Abstract
Background
Eosinophilic oesophagitis (EoE) is a chronic immune‐mediated or antigen‐mediated oesophageal disease characterised by symptoms related to oesophageal dysfunction and eosinophil‐predominant inflammation.
Objective
We aimed to estimate the incidence and prevalence of EoE in Denmark during the period 2008–2018.
Methods
Based on data from nationwide registers we identified cases of EoE using two definitions: a broad definition based solely on oesophageal biopsies registered in the Danish Pathology Register and a narrow definition also including symptoms of oesophageal dysfunction registered in the Danish National Patient Registry. The annual incidence and prevalence were standardised by sex and age in 5‐year intervals to the 2013 study population.
Results
From 2008 to 2011, the standardised incidence of EoE was stable, but from 2011 to 2018 it increased from 3.9 (95% CI 3.3–4.4) to 11.7 (95% CI 10.8–12.6) per 100,000 person‐years. Similar temporal trends were observed when using the narrow EoE definition. The increase in incidence was most pronounced in men and in individuals above 40 years of age. In children, the EoE incidence was a fourth of the incidence in adults aged 40–64 years: 4.4 (95% CI 3.2–5.6) versus 17.6 (95% CI 15.7–19.5) per 100,000 person‐years. The EoE incidence varied substantially across the five regions in Denmark. Overall, the biopsy rate as well as the proportion of oesophageal biopsies with detected eosinophilia increased during the study period.
Conclusion
This study of the entire population of Denmark during the period 2008 to 2018 shows that the incidence and prevalence of EoE is not yet plateauing and that EoE could be severely underdiagnosed, especially in children.
Keywords: eosinophilia, epidemiology, oesophagus, pathology
Key summary.
Summarise the established knowledge on this subject
The incidence of eosinophilic oesophagitis (EoE) has been increasing in the western world since the disease was defined in children in 1981, and in adults in 1993.
Estimates of EoE incidence and prevalence from unselected population‐based studies are needed to understand the current epidemiology of EoE and to improve timely diagnosis of EoE.
What are the significant and/or new findings of this study?
This Danish nationwide population‐based study shows a three‐fold increase in EoE incidence and prevalence from 2011 to 2018.
This increase was most pronounced in individuals above 40 years of age and in men.
The incidence and prevalence of EoE were substantially lower in children compared to adults and varied markedly across the five regions in Denmark.
This suggests that there are many undiagnosed and untreated patients with EoE, especially children, at risk of reduced quality of life and complications.
INTRODUCTION
Eosinophilic oesophagitis (EoE) is a clinicopathological disease of the oesophagus described for the first time in children in 1981, and in adults in 1993. 1 , 2 The first international definition was published in 2007 and was modified in 2011 and 2018. 3 , 4 , 5 The modifications of the EoE definition primarily regarded the status of gastro‐oesophageal reflux disease (GORD), which is now allowed as a comorbid condition to EoE. 4 Symptoms of EoE are dysphagia, food bolus obstruction, and other signs of oesophageal dysfunction. 3 Histologically, EoE is defined by 15+ eosinophils per high power field. 3 A meta‐analysis of 14 hospital‐based case series, 8 insurance database studies, and 7 administrative database studies reported a rising prevalence and incidence of EoE during 1976–2017. 6 However, estimates of EoE incidence and prevalence from unselected population‐based studies representing all EoE patients in a specific geographic area over a specific calendar period, including estimates in women and men and across different age groups are warranted to understand the current epidemiology of EoE.
The incidence of EoE in Denmark during the period 1997–2012 has been reported to be low compared to other European countries. 6 , 7 , 8 To improve the diagnosis of EoE, a biopsy protocol was instated in 2011 in the North Denmark Region 9 resulting in a substantial increase in EoE incidence. 10 However, it remains unknown whether this increase in incidence was confined to the North Denmark Region (representing approximately 1/10 of the Danish population corresponding to approximately 580,000 inhabitants) or if similar rising incidence rates were observed nationwide.
The aim of the present study was to estimate the incidence and prevalence of EoE in the Danish population from 2008 to 2018 and to examine temporal trends in prevalence and incidence according to age, sex, and region.
MATERIALS AND METHODS
Study population
Based on data from the Danish Civil Registration System, we defined a cohort of all individuals living in Denmark at some point between 1st of January 2008 and 31th of December 2018. 11 We linked this population to the Danish National Patient Register through the personal identification number assigned to all citizens at birth. The Danish National Patient Register holds information on all inpatient hospitalisations since 1977, and outpatient and emergency room contacts since 1995. 12 Based on International Classification of Disease (ICD)‐10 codes, we excluded individuals with diagnoses that could cause oesophageal eosinophilia, including malignancies, HIV, Crohn's disease, achalasia, eosinophilic gastritis/duodenitis, burn of oesophagus, and corrosion of oesophagus (for the full list of exclusions and their ICD‐10 codes, see Supplementary Table 1).
Information on age, sex, and region of address was obtained from the Danish Civil Registration System. In 2007, Denmark was divided into five regions: the Capital Region of Denmark, the Central Denmark Region, the North Denmark Region, Region Zealand, and the Region of Southern Denmark (Supplementary Figure 1). These regions are responsible for all health care, including hospital care in Denmark.
Identification of eosinophilic oesophagitis
To identify cases of EoE, we linked the study population to the Danish Pathology Register, which was established in 1997 and holds information on all pathology data in Denmark. 13 As previously described by Dellon et al, patients with oesophageal eosinophilia were identified as individuals with samples with a combination of the Systematised Nomenclature of Medicine (SNOMED) codes for oesophageal biopsies (T62xxx) and tissue eosinophilia (M47150). 8 Dellon et al added a criterion that individuals were classified as having EoE only if they had at least one ICD‐10 diagnosis of oesophageal dysfunction prior to the oesophageal biopsy. However, as the recording of symptoms show marked regional differences in Denmark, we included two definitions of EoE in the current study. A broad definition including all cases of oesophageal eosinophilia with no alternative cause of oesophageal eosinophilia, and a narrow definition including all cases of oesophageal eosinophilia with no alternative cause of oesophageal eosinophilia, and at least one registration of oesophageal dysfunction such as abdominal pain, dysphagia, oesophageal obstruction, or dyspepsia prior to the oesophageal biopsy (for the full list and ICD‐10 codes, see Supplementary Table 1). Thus, the latter definition corresponds to the “sensitive case definition” in Dellon et al. 8 The date of the pathology sample was used to define the date of diagnosis of EoE.
Statistical analysis
All individuals were followed from 1st of January 2008, birth or first address in Denmark, whichever came first, until 31st of December 2018, death, emigration, or a diagnosis of a competing cause of oesophageal eosinophilia (see above and Supplementary Table 1). The annual standardised incidence was estimated by direct standardisation as number of EoE cases per 100,000 person‐years and standardised by sex and age in 5‐year intervals to the 2013 study population.
In addition to estimating the annual standardised incidence, we also estimated the annual standardised prevalence of EoE during the study period. To estimate the total prevalence of EoE in Denmark, we estimated the number of individuals being diagnosed with EoE before 2008 and calculated the annual prevalence for a given year as the proportion of individuals who had ever received an EoE diagnosis by 31st of December of that year among all individuals living in Denmark by 31st of December of that year. All analyses were carried out in SAS 9.4.
Ethics
The study was approved by the Danish Data Protection Agency and data were analysed on a secure server at the Danish Health Data Authority. Ethical approval is not required for registry‐based research in Denmark.
RESULTS
During the study period 1st of January 2008 to 31st of December 2018, a total of 119,268 oesophageal biopsies were conducted on 81,255 individuals (Figure 1). Of these, 4011 individuals had a first diagnosis of EoE according to the broad EoE definition. Of the 4011 incident EoE patients, 2749 (69%) also had at least one recording of symptoms of oesophageal dysfunction at time of sampling, and thus fulfilled the narrow EoE definition (Figure 1). Demographic characteristics at time of diagnosis were similar between patients diagnosed with EoE according to the broad and narrow definition (Table 1). Among EoE patients, 70% were men as compared to 50% in the background population. A total of 8% of EoE patients were diagnosed before the age of 18 years and approximately 70% were 40 years or older at time of diagnosis with EoE (Table 1). Among EoE patients, 5% were born outside Denmark compared with 10% in the background population.
FIGURE 1.
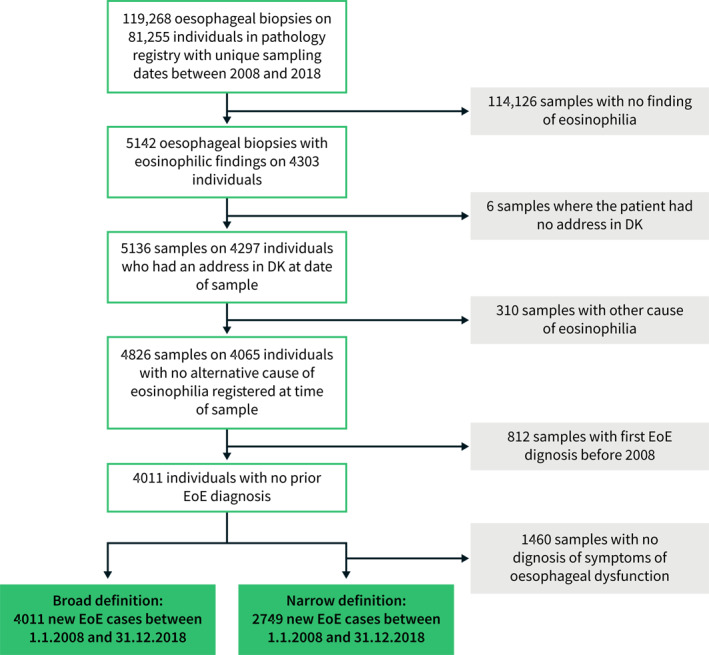
Flowchart illustrating identification of cases with oesophageal oesophagitis (EoE) in Denmark 2008–2018. Broad definition: EoE with no alternative cause of oesophageal eosinophilia. Narrow definition: EoE with no alternative cause of oesophageal eosinophilia and at least one diagnosis of oesophageal dysfunction
TABLE 1.
Demographic characteristics at time of diagnosis of eosinophilic oesophagitis 2008–2018 and for all residents in Denmark at 31 December 2013
| EoE cases, broad definition | EoE cases, narrow definition | Background population in 2013 a | ||
|---|---|---|---|---|
| Total | 4011 | 2749 | 5,606,705 | |
| Sex | Female | 1213 (30.2%) | 826 (30.0%) | 2,825,490 (50.4%) |
| Male | 2798 (69.8%) | 1923 (70.0%) | 2,781,215 (49.6%) | |
| Age | <18 years | 307 (7.7%) | 218 (7.9%) | 1,175,733 (21.0%) |
| 18–39 years | 858 (21.4%) | 666 (24.2%) | 1,522,386 (27.2%) | |
| 40–64 years | 1889 (47.1%) | 1264 (46.0%) | 1,882,431 (33.6%) | |
| 65+ years | 957 (23.9%) | 601 (21.9%) | 1,026,155 (18.3%) | |
| Region | Region Zealand | 629 (15.7%) | 407 (14.8%) | 8,15,240 (14.5%) |
| Region of Southern Denmark | 1034 (25.8%) | 843 (30.7%) | 1,200,027 (21.4%) | |
| The Capital Region of Denmark | 1179 (29.4%) | 612 (22.3%) | 1,739,395 (31.0%) | |
| The Central Denmark Region | 744 (18.5%) | 603 (21.9%) | 1,272,605 (22.7%) | |
| The North Denmark Region | 425 (10.6%) | 284 (10.3%) | 579,438 (10.3%) | |
| Municipal‐based area SES b | 0%–25% (most disadvantaged area) | 851 (21.2%) | 535 (19.5%) | 1,324,536 (23.6%) |
| 25%–49% | 979 (24.4%) | 702 (25.5%) | 1,338,695 (23.9%) | |
| 50%–75% | 997 (24.9%) | 745 (27.1%) | 1,274,170 (22.7%) | |
| 75%–100% (least disadvantaged area) | 1184 (29.5%) | 767 (27.9%) | 1,669,304 (29.8%) | |
| Urbanisation c | Densely populated areas | 1464 (36.5%) | 915 (33.3%) | 1,908,191 (34.0%) |
| Intermediate density area | 1224 (30.5%) | 866 (31.5%) | 1,816,698 (32.4%) | |
| Thinly populated areas | 1323 (33.0%) | 968 (35.2%) | 1,881,815 (33.6%) | |
| Country of birth | DK | 3790 (94.5%) | 2603 (94.7%) | 5,046,373 (90.0%) |
| Not DK | 221 (5.5%) | 146 (5.3%) | 560,332 (10.0%) |
Note: Broad definition: eosinophilic oesophagitis (EoE) with no alternative cause of oesophageal eosinophilia. Narrow definition: EoE with no alternative cause of oesophageal eosinophilia and at least one diagnosis of oesophageal dysfunction.
2013 was used as this was the midpoint of the study period.
Municipal‐based socio‐economic index (SES) is derived from an index developed by the Danish ministry of internal affairs (see noegletal.dk).
Municipal‐based urbanisation is classified according to the DEGURBA classification by Statistics Denmark.
In addition to incident EoE cases in the period 2008–2018, we also identified prevalent cases as of 1 January 2008, using the same criteria. A total of 506 prevalent EoE cases were identified according to the broad definition and 292 EoE cases according to the narrow definition.
Symptom recording and biopsy practice across Denmark
Symptom recording practice differed widely across the five regions in Denmark. In the Capital Region of Denmark, 51% of patients with oesophageal eosinophilia also had a recording of symptoms, whereas this number was 64% in Region Zealand, 66% in the North Denmark Region, 80% in the Central Denmark Region, and 81% in the Region of Southern Denmark.
The number of oesophageal biopsies per 100,000 citizens increased over the study period (Supplementary Figure 2), and the proportion of oesophageal biopsies where eosinophilia was detected also increased over the period (Supplementary Figure 3).
Incidence and prevalence of eosinophilic oesophagitis
During the period 2008–2011, the standardised incidence of EoE was stable, but from 2011 to 2018 it increased from 3.9 (95% CI 3.3–4.4) to 11.7 (95% CI 10.8–12.6) per 100,000 person‐years when using the broad EoE definition (Figure 2a). Likewise, the annual standardised prevalence of EoE increased slightly from 2008 to 2011, whereas it increased more steeply from 2011 to 2018 (Figure 2b). Accordingly, the prevalence of EoE (using the broad EoE definition) was 22.2 (95% CI 21.0–23.5) per 100,000 individuals in 2011 and 69.7 (95% CI 67.5–71.9) per 100,000 in 2018. When using the narrow EoE definition, the incidence and prevalence were lower, but temporal trends of standardised incidence and prevalence were similar (Figure 2).
FIGURE 2.
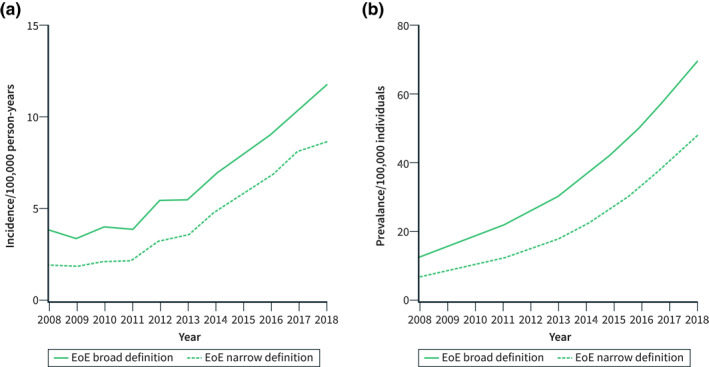
Standardised incidence (a) and prevalence (b) of oesophageal eosinophilia (EoE) in Denmark 2008–2018. Broad definition: EoE with no alternative cause of oesophageal eosinophilia. Narrow definition: EoE with no alternative cause of oesophageal eosinophilia and at least one diagnosis of oesophageal dysfunction
Incidence of eosinophilic oesophagitis stratified by age, sex, and region
Using the broad EoE definition, we stratified analyses by age, sex, and region. When stratifying by age, the standardised incidence rate of EoE increased substantially from 2011 to 2018 among individuals 40 years or older, whereas the increase was more modest among individuals younger than 40 years (Figure 3a). In 2018, the standardised incidence of EoE was 4.4 (95% CI 3.2–5.6) per 100,000 person‐years in individuals <18 years, 8.6 (95% CI 7.1–10.0) per 100,000 person‐years in individuals 18–39 years, 17.6 (95% CI 15.7–19.5) per 100,000 person‐years in individuals 40–64 years, and 14.2 (95% CI 11.9–16.5) per 100,000 person‐years in individuals 65 years or older.
FIGURE 3.
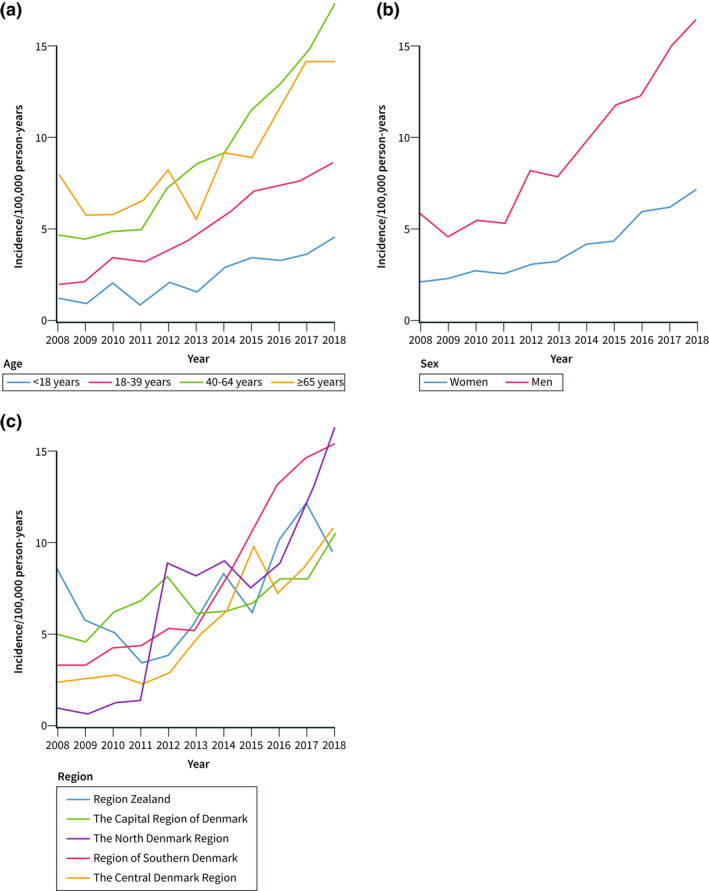
Standardised incidence of oesophageal eosinophilia (EoE) in Denmark 2008–2018 using the broad definition of EoE, that is EoE with no alternative cause of oesophageal eosinophilia. (a) Standardised incidence of EoE stratified by age groups, (b) Standardised incidence of EoE stratified by sex, and (c) Standardised incidence of EoE stratified by region
When stratifying by sex, a marked increase in the standardised incidence of EoE was observed in men, whereas a more modest increase was observed in women (Figure 3b). Accordingly, the standardised incidence of EoE increased from 5.9 (95% CI 4.9–6.9) to 16.5 (95% CI 15.0–18.0) per 100,000 person‐years in men and from 2.1 (95% CI 1.5–2.6) to 7.1 (95% CI 6.1–8.0) per 100,000 person‐years in women from 2008 to 2018.
The temporal trends of the standardised incidence of EoE also differed across regions in Denmark (Figure 3c). In the North Denmark Region, the standardised incidence of EoE was low from 2008 to 2011 but increased markedly from 2011 to 2012: 1.1 (95% CI 0.2–2.1) to 8.0 (95% CI 5.7–10.3) per 100,000 person‐years. In Region Zealand, the standardised incidence rate of EoE decreased from 2008 to 2011, and increased from 2011 to 2018, but to a lower extent compared to the other four regions (Figure 3c).
Prevalence of eosinophilic oesophagitis stratified by age, sex, and region
In accordance with the temporal trends for the standardised incidence of EoE, the annual standardised prevalence of EoE increased most in individuals of 40 years or older and in men (Figure 4a and b). In 2018, the standardised prevalence of EoE was 110.0 (95% CI 104.0–116.5) per 100,000 in individuals 65 years or older versus 18.7 (95% CI 16.5–21.1) per 100,000 in individuals <18 years of age. The prevalence of EoE was 98.4 (95% CI 94.9–102.1) per 100,000 in men versus 41.1 (95% CI 38.9–43.6) per 100,000 in women in 2018 (Figure 4b). The standardised prevalence of EoE increased in all five regions in Denmark, although with some differences in temporal trends (Figure 4c).
FIGURE 4.
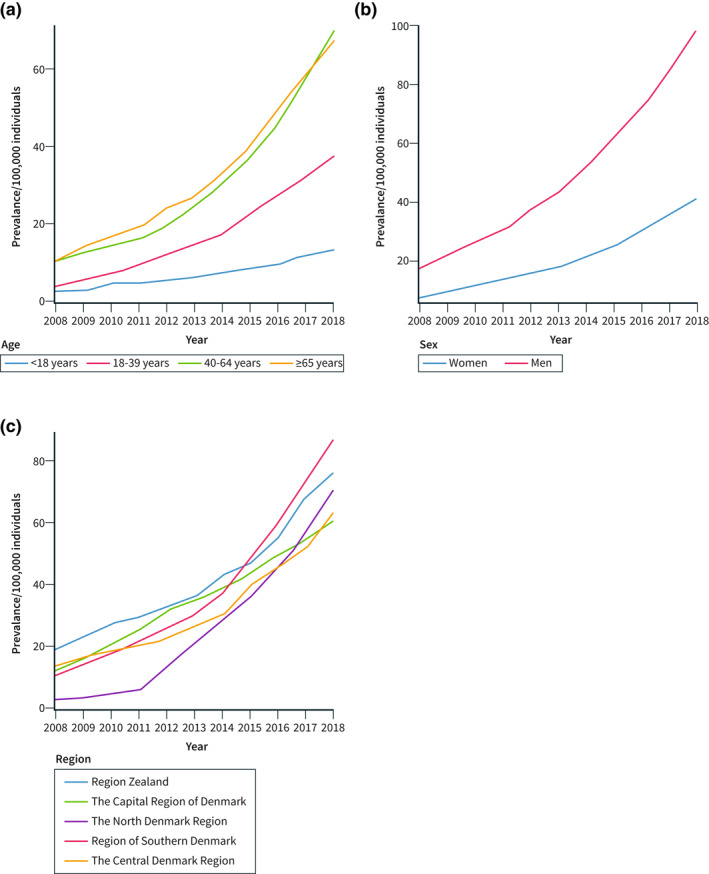
Prevalence of oesophageal eosinophilia (EoE) in Denmark 2008–2018 using the broad definition of EoE, that is EoE with no alternative cause of oesophageal eosinophilia. (a) Prevalence of EoE stratified by age groups, (b) Prevalence of EoE stratified by sex, and (c) Prevalence of EoE stratified by region
Paediatric eosinophilic oesophagitis
As shown in Figures 3 and 4, the incidence and prevalence of EoE were substantially lower in children compared to adults. Children constitute 21% of the Danish population (Table 1). However, only 8% (n = 322) of the EoE cases during the period 2008–2018 were observed in children (Table 2). As for adults, most cases were observed in males (Table 2). Substantial regional differences in the number of children diagnosed with EoE existed across the five regions. The Region of Southern Denmark which includes 21% of the Danish population diagnosed 37% of all EoE children in Denmark (Supplementary Table 1).
TABLE 2.
Paediatric eosinophilic oesophagitis cases in Denmark in 2008–2018
| Age group | All | Girls | Boys |
|---|---|---|---|
| 0–2 years, n (%) | 36 (100%) | 10 (27.8%) | 26 (72.2%) |
| 3–12 years, n (%) | 146 (100%) | 46 (31.5%) | 100 (68.5%) |
| 13–18 years, n (%) | 140 (100%) | 39 (27.9%) | 101 (72.1%) |
| Total | 322 | 95 | 227 |
DISCUSSION
This Danish nationwide population‐based study showed a three‐fold increase in EoE incidence and prevalence from 2011 to 2018. This increase was most pronounced in individuals above 40 years of age and in men. Among children, we observed a modest increase in EoE incidence and prevalence, but incidence and prevalence were much lower in children compared to adults. The incidence and prevalence of EoE increased in all five regions in Denmark, although with substantial differences in temporal trends.
The incidence of EoE has been increasing in the western world since the disease was defined. 6 As reported in the present study this development has taken place in Denmark too with a tripling of the incidence from 2011 to 2018. In the most recent meta‐analysis published in 2019, a total of 29 studies were included. These were based on hospital‐based case series (n = 14), insurance databases (n = 8), and administrative databases (n = 7) from North America (US and Canada), South America (Brazil) and Europe (The Netherlands, Denmark, Switzerland, and Spain). 6 The incidence and prevalence of EoE estimated by the meta‐analysis were lower than the incidence and prevalence observed in 2018 by the present study. 6 This likely reflects the continuous rising incidence and prevalence of EoE, and since the estimates from the meta‐analysis were derived based on many studies published before 2011, recent increases in the prevalence and incidence could not be accounted for. In contrast, our study provides estimates of EoE incidence and prevalence up until 2018 offering contemporary data to assist the planning of health care of patients with EoE.
The pronounced regional differences we found suggest that EoE is still underdiagnosed in Denmark. Our study cannot tell if the increasing incidence was caused by an increased awareness and detection of EoE or a true increase in the incidence of EoE. It has been shown in several comparable countries, that the EoE incidence has increased in centres with unchanged diagnosis culture regarding EoE, suggesting that the increase in EoE is not merely explained by improved detection. 14 , 15 , 16 , 17 This is likely to be a factor in Denmark too. On the other hand, up until 2011, Denmark had a very low incidence compared to other European countries 7 suggesting that the increase in EoE incidence observed from 2011 to 2018 may be partly explained by increased detection. During the study period, we observed an increase in the number of oesophageal biopsies per 100,000 citizens and an increase in the proportion of oesophageal biopsies where eosinophilia was detected suggesting that physicians need to biopsy even more patients than they do now to diagnose all EoE patients. Potential underdiagnosis of EoE is also supported by the documentation of a 10‐year diagnostic delay not showing a decreasing trend in the North Denmark Region. 10 Besides increasing recognition of EoE from clinicians, reasons for the increase are still largely unknown and several research projects are investigating the topic. The hygiene hypothesis has been suggested as a potential explanation. 18 According to this hypothesis, the increasing incidence of many autoimmune and allergic conditions in the western world are caused by living in cleaner environment with less exposure to microorganisms. 18 The influence of seasonal and genetic factors has also been investigated but remains unclear. 19 , 20 , 21 , 22
We observed the highest EoE incidence and prevalence and the largest increases in incidence and prevalence among individuals above 40 years of age. The lowest incidence and prevalence of EoE were observed in children, and the incidence and prevalence increased to a much lesser degree in children as compared to adults during the study period. This is in accordance with previous findings, where lower and stable incidence of EoE was also observed in children. 6 In the present study, we observed substantial regional differences in diagnosis of EoE in children. Despite including only 21% of the Danish population, the Region of Southern Denmark diagnosed 40% of all EoE children in Denmark during the study period. This finding is in accordance with findings from the DanEoE study documenting severe issues with diagnosing and treating EoE children in the North Denmark Region. 23 Furthermore, the expected incidence measured in the meta‐analyses by Navarro et al in 2017 was 6.6/100.000 person‐years which was higher than the data from the current study (1.2/100,000 person‐years in 2008 and 4.8/100,000 person‐years in 2018). 6 Collectively these findings imply that a substantial number of children with EoE may be undiagnosed in Denmark. Undiagnosed or insufficiently treated EoE may result in an increased risk of psychiatric comorbidity including anxiety and depression in both children and adults 24 and also correlates with stricture formation and poor quality of life. 25 Treating EoE is simple as the medication is efficient and has few side effects. 26 The high physical and psychological symptom burden of EoE combined with efficient treatment options makes EoE detection a “low hanging fruit” for improving life for these individuals.
We observed substantially higher EoE incidence and prevalence in men as compared to women. Over the 10‐year study period, the incidence and prevalence of EoE increased more in men compared to women. This difference was also evident in children where approximately 70% of the EoE cases were observed in boys correlating well with previous studies. 4 , 27
Despite Denmark being a small country with free access to health care, we observed substantial regional differences in EoE incidence and prevalence and in the number of oesophageal biopsies taken per 100,000 citizens. Many factors can have an impact. EoE is still a relatively new diagnosis, and the knowledge among physicians about what symptoms should trigger clinical suspicion of EoE may vary. Denmark is a small country with good infrastructure, free and equal access to medical care, and a high level of education among doctors. We would therefore not expect substantial differences in incidence of EoE across the five Danish regions. The observed marked differences strongly suggest that there are many undiagnosed and untreated patients, whom we know have a low quality of life and risk of complications. Many of these are children. Importantly, most of the general practitioners, paediatricians, and endoscopists who should discover and diagnose EoE never learned about EoE during their medical training, as the disease was not defined until after they started practicing. Therefore, improved knowledge dissemination and education in EoE is critical to improve the health and quality of life for the patients. A reason for the low detection of EoE in children could be the limited number of sites that could perform upper gastrointestinal endoscopy for children. Furthermore, parents may be reluctant to accept anaesthesia of children for the endoscopy. However, the lack of knowledge of EoE and its symptoms seem to be more important when discussing it with the paediatricians. In support of this, the Region of Southern Denmark has had a special interest in EoE and initially, the paediatric department in this region received children with EoE from other regions for treatment.
A major strength of our study is its unselected population‐based nature ensuring that selection bias is mitigated and that estimates are provided for both genders and for all age groups. However, it also has potential limitations, one of the most prominent being potential misclassification of EoE. Individuals with oesophageal eosinophilia were identified by using SNOMED codes registered in the Danish Pathology Register which has almost 100% coverage. 13 Validation of SNOMED codes for EoE has been done by Dellon et al 8 showing correct SNOMED coding in 90% of EoE cases. In this study, we excluded patients with other diseases that could cause oesophageal eosinophilia, but it is possible that some patients with EoE may in fact be patients with GORD. Dellon et al added a criterion including symptoms of oesophageal dysfunction to improve the specificity of EoE diagnosis. However, the registration of symptoms varied widely across the Danish regions. Whereas diagnostic codes are registered routinely by physicians in Denmark, symptom registration are not. Due to the differences in symptom registration across the regions, we chose to include both a broad definition (not including symptoms) and a narrow definition (including symptoms) of EoE. Likely, the broad definition of EoE overestimated the incidence and prevalence by including too many GORD patients, whereas the narrow definition underestimated EoE by excluding EoE patients where symptoms were not registered. In general, the differentiation of EoE and GORD is difficult, and the current consensus guideline allows EoE and GORD to overlap. Furthermore, the current EoE definition also accepts heartburn and chest pain as symptoms of EoE, rendering the differentiation between EoE and GORD difficult. We did not use the diagnosis code DK20.9D for EoE in the current study, as it was introduced in Denmark as late as 2014, and still not routinely used. Furthermore, recent studies have reported on asymptomatic oesophageal eosinophilia (aEE), which has endoscopic and histological findings similar to those of EoE, but does not have oesophageal dysfunction. 28 , 29 EoE and aEE may have the same pathogenesis, but it is still unclear if aEE should be treated or not, and further investigation is needed. 29 When using the broad definition, we may include patients with aEE, although most of the patients undergoing an upper gastrointestinal endoscopy will have upper gastrointestinal symptoms as an indication for the procedure. A limitation of our study is that in the cases where no symptoms were registered, we cannot know if the patients were truly asymptomatic or whether symptoms were not registered by the physician. Finally, our study cannot tell whether the observed increase in incidence of EoE reflects a true increase in the incidence of EoE and if so, what the underlying mechanism are.
In summary, regardless of EoE definition, the incidence and prevalence of EoE increased in Denmark, although with substantial regional and temporal trend differences. It remains unknown whether the increasing EoE incidence and prevalence represent increased awareness and detection of the disease or a true increase in the occurrence of EoE. However, the increasing biopsy rates in combination with an increasing proportion of eosinophilia in oesophageal biopsies, and large regional incidence differences suggest that we have not yet reached a stable incidence and prevalence of EoE, and that knowledge of EoE is unevenly distributed among Danish physicians. The incidence and prevalence of EoE were much lower in children compared to adults, were unevenly distributed across regions and increased only modestly during the study period. This suggests that EoE is underdiagnosed in Danish children.
In conclusion, this study of the entire population of Denmark during the period 2008 to 2018 showed an increasing incidence and prevalence of EoE, especially in men and in individuals above 40 years of age. Collectively, our findings suggest that the incidence and prevalence of EoE is not yet plateauing, and that EoE could be severely underdiagnosed, especially in children.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
The study was funded by The Danish National Research Foundation (grant no. DNRF148), Marie Pedersen and Jensine Heiberg's Foundation, Spiremidlerne, Center for Clinical Research, North Regional Hospital Hjørring, Denmark, and a research grant from Vifor Pharma and Dr. Falk Pharma. The funders had no role in the design, conduct, or reporting of the study.
Allin KH, Poulsen G, Melgaard D, Frandsen LT, Jess T, Krarup AL. Eosinophilic oesophagitis in Denmark: population‐based incidence and prevalence in a nationwide study from 2008 to 2018. United European Gastroenterol J. 2022;10(7):642–52. 10.1002/ueg2.12273
DATA AVAILABILITY STATEMENT
The study is based on data from the Danish nationwide registers (https://sundhedsdatastyrelsen.dk). The register data is protected by the Danish Act on Processing of Personal Data and are accessed through application to and approval from the Danish Data Protection Agency and the Danish Health Data Authority.
REFERENCES
- 1. Picus D, Frank PH. Eosinophilic esophagitis. AJR Am J Roentgenol. 1981;136(5):1001–3. [DOI] [PubMed] [Google Scholar]
- 2. Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38(1):109–16. [DOI] [PubMed] [Google Scholar]
- 3. Dellon ES, Liacouras CA, Molina‐Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018;155(4):1022–33. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. e6. [DOI] [PubMed] [Google Scholar]
- 5. Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–63. [DOI] [PubMed] [Google Scholar]
- 6. Navarro P, Arias Á, Arias‐González L, Laserna‐Mendieta EJ, Ruiz‐Ponce M, Lucendo AJ. Systematic review with meta‐analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population‐based studies. Aliment Pharmacol Ther. 2019;49(9):1116–25. [DOI] [PubMed] [Google Scholar]
- 7. Dellon ES, Erichsen R, Baron JA, Shaheen NJ, Vyberg M, Sorensen HT, et al. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population‐based estimates from Denmark. Aliment Pharmacol Ther. 2015;41(7):662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dellon ES, Erichsen R, Pedersen L, Shaheen NJ, Baron JA, Sørensen HT, et al. Development and validation of a registry‐based definition of eosinophilic esophagitis in Denmark. World J Gastroenterol. 2013;19(4):503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krarup AL, Drewes AM, Ejstrud P, Laurberg PT, Vyberg M. Implementation of a biopsy protocol to improve detection of esophageal eosinophilia: a Danish registry‐based study. Endoscopy. 2021;53(1):15–24. [DOI] [PubMed] [Google Scholar]
- 10. Melgaard D, Westmark S, Laurberg PT, Krarup AL. A diagnostic delay of 10 years in the DanEoE cohort calls for focus on education ‐ a population‐based cross‐sectional study of incidence, diagnostic process and complications of eosinophilic oesophagitis in the North Denmark Region. United European Gastroenterol J. 2021;9(6):688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–9. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;17(7):449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Publ Health. 2011;39(7 Suppl l):72–4. [DOI] [PubMed] [Google Scholar]
- 14. Giriens B, Yan P, Safroneeva E, Zwahlen M, Reinhard A, Nydegger A, et al. Escalating incidence of eosinophilic esophagitis in Canton of Vaud, Switzerland, 1993‐2013: a population‐based study. Allergy. 2015;70(12):1633–9. [DOI] [PubMed] [Google Scholar]
- 15. Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M, et al. Escalating incidence of eosinophilic esophagitis: a 20‐year prospective, population‐based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128(6):1349–50. e5. [DOI] [PubMed] [Google Scholar]
- 16. Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted county, Minnesota. Clin Gastroenterol Hepatol. 2009;7(10):1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warners MJ, de Rooij W, van Rhijn BD, Verheij J, Bruggink AH, Smout AJPM, et al. Incidence of eosinophilic esophagitis in The Netherlands continues to rise: 20‐year results from a nationwide pathology database. Neuro Gastroenterol Motil. 2018;30(1). [DOI] [PubMed] [Google Scholar]
- 18. Okada H, Kuhn C, Feillet H, Bach JF. The “hygiene hypothesis” for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lucendo AJ, Arias Á, Redondo‐González O, González‐Cervera J. Seasonal distribution of initial diagnosis and clinical recrudescence of eosinophilic esophagitis: a systematic review and meta‐analysis. Allergy. 2015;70(12):1640–50. [DOI] [PubMed] [Google Scholar]
- 20. Suryawala K, Palle S, Altaf M. Epidemiology, clinical presentation, and seasonal variation in the diagnosis of children with eosinophilic esophagitis in Oklahoma. South Med J. 2020;113(1):37–41. [DOI] [PubMed] [Google Scholar]
- 21. Leigh L, Spergel J. An in‐depth characterization of a large cohort of adult patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2019;122(1):65–72. [DOI] [PubMed] [Google Scholar]
- 22. Biedermann L, Straumann A, Greuter T, Schreiner P. Eosinophilic esophagitis—established facts and new horizons. Semin Immunopathol. 2021;43(3):319–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nielsen MH, Terkelsen JH, Kramme F, Sogaard P, Maeng M, Tayal B, et al. The incidence of eosinophilic oesophagitis among children in North Denmark region between 2007‐2017 is lower than expected. BMC Pediatr. 2022;22(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taft TH, Guadagnoli L, Edlynn E. Anxiety and depression in eosinophilic esophagitis: a scoping review and recommendations for future research. J Asthma Allergy. 2019;12:389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow‐up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125(6):1660–9. [DOI] [PubMed] [Google Scholar]
- 26. Gonsalves NP, Aceves SS. Diagnosis and treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collins CA, Palmquist J, Proudfoot JA, Qian A, Wangberg H, Khosh‐Hemmat E, et al. Evaluation of long‐term course in children with eosinophilic esophagitis reveals distinct histologic patterns and clinical characteristics. J Allergy Clin Immunol. 2019;144(4):1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kitamura H, Tanaka F, Nadatani Y, Otani K, Hosomi S, Kamata N, et al. Eosinophilic esophagitis and asymptomatic esophageal eosinophilia display similar immunohistological profiles. J Clin Biochem Nutr. 2021;68(3):246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki Y, Iizuka T, Hosoi A, Ochiai Y, Suzuki Y, Hayasaka J, et al. Clinicopathological differences between eosinophilic esophagitis and asymptomatic esophageal eosinophilia. Intern Med. 2022;61(9):1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The study is based on data from the Danish nationwide registers (https://sundhedsdatastyrelsen.dk). The register data is protected by the Danish Act on Processing of Personal Data and are accessed through application to and approval from the Danish Data Protection Agency and the Danish Health Data Authority.


