the Mann-Whitney test for continuous outcomes and the χ2 test (or Fisher’s exact test when appropriate) for quantitative data. Univariate and multivariate linear regression models were built to determine which variables were associated independently with the PGA, with the latter considered the dependent variable. The variables considered for the univariate linear regression model were extracted from the LBBR database and reflected data at the moment of patient inclusion: age, sex, anxiety, depression, PGA, SELENA-SLEDAI, hydroxychloroquine, any glucocorticoid treatment, glucocorticoid treatment >10 mg/day and any immunosuppressive treatment. All variables with a p value <0.10 in univariate analyses were entered in the multivariate models, and multivariate p values, standardised estimates and their CIs were computed after adjustment for the centre effect. All tests were bilateral and p values <0.05 were considered statistically significant. Statistical analyses were performed with the software JMP V.13 (SAS Institute, Cary, North Carolina, USA).
Results
Patient characteristics
A total of 350 patients (312 female, 89%) were included, with a median age at inclusion of 42 years (IQR: 34–52). The median SELENA-SLEDAI score at inclusion was 4 (IQR: 2–6). Of the patients, 272 (80%) were treated with hydroxychloroquine, 225 (64%) with glucocorticoids and 151 (43%) with an immunosuppressive agent or a biological therapy. The detailed characteristics and treatments of the 350 patients are shown in table 1.
Table 1.
Baseline characteristics of the 350 patients included in the PGA analysis
| Characteristics | Value |
| Age, years, median (IQR 25–75) | 42 (34–52), n=350 |
| Gender (female) | 312 (89), n=350 |
| Fatigue | 257 (73), n=350 |
| Severe fatigue | 144 (41), n=350 |
| Anxiety | 106 (33.5), n=316 |
| Depression | 36 (12), n=304 |
| SELENA-SLEDAI, median (IQR 25–75) | 4 (2–6), n=350 |
| Hydroxychloroquine | 272 (80), n=340 |
| Any GC | 225 (64), n=350 |
| GC >10 mg/day | 65 (19), n=339 |
| Any IS | 151 (43), n=350 |
All results are presented as n (%) unless stated otherwise.
‘n=’ means number of patients with available data.
GC, glucocorticoids; IQR 25–75, 25th–75th percentile IQR; IS, immunosuppressive agent; PGA, Physician Global Assessment; SELENA, Safety of Estrogens in Lupus Erythematosus National Assessment; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
Prevalence of fatigue, severe fatigue and analysis of fatigue subtypes
Of the 350 patients, 257 (73%) reported significant fatigue (table 2), including 219 (63%) with cognitive fatigue, 246 (70%) with motor fatigue and 144 (41%) with severe fatigue. Among the 257 patients with fatigue (table 2), the prevalence of anxiety (97 patients, 42%) and depression (34 patients, 15%) was significantly increased compared with those without fatigue (p<0.0001 and p=0.002, respectively). There was no significant difference regarding the median SELENA-SLEDAI score or treatments between those with and without fatigue (table 2). However, the PGA was higher (p=0.004) in patients with fatigue (median: 0.5, IQR: 0.2–1) versus those without fatigue (median: 0.3, IQR: 0.2–0.95), and this remained significant after adjustment for disease activity according to the SELENA-SLEDAI score (p=0.01). To improve our understanding of the relationship between the PGA and fatigue in patients with higher disease activity, we performed a subgroup analysis of patients with SELENA-SLEDAI ≥6 and confirmed the significant association between the PGA and fatigue in the univariate analysis (p=0.04) as well as in the multivariable analysis after adjustment for SELENA-SLEDAI (p=0.0489). Figure 1 shows the distribution of fatigue according to different PGA intervals.
Table 2.
Comparison of characteristics of patients with SLE with or without fatigue
| Characteristics | Patients with fatigue (n=257) |
Patients without fatigue (n=93) |
P value |
| Age, years, median (IQR 25–75) | 43 (35–54), n=257 | 41 (31–47), n=93 | 0.03 |
| Sex (female) | 230 (89), n=257 | 82 (88), n=93 | 0.70 |
| Anxiety | 97 (42), n=232 | 9 (11), n=84 | <0.0001 |
| Depression | 34 (15), n=224 | 2 (2), n=80 | 0.002 |
| PGA, median (IQR 25–75) | 0.5 (0.2–1), n=257 | 0.3 (0.2–0.95), n=93 | 0.004 |
| SELENA-SLEDAI, median (IQR 25–75) | 4 (1.5–6), n=257 | 2 (2–5), n=93 | 0.43 |
| Hydroxychloroquine | 198 (79), n=249 | 74 (81), n=91 | 0.76 |
| Any GC | 173 (67), n=257 | 52 (56), n=93 | 0.06 |
| GC >10 mg/day | 50 (20), n=250 | 15 (17), n=89 | 0.64 |
| Any IS | 114 (44), n=257 | 37 (40), n=93 | 0.46 |
P values in bold indicate significance.
All results are presented as n (%) unless stated otherwise.
‘n=’ means number of patients with available data.
IS agents include azathioprine or methotrexate or mycophenolate or cyclophosphamide or rituximab or belimumab.
GC, glucocorticoids; IQR 25–75, 25th–75th percentile IQR; IS, immunosuppressive agent; PGA, Physician Global Assessment; SELENA, Safety of Estrogens in Lupus Erythematosus National Assessment; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
Figure 1.
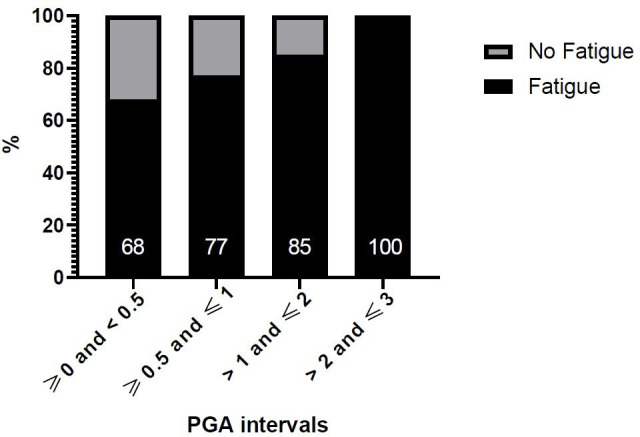
Prevalence of fatigue in patients according to Physician Global Assessment (PGA) intervals.
Variables associated with the PGA
In the univariate analysis (table 3), the variables associated with the PGA were age (p=0.03), fatigue (p=0.004), severe fatigue (p=0.005), motor fatigue (p=0.01), disease activity according to the SELENA-SLEDAI (p<0.0001), and use of glucocorticoids (p=0.0003) or immunosuppressive treatments (p=0.004). Neither anxiety (p=0.83) nor depression (p=0.13) was associated with the PGA.
Table 3.
Univariate and multivariate analyses of variables associated with the PGA
| Parameters | Univariate | Multivariate | |||
| P value | First model (any fatigue) | Second model (severe fatigue) | |||
| P value | Standardised estimate (CI) | P value | Standardised estimate (CI) | ||
| Age | 0.03 | 0.29 | −0.075 (−0.21 to 0.06) | 0.37 | −0.06 (−0.20 to 0.07) |
| Female | 1 | – | – | – | – |
| Any fatigue | 0.004 | 0.01 | 0.08 (0.02 to 0.14) | – | – |
| Severe fatigue | 0.005 | 0.03 | 0.06 (0.005 to 0.12) | ||
| Anxiety | 0.8 | – | – | – | – |
| Depression | 0.1 | – | – | – | – |
| SELENA-SLEDAI | <0.0001 | <0.0001 | 0.65 (0.49 to 0.82) | <0.0001 | 0.65 (0.49 to 0.82) |
| Hydroxychloroquine | 0.6 | – | – | – | – |
| Any GC | 0.0003 | 0.1 | −0.05 (−0.01 to 0.11) | 0.08 | 0.05 (−0.005 to 0.11) |
| Any IS | 0.004 | 0.2 | −0.04 (−0.02 to 0.09) | 0.17 | −0.04 (−0.02 to 0.09) |
Multivariate analysis adjusted for the evaluating centre. ‘–’: variables not entered in the multivariate model.
P values in bold indicate significance.
IS agents include azathioprine or methotrexate or mycophenolate or cyclophosphamide or rituximab or belimumab.
GC, glucocorticoids; IS, immunosuppressive agent; PGA, Physician Global Assessment; SELENA, Safety of Estrogens in Lupus Erythematosus National Assessment; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
Two multivariate models (table 3) were built to study the relationship between the PGA and fatigue. In the first model, the variables independently associated with the PGA were fatigue (p=0.01) and disease activity according to SELENA-SLEDAI (p<0.0001). In the second model, severe fatigue (p=0.03) and SELENA-SLEDAI (p<0.0001) remained independently associated with the PGA.
Discussion
Here, we analysed data from 350 patients included in the LBBR biobank and found that fatigue was independently associated with the PGA in two different multivariate models, regardless of disease activity assessed according to SELENA-SLEDAI. Therefore, fatigue may be a confounder of PGA ratings and has been shown to artificially increase the PGA, even by experienced clinicians. For instance, in the case of a patient with no disease activity but very high fatigue, which would artificially result in a PGA >0.5, the inappropriate inclusion of fatigue in the rating of the PGA would have made us considered that this patient was not in Definition Of Remission In Systemic lupus (DORIS) remission16 while he actually was. The detailed way to standardise the rating of the PGA in SLE has been recently described by our group in an international consensus paper.17
Fatigue is reported by two-thirds of patients with SLE and currently remains one of the most challenging manifestations.2 3 14 18 Fatigue can be related to general causes unrelated to SLE (eg, lifestyle, psychobehavioural causes including depression,8 14 medications, sleep or metabolic disorders), but also associated with disease activity or organ damage. In their recent categorisation of disease manifestations, Pisetsky et al7 distinguish between signs and symptoms undoubtedly related to disease activity, such as arthritis, vasculitis, nephritis (type 1 group) and those related to damage or to non-specific consequences of the chronic disease (type 2). In the second group are included symptoms such as fatigue, depression and anxiety, which are manifestations typically refractory to immunosuppressive therapy. Further supporting the lack of relationship between disease activity and type 2 symptoms, we recently described a cohort of 502 patients with lupus8 in which fatigue was common (68%) in patients with SLE without disease activity. Our current findings highlight the importance of differentiating type 1 from type 2 manifestations. In SLE, fatigue is associated independently with the PGA.
Compared with a glossary-based index, the main strength of the PGA is its ability to capture disease activity globally. Despite this, the PGA should not incorporate fatigue when unrelated to disease activity. This is even more crucial owing to the fact that the current definitions of remission and of low disease activity in SLE incorporate the PGA.9
The limitations of the study are largely inherent to its design. Fatigue ratings were collected as categorical data according to the validated thresholds and not as continuous numerical values and could therefore impact statistical modulation, but univariate and multivariate linear regression models were adapted appropriately. However, this limitation was presently compensated by the large number of cases available in the LBBR database and the collection of data by senior physicians specialised in the care of SLE. Fatigue remains a subjective feature and its quantification remains a matter of debate as different scales are available. None of the available routine fatigue evaluation scores was developed or validated in SLE. In the current study, fatigue was measured according to the FSMC, which evaluates motor and cognitive fatigue separately and provides an aggregated score for both. Moreover, we used the same definitions as previously used in the FATILUP studies from our research group. Also, the PGA was evaluated only by senior physicians specialised in the care of patients with SLE and using always the same visual analogue scale in a real-life perspective. Finally, these real-life results should be interpreted in the light of treatments, with 64% of patients receiving glucocorticoids and 43% receiving immunosuppressive agents, which might explain the relatively low median SELENA-SLEDAI score of the patients (4, 25th–75th percentile IQR: 2–6).
Altogether, our findings highlight the importance of taking into account only objectively active (type 1) manifestations when assessing the PGA in SLE. Doing this may avoid harmful and inappropriate treatment escalation and improve the standardisation of the PGA when assessing remission or lupus low disease activity state.
Footnotes
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual content and all authors approved the final version to be published. Study conception and design: PM, MP, EC, LA. Acquisition of data: PM, MP, EC, ZA, REV, AS, FM, GB, BB, VP, CF, H-ML, A-SK, JS, TM, LA. Analysis and interpretation of data: PM, MP, EC, ZA, REV, AS, FM, GB, BB, VP, CF, H-ML, A-SK, JS, TM, LA. Author responsible for the overall content as the guarantor: LA.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: LA is a consultant for Alexion, Amgen, AstraZeneca, AbbVie, Biogen, BMS, Boehringer Ingelheim, Cêmka, GSK, Janssen, Kezar, LFB, Lilly, Menarini France, Medac, Novartis, Oséus, Pfizer, Roche-Chugaï, Sêmeia and UCB. MP has received consultancy fees from GSK, AstraZeneca and Pfizer (unrelated to this study). ZA has received research grants from Amgen, AstraZeneca, GSK and Roche, and fees for consultancy from AstraZeneca, GSK and Kezar. AS has received grant research support from GSK, AstraZeneca, Pfizer, AbbVie and Novartis. JS has received consulting fees from Roche, Chugai, Bristol Myers Squibb, UCB, GSK, LFB, Actelion, Pfizer, MSD, Novartis, Amgen, AbbVie, Sandoz, Gilead, Lilly, Sanofi Genzyme, Janssen, Mylan, Galapagos and Sobi. REV has received consultancy fees from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, BMS, GSK, Janssen-Cilag, Hexal, Neutrolis, Novartis and Sanofi, and grant/research support from Amgen, BMS, Novartis and Pfizer (all unrelated to this study). TM is a consultant/advisor for GlaxoSmithKline, Amgen and AbbVie, and has received grant/research support from GlaxoSmithKline and Janssen. PM, EC, GB, BB, VP, A-SK and H-ML have no disclosures.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by the CHU Strasbourg Medical Ethical Committee under number CPP-IV-Est-08/02/2011 and complies with the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
References
- 1.Lopez R, Davidson JE, Beeby MD, et al. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology 2012;51:491–8. 10.1093/rheumatology/ker368 [DOI] [PubMed] [Google Scholar]
- 2.Felten R, Sagez F, Gavand P-E, et al. 10 most important contemporary challenges in the management of SLE. Lupus Sci Med 2019;6:e000303. 10.1136/lupus-2018-000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piga M, Arnaud L. The main challenges in systemic lupus erythematosus: where do we stand? J Clin Med 2021;10. 10.3390/jcm10020243. [Epub ahead of print: 11 01 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanouriakis A, Kostopoulou M, Alunno A. Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;2019:736–45. 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 5.Chessa E, Piga M, Floris A, et al. Use of physician global assessment in systemic lupus erythematosus: a systematic review of its psychometric properties. Rheumatology 2020;59:3622–32. 10.1093/rheumatology/keaa383 [DOI] [PubMed] [Google Scholar]
- 6.Chessa E, Piga M, Arnaud L. Physician global assessment in systemic lupus erythematosus: can we rely on its reliability? Ann Rheum Dis 2022;81:e79. 10.1136/annrheumdis-2020-217632 [DOI] [PubMed] [Google Scholar]
- 7.Pisetsky DS, Clowse MEB, Criscione-Schreiber LG, et al. A novel system to Categorize the symptoms of systemic lupus erythematosus. Arthritis Care Res 2019;71:735–41. 10.1002/acr.23794 [DOI] [PubMed] [Google Scholar]
- 8.Arnaud L, Mertz P, Amoura Z. Patterns of fatigue and association with disease activity and clinical manifestations in systemic lupus erythematosus. Rheumatol Oxf Engl. [DOI] [PubMed] [Google Scholar]
- 9.Mertz P, Schlencker A, Schneider M, et al. Towards a practical management of fatigue in systemic lupus erythematosus. Lupus Sci Med 2020;7:e000441. 10.1136/lupus-2020-000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornet A, Andersen J, Myllys K, et al. Living with systemic lupus erythematosus in 2020: a European patient survey. Lupus Sci Med 2021;8:e000469. 10.1136/lupus-2020-000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi ST, Kang JI, Park I-H, et al. Subscale analysis of quality of life in patients with systemic lupus erythematosus: association with depression, fatigue, disease activity and damage. Clin Exp Rheumatol 2012;30:665–72. [PubMed] [Google Scholar]
- 12.Azizoddin DR, Gandhi N, Weinberg S, et al. Fatigue in systemic lupus: the role of disease activity and its correlates. Lupus 2019;28:163–73. 10.1177/0961203318817826 [DOI] [PubMed] [Google Scholar]
- 13.Burgos PI, Alarcón GS, McGwin G, et al. Disease activity and damage are not associated with increased levels of fatigue in systemic lupus erythematosus patients from a multiethnic cohort: LXVII. Arthritis Rheum 2009;61:1179–86. 10.1002/art.24649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnaud L, Gavand PE, Voll R, et al. Predictors of fatigue and severe fatigue in a large international cohort of patients with systemic lupus erythematosus and a systematic review of the literature. Rheumatology 2019;58:987–96. 10.1093/rheumatology/key398 [DOI] [PubMed] [Google Scholar]
- 15.Penner IK, Raselli C, Stöcklin M, et al. The fatigue scale for motor and cognitive functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler 2009;15:1509–17. 10.1177/1352458509348519 [DOI] [PubMed] [Google Scholar]
- 16.van Vollenhoven R, Voskuyl A, Bertsias G, et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis 2017;76:554–61. 10.1136/annrheumdis-2016-209519 [DOI] [PubMed] [Google Scholar]
- 17.Piga M, Chessa E, Morand EF, et al. Physician global assessment international standardisation consensus in systemic lupus erythematosus: the PISCOS study. Lancet Rheumatol 2022;4:e441–9. 10.1016/S2665-9913(22)00107-2 [DOI] [PubMed] [Google Scholar]
- 18.Fonseca R, Bernardes M, Terroso G, et al. Silent burdens in disease: fatigue and depression in SLE. Autoimmune Dis 2014;2014:1–9. 10.1155/2014/790724 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.


