Abstract
Background
Real‐world data on clinical outcomes of ustekinumab in ulcerative colitis are lacking.
Objective
To assess short‐ and long‐term clinical outcomes of ustekinumab in ulcerative colitis.
Methods
Adult ulcerative colitis patients without previous colectomy starting ustekinumab treatment up until 11 December 2020 were identified through the Swedish Inflammatory Bowel Disease Register (SWIBREG). Prospectively recorded data were extracted from the SWIBREG. The primary outcome was persistence to ustekinumab 16 weeks after treatment initiation. Secondary outcomes included drug persistence beyond week 16, clinical remission (defined as a patient‐reported Mayo rectal bleeding subscore = 0 and stool frequency subscore ≤1), biochemical remission (defined as faecal‐calprotectin <250 μg/g) and changes in health‐related quality of life (HRQoL), as measured by the Short Health Scale (SHS). Logistic regression was used to identify potential predictors of ustekinumab persistence at 16 weeks.
Results
Of the 133 patients with ulcerative colitis, only three were naïve to biologics and tofacitinib. The persistence rates of ustekinumab were 115/133 (86%) at 16 weeks and 89/133 (67%) at last follow‐up, that is, after a median follow‐up of 32 (interquartile range 19–56) weeks. The clinical remission rates were 17% at 16 weeks and 32% at the last follow‐up. The corresponding rates for biochemical remission were 14% and 23%. The median faecal‐calprotectin concentration decreased from 740 μg/g at baseline to 98 μg/g at the last follow‐up (p < 0.01, n = 37). Improvement was seen in each dimension of the SHS between baseline and last follow‐up (p < 0.01 for each dimension, n = 46). Male sex was associated with ustekinumab persistence at 16 weeks (adjusted odds ratio = 4.00, 95% confidence interval: 1.35–11.83).
Conclusion
In this nationwide real‐world cohort of ulcerative colitis patients with prior drug failures, including other biologics and tofacitinib, ustekinumab was associated with high drug persistence rates and improvements in clinical, biochemical and HRQoL measures.
Keywords: anti‐tnf‐antibodies, inflammatory bowel disease, ulcerative colitis, ustekinumab
Key summary.
Established knowledge on this subject:
Ustekinumab is approved for the treatment of moderate‐to‐severe ulcerative colitis in adult patients with inadequate response, loss of response or intolerance to either conventional therapy or biologics.
Real‐world data on treatment outcomes of ustekinumab in ulcerative colitis are scarce and the relationship between ustekinumab treatment and health‐related quality of life (HRQoL) measures in a real‐world setting is unknown.
New findings of this study:
In our nationwide cohort of patients with ulcerative colitis and no previous colectomy, ustekinumab persistence rates were 86% at 16 weeks and 67% after a median follow‐up of 32 weeks.
Applying non‐response imputation, 17% were in clinical remission at week 16 and the corresponding figure at last follow‐up was 32%.
Almost all (98%) patients with ulcerative colitis treated with ustekinumab in Sweden have previously been exposed to biologics or tofacitinib.
Ustekinumab treatment was associated with improvements in clinical, biochemical and HRQoL outcomes.
INTRODUCTION
Ulcerative colitis is a chronic inflammatory bowel disease (IBD) usually characterised by rectal bleeding, fatigue, diarrhoea, abdominal pain and urgency. 1 The disease is associated with impaired HRQoL, loss of work productivity and increased morbidity. 2 , 3 , 4 Patients who fail or develop intolerance to conventional medical therapies (i.e., 5‐Aminosalicylates, corticosteroids and immunomodulators) are often treated with an anti‐tumour necrosis factor (TNF)‐alpha agent. 5 However, many patients do not respond to or experience secondary loss of response or intolerance to anti‐TNF treatment. 6 , 7 , 8 , 9 Remaining treatment options include colectomy and more recently approved targeted therapies that is, vedolizumab, ustekinumab and tofacitinib. 10
Targeted therapies have specific mechanisms of action. The humanised monoclonal antibody ustekinumab is directed to the common p40 subunit of interleukin‐12 and interleukin‐23. 10 , 11 Ustekinumab was granted marketing authorisation in September 2019 by the European Medicines Agency for the treatment of moderate‐to‐severe ulcerative colitis in adult patients with inadequate response, loss of response, or intolerance to either conventional therapy or biologics. 12 The efficacy and safety of ustekinumab were demonstrated in the large randomised UNIFI trial. 13 However, the understanding of the optimal use of ustekinumab and its outcomes in a real‐world setting is limited. 14 , 15 , 16 , 17 , 18 In addition, identifying clinical predictors of ustekinumab response is becoming increasingly important given the increasing number of approved drugs in ulcerative colitis.
To examine the short‐ and long‐term outcomes in ustekinumab‐treated patients with ulcerative colitis, we performed a nationwide study using prospectively recorded data from the Swedish Inflammatory Bowel Disease quality Register (SWIBREG), a national quality register. 19 In addition, we sought to identify potential predictors of persistence to ustekinumab after induction therapy, that is, 16 weeks after the initiation of treatment.
MATERIALS AND METHODS
Study design and setting
This investigation was a nationwide non‐interventional cohort study assessing clinical outcomes in patients with ulcerative colitis treated with ustekinumab based on information in SWIBREG. At the end of 2020, Sweden had a population of 10.4 million. 20 The Swedish healthcare system is tax‐funded and offers universal access, with prescription drugs provided free of charge above an annual threshold of 2350 Swedish krona [approximately €230]. Using the unique Swedish personal identity number, assigned to all legal Swedish residents, 21 individuals can be followed until emigration or death with virtually no loss of follow‐up.
Data source
Swedish Inflammatory Bowel Disease Register was established in 2005. In 2020, SWIBREG was comprised of almost 54,000 patients with IBD, including >85% of all IBD patients treated with immunomodulators, biologics or surgery in Sweden. 22 The register holds prospectively recorded information on demographics, clinical characteristics, disease activity, treatments and HRQoL measures (e.g., the Short‐Health Scale (SHS). 19 The validity of diagnoses and information about some specific phenotypes in the SWIBREG is high. 23 , 24
Study population
Patients with ulcerative colitis who received at least one intravenous infusion or injection of ustekinumab before the date of first data extraction (i.e., 11 December 2020) were identified through SWIBREG. Patients aged ≥18 years at the start of ustekinumab treatment without previous colectomy were eligible for inclusion. All patients were followed until termination of ustekinumab, emigration, death or end of follow‐up (i.e., 11 December 2020), whichever occurred first. Complementary information was extracted on 2 February 2021 to allow a minimum follow‐up of 16 weeks.
Information was extracted from SWIBREG on age, sex, smoking status and maximum disease extent according to the Montreal classification at baseline. 25 In addition, we extracted data on the patient‐reported Mayo score, comprising of the Mayo stool frequency and Mayo rectal bleeding subscore, 26 previous and ongoing medical treatment, colectomy, faecal‐calprotectin (f‐calprotectin), C‐reactive protein (CRP) and SHS at baseline and during follow‐up. The SHS captures four self‐reported dimensions of HRQoL in patients with IBD: symptom burden, functional status, disease‐related worry and general wellbeing. Each domain is scored on a six‐point scale from 0 (no problem) to 5 (worst imaginable state). The SHS has previously been validated against several other HRQoL instruments, including the IBD questionnaire (IBDQ). 27 Smoking status at baseline was defined as last reported information on smoking before or at the initiation of ustekinumab treatment. The reasons for discontinuation of the last targeted therapy (i.e. biologics and tofacitinib) were lack of or loss of response (termination because of primary non‐response or secondary loss of response), intolerance or other reasons (e.g., patient's request and pregnancy).
Outcomes
All outcomes were assessed at 16 weeks after the initiation of ustekinumab and at the last follow‐up. The primary outcome was the persistence rate of ustekinumab at week 16. Secondary outcomes were the persistence rate of ustekinumab at the last follow‐up and clinical remission, defined as a stool frequency subscore ≤1 and a rectal bleeding subscore of 0 in the patient‐reported Mayo score and corticosteroid‐free clinical remission for patients with concomitant corticosteroid treatment at baseline. Other secondary outcomes included biochemical remission (defined as f‐calprotectin <250 µg/g), CRP and f‐calprotectin levels and SHS scores compared to baseline. Data at baseline and 16 weeks after the initiation of ustekinumab treatment were included if recorded in SWIBREG within ±4 weeks of each time point.
Statistical analyses
Non‐normally distributed continuous variables are presented as medians and interquartile ranges (IQR). A Kaplan‐Meier curve was used to illustrate ustekinumab persistence over time. For clinical and biochemical remission status at 16 weeks and last follow‐up, we applied an intention‐to‐treat approach and reported rates were based on non‐responder imputation where missing data and discontinuation of ustekinumab were classified as treatment failure, regardless of the reason for discontinuation. For comparisons of f‐calprotectin, CRP, patient‐reported Mayo score and SHS between baseline and follow‐up, we performed pairwise assessments, using the Wilcoxon matched‐pairs signed‐rank test. For clarity purposes, the number of individuals with complete data is reported in brackets for each analysis. For CRP and f‐calprotectin levels below the lowest limit of detection (LOD), values were substituted with LOD/√2. 28 Finally, predictors of ustekinumab persistence at 16 weeks after the initiation of treatment were assessed using univariable and multivariable logistic regression and reported as odds ratios (ORs) and adjusted ORs (aORs), respectively, with 95% confidence intervals. Covariates (sex, age, disease extent, concurrent medical treatment) were selected based on potential biological associations with the outcome. Hence, in the multivariable models, variables were selected on basis of their potential relevance to the outcome rather than based on statistical significance levels. All tests were two‐tailed, and p‐values <0.05 were considered statistically significant. SPSS version 22 (IBM Corp., Armonk, NY) and R (version 4.1.1, R Core Team, New Zealand) with ggplot2 was used for statistical analysis. 29
Ethical consideration
This study was approved by the Swedish Ethical review Authority (2014/375‐31 and 2020–05060).
RESULTS
Some 133 ulcerative colitis patients, aged ≥18 years, without previous colectomy, who initiated on ustekinumab treatment were included in the analyses. Demographics and clinical characteristics at baseline are presented in Table 1. All but six patients had been treated with at least one anti‐TNF agent before the initiation of ustekinumab therapy, and only three were naïve to both biologics and tofacitinib.
TABLE 1.
Baseline characteristics of ustekinumab‐treated patients with ulcerative colitis and no previous colectomy (n = 133)
| Sex, male, n (%) | 80 (60) |
| Median age at start of ustekinumab, years (IQR) | 38 (28–48) |
| Median disease duration, years (IQR) | 7 (3–12) |
| Current smoker, n (%) a | 8 (6) |
| Disease extent, n (%) b | |
| Proctitis | 3 (2) |
| Left‐sided colitis | 34 (26) |
| Extensive colitis | 94 (71) |
| Median patient‐reported Mayo score (IQR) c | 4 (3–6) |
| Median faecal‐calprotectin, µg/g (IQR) d | 962 (384–1590) |
| Concomitant medication at baseline, n (%) | |
| 5‐Aminosalicylates | 71 (53) |
| Corticosteroids | 33 (25) |
| Immunomodulators | 36 (27) |
| Previous targeted therapy, n (%) | |
| ≥1 Ant‐TNF agent | 127 (95) |
| ≥2 Anti‐TNF agent | 62 (49) |
| ≥3 Anti‐TNF agent | 11 (9) |
| Vedolizumab | 88 (66) |
| Tofacitinib | 15 (11) |
| Reasons for termination of last targeted therapy, n (%) e | |
| Primary non‐response | 19 (15) |
| Secondary loss of response | 70 (55) |
| Adverse drug reaction | 4 (3) |
| Other | 1 (1) |
Abbreviations: IQR, interquartile range; TNF, tumour necrosis factor.
Immunomodulators were defined as thiopurines and methotrexate. Targeted therapy included anti‐TNF agents, vedolizumab and tofacitinib.
Data were missing for:
n = 21.
n = 2.
n = 52.
n = 72.
n = 21 patients.
Outcome at 16 weeks
The ustekinumab persistence rate at 16 weeks was 115/133 patients (86%). In total, 22/133 patients (17%) were in clinical remission, and 19/133 (14%) were in biochemical remission (Figure 1). However, 79/133 (59%) patients lacked data on clinical remission status at 16 weeks, and 80/133 (60%) on biochemical remission status. In total, 23 patients were on concomitant corticosteroid treatment at baseline, and five (22%) of these patients were in corticosteroid‐free clinical remission at 16 weeks, whereas information on clinical remission status was lacking for 10 patients. Of the patients with information on patient‐reported Mayo scores at both baseline and at 16 weeks, the patient‐reported Mayo score decreased from 4 (IQR 3–5) to 3 (IQR 2–4) (p = 0.03, n = 31). Compared to baseline, f‐calprotectin decreased from 875 μg/g (IQR 193–1561) to 239 μg/g (IQR 56–908) at 16 weeks (p = 0.04, n = 28). No statistically significant change in median CRP levels was observed between baseline [2.8 mg/L (IQR 2.0–8.0)] and week 16 [2.8 mg/L (IQR 1.0–4.5)] (p = 0.32, n = 29). A significant improvement in the “functional status” domain of the self‐reported SHS was observed (p = 0.02, Table 2), as well as a non‐significant amelioration in the remaining three domains (Table 2, n = 25).
FIGURE 1.
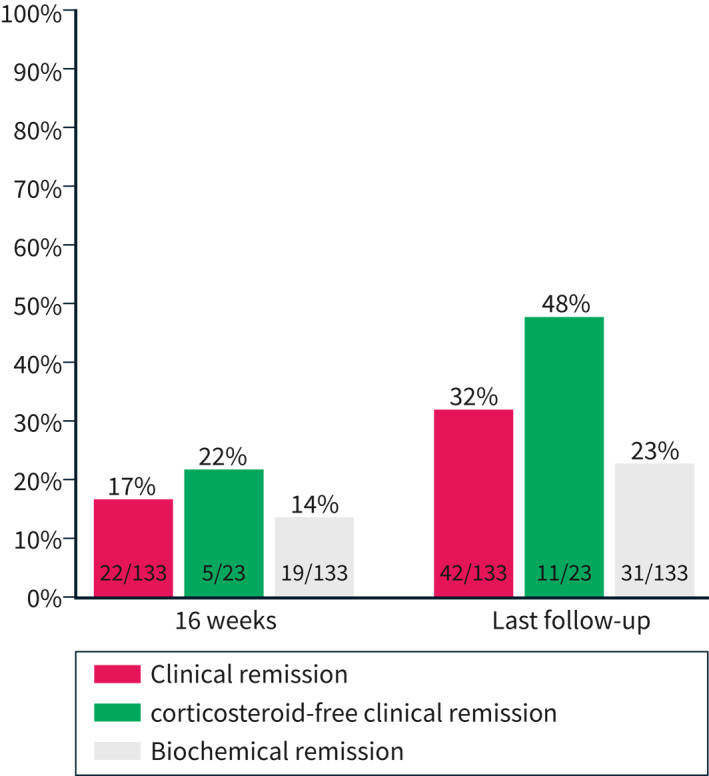
The proportion of ulcerative colitis patients with clinical and biochemical remission at week 16 and the last follow‐up
TABLE 2.
Short health scale (SHS) at baseline, 16 weeks and the last follow‐up in patients with ulcerative colitis
| Baseline (n = 82) | 16 weeks (n = 25) | p‐value | Last follow‐up (n = 46) | p‐value | |
|---|---|---|---|---|---|
| Symptom burden, median (IQR) | 2 (1–3) | 1 (1–2) | 0.08 | 1 (0–2) | <0.01 |
| Functional status, median (IQR) | 2 (1–3) | 1 (1–2) | 0.02 | 1 (1–2) | <0.01 |
| Disease‐related worry, median (IQR) | 2 (1–3) | 1 (1–2) | 0.09 | 1 (1–3) | <0.01 |
| General wellbeing, median (IQR) | 2 (2–3) | 2 (1–2) | 0.45 | 2 (1–2) | <0.01 |
Note: Comparisons between baseline and 16 weeks (n = 25), baseline and the last follow‐up (n = 46), were performed using the Wilcoxon matched‐pairs signed‐rank test. Bold values denote statistical significance at the p < 0.05 level.
Abbreviation: IQR, interquartile range.
Outcome at the last follow‐up
The rate of persistence of ustekinumab treatment was 89/133 (67%) patients after a median follow‐up of 32 (IQR 19–56) weeks (Figure 2). A stratified post‐hoc analysis by sex is shown in Supplementary Figure S1. Reasons for termination of ustekinumab treatment (missing data n = 6) were lack of or loss of response (n = 32), intolerance (n = 2) and other reasons (n = 4). Information on clinical remission at the last follow‐up was missing for 27/133 (20%) patients. Moreover, 31/133 (23%) patients were in biochemical remission, whereas 39/133 (29%) lacked data on this outcome. Of the 23 patients with corticosteroid therapy at baseline, 11 (48%) were in corticosteroid‐free clinical remission at the last follow‐up (Figure 1). The patient‐reported Mayo score decreased from 4 (IQR 3–5) at baseline to 2 (IQR 2–4) at the last follow‐up (p < 0.01, n = 47). The corresponding median f‐calprotectin concentrations decreased from 740 μg/g (IQR 278–1510) to 98 μg/g (IQR 32–663) (p < 0.01, n = 37). No statistically significant change in median CRP levels was observed between baseline (2.8 mg/L, IQR 1.0–6.1) and the last follow‐up (2.8 mg/L, IQR 1.0–5.3) (p = 0.54, n = 43). Compared to baseline, statistically significant improvements were seen in all four domains of the SHS at the last follow‐up (n = 46) (Table 2).
FIGURE 2.

Kaplan‐Meier curve illustrating ustekinumab persistence in 133 patients with ulcerative colitis. Median follow‐up time 32 weeks (interquartile range 19–56)
Predictors of ustekinumab persistence at 16 weeks
Univariable analyses of predictors showed that male sex was significantly associated with persistence to ustekinumab at 16 weeks (OR = 3.61, 95% CI: 1.26–10.33) (Table 3) and the association remained significant in multivariable analyses (aOR = 4.00, 95%CI: 1.35–11.83) (Table 3). The analyses did not include exposure to biologics and tofacitinib at baseline, as only three patients were naïve to such treatment.
TABLE 3.
Baseline predictors of ustekinumab persistence at 16 weeks in patients with ulcerative colitis (n = 133)
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| Sex (male) | 3.61 (1.26–10.33) | 0.02 | 4.00 (1.35–11.83) | 0.01 |
| Age | 0.98 (0.95–1.02) | 0.30 | 0.98 (0.94–1.02) | 0.29 |
| Disease extent | ||||
| Proctitis or left‐sided colitis | Reference | Reference | ||
| Extensive colitis | 1.24 (0.43–3.59) | 0.69 | 1.15 (0.38–3.52) | 0.80 |
| Concurrent medical therapy | ||||
| 5‐Aminosalicylates | 1.51 (0.56–4.12) | 0.42 | 1.51 (0.52–4.40) | 0.45 |
| Corticosteroids | 1.18 (0.36–3.87) | 0.79 | 1.04 (0.29–3.72) | 0.95 |
| Immunomodulators a | 2.01 (0.55–7.41) | 0.29 | 1.94 (0.49–7.67) | 0.35 |
Note: Bold values denote statistical significance at the p < 0.05 level.
Abbreviations: CI, confidence intervals; OR, odds ratio.
Thiopurines or methotrexate.
DISCUSSION
In this nationwide real‐world study, based on prospectively collected data from SWIBREG, we observed a high ustekinumab persistence rate in patients with ulcerative colitis, both after induction treatment (i.e. 16 weeks) and after a median follow‐up of 32 weeks. The observed persistence rates are supported by improvements in f‐calprotectin levels, patient‐reported Mayo scores and HRQoL measures in patients who continued ustekinumab treatment. Collectively, these real‐world findings indicate that ustekinumab treatment is associated with improved clinical outcomes, even though most patients had previously experienced treatment failure of biologics or tofacitinib.
The drug persistence rate of 67% at the last follow‐up in this study can be compared to the results of the randomised phase III UNIFI trial. In that trial, 87% of the patients with ulcerative colitis who were re‐randomised to ustekinumab maintenance treatment and completed the trial were still treated with ustekinumab after 1 year 13 In contrast to our real‐world study, only patients who responded to induction therapy with intravenous ustekinumab continued with maintenance therapy in the pivotal trial. 13 In addition, 49% of patients enrolled in the UNIFI trial were naïve to biologics, whereas only 2% of patients in our cohort had not previously been treated with biologics or tofacitinib. These differences may in part explain the observed difference in ustekinumab persistence rate in our cohort compared to the UNIFI maintenance trial.
Few real‐world studies have examined clinical outcomes of ustekinumab treatment in patients with ulcerative colitis. 14 , 15 , 16 , 17 , 18 In line with our result, improvements in clinical outcomes, such as the partial Mayo score, have previously been reported. 14 , 15 , 16 , 18 Amiot et al. observed an improvement in the partial Mayo score and CRP concentrations 12–16 weeks after initiating ustekinumab treatment in 103 French patients with ulcerative colitis. 14 Compared to our data, in which 14% had discontinued ustekinumab at 16 weeks, only two of the 103 French patients terminated ustekinumab before week 16. The disparity in ustekinumab persistence rates may indicate differences in overall disease severity between the cohorts, although most patients in the French cohort had also failed multiple biologics. On the other hand, the authors reported a persistence rate of only 56% after 52 weeks in a recent follow‐up, 18 suggesting that differences in early treatment management may help explain the disparity at 16 weeks. Consistent with our results, a drug persistence rate of 64% was observed in 95 ulcerative colitis patients in the ENEIDA register after a median follow‐up of 31 (IQR 18–59) weeks. 15 The Spanish ENEIDA register holds information on 69,000 IBD patients and shares some features with SWIBREG, including data on demographics, clinical characteristics and treatments. 30 Also, the clinical remission rates seemed to be comparable between the Spanish (33%) and Swedish cohorts (32%) despite that the assessment of remission status in the Spanish study was restricted to patients followed for at least 52 weeks 15 Chiapetta et al. reported improvements in the partial Mayo score and CRP in a cohort of 68 Italian patients treated with ustekinumab, of whom 38 were followed for 52 weeks 16
Reported real‐world ustekinumab persistence rates 14 , 15 , 16 , 18 are promising and warrant further studies to identify clinical predictors of response and remission. In the multivariable analysis of predictors of treatment persistence at 16 weeks only male sex remained statistically significant. Amiot et al. did not include sex in their analyses of potential predictors of steroid‐free clinical remission at 12–16 weeks after the initiation of ustekinumab. 14 However, a similar influence of male sex has been reported in an Australian cohort of Crohn's disease patients treated with ustekinumab. 31 A biological mechanism underlying the difference in ustekinumab retention rates between males and females has not been identified. In theory, some women may stop treatment because of pregnancy, but in the present study none of the patients discontinued ustekinumab for this reason. Inconsistent results are reported in the literature, although a lower drug persistence rate has been observed in female IBD patients receiving vedolizumab in Sweden 32 and some cohorts of patients with Crohn's disease treated with golimumab. 33 In an additional analysis in which ustekinumab persistence was assessed beyond week 16 the difference seemed to diminish.
This study represents the largest real‐world cohort of patients with ulcerative colitis treated with ustekinumab and is the first to provide new data describing the relationship between HRQoL and ustekinumab treatment. Patients with IBD, including ulcerative colitis, commonly experience reduced quality of life compared to healthy individuals, 34 and improvement of the HRQoL is one of the primary treatment goals. Therefore, it is essential to examine whether treatments improve HRQoL outside of the setting of a placebo‐controlled trial, where the inclusion is restricted to a selected group of eligible patients. Using the SHS, we noted an improvement in HRQoL after a median follow‐up of 32 weeks in patients who continued ustekinumab treatment. We also observed a significant improvement in the “functional status” domain of the SHS already at 16 weeks. These novel findings strengthen our study and are reassuring for patients with ulcerative colitis who start ustekinumab in clinical practice. Another strength of this study is that we included all the ustekinumab‐treated patients with ulcerative colitis in Sweden, as documented in SWIBREG, representing university and regional hospitals. The use of SWIBREG (a nationwide quality register) and that the data were prospectively reported to the register enhance the generalisability of our findings. The lack of a control group represents a major limitation of the study and precludes the possibility of drawing firm conclusions about ustekinumab's effects. In contrast to randomised clinical trials in which the study protocol predetermines the duration, the period to the most recent follow‐up varied between the patients in our study. This design may limit the generalisability of some findings. Compared to the life‐long disease, the median follow‐up of 32 weeks was relatively short. Because of the observational design, assessment of treatment outcomes was not compulsory and a considerable proportion of patients lacked data on various outcomes during follow‐up. Reported remission rates may underestimate the actual rates because we applied an intention‐to‐treat approach, and reported rates were based on non‐responder imputation, where missing data were treated as treatment failure. We cannot exclude that patients with missing data differ from those with complete data coverage; patients with severe disease are more likely to be frequently followed by the treating physician and thus more likely to have data reported during follow‐up. The analyses of changes in clinical disease activity, inflammatory markers and HRQoL were based on paired observations at baseline and during follow‐up. This methodology reduced the number of patients with available data and may have introduced bias and rendered low statistical power. Endoscopy is considered the gold standard for the assessment of disease activity in ulcerative colitis. However, in clinical practice the rate of endoscopic examinations is often lower than in clinical trials because of patient preference. Therefore, proxies of endoscopic activity, such as f‐calprotectin, are often used to assess disease activity in clinical practice. Data on endoscopic activity were insufficient to allow any meaningful analyses. However, data on f‐calprotectin at baseline and at last follow‐up were available for approximately one third of all patients. In our cohort, 23% were in biochemical remission, defined as an f‐calprotectin <250 µg/g, at the last follow‐up. In a previous study of 39 patients with ulcerative colitis an f‐calprotectin of >250 µg/g had a specificity of 100% and sensitivity of 71% to identify endoscopically active disease. 35
In conclusion, this nationwide real‐world study demonstrates that patients treated with ustekinumab had a relatively high drug persistence rate, even though almost all patients had failed biologics or tofacitinib. Treatment with ustekinumab was associated with improvements in clinical, biochemical and HRQoL measures, supporting its role as a valid treatment in ulcerative colitis.
AUTHOR CONTRIBUTIONS
Guarantor: Jonas Halfvarson had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Joel Thunberg, Olle Björkqvist, Carl Eriksson and Jonas Halfvarson. Acquisition of data: Jonas Halfvarson, Ola Olén, Jonas F Ludvigsson. Draughting of the manuscript: Joel Thunberg, Olle Björkqvist, Carl Eriksson and Jonas Halfvarson. Statistical analysis: Joel Thunberg, Carl Eriksson and Jonas Halfvarson. Interpretation of data and critical revision of the manuscript for important intellectual content: All authors.
CONFLICTS OF INTEREST
Joel Thunberg has nothing to declare. Olle Björkqvist has nothing to declare. Charlotte R. H. Hedin has received speaker fees from Takeda, Ferring, AbbVie, and Janssen, and consultancy fees from Pfizer. She has acted as local principal investigator for clinical trials for Janssen and GlaxoSmithKline. She is PI on projects at the Karolinska Institutet partly by investigator‐initiated grants from Takeda and Tillotts. None of these activities have any relation to the present study. Anders Forss has served as a speaker for Janssen. Charlotte Söderman has nothing to declare. Daniel Bergemalm reports personal fees from Ferring, Takeda, Janssen and BMS outside the submitted work. Jonas F Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). That study has received funding from the Janssen corporation. Ola Olén has been PI on projects at Karolinska Institutet partly financed by investigator‐initiated grants from Janssen and Ferring and reported a grant from Pfizer in a national safety monitoring programme. None of these studies have any relation to the present study. Karolinska Institutet has received fees for lectures and participation on advisory boards by OO from Janssen, Ferring, Takeda and Pfizer on topics not related to the present study. Henrik Hjortswang has served on the advisory board to AbbVie, Fresenius Kabi, Janssen, Norgine, Pfizer, Takeda and Tillotts. Henrik Hjortswang has served as a speaker for AbbVie, Janssen, Takeda and Tillotts. Hans Strid has served as a speaker or as an advisory board member for AbbVie, Ferring, Janssen, Pfizer, Takeda, Gilead and Tillotts Pharma. Carl Eriksson received grant support/lecture fee/advisory board from Takeda, Janssen Cilag, Pfizer and AbbVie. Jonas Halfvarson has served as a speaker, consultant or advisory board member: AbbVie, Aqilion, Celgene, Celltrion, Dr Falk Pharma and the Falk Foundation, Ferring, Galapagos, Gilead, Index Pharma, Janssen, Lincs, MSD, Novartis, Olink Proteomics, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, and received grant support from Janssen, MSD and Takeda.
ETHICAL APPROVAL
This study was approved by the Swedish Ethical Review Authority (registration number 2014/375‐31 and 2020‐05060) on 26 October 2020.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
We acknowledge the members of the SWIBREG study group: Olof Grip: Department of Gastroenterology, Skåne University Hospital, Malmö, Sweden. Susanna Jäghult: Södersjukhuset, Karolinska Institutet, Stockholm Sweden. Pär Myrelid: Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden; Department of Surgery, County Council of Östergötland, Linköping, Sweden. Adam Carstens: Department of Internal Medicine, Ersta Hospital, Stockholm, Sweden. Michael Eberhardson: Department of Gastroenterology and Hepatology, Linköping University, Linköping, Sweden. Martin Rejler: Department of Medicine, Höglandssjukhuset Eksjö, Region Jönköping County Council, Jönköping, Sweden; Jönköping Academy for Improvement of Health and Welfare, Jönköping University, Jönköping, Sweden. Caroline Nordenvall: Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; Department of Colorectal Cancer, Karolinska University Hospital, Stockholm, Sweden. Pontus Karling: Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden. Malin Olsson: Department of Surgery, County Council of Östergötland, Linköping, Sweden. Ulrika L. Fagerberg: Centre for Clinical Research, Västmanland Hospital, Västerås, Sweden and Uppsala University, Uppsala, Sweden; Department of Pediatrics, Västmanland Hospital, Sweden; Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden. This work was funded by Janssen Cilag as an investigator‐initiated study (CNTO1275UCO0001); the Regional Agreement on Medical Training and Clinical Research between Region Örebro County and Örebro University: ALF (grant number OLL‐836791 to CE and grant number OLL‐961742 to OB); The study design, the interpretation of the data and draughting of the manuscript were made by the authors without contribution from any of the funding organisations.
Thunberg J, Björkqvist O, Hedin CRH, Forss A, Söderman C, Bergemalm D, et al. Ustekinumab treatment in ulcerative colitis: real‐world data from the Swedish inflammatory bowel disease quality register. United European Gastroenterol J. 2022;10(7):633–41. 10.1002/ueg2.12275
Collaborators are listed in the acknowledgement section.
DATA AVAILABILITY STATEMENT
No additional data are available due to the Swedish data protection regulation.
REFERENCES
- 1. Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European federation of Crohn’s and ulcerative colitis associations (EFCCA) patient survey. J Crohn's Colitis. 2007;1(1):10–20. 10.1016/j.crohns.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 3. Nordin K, Påhlman L, Larsson K, Sundberg‐Hjelm M, Lööf L. Health‐related quality of life and psychological distress in a population‐based sample of Swedish patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;37(4):450–7. 10.1080/003655202317316097 [DOI] [PubMed] [Google Scholar]
- 4. Neovius M, Arkema EV, Blomqvist P, Ekbom A, Smedby KE. Patients with ulcerative colitis miss more days of work than the general population, even following colectomy. Gastroenterology. 2013;144(3):536–43. 10.1053/j.gastro.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 5. Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology : Off. J Am Gastroenterol Assoc. 2015;148(5):1035–58. 10.1053/j.gastro.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 6. Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology : Off J Am Gastroenterol Assoc. 2007;132(1):52–65. 10.1053/j.gastro.2006.11.041 [DOI] [PubMed] [Google Scholar]
- 7. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–9. 10.1016/s0140-6736(02)08512-4 [DOI] [PubMed] [Google Scholar]
- 8. Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human anti–tumor necrosis factor monoclonal antibody (Adalimumab) in Crohn’s disease: the CLASSIC‐I trial. Gastroenterology : Off. J Am Gastroenterol Assoc. 2006;130(2):323–33. 10.1053/j.gastro.2005.11.030 [DOI] [PubMed] [Google Scholar]
- 9. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76. 10.1056/nejmoa050516 [DOI] [PubMed] [Google Scholar]
- 10. Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohn's Colitis. 2022;16(1):2–17. 10.1093/ecco-jcc/jjab178 [DOI] [PubMed] [Google Scholar]
- 11. Benson JM, Peritt D, Scallon BJ, Heavner GA, Shealy DJ, Giles‐Komar JM, et al. Discovery and mechanism of ustekinumab. mAbs. 2011;3(6):535–45. 10.4161/mabs.3.6.17815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stelara ema.europa.eu. https://www.ema.europa.eu/en/medicines/human/EPAR/stelara%23
- 13. Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14. 10.1056/nejmoa1900750 [DOI] [PubMed] [Google Scholar]
- 14. Amiot A, Filippi J, Abitbol V, Cadiot G, Laharie D, Serrero M, et al. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: a GETAID multicentre real‐world cohort study. Aliment Pharmacol Ther. 2020;51(11):1039–46. 10.1111/apt.15717 [DOI] [PubMed] [Google Scholar]
- 15. Chaparro M, Garre A, Iborra M, Sierra‐Ausín M, Barreiro‐De Acosta M, Fernández‐Clotet A, et al. Effectiveness and safety of ustekinumab in ulcerative colitis: real‐world evidence from the ENEIDA registry. J Crohn's Colitis. 2021;15(11):1846–51. 10.1093/ecco-jcc/jjab070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiappetta MF, Viola A, Mastronardi M, Turchini L, Carparelli S, Orlando A, et al. One‐year effectiveness and safety of ustekinumab in ulcerative colitis: a multicenter real‐world study from Italy. Expet Opin Biol Ther. 2021;21(11):1–7. 10.1080/14712598.2021.1981855 [DOI] [PubMed] [Google Scholar]
- 17. Dalal RS, Esckilsen S, Barnes EL, Pruce JC, Marcus J, Allegretti JR, et al. Predictors and outcomes of ustekinumab dose intensification in ulcerative colitis: a multicenter cohort study. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fumery M, Filippi J, Abitbol V, Biron A, Laharie D, Serrero M, et al. Effectiveness and safety of ustekinumab maintenance therapy in 103 patients with ulcerative colitis: a GETAID cohort study. Aliment Pharmacol Ther. 2021;54(7):944–51. 10.1111/apt.16544 [DOI] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Andersson M, Bengtsson J, Eberhardson M, Fagerberg UL, Grip O, et al. Swedish inflammatory bowel disease register (SWIBREG) – a nationwide quality register. Scand J Gastroenterol. 2019;54(9):1089–101. 10.1080/00365521.2019.1660799 [DOI] [PubMed] [Google Scholar]
- 20. Befolkningsstatistik i sammandrag 1960–2020 SCB [cited 2022. https://www.scb.se/hitta‐statistik/statistik‐efter‐amne/befolkning/befolkningens‐sammansattning/befolkningsstatistik/pong/tabell‐och‐diagram/helarsstatistik‐‐riket/befolkningsstatistik‐i‐sammandrag/
- 21. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–67. 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. SWIBREG . Swedish inflammatory bowel disease registry; 2021. http://www.swibreg.se/
- 23. Jakobsson GL, Sternegård E, Olén O, Myrelid P, Ljung R, Strid H, et al. Validating inflammatory bowel disease (IBD) in the Swedish national patient register and the Swedish quality register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52(2):216–21. 10.1080/00365521.2016.1246605 [DOI] [PubMed] [Google Scholar]
- 24. Shrestha S, Olén O, Eriksson C, Everhov ÅH, Myrelid P, Visuri I, et al. The use of ICD codes to identify IBD subtypes and phenotypes of the Montreal classification in the Swedish national patient register. Scand J Gastroenterol. 2020;55(4):430–5. 10.1080/00365521.2020.1740778 [DOI] [PubMed] [Google Scholar]
- 25. Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal world congress of gastroenterology. Can J Gastroenterol. 2005;19(Suppl a):5A–36A. 10.1155/2005/269076 [DOI] [PubMed] [Google Scholar]
- 26. Jairath V, Khanna R, Zou GY, Stitt L, Mosli M, Vandervoort MK, et al. Development of interim patient‐reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment Pharmacol Ther. 2015;42(10):1200–10. 10.1111/apt.13408 [DOI] [PubMed] [Google Scholar]
- 27. Hjortswang H, Järnerot G, Curman B, Sandberg‐Gertzén H, Tysk C, Blomberg B, et al. The short health scale: a valid measure of subjective health in ulcerative colitis. Scand J Gastroenterol. 2006;41(10):1196–203. 10.1080/00365520600610618 [DOI] [PubMed] [Google Scholar]
- 28. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. 10.1080/1047322x.1990.10389587 [DOI] [Google Scholar]
- 29. Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer‐Verlag; 2016. [Google Scholar]
- 30. Gutiérrez A, Zapater P, Ricart E, González‐Vivó M, Gordillo J, Olivares D, et al. Immigrant IBD patients in Spain are younger, have more extraintestinal manifestations and use more biologics than native patients. Front Med. 2022;9. 10.3389/fmed.2022.823900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chien TH, Puig A, Khuong T, Kouhkamari MH, Che S, Huang TH.‐W, et al. An Australian real‐world study of treatment persistence of ustekinumab in Crohn’s disease. Biol Targets & Ther. 2021;15:237–45. 10.2147/btt.s310076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eriksson C, Marsal J, Bergemalm D, Vigren L, Björk J, Eberhardson M, et al. Long‐term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish national quality registry for inflammatory bowel disease (SWIBREG). Scand J Gastroenterol. 2017;52(6‐7):722–9. 10.1080/00365521.2017.1304987 [DOI] [PubMed] [Google Scholar]
- 33. Rundquist S, Eriksson C, Nilsson L, Angelison L, Jäghult S, Björk J, et al. Clinical effectiveness of golimumab in Crohn’s disease: an observational study based on the Swedish national quality registry for inflammatory bowel disease (SWIBREG). Scand J Gastroenterol. 2018;53(10‐11):1257–63. 10.1080/00365521.2018.1519597 [DOI] [PubMed] [Google Scholar]
- 34. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO‐ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohn's Colitis. 2019;13(2):144–64K. 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 35. DʼHaens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2218–24. 10.1002/ibd.22917 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
No additional data are available due to the Swedish data protection regulation.


