Abstract
SARS-CoV-2 variants have posed significant challenges to the hopes of using ancestral strain-based vaccines to address the risk of breakthrough infection by variants. We designed and developed a bivalent vaccine based on SARS-CoV-2 Alpha and Beta variants (named SCTV01C). SCTV01C antigens were stable at 25 oC for at least 6 months. In the presence of a squalene-based oil-in-water adjuvant SCT-VA02B, SCTV01C showed significant protection efficacy against antigen-matched Beta variant, with favorable safety profiles in rodents. Notably, SCTV01C exhibited cross-neutralization capacity against Omicron subvariants (BA.1, BA.1.1, BA.2, BA.3, and BA.4/5) in mice, superior to a WT (D614G)-based vaccine, which reinforced our previously published findings that SCTV01C exhibited broad-spectrum neutralizing potencies against over a dozen pre-Omicron variants and the Omicron BA.1 variant. In summary, variant-based multivalent protein vaccine could be a platform approach to address the challenging issues of emerging variants, vaccine hesitancy and the needs of affordable and thermal stable vaccines.
Keywords: SARS-CoV-2 variant, Multivalent protein vaccine, Thermal stability, Cross-neutralization, Omicron subvariants
1. Introduction
As of 8 October 2021, fifteen commercial vaccines based on ancestral strain of SARS-CoV-2, namely first-generation COVID-19 vaccines, have been approved in multiple countries (Huang et al., 2021). However, insufficient vaccine manufacturing capacity, unequal distribution of vaccine supplies (Irwin and Nkengasong, 2021; Wouters et al., 2021; United Nations, 2021), challenges in vaccine storage and transportation at ultra-low temperatures due to the lack of thermal stability (Cao and Gao, 2021; Uddin and Roni, 2021), and vaccine hesitancy caused by factors such as lack of confidence in vaccine efficacy and new vaccine technology, as well as concerns about safety issues (Wouters et al., 2021; Ipsos, 2021; Siu et al., 2022), have limited COVID-19 vaccination in both developed and developing countries.
Traditional protein-based vaccines have been used for decades in protection against viral infection (Hansson et al., 2000; Nascimento and Leite, 2012; Pollet et al., 2021). Squalene-based oil-in-water adjuvants, such as MF59 and AS03, have shown over 20 years of outstanding safety profiles in both adults and children (Tsai, 2013; Cruz-Valdez et al., 2018; Kavian et al., 2020; Yoo et al., 2018). The combination of a classical protein-based vaccine with a squalene-based adjuvant could potentially generate strong immunogenicity with favorable safety profiles, which could offer an additional solution to overcome vaccine hesitancy.
On the other hand, even in countries reaching over 80% vaccination rate, no herd immunity has been confirmed to date. The continuous emergence of new variants makes the task even more challenging to reach. Meta-analysis demonstrated that, sera from ancestral strain-infected or previously vaccinated people showed reduced neutralization titers to the Alpha, Beta, Gamma, and Delta variants (1.6-, 8.8-, 3.5-, and 3.9-fold reduction, respectively), when compared with those to the ancestral virus (Leier et al., 2021). The newly emerged Omicron variant (B.1.1.529), made up of several subvariants (BA.1, BA.1.1, BA.2, BA.3, and the most recently emerged BA.4 and BA.5) (GISAID, 2022; Bai et al., 2021; Desingu et al., 2022; Cao et al., 2022), sparked another wave of global pandemic due to markedly diminished efficacy of first-generation vaccines (Dejnirattisai et al., 2022; Lu et al., 2021; Ikemura et al., 2021).The long-term waning of immunity evoked by first-generation vaccines, as well as reduced recognition of SARS-CoV-2 variant strains by antibodies induced by vaccines based on ancestral strain, both played roles in decreased neutralizing activities and protection efficacy against emerging variants.
Proof-of-concept studies indicated that immunogens based on heterologous domains from different Sarbecoviruses (Martinez et al., 2021) or conserved T cell epitopes (Nathan et al., 2021) can benefit the design of universal Sarbecovirus vaccines with the capacity to elicit broad B cell or T cell responses against Sarbecoviruses. Despite some recent progresses, the development of a universal SARS-CoV-2 vaccine might meet with various challenges and could be time-consuming to gain approval. Alternatively, multivalent vaccines could be a quick solution to compensate the limited cross-neutralizing activities elicited by monovalent vaccines (Wang et al., 2022), since diverse coverage on the key mutation sites could be offered by the combination of antigens based on different variants (Reed et al., 2021; Chalkias et al., 2022; Kalnin et al., 2022).
In the present study, we designed and developed a thermostable bivalent recombinant protein vaccine SCTV01C, based on the extracellular domain of spike protein (S-ECD) of SARS-CoV-2 variants Alpha and Beta. Multiple Phase II clinical trials are on-going to further evaluate its safety and immunogenicity in human (NCT05148091, NCT05043311, NCT05043285). Interim analysis indicated that two doses of SCTV01C in naïve population induced superb neutralizing antibody titers against various variants including Omicron, while adverse event rates were similar to those induced by inactivated vaccines, and hence SCTV01C could potentially become the first vaccine to show cross-protective efficacy against Omicron with only two doses of immunization (manuscript in preparation).
2. Materials and methods
2.1. Vaccine antigen production
The extracellular domain of SARS-CoV-2 spike proteins were fused to a T4 Foldon to stabilize the conformation of the trimeric protein (Meier et al., 2004). Antigens were produced as previously described (Wang et al., 2022).
2.2. Transmission electron microscopy (TEM)
Electron microscopy was performed with a Hitachi JEM1200 electron microscope at 200 kV (JEM1200, JEOL, Japan). SCTV01C antigens were diluted to 0.3 mg/mL in formulation buffer. Samples were loaded to the copper net and stained with uranyl acetate for 90 s. Images were acquired under the transmission electron microscope.
2.3. Kinetics of SCTV01C antigen binding to human angiotensin converting enzyme-2 (ACE-2)
Binding kinetics of SCTV01C antigen to human ACE2 receptor were determined on OCTET RED96e (ForteBio). hACE2-Fc (10 μg/mL) (10108-H02H, Sino Biological) was immobilized on ProA Sensor. After baseline, SCTV01C antigens were serially diluted over a range of 37.0–296.3 nM (associate for 300 s, and dissociation for 300 s). Data analysis were carried out with Data Analysis 11.1.
2.4. SEC-HPLC and reduced SDS-PAGE
The purity of SCTV01C antigens was analyzed by Size-Exclusion high-performance liquid chromatograph (SEC-HPLC). Agilent 1260 Infinity HPLC and TSK gel G3000 SWxL column (7.8 × 300 mm, 5 μm) were used with a column temperature of 25 °C. As the mobile phase, SEC buffer (200 mM disodium hydrogen phosphate, 100 mM arginine, 1% isopropanol, pH 6.5) was used with ultraviolet detection at OD280 nm (flow rate: 0.15 ml/min).
The sizes and purities of SCTV01C antigens were further verified by reduced SDS-PAGE. Samples were prepared by mixing 5 μg of SCTV01C antigens (S-ECD of B.1.1.7 or B.1.351) with loading buffer, and incubated in 70 °C for 10 min. Samples were further analyzed on a 7.5% NuPAGE® gel (Thermo Fisher) under reducing conditions.
2.5. Thermal stability measurement
Differential Scanning Fluorimetry (DSF, UNCLE-0330, Unchained Labs, Pleasanton, CA) was used for assessment of the melting temperature (Tm) and aggregation temperature (Tagg) of SCTV01C antigen. SCTV01C solution (9 μL) was heated from 25 °C to 95 °C (0.3 °C/min). The barycentric mean (BCM) curve was generated through measurement of temperature-dependent intrinsic fluorescence spectra. Intrinsic Protein Fluorescence (IPF) (excitation: 266 nm, emission: 280–450 nm) was monitored for measurement of Tm. The aggregation curve was generated through measurement of temperature-dependent intensity of static light scattering (SLS) (excitation: 266 nm). Static Light Scattering (SLS) at 473 nm was monitored for measurement of Tagg. Real-time stability of SCTV01C antigens at 25oC was evaluated by SEC-HPLC, relative antigen-specific IgG titer and relative pseudovirus neutralizing activities.
2.6. Vaccine immunization and immunogenicity assessment
All experimental procedures of animal studies were approved by the Committee of Laboratory Animal Welfare and Ethics of the National Institutes for Food and Drug Control [No. 2020(B)011]. Balb/c mice (female, 6–8 weeks old), C57BL/6 mice (female, 6–8 weeks old) and SD rats (200–220g, female) were provided by Beijing Vital River Laboratory Animal Technology Co (Beijing, China). C57BL/6 hACE2 transgenic mice (female, 6–8 weeks old) were provided by National Institutes for Food and Drug Control. Animals were intramuscularly immunized. Dose levels and schedules of immunization are indicated in figure legends for each experiment. Neutralizing antibody titers (NTs) against antigen-matched pseudoviruses (PSVs) and antigen-specific IgG titers were analyzed as previously described (Wang et al., 2022) at indicated time points. Based on our previous study focused on cross-neutralizing capacity of SCTV01C against pre-Omicron variants and Omicron BA.1 (Wang et al., 2022), neutralizing potency of vaccinated mouse sera were further subjected to assessment of cross-neutralizing activities against Omicron subvariants BA.1, BA.1.1, BA.2, BA.3, and BA.4/5. Mutation sites in S-ECD of pseudoviruses based on SARS-CoV-2 variant strains are shown in Table S1 (Supplementary Table S1).
2.7. SARS-CoV-2 challenge in mice
Mouse SARS-CoV-2 challenge studies, performed in biosafety level 3 (BSL-3) facilities were conducted at Chinese Academy of Military and Medical Sciences. Sera were collected on Day 28 for antibody detection. Two weeks after the second shot, animals were intranasally inoculated with either 4 × 106 pfu/mL of Wuhan-Hu-1 strain (30 μL/dose) (Genome number: GWHACBB01000000) or 4 × 105 pfu/mL B.1.351 strain (30 μL/dose) (CSTR: 16698.06. NPRC2.062100001). Lung tissues were collected 5 days post virus infection. Total RNA was extracted, followed by measurement of SARS-CoV-2 subgenomic RNA (sgRNA) loads and genomic RNA (gRNA) loads in lung tissues by RT-qPCR (Takara), with primers and probes listed in Table S2 (Supplementary Table S2).
2.8. Histopathological analysis
Mice were euthanized at 5 dpi. Lung tissues were fixed with 4% paraformldehyde (Sigma-Aldrich, St. Louis, MO), followed by paraffin embedding, and sectioned into 5-μm slices. Three sections from each animal were used for hematoxylin and eosin (H&E) staining, and subjected to histological examination with KF-PRO-120 Digital Pathology Slide Scanner (KFBIO) in a blinded fashion. Sections were scored for degeneration of alveolar septum thickening, infiltration of inflammatory cells, alveolar exudation, as well as intra-alveolar hemorrhage with a severity scale of 0–4, as previously reported (Sun et al., 2020).
2.9. Statistical analysis
GraphPad Prism 8.2.1 software was used for statistical analysis. Comparison of the means between groups was carried out using Student's t-test or one-way ANOVA as indicated in figure legends. Antibody titers were log transformed for statistical analysis.
3. Results
3.1. Design and characterization of SCTV01C
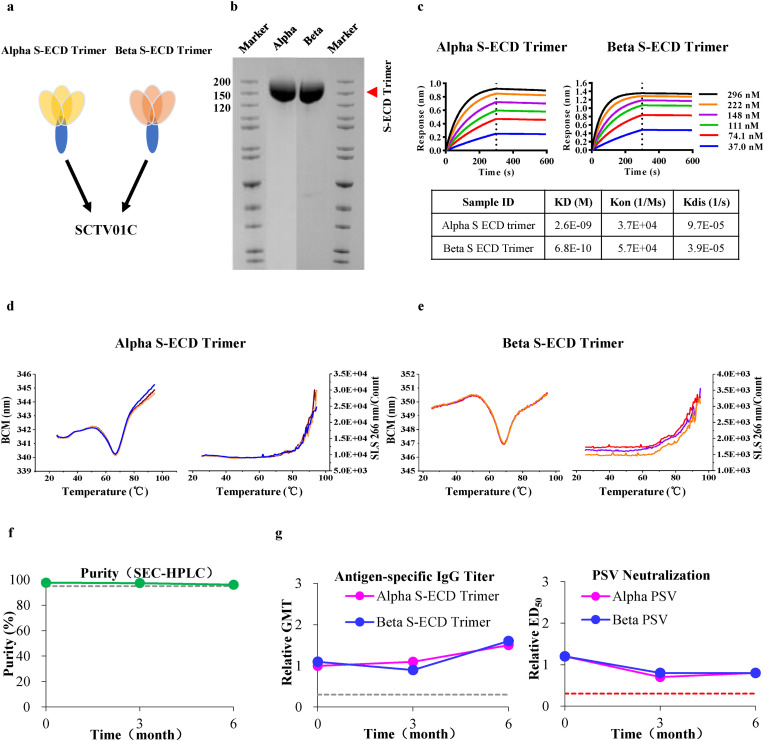
SCTV01C is a bivalent vaccine based on trimeric S-ECD (1–1208 aa) of SARS-CoV-2 variants Alpha and Beta (mixed at a ratio of 1:1) with a squalene-based oil-in-water adjuvant SCT-VA02B (Fig. 1 a). Reduced SDS-PAGE analysis confirmed the trimeric Alpha S-ECD and Beta S-ECD with an apparent molecular weight around 150 kD (Fig. 1b), consistent with the theoretical value. SEC-HPLC analysis further demonstrated a purity of 99.2% and 98.2% for trimeric Alpha S-ECD and Beta S-ECD, respectively (Supplementary Fig. S1a). Binding affinities of SCTV01C antigens to hACE2 were analyzed by surface plasmon resonance (SPR). As shown in Fig. 1c, the affinity (KD) of purified trimeric Alpha S-ECD and Beta S-ECD to hACE2 was 2.6 nM and 0.68 nM, respectively. Transmission electron microscope demonstrated its uniform 12 × 20 nm pre-fusion trimeric nanoparticles (Supplementary Fig. S1b). Notably, both antigens displayed an outstanding thermal stability under stressed conditions. SCTV01C antigens maintained at a stable state below 63 °C and only polymerized at a temperature beyond 72 °C (Fig. 1d and e). Further real-time stability assessment showed good stability of SCTV01C for at least 6 months at 25 °C (Fig. 1f). In addition, immunization with SCTV01C maintained at least 6 months at 25 °C still can induce similar humoral immune responses (antigen-specific IgG titer) and neutralizing activities (ED50) in Balb/c mice compared to reference (Fig. 1g).
Fig. 1.
Physical and Chemical Characterization of SCTV01C. (a) Schematic diagram of SCTV01C antigens. (b) SDS-PAGE analysis of SCTV01C antigens. (c) Binding affinity of SCTV01C antigens to human ACE2. (d,e) Thermal stability of SCTV01C antigens analyzed by Differential Scanning Fluorimetry (DSF). (f) Real-time stability of SCTV01C antigens at 25 oC evaluated by SEC-HPLC. (g) Balb/c mice (n = 10) were vaccinated with a single dose of SCTV01C (2 μg/dose for left panel; five dose levels for right panel: 0.5, 1, 2, 4 and 8 μg/dose). Sera were collected 4 weeks later for detection of relative antigen-specific IgG titer and relative pseudovirus neutralizing activities. Dashed line represents the value of criterion.
3.2. Protection efficacy of SCTV01C in hACE2 mice
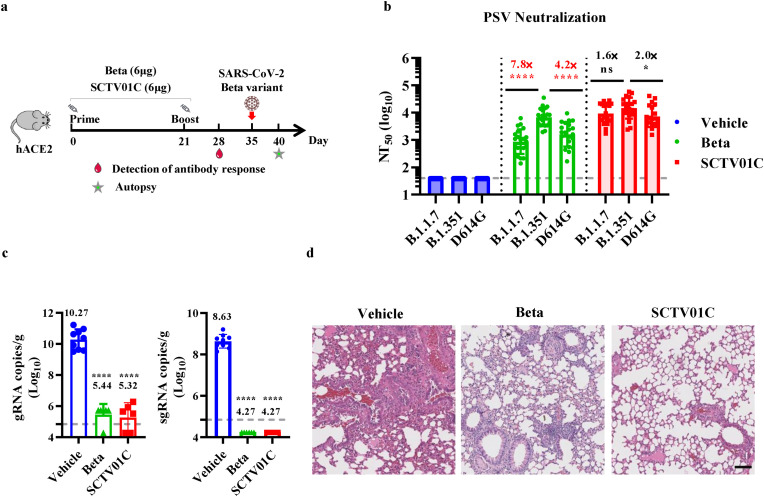
Previous immunogenicity studies in mice have demonstrated that SCTV01C elicited potent humoral and cellular immune responses in mice and showed advantages over monovalent vaccines against various variant strains of SARS-CoV-2 (Wang et al., 2022). In the present study, we further evaluate the protective efficacy elicited by SCTV01C vaccination against challenge with antigen-matched SARS-CoV-2 Beta variant. C57BL/6 hACE2 mice were immunized twice with SCTV01C, in comparison with the Beta monovalent vaccine, on Day 0 and Day 21 (n = 10/group). Antibody detection was carried out on Day 28. Mice were challenged on Day 35 via intranasal inoculation with 4 × 105 plaque-forming unit (pfu) of SARS-CoV-2 Beta variant. Mice were sacrificed 5 days post-infection (dpi), and subjected to lung tissue collection for viral RNA level determination and histopathological analysis (Fig. 2 a). Neutralizing antibody titers against D614G, B.1.1.7 or B.1.351 based pseudoviruses were evaluated. The result indicated that SCTV01C immune sera exhibited balanced and higher neutralizing antibody titers (NT50) against the three variants tested (B.1.351 NT50 = 14547; B.1.1.7 NT50 = 9191; D614G NT50 = 7264) (Fig. 2b). In contrast to the bivalent vaccine SCTV01C, a relatively higher variation (4 to 7-fold) in NT50 against the three variants was observed in monovalent vaccine (Beta) immune sera (B.1.351 NT50 = 6714; B.1.1.7 NT50 = 861; D614G NT50 = 1612) (Fig. 2b). The qRT-PCR results from lung tissues showed that a sharp and statistically significant reduction in genomic RNA (gRNA) was observed in SCTV01C-immunized mice (105.32 copies/g) when compared with vehicle-treated animals (1010.27 copies/g). Similarly, there was no detectable gRNA in those from SCTV01C vaccinated mice, however an average of 108.63 copies/g gRNA was detected in the lung tissues from vehicle-treated mice (Fig. 2c). The Beta monovalent vaccine also reduced viral loads in lung tissues, as indicated by gRNA (105.44 copies/g) and sgRNA analysis (Fig. 2c). Histopathological changes in the lung tissues collected at 5 dpi were evaluated using hematoxylin and eosin staining. The lung tissues from vehicle-treated mice exhibited features of interstitial pneumonia, indicated by thickening of the alveolar septum, increased infiltration of inflammatory cells, and fluid leakage out of the pulmonary vasculature. In contrast, vaccination with SCTV01C or the B.1.351 monovalent vaccine significantly improved the interstitial pneumonia (Fig. 2d).
Fig. 2.
Immunogenicity analysis and protective efficacy against SARS-CoV-2 Beta infection after immunization with Beta monovalent or SCTV01C vaccine in hACE2 transgenic mice. (a) Scheme of immunizations, sera collection, virus challenge, and tissue processing. (b) Serum neutralizing antibody titers (NT50) against D614G, B.1.351 and B.1.1.7 pseudovirus were analyzed one week after the second dose. (c) Subgenomic RNA (sgRNA) (right panel) loads and genomic RNA (gRNA) loads (left panel) in lung tissues of mice were measured by RT-qPCR at 5 dpi. (d) Histopathological analysis of lungs at 5 dpi. Data are shown as GMT±SD. Scale bar: 200 μm. (b) and mean ± SEM (c). GMT: geometric mean titer. SD: standard deviation. NTs: neutralizing antibody tilters. One-way ANOVA was used for comparison. *p ≤ 0.05, ****p ≤ 0.0001, ns means not statistically significant.
3.3. Cross-neutralization capacity of SCTV01C against Omicron subvariants in comparison with ancestral strain-based vaccine in Balb/c and C57BL/6 mice
The rise of Omicron variant (B.1.1.529 or BA.1), with its subvariants (BA.1.1, BA.2, BA.3, and BA.4/5), posed a new public health challenge due to higher transmissibility and severe resistance to first-generation vaccines (Dejnirattisai et al., 2022; Lu et al., 2021; Ikemura et al., 2021). As demonstrated by our previous study, SCTV01C exhibited superior cross-neutralization potencies against pre-Omicron variants and the Omicron BA.1 variant, relative to its corresponding monovalent vaccines (i.e., B.1.1.7-specifc and B.1.351-specific monovalent vaccines) (Wang et al., 2022). In the present study, we compared the cross-neutralizing potencies against more recent Omicron subvariants by vaccination with SCTV01C or a D614G-matched vaccine, mimicking the breadth of cross-neutralization activities elicited by first-generation vaccines.
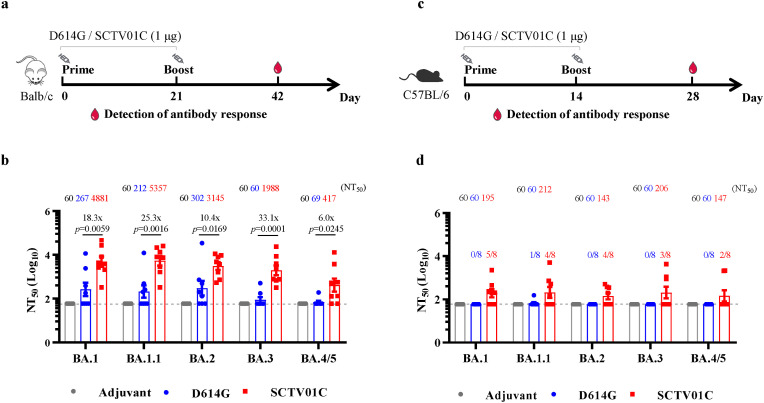
Balb/c mice were intramuscularly immunized with either D614G-matched monovalent vaccine or SCTV01C (1 μg/dose) with a 2-dose regimen (Fig. 3 a). Neutralizing antibody titers (NTs) against pseudoviruses (PSVs) based on Omicron subvariants (BA.1, BA.1.1, BA.2, BA.3, and BA.4/5) were evaluated. As shown in Fig. 3b, three weeks after the second immunization, NT50 of SCTV01C vaccinated sera against BA.1, BA.1.1, BA.2, and BA.3 reached 103 (NT50 GMT: 4881 for BA.1; 5357 for BA.1.1; 3145 for BA.2; and 1988 for BA.3). The D614G vaccine showed relatively low neutralizing capacity against Omicron BA.1, BA.1.1, BA.2 or BA.3 (NT50 GMT: 267 for BA.1; 212 for BA.1.1; and 302 for BA.2) and could hardly induce neutralizing antibody responses against BA.3. Significant differences between D614G vaccine and SCTV01C in NT50 were observed (p = 0.0059 for BA.1; p = 0.0016 for BA.1.1, p = 0.0169 for BA.2; p = 0.0001 for BA.3) (Fig. 3b). The most recent Omicron subvariant BA.4, more resistant to vaccines, is causing the present wave of SARS-CoV-2 infection (Tuekprakhon et al., 2022; Hachmann et al., 2022; Yamasoba et al., 2022). Regarding neutralizing activities against Omicron BA.4/5, D614G vaccinated sera elicited nearly no detectable neutralizing antibodies, while a moderate neutralizing capacity of SCTV01C against BA.4/5 was observed (NT50 GMT: 417) (Fig. 3b).
Fig. 3.
Cross-neutralization capacity of SCTV01C immunized mouse sera against Omicron subvariants. (a) Balb/c mice were intramuscularly injected with D614G monovalent vaccine or SCTV01C (1 μg/dose) twice on Day 0 and Day 21 (n = 8/group). (b) 21 days after the second shot, moue sera were used to detect NTs against Omicron PSVs. (c) C57BL/6 mice were intramuscularly injected with D614G monovalent vaccine or SCTV01C (1 μg/dose) twice with a 2-week interval (n = 8/group). (d) Two weeks after the second immunization, sera were used to detect NTs against Omicron PsVs. Data are shown as GMT mean ± SD. GMT: geometric mean titer. SD: standard deviation. NTs: neutralizing antibody tilters. Comparisons were performed by One-way ANOVA.
The above findings were further validated in C57BL/6 mice, mimicking individuals with mild to moderate humoral immune responses after vaccination. Mice were intramuscularly injected with either D614G-matched monovalent vaccine or SCTV01C (1 μg/dose) twice with a 2-week interval (Fig. 3c). Mouse sera were collected 2 weeks after the boost immunization for measurement of NT50 against Omicron subvariants. Neutralizing activities against all tested PSVs in mouse sera immunized with the D614G vaccine were nearly undetectable. Notably, SCTV01C vaccination induced neutralizing antibody responses against BA.1, BA.1.1, BA.2, BA.3, and BA.4/5 PSVs (Fig. 3d). Elevated neutralizing potency of SCTV01C against various Omicron subvariants was observed after a third shot, while no further increase in neutralizing antibody titers was observed in D614G monovalent vaccine immunized mouse sera (Supplementary Fig. S2).
The immunogenicity of D614G monovalent vaccine or SCTV01C is comparable, as indicated by neutralizing antibody responses elicited by each vaccine against antigen-matched pseudoviruses (Supplementary Table S3). Overall, the SCTV01C immunization regimen induced more potent cross-neutralizing antibody responses across all the Omicron variants tested, when compared with the D614G vaccine mimicking SARS-CoV-2 ancestral strain-based first-generation vaccines.
In addition, as indicated by exploratory studies based on C57BL/6 mice and Cynomolgus monkeys for comparison of humoral immune responses elicited by the S-ECD component and the T4 foldon in SCTV01C antigens, T4 foldon-specific IgG titers were 1–2 orders of magnitude lower than the S-ECD-specific IgG titers (Supplementary Fig. S3).
3.4. Dose-response exploration of SCTV01C in SD rats
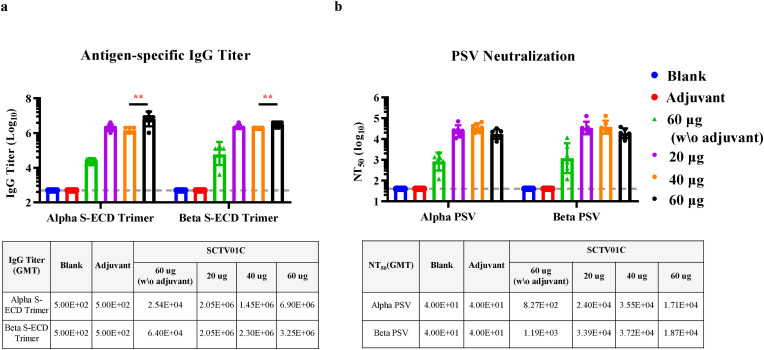
To explore the dose-response of SCTV01C antigen, SD rats were immunized with SCTV01C at 3 different dose levels (20, 40, or 60 μg/dose) in the presence of SCT-VA02B adjuvant (10 mg/dose) with a 2-week interval. Two weeks after the second immunization, the antigen-specific IgG titers reached 106 in all SCT-VA02B-adjuvanted groups (GMT = 2048000, 1448155, 6901995 for the B.1.1.7 antigen; 2298802, 2048000, 3250997 for the B.1.351 antigen). Especially, a significantly elevated IgG titer was observed when 60 μg SCTV01C antigens were used. However, in the absence of SCT-VA02B adjuvant, more than 100-fold decrease in total IgG titers was observed (Fig. 4 a). Likewise, the NT50 against pseudovirus based on B.1.1.7 and B.1.351 reached 104 in all SCT-VA02B-adjuvanted groups (B.1.1.7 NT50 = 23995, 35502 and 17081; B.1.351 NT50 = 33854, 37202 and 18732), with no significant differences between each group. However, more than 15-fold decrease in NT50 was observed when SCTV01C antigens were used for immunization without adjuvant (Fig. 4b). These data demonstrated that SCTV01C can induce potent humoral immune responses when antigens were used ranging from 20 μg to 60 μg in the presence of 10 mg SCT-VA02B adjuvant, which could further augment the humoral immune responses induced by SCTV01C antigen alone.
Fig. 4.
Dose-effect relationship of SCTV01C antigen on humoral immune responses in SD rats. SD rats were injected with saline (Blank), 10 mg SCT-VA02B (Adjuvant), SCTV01C antigen (60 μg/dose), or SCTV01C antigen (20, 40, or 60 μg/dose) with 10 mg SCT-VA02B adjuvant with a 2-week interval (n = 6/group). Three weeks after the second immunization, humoral immune responses were analyzed based on antigen-specific IgG titers (a) and pseudovirus neutralizing antibody titers (b). Data are shown as GMT±SD. Comparisons were performed by Student's t-test (unpaired, two tail). **p ≤ 0.001.
3.5. Safety evaluation of SCTV01C in SD rats
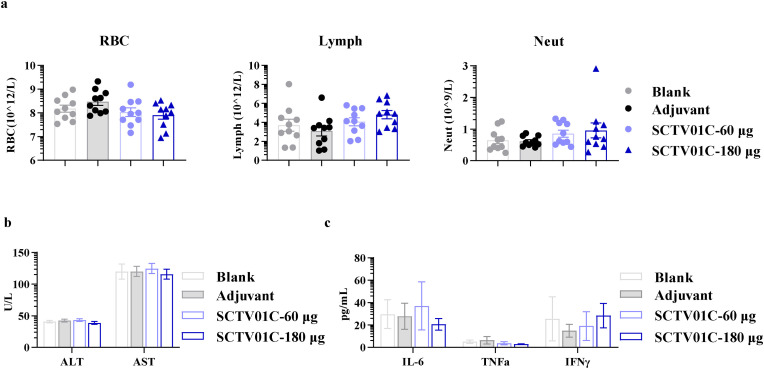
A repeat-dose toxicity study of SCTV01C was carried out in SD rats (JOINN Laboratories, Beijing, China). Animals were vaccinated with four doses of SCTV01C (60 or 180 μg/dose, 2-week interval). All immunized animals developed a robust immunogenicity. No obvious systemic toxicity or immunotoxicity was observed when animals were exposed to SCTV01C at dose levels up to 180 μg/dose. Two weeks after the last immunization, peripheral blood samples were subjected to analysis of blood cell counting, blood biochemistry index, and inflammatory cytokines. No abnormal signs were observed on these concerned items (Fig. 5 , a-c).
Fig. 5.
Safety evaluation of SCTV01C in SD rats. SD rats were injected with saline (Blank), 10 mg SCT-VA02B (Adjuvant), or SCTV01C (60 μg/dose or 180 μg/dose, in the presence of 10 mg or 30 mg SCT-VA02B adjuvant) on Day 0, 14, 28 and 42 (n = 10/group). Peripheral blood counts (a), biochemical indexes (b) and inflammatory cytokines (c) were detected on Day 57. Data are shown as mean ± SEM. RBC, red blood cells. Lymph, lymphocytes. Neut, neutrophils. ALT, Alanine aminotransferase. AST, Aspartate aminotransferase.
4. Discussion
In this study, the bivalent SARS-CoV-2 vaccine SCTV01C elicited robust neutralizing activities and exhibited excellent protection efficacy against challenge by antigen-matched SARS-CoV-2 Beta variant in a mouse model. Notably, SCTV01C also induced cross-neutralization activities against subvariants of the newly emerged Omicron variant, superior to the D614G-matched vaccine, mimicking the breadth of cross-neutralization activities elicited by first-generation vaccines (Fig. 3). Recently, Chalkias et al. also have reported that a booster dose of the clinical stage bivalent mRNA vaccine candidate (WT and Beta spike proteins) showed advantages over the WT booster dose in human, as indicated by more potent humoral immune responses against antigen-matched WT strain and the Beta variant, as well as antigen-mismatched Delta and Omicron BA.1 variants (Chalkias et al., 2022). Beta variant based monovalent vaccine also showed potential advantages over D614G or WT vaccines in protection efficacy against Omicron BA.1 in the Phase 3 VAT02 study (Launay et al., 2022).
So far, cross-neutralization potency elicited by monovalent or multivalent vaccines based on Beta variant against newly emerged Omicron subvariants have not been elaborated in either clinical or preclinical studies. Our data indicated potential advantages of Beta variant-based multivalent vaccine in curtailing risks posed by new subvariants of the Omicron variant, as demonstrated by more potent cross-neutralizing antibody responses against Omicron BA.1, BA.1.1, BA.2, BA.3 and BA.4/5, when compared with the D614G vaccine (Fig. 3). Therefore, the variant-based bivalent vaccine SCTV01C could play important roles in defending against existing and future emerging variants. One limitation of the study is that the cross-protection capacity of SCTV01C against more recent SARS-CoV-2 variants, such as Delta and members of the Omicron family, was not elaborated in this study due to the lack of Animal Biosafety Level 3 (ABSL-3) facilities.
Increasing vaccination rate with highly effective vaccines is an important approach to build herd immunity and terminate the current COVID-19 pandemic. Although the vaccination rate in high income countries reaching over 50%, a vast majority of people, from lower-middle-income countries (LMICs) or low-income countries (LICs), remain unvaccinated (Maxmen, 2021). Availability of COVID-19 vaccines, as well as no requirements of ultra-low-temperature for vaccine storage and transportation, contributed to vaccination coverage, especially in LICs and LMICs (Cao and Gao, 2021; Uddin and Roni, 2021). The outstanding thermal stability of SCTV01C, demonstrated by no significant loss of purity and immunogenicity at 25 oC for at least 6 months (Fig. 1f and g), is well suited for mass vaccination in developing countries lacking advanced facilities and infrastructure. SCTV01C can be manufactured with standard existing large scale CHO cell culture, and hence can be easily produced in large quantities to meet the demand for increased vaccine coverage of people at risk for COVID-19 in LICs and LMICs.
In developed countries where vaccine supply is no longer an issue, suboptimal vaccine coverage might due to vaccine hesitance caused by various reasons, including distrust in newly developed vaccine technology, concerns on adverse events caused by vaccination, and lack of confidence in vaccine efficacy (Wouters et al., 2021; Ipsos, 2021; Siu et al., 2022). For example, mRNA vaccines have shown promising clinical efficacies in protection against infection by early strains of SARS-CoV-2 and reduction in disease severity, however, hesitance towards vaccination remains due to lack of long-term safety data, as well as rare but potentially severe side effects (Mevorach et al., 2021; Witberg et al., 2021; Montgomery et al., 2021; Barda et al., 2021). Spike protein-based vaccine with the newly developed Matrix-M™ adjuvant showed high protection efficacy with safety issues similar to mRNA vaccines (Heath et al., 2021). Inactivated vaccines showed relatively low adverse event rates but concern remains about the suboptimal antibody responses and protection efficacy (Li et al., 2022). Some people were reluctant to adenoviral vector-based vaccine despite evidences for its clinical efficacy (Sadoff et al., 2021; Voysey et al., 2021; Scully et al., 2021; Schultz et al., 2021; Pavord et al., 2021). Therefore, development of vaccines, with potent immunogenicity and efficacy and low side effects, could offer additional options to people with vaccine hesitancy, and would be helpful to increase vaccination rate. Preclinical safety assessment of SCTV01C indicated that no adverse safety issues were observed in SD rats vaccinated with a 4-dose regimen of SCTV01C at a dose level up to 180 μg (Fig. 5).
Phase II clinical trials are in progress to evaluate the safety and immunogenicity profiles, as well as efficacy of SCTV01C in unvaccinated populations or as booster doses. Interim analysis results indicated that SCTV01C showed similar safety profiles to inactivated vaccines and induced potent neutralizing antibodies to variants including the Omicron variant (manuscript in preparation).
5. Conclusions
In this study, preclinical assessments of a bivalent S-trimer vaccine SCTV01C with a squalene-based oil-in-water adjuvant SCT-VA02B were conducted. Our data demonstrated that SCTV01C exhibited outstanding thermal stability (over 6 months at 25 oC), significant protection efficacy in a mouse challenge model, superior cross-neutralizing capacity against newly emerged subvariants of the Omicron variant, as well as favorable safety profiles.
This study demonstrated that multivalent vaccines composed of high quality and thermal stable S-trimer protein antigens and SCT-VA02B adjuvant could play significant roles in helping to curb the current COVID-19 pandemic as well as become an important platform for vaccine development against SARS-CoV-2 variants emerging in the future.
CRediT authorship contribution statement
Rui Wang: Investigation, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. Xun Huang: Investigation, Data curation, Writing – original draft. Tianshu Cao: Investigation, Data curation, Writing – original draft. Chunyun Sun: Project administration, Validation, Writing – original draft, Writing – review & editing. Dan Luo: Investigation, Data curation, mouse challenge studies. Hongying Qiu: Investigation, Data curation, mouse challenge studies. Mei Wu: Investigation, Data curation, mouse challenge studies. Xingyao Huang: Investigation, Data curation, mouse challenge studies. Chulin Yu: Investigation, Data curation, immunogenicity studies. Jing Li: Investigation, Data curation, immunogenicity studies. Desheng Kong: Investigation, Data curation, immunogenicity studies. Juan Ma: Investigation, Data curation, immunogenicity studies. Xiao Zhang: Investigation, Data curation, safety evaluation studies. Ping Hu: Investigation, Data curation, antigen-related biochemical studies. Yanjing Zhang: Resources. Chunxia Luo: Resources. Hui Zhao: Investigation, Validation, mouse challenge studies. Yuchang Li: Investigation, Validation, mouse challenge studies. Yongqiang Deng: Visualization, Validation, Writing – review & editing. Chengfeng Qin: Supervision, Funding acquisition, Writing – review & editing. Liangzhi Xie: Conceptualization, Supervision, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Rui Wang, Chunyun Sun, Chulin Yu, Jing Li, Desheng Kong, Juan Ma, Xiao Zhang, Ping Hu, Yanjing Zhang, Chunxia Luo and Liangzhi Xie are employees of Sinocelltech Ltd. and have ownership or potential stock option of the company.
Acknowledgements
This work was funded by Sinocelltech with grant support from Beijing Municipal Science and Technology Program (No. Z211100002521026) (China), National Science Fund for Distinguished Young Scholars (No. 81925025) (China), Innovative Research Group Project of the National Natural Science Foundation of China (No. 81621005), and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2019-I2M-5-049). We thank Hongpeng Huang for assistance in data analysis. We thank Jianbo Lu for assistance in pseudovirus production. We thank Sai Cao and Xiaokai Wang for assistance in antigen production.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virol.2022.09.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bai Y., Du Z., Xu M., Wang L., Wu P., Lau E.H., et al. International risk of SARS-CoV-2 Omicron variant importations originating in South Africa. medRixv. 2021 doi: 10.1101/2021.12.07.21267410. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Gao G.F. mRNA vaccines: a matter of delivery. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.R., Yisimayi A., Jian F., Song W., Xiao T., Wang L., et al. BA. 2.12. 1, BA. 4 and BA. 5 escape antibodies elicited by Omicron infection. bioRxiv. 2022 doi: 10.1101/2022.04.30.489997. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkias S., Eder F., Essink B., Khetan S., Nestorova B., Feng J., et al. Safety, immunogenicity and antibody persistence of a bivalent beta-containing booster vaccine. Res. Square. 2022 April:1–55. doi: 10.1038/s41591-022-02031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Valdez A., Valdez-Zapata G., Patel S.S., Castelli F.V., Garcia M.G., Jansen W.T., et al. MF59-adjuvanted influenza vaccine (FLUAD®) elicits higher immune responses than a non-adjuvanted influenza vaccine (Fluzone®): a randomized, multicenter, Phase III pediatric trial in Mexico. Hum. Vaccines Immunother. 2018;14(2):386–395. doi: 10.1080/21645515.2017.1373227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Shaw R.H., Supasa P., Liu C., Stuart A.S., Pollard A.J., et al. Reduced neutralisation of SARS-CoV-2 omicron B. 1.1. 529 variant by post-immunisation serum. Lancet. 2022;399(10321):234–236. doi: 10.1016/S0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desingu P.A., Nagarajan K., Dhama K. Emergence of Omicron third lineage BA. 3 and its importance. J. Med. Virol. 2022;94(5):1808–1810. doi: 10.1002/jmv.27601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISAID . 2022. Tracking of Variants.https://www.gisaid.org/hcov19-variants/ assessed 17 07 2021. [Google Scholar]
- Hachmann N., Miller J., Collier A., Ventura J., Yu J., Rowe M., et al. 2022. Neutralization Escape by the SARS-CoV-2 Omicron Variants BA. 2.12. 1 and BA. 4/BA. 5. medRxiv Preprint. [DOI] [Google Scholar]
- Hansson M., Nygren P.A., Ståhl S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000;32(2):95–107. doi: 10.1042/ba20000034. [DOI] [PubMed] [Google Scholar]
- Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.Y., Wang S.H., Tang Y., Sheng W., Zuo C.J., Wu D.W., et al. Landscape and progress of global COVID-19 vaccine development. Hum. Vaccines Immunother. 2021;17(10):3276–3280. doi: 10.1080/21645515.2021.1945901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura N., Hoshino A., Higuchi Y., Taminishi S., Inaba T., Matoba S.J.M. SARS-CoV-2 Omicron variant escapes neutralization by vaccinated and convalescent sera and therapeutic monoclonal antibodies. medRxiv. 2021 doi: 10.1101/2021.12.13.21267761. Preprint. [DOI] [Google Scholar]
- Ipsos . Ipsos; 2021. Global attitudes: COVID-19 Vaccines.https://www.ipsos.com/en-ro/global-attitudes-covid-19-vaccine-january-2021 assessed 10 05 2021. [Google Scholar]
- Irwin A., Nkengasong J. What it will take to vaccinate the world against COVID-19. Nature. 2021;592(7853):176–178. doi: 10.1038/d41586-021-00727-3. [DOI] [PubMed] [Google Scholar]
- Kalnin K.V., Plitnik T., Kishko M., Huang D., Raillard A., Piolat J., et al. Pan-SARS neutralizing responses after third boost vaccination in non-human primate immunogenicity model. Vaccine. 2022;40(9):1289–1298. doi: 10.1016/j.vaccine.2022.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavian N., Hachim A., Li A.P., Cohen C.A., Chin A.W., Poon L.L., et al. Assessment of enhanced influenza vaccination finds that FluAd conveys an advantage in mice and older adults. Clin Transl immunol. 2020;9(2):e1107. doi: 10.1002/cti2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay O., Cachanado M., Nguyen L.B.L., Ninove L., Lachâtre M., Ghezala I.B., et al. Immunogenicity and safety of Beta adjuvanted recombinant booster vaccine. medRxiv. 2022 doi: 10.1101/2022.05.25.22274904. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leier H.C., Bates T.A., Lyski Z.L., McBride S.K., Lee D.X., Coulter F.J., et al. Previously infected vaccinees broadly neutralize SARS-CoV-2 variants. medRxiv. 2021 doi: 10.1101/2021.04.25.21256049. Preprint. [DOI] [Google Scholar]
- Li C., Li A., Bi H., Hu J., Yang F., Zhou T., et al. Immunogenicity and safety of the CoronaVac inactivated SARS-CoV-2 vaccine in people with underlying medical conditions: a retrospective study. medRxiv. 2022 doi: 10.1101/2022.04.28.22274402. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Mok B.W.Y., Chen L.L., Chan J.M.C., Tsang O.T.Y., Lam B.H.S., et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin. Infect. Dis. 2021 Dec doi: 10.1093/cid/ciab1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D.R., Schäfer A., Leist S.R., De la Cruz G., West A., Atochina-Vasserman E.N., et al. Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice. Science. 2021;373(6558):991–998. doi: 10.1126/science.abi4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxmen A. The fight to manufacture COVID vaccines in lower-income countries. Nature. 2021;597(7877):455–457. doi: 10.1038/d41586-021-02383-z. [DOI] [PubMed] [Google Scholar]
- Meier S., Güthe S., Kiefhaber T., Grzesiek S. Foldon, the natural trimerization domain of T4 fibritin, dissociates into a monomeric A-state form containing a stable β-hairpin: atomic details of trimer dissociation and local β-hairpin stability from residual dipolar couplings. J. Mol. Biol. 2004;344(4):1051–1069. doi: 10.1016/j.jmb.2004.09.079. [DOI] [PubMed] [Google Scholar]
- Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., et al. Myocarditis after BNT162b2 mRNA vaccine against covid-19 in Israel. N. Engl. J. Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J., Ryan M., Engler R., Hoffman D., McClenathan B., Collins L., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento I.P., Leite L. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012;45(12):1102–1111. doi: 10.1590/S0100-879X2012007500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan A., Rossin E.J., Kaseke C., Park R.J., Khatri A., Koundakjian D., et al. Structure-guided T cell vaccine design for SARS-CoV-2 variants and sarbecoviruses. Cell. 2021;184(17):4401–4413. doi: 10.1016/j.cell.2021.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N. Engl. J. Med. 2021;385(18):1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet J., Chen W.H., Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021;170:71–82. doi: 10.1016/j.addr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C.C., Schultheis K., Andrade V.M., Kalia R., Tur J., Schouest B., et al. Design, immunogenicity and efficacy of a Pan-SARS-CoV-2 synthetic DNA vaccine. bioRxiv. 2021 doi: 10.1101/2021.05.11.443592. Preprint. [DOI] [Google Scholar]
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N. Engl. J. Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu J.Y., Cao Y., Shum D.H. Perceptions of and hesitancy toward COVID-19 vaccination in older Chinese adults in Hong Kong: a qualitative study. BMC Geriatr. 2022;22(1):1–16. doi: 10.1186/s12877-022-03000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.H., Chen Q., Gu H.J., Yang G., Wang Y.X., Huang X.Y., et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28(1):124–133. doi: 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.F. Fluad®-MF59®-adjuvanted influenza vaccine in older adults. Infect. Chemother. 2013;45(2):159–174. doi: 10.3947/ic.2013.45.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuekprakhon A., Huo J., Nutalai R., Dijokaite-Guraliuc A., Zhou D., Ginn H.M., et al. Further antibody escape by Omicron BA. 4 and BA. 5 from vaccine and BA. 1 serum. bioRivx. 2022 doi: 10.1101/2022.05.21.492554. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.N., Roni M.A. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines. 2021;9(9):1033. doi: 10.3390/vaccines9091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations . 2021. Unequal Vaccine Distribution Self-Defeating, World Health Organization Chief Tells Economic and Social Council,s Special Ministerial Meeting.https://www.un.org/press/en/2021/ecosoc7039.doc.htm accessed 10 05 2021. [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Sun C., Ma J., Yu C., Kong D., Chen M., et al. A bivalent COVID-19 vaccine based on Alpha and Beta variants elicits potent and broad immune responses in mice against SARS-CoV-2 variants. Vaccines. 2022;10(5):702. doi: 10.3390/vaccines10050702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after Covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Larson H.J., Teerawattananon Y., et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasoba D., Kosugi Y., Kimura I., Fujita S., Uriu K., Ito J., et al. Sensitivity of novel SARS-CoV-2 Omicron subvariants, BA. 2.11, BA. 2.12. 1, BA. 4 and BA. 5 to therapeutic monoclonal antibodies. bioRxiv. 2022 doi: 10.1101/2022.05.03.490409. (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B.W., Kim C.O., Izu A., Arora A.K., Heijnen E. Phase 4, post-marketing safety surveillance of the MF59-adjuvanted influenza vaccines Fluad® and Vantaflu® in South Korean subjects aged≥ 65 years. Infect. Chemother. 2018;50(4):301–310. doi: 10.3947/ic.2018.50.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.