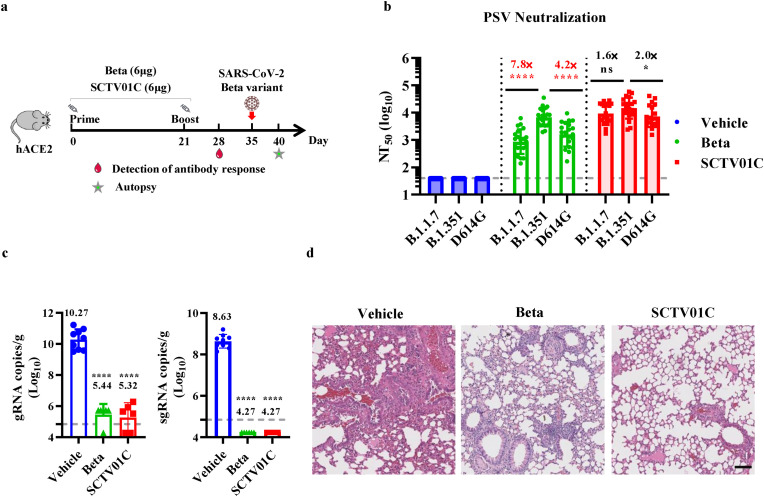
Fig. 2.
Immunogenicity analysis and protective efficacy against SARS-CoV-2 Beta infection after immunization with Beta monovalent or SCTV01C vaccine in hACE2 transgenic mice. (a) Scheme of immunizations, sera collection, virus challenge, and tissue processing. (b) Serum neutralizing antibody titers (NT50) against D614G, B.1.351 and B.1.1.7 pseudovirus were analyzed one week after the second dose. (c) Subgenomic RNA (sgRNA) (right panel) loads and genomic RNA (gRNA) loads (left panel) in lung tissues of mice were measured by RT-qPCR at 5 dpi. (d) Histopathological analysis of lungs at 5 dpi. Data are shown as GMT±SD. Scale bar: 200 μm. (b) and mean ± SEM (c). GMT: geometric mean titer. SD: standard deviation. NTs: neutralizing antibody tilters. One-way ANOVA was used for comparison. *p ≤ 0.05, ****p ≤ 0.0001, ns means not statistically significant.