Abstract
Acute kidney injury (AKI) is a significant clinical complication with a substantial impact on morbidity and mortality, for which therapeutic options remain limited. The Hippo signaling pathway is an evolutionarily conserved pathway implicated in cell proliferation, dedifferentiation, and apoptosis via phosphorylation and inactivation of its downstream effectors Yes-associated protein (YAP)/ transcriptional co-activator with PDZ-binding motif (TAZ). Recent studies have revealed that the Hippo pathway plays a pivotal role in the pathogenesis and repair of AKI. The Hippo pathway can mediate renal dysfunction through modulation of mitochondrial apoptosis under AKI conditions. Transient activation of YAP/TAZ in the acute phase of AKI may benefit renal recovery and regeneration, whereas persistent activation of YAP/TAZ in severe AKI may lead to maladaptive repair and transition to chronic kidney disease. This review aims to summarize recent findings on the associations between the Hippo pathway and AKI and to identify new therapeutic targets and strategies for AKI.
Keywords: Hippo pathway, Acute kidney injury (AKI), Maladaptive repair, Fibrosis, Chronic kidney disease (CKD)
INTRODUCTION
Acute kidney injury (AKI) remains a major medical challenge with high morbidity, mortality, and healthcare costs (Zuk & Bonventre, 2019). Apart from supportive treatment, few preventive and therapeutic options currently exist (Lameire et al., 2013). Among survivors, long-term outcomes of AKI include development of chronic kidney disease (CKD), end-stage renal disease (ESRD), and death (Lameire et al., 2013; Venkatachalam et al., 2015; Zuk & Bonventre, 2019). Incomplete recovery from AKI, also called maladaptive repair, can lead to long-term functional deficits and CKD. The progression of adaptive to maladaptive kidney repair in AKI leading to CKD can be attributed to G2/M cell cycle arrest, profibrotic cytokine secretion, myofibroblast generation, extracellular matrix (ECM) production, and inflammation (Ferenbach & Bonventre, 2015; Yu & Bonventre, 2020).
The mammalian Hippo pathway is a serine/threonine kinase cascade composed of mammalian sterile 20-like 1/2 (MST1/2), large tumor suppressor homolog 1/2 (LATS1/2), and Yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ) (Zhao et al., 2011b). It regulates cell growth and fate determination, organ size, and regeneration through the phosphorylation and inactivation of its downstream effectors YAP/TAZ (Ma et al., 2019). Recent studies have implicated the Hippo pathway in the pathogenesis and repair of AKI and transition to CKD. In this review, we summarize current findings on the correlations between the Hippo pathway and kidney injury and shed some light on future treatment for acute or chronic kidney diseases.
HIPPO PATHWAY IN MAMMALS: A KINASE CASCADE
The Hippo pathway is highly evolutionarily conserved in mammals and consists of a three-step kinase cascade of MST1/2 (also called STK4/3), LATS1/2, and YAP/TAZ (also called WWTR1). Upon Hippo pathway activation, Salvador homolog 1 (Sav1) interacts with and is phosphorylated by MST1/2, thereby acting as a partner of MST1/2 to promote phosphorylation of LATS1/2 (Callus et al., 2006; Tapon et al., 2002). LATS1/2 are activated by MST1/2 through multiple mechanisms: MST1/2 can directly phosphorylate LATS1/2 at the C-terminal hydrophobic motif (Thr1079 for LATS1 and Thr1041 for LATS2), promoting LATS1/2 autophosphorylation at its activation loop. Furthermore, MST1/2-phosphorylated Mps one binder 1 (MOB1) binds to the autoinhibitory domain of LATS1/2, leading to full activation of LATS1/2 (Callus et al., 2006; Chan et al., 2005; Praskova et al., 2004; Wu et al., 2003). Neurofibromin 2 (NF2) can also directly interact with and recruit LATS1/2 to the plasma membrane for phosphorylation by MST1/2 (Yin et al., 2013; Zhang et al., 2010). Activated LATS1/2 then phosphorylate serine on the YAP/TAZ HXRXXS motifs (Hao et al., 2008; Lei et al., 2008; Liu et al., 2010; Zhao et al., 2007, 2010). Phosphorylation of YAP Ser127 and TAZ Ser89 generates 14-3-3 binding sites, and binding to 14-3-3 results in cytoplasmic sequestration (Dong et al., 2007; Zhao et al., 2007). In addition, phosphorylation of YAP Ser381 and TAZ Ser311 leads to subsequent phosphorylation by casein kinase 1 (CK1) and activation of phosphodegron, resulting in the recruitment of SCFbeta-TRCP E3 ubiquitination ligase and proteasomal degradation of both YAP and TAZ (Liu et al., 2010; Zhao et al., 2010).
When upstream kinases of the Hippo pathway are inactivated, YAP/TAZ are dephosphorylated and activated. As transcriptional co-activators, YAP/TAZ enter the nucleus and bind with TEAD family transcription factors (TEAD 1–4) to induce the expression of target genes (Zhao et al., 2008), such as CTGF, Cyr61, ANKRD1, DIAPH3, amphiregulin, and survivin, thereby improving cell proliferation and inhibiting cell death (Bai et al., 2012; Rinschen et al., 2017). Vestigial-like family member 4 (VGLL4) competes with YAP/TAZ for TEAD binding in the nucleus and represses target gene expression (Figure 1) (Jiao et al., 2014; Zhang et al., 2014). The association between the Hippo pathway and AKI has attracted increasing attention, and the effects of Hippo pathway component activation on AKI are summarized in Table 1.
Figure 1.
Hippo pathway in mammalian cells
When Hippo signaling is inactive, YAP/TAZ enters the nucleus and competes with VGLL4 for TEAD binding, thus inducing target gene transcription. When Hippo signaling is active, YAP/TAZ is phosphorylated by LATS1/2, leading to 14-3-3-mediated YAP and TAZ cytoplasmic retention and SCF-mediated YAP/TAZ polyubiquitination and degradation. Soluble factors, mainly including ligands for GPCRs, act through Rho GTPases and F-actin to regulate LATS1/2 activity. Mechanical stress regulates Hippo pathway by adjusting Rho GTPases activity and F-actin dynamics. Both apical and basolateral spectrin networks function as sensors for mechanical stress in Hippo pathway regulation. Arrows and blunt ends indicate activation and inhibition, respectively. Dashed lines indicate unknown mechanisms.
Table 1. Possible role of Hippo pathway component activation in AKI.
| Component | Model | Species | Effect | Role | References |
| AKI: Acute kidney injury; I/R injury: Ischemia-reperfusion injury; AA: Aristolochic acid; UA: Uranyl acetate; UUO: Unilateral ureter obstructive. | |||||
| MST1 | I/R injury | Mouse | MST1 up-regulation accelerates progression of AKI via activation of mitochondrial fission. | Detrimental | Li et al., 2019 |
| I/R injury | Mouse | Genetic ablation of MST1 attenuates tubular epithelia apoptosis and kidney injury by repressing mitochondrial fission. | Detrimental | Feng et al., 2018a | |
| SAV1 | UUO | Mouse | SAV1 depletion in RTECs exhibits aggravated renal TIF. | Protective | Seo et al., 2016 |
| AA | Mouse | SAV1 loss results in up-regulated expression of profibrotic genes and TIF. | Protective | Leung et al., 2017 | |
| TAZ | I/R injury | Mouse | TAZ expression ameliorates tubular injury and reduces apoptosis in kidney after I/R injury. | Protective | Wu et al., 2020 |
| AA | Mouse | TAZ is activated in fibrosis through TGF-β1-dependent mechanisms and sustained TAZ signaling promotes epithelial maladaptive repair. | Protective | Anorga et al., 2018 | |
| YAP | UA | Rat | Transient overexpression of YAP in renal epithelia recovered from AKI inhibits apoptosis and results in acquired cytoresistance. | Protective | Iwakura et al., 2017 |
| I/R injury | Mouse | YAP activation after AKI mediates renal epithelial cell regeneration. | Protective | Chen et al., 2018 | |
| I/R injury | Rat | Transient YAP activation may promote repair of injured epithelia. Constant YAP activation may lead to interstitial fibrosis and abnormal renal tubule differentiation. |
Transient: Protective Persistent: Detrimental |
Xu et al., 2016 | |
| I/R injury | Mouse | YAP activation in post-acute phase of AKI is implicated in renal dysfunction and TIF. | Persistent: Detrimental |
Xu et al., 2021 | |
| I/R injury | Mouse | Tubular YAP persistent activation is associated with maladaptive repair and interstitial macrophage infiltration. | Persistent: Detrimental |
Zheng et al., 2021 | |
| YAP/TAZ | I/R injury/UUO | Mouse | YAP/TAZ activation promotes macrophage M2 polarization and contributes to kidney fibrosis. | Detrimental | Feng et al., 2018b |
| UUO | Mouse | YAP/TAZ knockout suppresses UUO-induced ECM deposition, myofibroblast accumulation and TIF. | Detrimental | Liang et al., 2017 | |
HIPPO PATHWAY REGULATION
In mammalian cells, the Hippo pathway is regulated by a variety of intrinsic and extrinsic factors. Cell polarity, cell-cell junctions, soluble factors, mechanical cues, and metabolic states, such as cellular energy and oxygen stress, can modulate Hippo pathway activity (Boggiano & Fehon, 2012; Meng, 2016; Mo et al., 2015; Zhao et al., 2011b). In particular, mechanical cues, such as the ECM and cellular geometry, are potent regulators (Dupont et al., 2011). For example, YAP/TAZ can be activated when cells are cultured on a matrix with high stiffness or large surface area. In addition, high density of round and compact cells can lead to YAP/TAZ activation. Conversely, low density of flat and loose cells can result in Hippo pathway inactivation, allowing YAP/TAZ to enter the nucleus and promote transcription of target genes (Aragona et al., 2013; Wada et al., 2011). Both apical and basolateral spectrin networks play regulatory roles in the Hippo pathway and function as sensors of mechanical cues (Deng et al., 2015; Fletcher et al., 2015).
Soluble factors act primarily through G protein-coupled receptors (GPCRs) in the regulation of the Hippo pathway. GPCRs are the largest family of plasma membrane receptors, linking the Hippo pathway to a broad range of upstream signals. GPCR ligands that signal through Gα12/13 or Gαq/11, such as lysophosphatidic acid (LPA), angiotensin II, and thrombin, increase YAP/TAZ nuclear entry by activating Rho GTPases; whereas ligands that signal through Gαs, such as epinephrine and glucagon, can activate LATS1/2 and repress YAP/TAZ via the AC-cAMP-PKA pathway (Yu et al., 2012, 2013; Zhou et al., 2015). Rho GTPases and actin cytoskeleton remodeling are key mediators of GPCR ligands and mechanical cues in the regulation of the Hippo pathway (Figure 2) (Dupont et al., 2011; Zhao et al., 2012).
Figure 2.
YAP/TAZ induced negative feedback loop in regulating Hippo pathway homeostasis
YAP/TAZ in complex with TEADs directly induce LATS2 and NF2 transcription and indirectly stimulate LATS1/2 kinase activity through NF2 and AMOTL2 up-regulation. YAP/TAZ-induced LATS activation can increase AMOT phosphorylation and protein accumulation. AMOT can directly bind to and inhibit YAP/TAZ and indirectly activate LATS. YAP/TAZ is also reported to up-regulate MST1.
In addition to GPCR ligands, the Hippo pathway also participates in crosstalk with Wingless/Ints (Wnt) (Bernascone & Martin-Belmonte, 2013), transforming growth factor (TGF)-β (Feng et al., 2018b; Seo et al., 2016), bone morphogenetic proteins (BMPs), Notch, Hedgehog (Hh) (Hansen et al., 2015), Src (Kim et al., 2019), Rac-GTPase activating protein 1 (RacGAP1) (Zhou et al., 2020), Gsk3β-p53 (Li et al., 2019), and EGF receptor (EGFR)-phosphatidylinositol 3-kinase (Pl3K)-Akt (Chen et al., 2018), which modulate YAP/TAZ activities and collectively control tissue growth and development. The EGFR-PI3K-Akt pathway and RacGAP1 can help renal tubular epithelial cells (RTECs) recover from acute injury by activating YAP (Chen et al., 2018; Zhou et al., 2020), whereas Wnt5a can exacerbate TGF-β1-induced macrophage M2 polarization and renal fibrosis through YAP/TAZ up-regulation (Feng et al., 2018b). These Hippo pathway regulatory factors may provide potential drug targets for the treatment of kidney injury.
NEGATIVE FEEDBACK LOOP OF HIPPO PATHWAY
Long-term hyperactivation of YAP/TAZ can lead to massive tissue overgrowth, enhanced stem cell-like properties that promote tumorigenic potential, and increased metastasis by epithelial-mesenchymal transition (EMT) (Harvey et al., 2013; Lei et al., 2008). Therefore, YAP/TAZ activity must be tightly controlled to maintain tissue homeostasis. In mammalian cells, the Hippo pathway exhibits a negative feedback mechanism. YAP/TAZ and TEAD complexes directly induce the transcription of NF2 and LATS2, and indirectly stimulate LATS1/2 kinase activity by increasing NF2 protein abundance. A reciprocal negative regulation also exists between YAP and TAZ dependent on LATS and proteasomal degradation (Moroishi et al., 2015; Park et al., 2016). YAP/TAZ-induced LATS activation can lead to increased angiomotin (AMOT) phosphorylation and protein accumulation. AMOT can directly inhibit YAP/TAZ and indirectly activate LATS, thus contributing to the negative feedback loop (Zhao et al., 2011a). Furthermore, MST1 and AMOT-like 2 (AMOTL2) are up-regulated by YAP/TAZ, and AMOTL2 can also activate LATS (Kim et al., 2016; Paramasivam et al., 2011; Park et al., 2016). This negative feedback loop is essential for maintaining proper transient activation of YAP/TAZ in response to stimuli and preventing tumorigenesis. Aberrant increase of O-GlcNAcylation on LATS2 can disrupt the LATS-mediated negative feedback loop, leading to YAP/TAZ hyperactivation and carcinogenesis (Kim et al., 2020).
RENAL DYSFUNCTION CAN BE EXACERBATED BY HIPPO PATHWAY-MEDIATED MITOCHONDRIAL FISSION FOLLOWING AKI
The kidney is second only to the heart in mitochondrial count and oxygen consumption (Duann & Lin, 2017). Mitochondria undergo significant changes after AKI, which affects the pathophysiology and recovery of renal function (Baligand et al., 2017; Funk & Schnellmann, 2012; Lan et al., 2016). Persistent disruption of mitochondrial homeostasis and sustained tubular damage can result in progression to CKD (Jiang et al., 2020). The pathogenic mechanisms of mitochondrial dysfunction include mitochondrial DNA damage (Lee & Back, 2017), mitophagy disturbance (Duann et al., 2016), and biosynthesis disorder (Xiao et al., 2017). RTECs are rich in mitochondria and the proximal tubule is especially vulnerable to mitochondrial damage (Hall et al., 2009). Mitochondrial apoptosis is considered the main reason for reperfusion-mediated RTEC apoptosis under renal ischemia/reperfusion (I/R) injury, attributable to mitochondrial fission (Perry et al., 2018; Wang et al., 2020b).
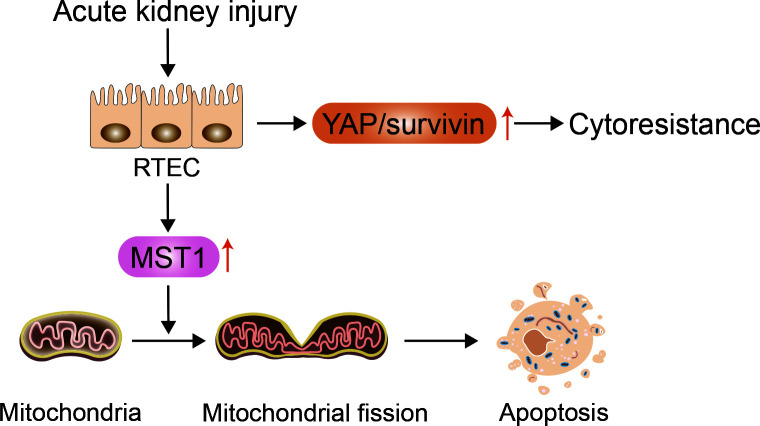
The core proteins that mediate mitochondrial fission and fusion include dynamin-related protein 1 (Drp1), mitofusin (Mfn), and optic atrophy 1 (OPA1). Drp1 mediates mitochondrial fission, whereas Mfn and OPA1 mediate fusion of the outer and inner membranes, respectively (Lee & Yoon, 2016). Li et al. (2019) demonstrated that MST1 is markedly up-regulated after renal I/R injury and that higher MST1 expression is associated with Gsk3β-p53 axis activation, which induces mitochondrial fission by Drp1 phosphorylation and F-actin assembly. Mitochondrial fission contributes to mitochondrial potential reduction, reactive oxygen species (ROS) production, mitochondrial permeability transition pore (mPTP) opening, cytochrome c (cyt c) release, and caspase-9-related mitochondrial apoptosis initiation, leading to RTEC apoptosis and renal dysfunction. Feng et al. (2018a) also showed that MST1 expression is up-regulated in response to renal I/R injury and that genetic ablation of MST1 improves renal function, alleviates reperfusion-mediated RTEC apoptosis, and attenuates kidney vulnerability to I/R injury. Moreover, increased MST1 blocks the AMPK-YAP pathway, thereby diminishing OPA1 expression, repressing mitophagy, and activating mitochondrial apoptosis in reperfused kidneys. Reactivation of the AMPK-YAP-OPA1 signaling pathway provides a survival advantage for RTECs in the context of renal I/R injury by repressing mitochondrial fission (Figure 3). Based on these findings, we speculate that the Hippo pathway plays a vital role in the pathogenesis of AKI and that increased MST1 expression can mediate mitochondrial damage and RTEC apoptosis by modulating mitochondrial fission.
Figure 3.
Increase in YAP and MST1 following AKI can result in RTEC cytoresistance and apoptosis, respectively
RTECs suffering from acute injury transiently overexpress YAP and its downstream mediator survivin, resulting in acquired cytoresistance and apoptosis inhibition. MST1 is also markedly up-regulated after AKI and can mediate RTEC apoptosis through mitochondrial fission.
YAP OVEREXPRESSION IN RTECs CAN RESULT IN ACQUIRED CYTORESISTANCE AFTER AKI
Animals recovering from previous AKI are resistant to subsequent renal challenge with the same agent, termed acquired resistance (Honda et al., 1987). The emergence of acquired cytoresistance after AKI can serve to protect the kidneys from further ischemia or nephrotoxicity (Zager, 2013). This is closely associated with heat shock proteins (Furuya et al., 1997; Mizuno et al., 1997), hypoxia-inducible factor (HIF)-1α (Ma et al., 2009), heme oxygenase-1 (Nath, 2014), cyclin-dependent kinase inhibitor (CDKI) (Nishioka et al., 2014; Price et al., 2004), growth/proliferation factors (Sun et al., 2011), and DNA repair-related factors (Miyaji et al., 2001; Sano et al., 2000).
Iwakura et al. (2017) demonstrated that rat renal proximal tubule epithelial cells (RPTCs) that have recovered from uranyl acetate (UA)-induced AKI can develop cytoresistance to subsequent UA treatment. These cells transiently overexpress YAP and its downstream mediator survivin, which may inhibit apoptosis and increase acquired cytoresistance. These effects may be correlated with enhanced G1 arrest via modulation of cell-cycle regulating factors, including cyclin D1, p21, and p27 (Iwakura et al., 2016), and persist until YAP/survivin expression levels return to baseline (Figure 3). Furthermore, YAP overexpression leads to hyperplasia in RPTCs, while YAP regression leads to a gradual decline in RPTCs with enhanced cell cycle arrest. Based on these findings, considerable attention should be given to the relationship between RTEC cytoresistance and cellular recovery with YAP/survivin expression in AKI treatment.
TRANSIENT YAP/TAZ ACTIVATION PROMOTES RTEC ADAPTIVE REPAIR IN MILD/MODERATE AKI
Due to the high abundance of mitochondria, the metabolically active proximal tubule, especially the S3 segment, is a primary target of injury in AKI (Munshi et al., 2011). Renal stem cells can be a cell source for maintaining normal kidney cell turnover (Alexandre et al., 2009; Bruno et al., 2009; Morigi et al., 2008). Mouse kidney progenitor cells (MKPCs) are a unique renal stem cell population, which can contribute to renal repair and survival after ischemic injury (Lee et al., 2010). Notably, intrarenal injection of MKPCs isolated from the medullary and papillary interstitium in ischemic-injured mice can rescue renal damage, with some MKPCs forming vessels with red blood cells inside and some incorporating into renal tubules 7 days after injury. Intrinsic RTEC dedifferentiation, proliferation, and migration can also contribute to renal regeneration after AKI (Duffield & Humphreys, 2011; Lin, 2006). Surviving RTECs can regenerate and regain normal kidney function if the injury is mild and transient, but may exhibit maladaptive repair if the injury is severe and recurring, leading to renal fibrosis (Figure 4) (Ferenbach & Bonventre, 2015; Qi & Yang, 2018; Yang et al., 2011). Recent studies have demonstrated the pivotal role of the Hippo signaling pathway in RTEC recovery and regeneration after AKI.
Figure 4.
Schematic of Hippo pathway mediating renal repair after AKI
Mild/moderate kidney injury results in transient YAP/TAZ activation, which promotes adaptive repair and leads to complete renal recovery. Whereas severe/repeated kidney injury results in persistent YAP/TAZ activation, which causes kidney maladaptive/incomplete repair and tubulointerstitial fibrosis through EMT, fibroblast and myofibroblast transition, macrophage M2 phenotype polarization and infiltration, RTEC hypertrophy, and G2/M cell cycle arrest. This may eventually develop into CKD and ESRD.
Chen et al. (2018) reported enhanced YAP expression and nuclear distribution in the RPTCs of kidneys in patients with AKI and mice with I/R AKI. Furthermore, YAP antagonist verteporfin (VP)-treated and RPTC-specific YAP knockout mice both showed more severe kidney injury compared with the controls, indicating that EGFR-Pl3k-Akt signaling mediates YAP activation and subsequent target gene cyclin D and amphiregulin (AREG) expression. Cyclin D can induce the phosphorylation of the retinoblastoma protein (Rb), thus activating cyclin-dependent kinase (CDK) 4 and 6, which are important for G1 to S transition during cell proliferation (Hamilton & Infante, 2016; Musgrove et al., 2011). In addition, AREG acts as an EGFR ligand to further initiate EGFR-Akt-dependent YAP activation, thereby promoting adaptive repair of the kidney after AKI. Of note, Chen et al. (2018) also suggested that YAP, but not TAZ, is critical for renal recovery from I/R injury as histological recovery is delayed in the YAP/TAZ double knockout mice but not in the TAZ single knockout mice. In contrast, Wu et al. (2020) reported a beneficial role of tubular TAZ in ischemic AKI, inconsistent with the findings of Chen et al. (2018). They demonstrated that in vivo treatment with chloroquine, which can enhance TAZ expression in RPTCs, preserves renal function, ameliorates tubular injury, and reduces apoptosis in the renal cortex after I/R injury. In contrast, both TAZ antagonist VP treatment and TAZ knockdown exaggerate tubular apoptosis and renal failure after I/R injury. Further studies are essential to clarify the role of TAZ in RTECs after AKI and illustrate the potential therapeutic targets against AKI.
Transiently enhanced and evenly distributed YAP levels in the cytoplasm and nucleus are reported in mild I/R AKI rat models (Xu et al., 2016). Dedifferentiation markers, including vimentin, α-smooth muscle actin (α-SMA), and proliferating-cell nuclear antigen (PCNA), show similar expression patterns to YAP during recovery after AKI, whereas quiescent tubular markers, including Na+/K+-ATPase and aquaporin 1 (AQP1), are decreased or absent, indicating that YAP may promote the proliferation and redifferentiation of reconstituted epithelia after acute I/R AKI. Moreover, suppression of YAP1 by the N6-methyladenosine mRNA methylase METTL4 promotes renal I/R injury progression (Xu et al., 2020b). Inhibition of YAP/TAZ degradation and nuclear factor-κB (NF-κB) translocation through Rictor/mTORC2 signaling is crucial for preventing renal inflammation and lipopolysaccharide (LPS)-induced AKI, thus revealing the potent effects of YAP/TAZ on alleviation of AKI severity (Figure 4) (Gui et al., 2020).
PERSISTENT YAP/TAZ ACTIVATION IN SEVERE AKI LEADS TO MALADAPTIVE REPAIR AND CKD
The Hippo pathway may exhibit bidirectional functions during I/R AKI repair. Xu et al. (2016) demonstrated that YAP mainly elicits beneficial effects on the proliferation of injured RTECs during repair, but persistent activation of YAP can impede the redifferentiation of dedifferentiated tubular cells and promote kidney fibrosis. Notably, they found more pronounced YAP activation in rats four weeks after I/R injury in the incomplete repair (I/R 45 min) group than in the complete repair (I/R 30 min) group. Furthermore, connective tissue growth factor (CTGF), fibrotic protein mRNA (including type I, III, and IV collagen), and RTPCs in the G2/M-phase were up-regulated with YAP increase, indicating kidney fibrosis exacerbation. In addition, the incomplete repair (I/R 45 min) group treated with YAP agonist digitoxin showed more severe tubulointerstitial fibrosis (TIF) compared with the control group. Xu et al. (2021) also implicated YAP activation in the post-acute phase of AKI in renal dysfunction and TIF, although it elicits a beneficial effect in the acute phase. Transcription factor KLF4 can cause persistent activation of YAP, while inhibition of the KLF4-YAP pathway can attenuate IR-induced renal function deterioration and slow TIF progress by decreasing the expression levels of TGF-β and CTGF. Zheng et al. (2021) confirmed that severe ischemic AKI can induce tubular maladaptive repair and TIF by persistently activating YAP. YAP activation can further cause monocyte chemoattractant protein 1 (MCP-1) overexpression and promote renal macrophage inflammation, a driving force for progressive TIF and AKI-CKD transition.
Moreover, down-regulation of YAP can significantly suppress chronic renal fibrosis. Both in vivo and in vitro studies report that miR-101a can potentially slow chronic renal fibrosis by blockade of the YAP-TGF-β-Smad signaling pathway via KDM3A (Ding et al., 2020). Kindlin-2 depletion reduces MOB1 degradation and alleviates renal fibrosis via activation of Hippo/YAP signaling in Kindlin-2 knockout mice with unilateral ureteral obstruction (UUO) (Song et al., 2019). Most recently, knockdown of YTHDF1, a modulator of m6A methylation, was shown to alleviate progression of renal fibrosis in both cultured cells induced by TGF-β administration and UUO model mice via down-regulation of YAP (Xing et al., 2022). In conclusion, appropriate modulation of the Hippo signaling pathway, especially intervention in YAP overexpression during repair, may be a potent preventive target in AKI-CKD transition after I/R injury.
Kidney fibrosis can be induced through Hippo pathway-mediated EMT
The severity and frequency of RPTC injury can determine whether the repair mechanism leads to complete recovery or maladaptive repair (Takaori et al., 2016). Maladaptive repair, i.e., renal fibrosis, is characterized by cell cycle arrest at the G2/M phase and a senescence-associated secretory phenotype (SASP). RTECs may turn to a mesenchymal phenotype via EMT after injury, which is a potential source of profibrotic cytokines and ECM deposition, thus leading to gradual aggravation of renal fibrosis (Qi & Yang, 2018).
EMT is the process by which a polarized epithelial cell adopts a mesenchymal cell phenotype, and includes enhanced migratory ability, invasiveness, resistance to apoptosis, and secretion of ECM (Kalluri & Weinberg, 2009). EMT is manifested by a decrease in epithelial markers, such as E-cadherin and zonula occludens-1 (ZO-1), and the acquisition of mesenchymal proteins, including vimentin, α-SMA, fibronectin, and collagen I (Liu, 2010). Anorga et al. (2018) reported that renal injury can result in elevated TGF-β1 expression, leading to TAZ protein induction and nuclear accumulation. Stable TAZ overexpression in RTECs promotes fibrotic factor expression (including CTGF, fibronectin, and plasminogen activator inhibitor (PAI)-1)), dedifferentiation (as evident by altered morphology, increased mesenchymal marker expression, and loss of epithelial characteristics), and growth inhibition via Smad3-dependent CTGF up-regulation. Seo et al. (2016) found that TEC-specific deletion of Sav1 can increase TAZ protein expression and YAP1 nuclear localization after UUO, resulting in TGF-β and TGF-β receptor II up-regulation and aberrant activation of Wnt/β-catenin signaling, thereby aggravating renal TIF and enhancing EMT-like phenotype changes. Sav1 loss can also induce Stat3 activation and SASP with the accumulation of collagen and vimentin, leading to a fibrosis phenotype that can be further enhanced by AKI with aristolochic acid (AA) (Leung et al., 2017). Treatment with VP (YAP inhibitor) inhibits the activation of genes associated with senescence, SASP, and Stat3, and impedes the development of fibrosis (Leung et al., 2017). Therefore, inhibition of the Hippo pathway may mediate the occurrence of EMT, and YAP/TAZ may serve as a new therapeutic target for chronic renal fibrosis progress after AKI.
Crosstalk between Hippo pathway and fibroblasts/myofibroblasts
Myofibroblasts are important in ECM production during TIF. Myofibroblasts are rare in normal kidneys but markedly increase during chronic fibrosis, with possible precursors including interstitial stromal cells, such as fibroblasts, pericytes, and mesenchymal stem cells (MSC), as well as bone marrow-derived cells and endothelial cells. Current evidence suggests that stromal cells are the major origin of myofibroblasts (Iwano et al., 2002; LeBleu et al., 2013; Yuan et al., 2019). Whether epithelial cells can turn into myofibroblasts has been debated for many years. However, recent studies suggest that epithelial cells contribute little to the myofibroblast population after AKI (Humphreys et al., 2010; Koesters et al., 2010; LeBleu et al., 2013). Myofibroblasts are the major source of ECM proteins, cross-linking enzymes, and inhibitors of matrix-degrading metalloproteinases, characterized by the expression of the classic marker α-SMA (Chen et al., 2020; Gewin, 2018).
Upon insult, maladaptive RTECs cause a switch in the microenvironment of the tubulointerstitial area where myofibroblasts are activated (Qi & Yang, 2018). Resident fibroblasts proliferate and transition into myofibroblasts by profibrotic factors, such as CTGF, which are synthesized and released from injured RPTCs through a YAP activation-dependent pathway (Chen et al., 2020). Szeto et al. (2016) suggested that soft ECM inhibits while stiff ECM augments TGF-β-induced Smad2/3 nuclear accumulation mediated by YAP/TAZ, leading to fibroblast-myofibroblast transition and renal fibrosis. Furthermore, treating cells and mice with VP can reduce YAP/TAZ levels and block TGF-β-induced myofibroblast activation. TGF-β1 can also up-regulate YAP/TAZ expression through mTORC2 signaling-stimulated YAP/TAZ transcriptional activation, thereby stimulating fibroblast activation (Gui et al., 2018). Liang et al. (2017) demonstrated that fibroblasts cultured on a stiff matrix transform into myofibroblasts in a process dependent on YAP activation, even in the absence of TGF-β. Furthermore, Gli1+ cell-specific knockout of YAP/TAZ in mice suppresses UUO-induced ECM deposition, myofibroblast accumulation, and TIF. Therefore, YAP acts as a tissue mechanosensor in the kidney and can be activated by ECM to transform fibroblasts into myofibroblasts. The interaction between YAP/TAZ and ECM forms a positive feedback loop, resulting in kidney fibrosis.
Correlation between Hippo pathway and G2/M cell cycle arrest in renal fibrosis
Cell cycle arrest in RTECs during maladaptive repair is an important driver of kidney fibrogenesis (Yang et al., 2011). In uninjured kidneys, most RTECs are quiescent and remain in the G0 phase of the cell cycle. Upon injury, cells may enter the cell cycle to replace cells lost during the insult, with some cells arrested in either the G1 or G2 phase to allow time for DNA damage repair (Gewin, 2018). Accumulation of cells arrested in the G2/M growth phase is a common feature of progressive fibrotic kidney disease, resulting in the acquisition of a pathogenic phenotype characterized by sustained synthesis and secretion of profibrotic factors (Bonventre, 2014; Ferenbach & Bonventre, 2015).
As YAP/TAZ are involved in cell proliferation and survival, Yap activation can promote cell cycle progression (Chen et al., 2018; Mizuno et al., 2012). Thus, whether YAP/TAZ are associated with G2/M phase cell cycle arrest warrants investigation. Anorga et al. (2018) found that TAZ-overexpressing epithelial cells exhibit epithelial growth inhibition and G2/M cell cycle arrest after injury, consistent with the enhanced expression of growth-arrest gene p21, conferring a fibrotic phenotype. Down-regulation of YAP in human laryngeal carcinoma Hep-2 cells induces G2/M cell cycle arrest, reduces G0/G1 phase percentage, and increases apoptosis, indicating that YAP inactivation is related to G2/M arrest (Tang et al., 2019). However, other studies have shown that tubular maladaptive repair in the kidney is associated with YAP activation and that up-regulation of YAP induces an increase in the G2/M- and S-phases and a decrease in the G0/G1-phase in HK-2 cells, thus mediating kidney fibrosis after acute injury (Xu et al., 2016; Zheng et al., 2021). Clearly, these two hypotheses conflict with each other and there may exist a feedback regulation against G2/M phase arrest by triggering YAP activation in the kidney. Furthermore, Gerhardt et al. (2021) suggested the Hippo is involved in early injured RPTCs in mice after moderate AKI based on up-regulation of TEAD1 regulon. They also reported up-regulation of regulators involved in YAP-inactivation and Hippo signaling (e.g., Sav1, Stk3), as well as effectors of the transcriptional response after Hippo silencing (e.g., Yap1, Tead 1) and YAP-target genes such as Axl and Ctgf. In particular, they identified Vcam1+/Ccl2+ RPTCs at a late injury stage with marked activation of NF-κB-, TNF-, and AP-1-signaling pathways. This population of RPTCs showed features of SASP but did not exhibit G2/M cell cycle arrest, distinct from other reports of maladaptive RPTCs following kidney injury. However, the exact associations between YAP/TAZ and G2/M cell cycle arrest and whether G2/M cell cycle arrest exists in RPTCs that fail to repair after injury require further investigation.
Function of Hippo pathway in macrophage and inflammation regulation
AKI is typically accompanied by immune activation and inflammation, which involve macrophage infiltration. Recruited macrophages exert versatile effects on kidney repair after injury depending on their polarization form: i.e., proinflammatory M1 phenotype and reparative M2 phenotype. The predominant M1 phenotype in the early phase of AKI produces proinflammatory cytokines and mediators, which, in turn, exacerbate inflammation (Ferenbach & Bonventre, 2015; Qi & Yang, 2018). The M2 polarization of macrophages in the resolution phase of AKI is beneficial for renal recovery. However, the M2 phenotype may exert a pro-fibrotic influence by promoting TIF, expressing matrix metalloproteases, and secreting pro-fibrotic factors (Gewin et al., 2017). Therefore, persistent activation of M2 macrophages is instrumental in maladaptive repair and fibrosis in CKD (Baek, 2019).
Hippo-YAP pathway activation after ischemic AKI is associated with tubular maladaptive repair and interstitial macrophage infiltration mediated by MCP-1 up-regulation, while inhibition of YAP nuclear translocation by VP attenuates macrophage infiltration and TIF (Zheng et al., 2021). VP treatment in UUO-induced AKI mice also decreases macrophage recruitment and cell adhesion molecule expression (Jin et al., 2020). Tubular cell-specific knockout of MST1/2 promotes the expression of inflammatory factors and macrophage marker F4/80; however, expression returns to physiological levels after YAP deletion in MST1/2 double knockout mice (Xu et al., 2020a). These data suggest that YAP may play a pivotal role in chronic inflammation during maladaptive repair after renal injury.
Feng et al. (2018b) demonstrated that YAP/TAZ induction mediates Wnt5a and TGF-β1-promoted macrophage M2 polarization and contributes to renal fibrosis after UUO or I/R injury in mice, whereas specific ablation of the TAZ gene in macrophages can markedly decrease the M2 phenotype and kidney fibrosis. However, further studies are needed to decipher the exact mechanism by which YAP/TAZ regulate macrophage M2 polarization.
Hippo-YAP pathway activation can lead to renal hypertrophy through RTEC enlargement
Renal hypertrophy is characterized by increased organ size due to cell enlargement rather than cell proliferation (Fine & Norman, 1989). In the kidney, the growth of remaining renal tissue in response to renal tissue loss is known as renal compensatory hypertrophy (RCH) and can help restore normal kidney function following disease or loss of kidney tissue (Sugaya et al., 2000). As the proximal tubule forms the bulk of kidney mass, this nephron segment contributes the most to renal hypertrophy (Hayslett et al., 1968).
In renal epithelial cells, increased nuclear accumulation and transcriptional activity of YAP can trigger activation of the Akt/mTOR signaling pathway and its downstream target S6K1. This leads to silencing of the peripheral tight junction protein zona occludens 2 (ZO-2), which induces an increase in cell size and a higher protein/DNA ratio, thereby triggering RCH (Domínguez-Calderón et al., 2016). ZO-2 is also a positive regulator of the Hippo pathway as it associates with LATS1 and functions as a pathway scaffold (González-González et al., 2021). However, chronic cell expansion beyond optimal conditions may present certain disadvantages, and hemodynamic and metabolic hyperfunction of residual nephrons may prove maladaptive over time (Lafferty & Brenner, 1990; Nath, 1992). Excessive RCH can lead to tubular atrophy, interstitial fibrosis, and progressive decline in renal function (Hostetter, 1995). Therefore, persistent YAP activation after AKI may also cause renal hypertrophy and accelerate progression towards CKD (Figure 4).
POTENTIAL ROLE OF HIPPO PATHWAY IN FERROPTOSIS AFTER AKI
Ferroptosis is characterized by intracellular iron accumulation and lipid peroxidation during cell death (Dixon et al., 2012). The characteristics of ferroptosis differ from necrosis, apoptosis, and autophagy in cell morphology and function (Xie et al., 2016). The morphology of ferroptotic cells includes mitochondrial changes, such as reduced volume, decreased mitochondrial cristae, and increased membrane density (Wang et al., 2021). However, the nucleus remains normal, with no chromatin agglutination (Wang et al., 2020a). Following ferroptosis, intracellular glutathione (GSH) consumption and glutathione peroxidase 4 (GPX4) inactivation occur (Li et al., 2020). Recent studies have shown that ferroptosis participates in various pathological models of AKI and classical ferroptosis inhibitors, such as ferrostatin-1 and liproxstatin-1, are capable of interfering with key molecules in the ferroptosis signaling pathway to resist AKI (Hu et al., 2019; Martin-Sanchez et al., 2017; Skouta et al., 2014; Zilka et al., 2017). GPX4 deletion in mice can spontaneously trigger AKI (Friedmann Angeli et al., 2014), whereas GPX4 up-regulation prevents AKI (Zhang et al., 2021).
A growing number of studies have revealed the relationship between the Hippo pathway and ferroptosis in cancer cells. Yang & Chi (2020) suggested that triggering ferroptosis may have therapeutic potential for TAZ-activated tumors. Ferroptosis sensitivity in renal and ovarian cells is regulated by cell density through the TAZ-EMP-NOX4 and TAZ-ANGPTL4-NOX2 pathways, respectively (Yang et al., 2019, 2020). TAZ removal confers ferroptosis resistance, while overexpression of the constitutively active form of TAZ (TAZS89A) sensitizes cells to ferroptosis. YAP exerts a similar function in cancer cell ferroptosis, with knockdown of YAP or its direct target gene S-phase kinase-associated protein 2 (SKP2) shown to robustly protect cells and abolish lipid peroxidation during erastin-induced ferroptosis (Yang et al., 2021). However, despite current evidence supporting the connection between Hippo pathway effectors and ferroptosis in cancer, there is a lack of research on the role of the Hippo pathway in ferroptosis during AKI progression. Moreover, the specific role of ferroptosis in AKI caused by different etiologies remains unclear. Thus, further exploration in this field is necessary for the clinical treatment of AKI.
HIPPO PATHWAY MAY CONTRIBUTE TO KIDNEY CANCER DEVELOPMENT IN AKI
Renal cell carcinoma (RCC), which is derived from RTECs, accounts for 85% of all renal malignancies (Cinar et al., 2021; Kovacs et al., 1997). Emerging evidence suggests that dysregulation of Hippo-YAP1 signaling plays a significant role in the etiology of aggressive kidney cancer (Han, 2019). YAP1 protein accumulates in the nuclei of clear cell RCC (ccRCC) cells, although normal kidney cells primarily express the cytoplasmic YAP1 protein (Godlewski et al., 2018). Similarly, nuclear YAP1 is considerably higher in ccRCC cells than in unaltered proximal kidney cortices (Rybarczyk et al., 2017). Up-regulation of YAP1 in ccRCC patients is also associated with poorer clinical outcomes (Chen et al., 2014). Thus, nuclear YAP1 appears to play an oncogenic role in ccRCC cells, promoting cell proliferation and survival (Rybarczyk et al., 2017).
Recent studies have identified AKI as a risk factor for renal cancer development (Kusmartsev, 2021). Based on data collected from several multicenter studies, Peired et al. (2020) found that AKI can significantly increase the risk of papillary RCC (pRCC) development and tumor relapse in humans. They also induced I/R injury in wild-type mice and examined their kidneys at different timepoints and found that hypoxia promotes the undifferentiated cell state in various stem and precursor populations by activating Notch-responsive promoters and increasing expression of genes downstream of Notch, driving a hypoxia-inducible factor HIF1a-to-HIF2a switch favoring transformation into cancer stem cells and supporting tumor growth. Notably, YAP/TAZ activity is linked to oxygen availability (Yu et al., 2015). Under hypoxic conditions, HIF1 stimulates the expression of SIAH1/2, two E3 ubiquitin ligases. SIAH1/2 then promote ubiquitination and degradation of LATS2, leading to YAP/TAZ activation (Ma et al., 2015; Xiang et al., 2014). In addition, HIF1 directly induces the transcription of TAZ (Xiang et al., 2014), and YAP interacts with and stabilizes HIF1 to enhance the transcription of HIF1 target genes (Ma et al., 2015). Furthermore, there are two main modalities of YAP/TAZ-Notch signaling crosstalk: i.e., YAP/TAZ-mediated transcriptional regulation of Notch ligands or receptors and transcriptional co-regulation of common direct target genes by YAP/TAZ and the Notch intracellular domain (NICD) (Totaro et al., 2018). Whether the Hippo pathway exerts a function in pRCC development after AKI deserves further clarification.
Zhou et al. (2021) also investigated the relationship between AKI-associated systemic inflammation and risk of kidney cancer development. They subjected genetically modified mice with Cre-mediated deletion of the Trp53 and Pten genes in Ggt1-expressing RPTCs (GPPY mice) to I/R injury to induce acute injury in one kidney. Their results showed that I/R injury-induced AKI promoted the formation of ccRCC in the contralateral uninjured kidney, but not in the kidney subjected to I/R injury. A significant increase in tissue infiltration of neutrophils and fibroblasts was also observed, associated with the up-regulation of inflammatory factors CXCL1, CXCL13, TIMP1, and ILRα, with CXCL1 displaying the highest levels of up-regulation locally and systemically. Notably, CXCL1 expression was predominantly associated with dysplastic epithelial cells in the I/R-injured kidney, but not in the contralateral uninjured kidney. Up-regulation of CXCR2, which serves as a natural receptor for CXCL1, was also observed in stromal fibroblasts, mostly in the uninjured kidney. Administration of anti-CXCR2 antibodies blocked CXCL1/CXCR2 signaling, leading to a reduction in the recruitment of neutrophils, fibroblasts, and most importantly, RCC incidence. Chen et al. (2018) also demonstrated an increase in YAP expression and YAP-TEAD interaction in RPTCs from post-I/R-injured mouse kidneys. Activation of TEAD1 can lead to an increase in the expression of its target, CXCL1, i.e., chemokine-mediated neutrophil recruitment (Kusumanchi et al., 2021). Zheng et al. (2021) also proved that renal CXCL2 and CXCR2 are markedly increased in post-AKI mice through tubular YAP activation. Hence, growing evidence suggests that the Hippo pathway is involved in the positive association between ccRCC formation and AKI-induced systemic inflammation.
CONCLUSIONS AND FUTURE PERSPECTIVES
Research published over the last several years has revealed the significant role that the Hippo signaling pathway plays in pathological changes of AKI and subsequent repair. During renal pathological injury in AKI, MST1, the upstream factor of the Hippo pathway, mediates RTEC apoptosis by inducing mitochondrial fission, thereby aggravating tubular damage. Transient overexpression of YAP and its downstream mediator survivin in the aftermath of AKI can result in acquired cytoresistance in RPTCs to protect the kidney against further ischemia or nephrotoxicity. Moreover, the Hippo pathway elicits both beneficial and detrimental effects on recovery after AKI. If the injury is mild/moderate, downstream factors YAP/TAZ can be transiently activated, leading to complete/adaptive renal repair. However, if the injury is severe and recurring, the constant increase and activation of YAP/TAZ can lead to incomplete/maladaptive renal repair and progressive fibrotic CKD via several mechanisms, including EMT, fibroblast to myofibroblast conversion, G2/M phase cell cycle arrest, macrophage infiltration, and renal hypertrophy. Although ferroptosis is implicated in various pathological models of AKI and Hippo pathway effectors are highly associated with ferroptosis in cancer cells, whether the Hippo pathway is involved in ferroptosis in AKI progression requires further investigation. In addition, while AKI is a risk factor of kidney cancer development, including ccRCC and pRCC, whether the Hippo pathway participates in this process remains to be clarified. Hence, despite compelling evidence, the correlation between the Hippo pathway and kidney injury still requires additional research, and the potential mechanisms and signaling pathways need to be further elucidated. Furthermore, pharmacological manipulation of the Hippo pathway for the treatment of AKI is uncertain and requires future exploration.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82070718; 81770712) and Shanghai Science and Technology Innovation Natural Foundation (20ZR1444700)
Contributor Information
Hui-Juan Wu, Email: hjwu@shmu.edu.cn.
Jun Liu, Email: liujun-sgh@sjtu.edu.cn.
References
- 1.Alexandre CS, Volpini RA, Shimizu MH, Sanches TR, Semedo P, Di Jura VL, et al Lineage-negative bone marrow cells protect against chronic renal failure. Stem Cells. 2009;27(3):682–692. doi: 10.1634/stemcells.2008-0496. [DOI] [PubMed] [Google Scholar]
- 2.Anorga S, Overstreet JM, Falke LL, Tang JQ, Goldschmeding RG, Higgins PJ, et al Deregulation of Hippo-TAZ pathway during renal injury confers a fibrotic maladaptive phenotype. The FASEB Journal. 2018;32(5):2644–2657. doi: 10.1096/fj.201700722R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 4.Baek JH The impact of versatile macrophage functions on acute kidney injury and its outcomes. Frontiers in Physiology. 2019;10:1016. doi: 10.3389/fphys.2019.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai HB, Zhang NL, Xu Y, Chen Q, Khan M, Potter JJ, et al Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56(3):1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baligand C, Qin HC, True-Yasaki A, Gordon JW, Von Morze C, Santos JD, et al Hyperpolarized 13C magnetic resonance evaluation of renal ischemia reperfusion injury in a murine model. NMR in Biomedicine. 2017;30(10):e3765. doi: 10.1002/nbm.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernascone I, Martin-Belmonte F Crossroads of wnt and Hippo in epithelial tissues. Trends in Cell Biology. 2013;23(8):380–389. doi: 10.1016/j.tcb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Boggiano JC, Fehon RG Growth control by committee: intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Developmental Cell. 2012;22(4):695–702. doi: 10.1016/j.devcel.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonventre JV Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney International Supplements. 2014;4(1):39–44. doi: 10.1038/kisup.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology. 2009;20(5):1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callus BA, Verhagen AM, Vaux DL Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. The FEBS Journal. 2006;273(18):4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan EHY, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HHW The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24(12):2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 13.Chen JC, Wang XY, He Q, Bulus N, Fogo AB, Zhang MZ, et al YAP activation in renal proximal tubule cells drives diabetic renal interstitial fibrogenesis. Diabetes. 2020;69(11):2446–2457. doi: 10.2337/db20-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JC, You HZ, Li Y, Xu Y, He Q, Harris RC EGF receptor-dependent YAP activation is important for renal recovery from AKI. Journal of the American Society of Nephrology. 2018;29(9):2372–2385. doi: 10.1681/ASN.2017121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen KH, He J, Wang DL, Cao JJ, Li MC, Zhao XM, et al Methylation-associated inactivation of LATS1 and its effect on demethylation or overexpression on YAP and cell biological function in human renal cell carcinoma. International Journal of Oncology. 2014;45(6):2511–2521. doi: 10.3892/ijo.2014.2687. [DOI] [PubMed] [Google Scholar]
- 16.Cinar B, Alp E, Al-Mathkour M, Boston A, Dwead A, Khazaw K, et al The Hippo pathway: an emerging role in urologic cancers. American Journal of Clinical And Experimental Urology. 2021;9(4):301–317. [PMC free article] [PubMed] [Google Scholar]
- 17.Deng H, Wang W, Yu JZ, Zheng YG, Qing Y, Pan DJ Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. eLife. 2015;4:e06567. doi: 10.7554/eLife.06567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding H, Xu YY, Jiang N Upregulation of miR-101a suppresses chronic renal fibrosis by regulating KDM3A via blockade of the YAP-TGF-β-smad signaling pathway. Molecular Therapy-Nucleic Acids. 2020;19:1276–1289. doi: 10.1016/j.omtn.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domínguez-Calderón A, Ávila-Flores A, Ponce A, López-Bayghen E, Calderón-Salinas JV, Reyes JL, et al ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway. Molecular Biology of the Cell. 2016;27(10):1581–1595. doi: 10.1091/mbc.E15-08-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong JX, Feldmann G, Huang JB, Wu SA, Zhang NL, Comerford SA, et al Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duann P, Lianos EA, Ma JJ, Lin PH Autophagy, innate immunity and tissue repair in acute kidney injury. International Journal of Molecular Sciences. 2016;17(5):662. doi: 10.3390/ijms17050662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duann P, Lin PH. 2017. Mitochondria damage and kidney disease. In: Santulli G. Mitochondrial Dynamics in Cardiovascular Medicine. Cham: Springer, 529–551.
- 24.Duffield JS, Humphreys BD Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney International. 2011;79(5):494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- 25.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 26.Feng JX, Li HY, Zhang YF, Wang Q, Zhao SL, Meng P, et al Mammalian STE20-like kinase 1 deletion alleviates renal ischaemia-reperfusion injury via modulating mitophagy and the AMPK-YAP signalling pathway. Cellular Physiology and Biochemistry. 2018a;51(5):2359–2376. doi: 10.1159/000495896. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y, Liang Y, Zhu X, Wang M, Gui Y, Lu Q, et al The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. Journal of Biological Chemistry. 2018b;293(50):19290–19302. doi: 10.1074/jbc.RA118.005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferenbach DA, Bonventre JV Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nature Reviews Nephrology. 2015;11(5):264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine LG, Norman J Cellular events in renal hypertrophy. Annual Review of Physiology. 1989;51:19–32. doi: 10.1146/annurev.ph.51.030189.000315. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, Thompson BJ The Spectrin cytoskeleton regulates the Hippo signalling pathway. The EMBO Journal. 2015;34(7):940–954. doi: 10.15252/embj.201489642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biology. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funk JA, Schnellmann RG Persistent disruption of mitochondrial homeostasis after acute kidney injury. American Journal of Physiology-Renal Physiology. 2012;302(7):F853–F864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuya R, Kumagai H, Hishida A Acquired resistance to rechallenge injury with uranyl acetate in LLC-PK1 cells. Journal of Laboratory and Clinical Medicine. 1997;129(3):347–355. doi: 10.1016/S0022-2143(97)90183-9. [DOI] [PubMed] [Google Scholar]
- 34.Gerhardt LMS, Liu J, Koppitch K, Cippà PE, Mcmahon AP Single-nuclear transcriptomics reveals diversity of proximal tubule cell states in a dynamic response to acute kidney injury. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(27):e2026684118. doi: 10.1073/pnas.2026684118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gewin L, Zent R, Pozzi A Progression of chronic kidney disease: too much cellular talk causes. damage. Kidney International. 2017;91(3):552–560. doi: 10.1016/j.kint.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gewin LS. 2018. Renal fibrosis: primacy of the proximal tubule. Matrix Biology, 68–69: 248–262.
- 37.Godlewski J, Kiezun J, Krazinski BE, Kozielec Z, Wierzbicki PM, Kmiec Z The immunoexpression of YAP1 and LATS1 proteins in clear cell renal cell carcinoma: impact on patients' survival. Biomed Research International. 2018;2018:2653623. doi: 10.1155/2018/2653623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González-González L, Gallego-Gutiérrez H, Martin-Tapia D, Avelino-Cruz JE, Hernández-Guzmán C, Rangel-Guerrero SI, et al ZO-2 favors Hippo signaling, and its re-expression in the steatotic liver by AMPK restores junctional sealing. Tissue Barriers. 2021;10(2):1994351. doi: 10.1080/21688370.2021.1994351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gui Y, Hou Q, Lu QM, Dai CS, Li JZ Loss of rictor in tubular cells exaggerates lipopolysaccharide induced renal inflammation and acute kidney injury via Yap/Taz-NF-κB axis. Cell Death Discovery. 2020;6:40. doi: 10.1038/s41420-020-0274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gui Y, Li JZ, Lu QM, Feng Y, Wang MJ, He WC, et al Yap/Taz mediates mTORC2-stimulated fibroblast activation and kidney fibrosis. Journal of Biological Chemistry. 2018;293(42):16364–16375. doi: 10.1074/jbc.RA118.004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall AM, Unwin RJ, Parker N, Duchen MR Multiphoton imaging reveals differences in mitochondrial function between nephron segments. Journal of the American Society of Nephrology. 2009;20(6):1293–1302. doi: 10.1681/ASN.2008070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton E, Infante JR Targeting CDK4/6 in patients with cancer. Cancer Treatreatment Reviews. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Han YY Analysis of the role of the Hippo pathway in cancer. Journal of Translational Medicine. 2019;17(1):116. doi: 10.1186/s12967-019-1869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen CG, Moroishi T, Guan KL YAP and TAZ: a nexus for Hippo signaling and beyond. Trends in Cell Biology. 2015;25(9):499–513. doi: 10.1016/j.tcb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao YW, Chun A, Cheung K, Rashidi B, Yang XL. 2008. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. Journal of Biological Chemistry, 283(9): 5496–5509.
- 46.Harvey KF, Zhang XM, Thomas DM The Hippo pathway and human cancer. Nature Reviews Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 47.Hayslett JP, Kashgarian M, Epstein FH Functional correlates of compensatory renal hypertrophy. Journal of Clinical Investigation. 1968;47(4):774–782. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honda N, Hishida A, Ikuma K, Yonemura K Acquired resistance to acute renal failure. Kidney International. 1987;31(6):1233–1238. doi: 10.1038/ki.1987.136. [DOI] [PubMed] [Google Scholar]
- 49.Hostetter TH Progression of renal disease and renal hypertrophy. Annual Review of Physiology. 1995;57:263–278. doi: 10.1146/annurev.ph.57.030195.001403. [DOI] [PubMed] [Google Scholar]
- 50.Hu ZX, Zhang H, Yang SK, Wu XQ, He D, Cao K, et al Emerging role of ferroptosis in acute kidney injury. Oxidative Medicine and Cellular Longevity. 2019;2019:8010614. doi: 10.1155/2019/8010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American Journal of Pathology. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwakura T, Fujigaki Y, Fujikura T, Ohashi N, Kato A, Yasuda H Acquired resistance to rechallenge injury after acute kidney injury in rats is associated with cell cycle arrest in proximal tubule cells. American Journal of Physiology-Renal Physiology. 2016;310(9):F872–F884. doi: 10.1152/ajprenal.00380.2015. [DOI] [PubMed] [Google Scholar]
- 53.Iwakura T, Fujigaki Y, Fujikura T, Tsuji T, Ohashi N, Kato A, et al Cytoresistance after acute kidney injury is limited to the recovery period of proximal tubule integrity and possibly involves Hippo-YAP signaling. Physiological Reports. 2017;5(11):e13310. doi: 10.14814/phy2.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwano M, Plieth D, Danoff TM, Xue CS, Okada H, Neilson EG Evidence that fibroblasts derive from epithelium during tissue fibrosis. The Journal of Clinical Investigation. 2002;110(3):341–350. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang MZ, Bai M, Lei J, Xie YF, Xu S, Jia ZJ, et al Mitochondrial dysfunction and the AKI-to-CKD transition. American Journal of Physiology-Renal Physiology. 2020;319(6):F1105–F1116. doi: 10.1152/ajprenal.00285.2020. [DOI] [PubMed] [Google Scholar]
- 56.Jiao S, Wang HZ, Shi ZB, Dong AM, Zhang WJ, Song XM, et al A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25(2):166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 57.Jin JX, Wang T, Park W, Li WJ, Kim W, Park SK, et al Inhibition of yes-associated protein by verteporfin ameliorates unilateral ureteral obstruction-induced renal tubulointerstitial inflammation and fibrosis. International Journal of Molecular Science. 2020;21(21):8184. doi: 10.3390/ijms21218184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalluri R, Weinberg RA The basics of epithelial-mesenchymal transition. The Journal Of Clinical Investigation. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim DH, Choi HI, Park JS, Kim CS, Bae EH, Ma SK, et al Src-mediated crosstalk between FXR and YAP protects against renal fibrosis. The FASEB Journal. 2019;33(10):11109–11122. doi: 10.1096/fj.201900325R. [DOI] [PubMed] [Google Scholar]
- 60.Kim E, Kang JG, Kang MJ, Park JH, Kim YJ, Kweon TH, et al O-GlcNAcylation on LATS2 disrupts the Hippo pathway by inhibiting its activity. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(25):14259–14269. doi: 10.1073/pnas.1913469117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim M, Kim M, Park SJ, Lee C, Lim DS Role of angiomotin-like 2 mono-ubiquitination on YAP inhibition. EMBO Reports. 2016;17(1):64–78. doi: 10.15252/embr.201540809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, et al Tubular overexpression of transforming growth factor-β1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. The American Journal of Pathology. 2010;177(2):632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, et al The Heidelberg classification of renal cell tumours. Journal of Pathology. 1997;183(2):131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 64.Kusmartsev S Acute Kidney Injury-induced systemic inflammation and risk of kidney cancer formation. Cancer Research. 2021;81(10):2584–2585. doi: 10.1158/0008-5472.CAN-21-0807. [DOI] [PubMed] [Google Scholar]
- 65.Kusumanchi P, Liang TB, Zhang T, Ross RA, Han S, Chandler K, et al Stress-responsive gene FK506-binding protein 51 mediates alcohol-induced liver injury through the Hippo pathway and chemokine (C-X-C Motif) ligand 1 signaling. Hepatology. 2021;74(3):1234–1250. doi: 10.1002/hep.31800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lafferty HM, Brenner BM Are glomerular hypertension and "hypertrophy" independent risk factors for progression of renal disease. Seminars in Nephrology. 1990;10(3):294–304. [PubMed] [Google Scholar]
- 67.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al Acute kidney injury: an increasing global concern. The Lancet. 2013;382(9887):170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 68.Lan RP, Geng H, Singha PK, Saikumar P, Bottinger EP, Weinberg JM, et al Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. Journal of the American Society of Nephrology. 2016;27(11):3356–3367. doi: 10.1681/ASN.2015020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeBleu VS, Taduri G, O'Connell J, Teng YQ, Cooke VG, Woda C, et al Origin and function of myofibroblasts in kidney fibrosis. Nature Medicine. 2013;19(8):1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee H, Yoon Y Mitochondrial fission and fusion. Biochemical Society Transactions. 2016;44(6):1725–1735. doi: 10.1042/BST20160129. [DOI] [PubMed] [Google Scholar]
- 71.Lee HY, Back K. 2017. Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. Journal of Pineal Research, 62(2): e12379.
- 72.Lee PT, Lin HH, Jiang ST, Lu PJ, Chou KJ, Fang HC, et al Mouse kidney progenitor cells accelerate renal regeneration and prolong survival after ischemic injury. Stem Cells. 2010;28(3):573–584. doi: 10.1002/stem.310. [DOI] [PubMed] [Google Scholar]
- 73.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, et al TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the Hippo pathway. Molecular and Cellular Biology. 2008;28(7):2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leung JY, Wilson HL, Voltzke KJ, Williams LA, Lee HJ, Wobker SE, et al Sav1 loss induces senescence and Stat3 activation coinciding with tubulointerstitial fibrosis. Molecular and Cellular Biology. 2017;37(12):e00565–16. doi: 10.1128/MCB.00565-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li HY, Feng JX, Zhang YF, Feng JX, Wang Q, Zhao SL, et al Mst1 deletion attenuates renal ischaemia-reperfusion injury: the role of microtubule cytoskeleton dynamics, mitochondrial fission and the GSK3β-p53 signalling pathway. Redox Biology. 2019;20:261–274. doi: 10.1016/j.redox.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, et al Ferroptosis: past, present and future. Cell Death & Disease. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang M, Yu M, Xia RH, Song K, Wang J, Luo JL, et al Yap/Taz deletion in Gli+ cell-derived myofibroblasts attenuates fibrosis. Journal of the American Society of Nephrology. 2017;28(11):3278–3290. doi: 10.1681/ASN.2015121354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin FM. 2006. Stem cells in kidney regeneration following acute renal injury. Pediatric Research, 59(4 Pt 2): 74R–78R.
- 79.Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, et al The Hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFβ-TrCP E3 ligase. Journal of Biological Chemistry. 2010;285(48):37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu YH New insights into epithelial-mesenchymal transition in kidney fibrosis. Journal of the American Society of Nephrology. 2010;21(2):212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma B, Chen Y, Chen L, Cheng HC, Mu CL, Li J, et al Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nature Cell Biology. 2015;17(1):95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- 82.Ma DQ, Lim T, Xu J, Tang H, Wan YJ, Zhao HL, et al Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1α activation. Journal of the American Society of Nephrology. 2009;20(4):713–720. doi: 10.1681/ASN.2008070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma SH, Meng ZP, Chen R, Guan KL The Hippo pathway: biology and pathophysiology. Annual Review of Biochemistry. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 84.Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Niño MD, et al Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. Journal of the American Society of Nephrology. 2017;28(1):218–229. doi: 10.1681/ASN.2015121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meng ZP, Moroishi T, Guan KL Mechanisms of Hippo pathway regulation. Genes & Development. 2016;30(1):1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyaji T, Kato A, Yasuda H, Fujigaki Y, Hishida A Role of the increase in p21 in cisplatin-induced acute renal failure in rats. Journal of American Society of Nephrology. 2001;12(5):900–908. doi: 10.1681/ASN.V125900. [DOI] [PubMed] [Google Scholar]
- 87.Mizuno S, Fujita K, Furuy R, Hishid A, Ito H, Tashim Y, et al. 1997. Association of HSP73 with the acquired resistance to uranyl acetate-induced acute renal failure. Toxicology, 117(2–3): 183–191.
- 88.Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, et al YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31(49):5117–5122. doi: 10.1038/onc.2012.5. [DOI] [PubMed] [Google Scholar]
- 89.Mo JS, Meng ZP, Kim YC, Park HW, Hansen CG, Kim S, et al Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nature Cell Biology. 2015;17(4):500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, et al Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26(8):2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 91.Moroishi T, Park HW, Qin BD, Chen Q, Meng ZP, Plouffe SW, et al A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes & Development. 2015;29(12):1271–1284. doi: 10.1101/gad.262816.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munshi R, Hsu C, Himmelfarb J Advances in understanding ischemic acute kidney injury. BMC Medicine. 2011;9:11. doi: 10.1186/1741-7015-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL Cyclin D as a therapeutic target in cancer. Nature Reviews Cancer. 2011;11(8):558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 94.Nath KA Tubulointerstitial changes as a major determinant in the progression of renal damage. American Journal of Kidney Diseases. 1992;20(1):1–17. doi: 10.1016/S0272-6386(12)80312-X. [DOI] [PubMed] [Google Scholar]
- 95.Nath KA Heme oxygenase-1 and acute kidney injury. Current Opinion in Nephrology and Hypertension. 2014;23(1):17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishioka S, Nakano D, Kitada K, Sofue T, Ohsaki H, Moriwaki K, et al The cyclin-dependent kinase inhibitor p21 is essential for the beneficial effects of renal ischemic preconditioning on renal ischemia/reperfusion injury in mice. Kidney International. 2014;85(4):871–879. doi: 10.1038/ki.2013.496. [DOI] [PubMed] [Google Scholar]
- 97.Paramasivam M, Sarkeshik A, Yates III JR, Fernandes MJG, McCollum D Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Molecular Biology of the Cell. 2011;22(19):3725–3733. doi: 10.1091/mbc.e11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park GS, Oh H, Kim M, Kim T, Johnson RL, Irvine KD An evolutionarily conserved negative feedback mechanism in the Hippo pathway reflects functional difference between LATS1 and LATS2. Oncotarget. 2016;7(17):24063–24075. doi: 10.18632/oncotarget.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peired AJ, Antonelli G, Angelotti ML, Allinovi M, Guzzi F, Sisti A, et al Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Science Translational Medicine. 2020;12(536):eaaw6003. doi: 10.1126/scitranslmed.aaw6003. [DOI] [PubMed] [Google Scholar]
- 100.Perry HM, Huang LP, Wilson RJ, Bajwa A, Sesaki H, Yan Z, et al Dynamin-related protein 1 deficiency promotes recovery from AKI. Journal of the American Society of Nephrology. 2018;29(1):194–206. doi: 10.1681/ASN.2017060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. The Biochemical Journal. 2004;381(2):453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Price PM, Safirstein RL, Megyesi J Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. American Journal of Physiology-Renal Physiology. 2004;286(2):F378–F384. doi: 10.1152/ajprenal.00192.2003. [DOI] [PubMed] [Google Scholar]
- 103.Qi RC, Yang C Renal tubular epithelial cells: the neglected mediator of tubulointerstitial fibrosis after injury. Cell Death & Disease. 2018;9(11):1126. doi: 10.1038/s41419-018-1157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rinschen MM, Grahammer F, Hoppe AK, Kohli P, Hagmann H, Kretz O, et al YAP-mediated mechanotransduction determines the podocyte's response to damage. Science Signaling. 2017;10(474):eaaf8165. doi: 10.1126/scisignal.aaf8165. [DOI] [PubMed] [Google Scholar]
- 105.Rybarczyk A, Klacz J, Wronska A, Matuszewski M, Kmiec Z, Wierzbicki PM Overexpression of the YAP1 oncogene in clear cell renal cell carcinoma is associated with poor outcome. Oncology Reports. 2017;38(1):427–439. doi: 10.3892/or.2017.5642. [DOI] [PubMed] [Google Scholar]
- 106.Sano K, Fujigaki Y, Miyaji T, Ikegaya N, Ohishi K, Yonemura K, et al Role of apoptosis in uranyl acetate-induced acute renal failure and acquired resistance to uranyl acetate. Kidney International. 2000;57(4):1560–1570. doi: 10.1046/j.1523-1755.2000.00777.x. [DOI] [PubMed] [Google Scholar]
- 107.Seo E, Kim WY, Hur J, Kim H, Nam SA, Choi A, et al The Hippo-salvador signaling pathway regulates renal tubulointerstitial fibrosis. Scientific Reports. 2016;6:31931. doi: 10.1038/srep31931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skouta R, Dixon SJ, Wang JL, Dunn DE, Orman M, Shimada K, et al Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. Journal of the American Chemical Society. 2014;136(12):4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song JG, Wang TZ, Chi XC, Wei XF, Xu SD, Yu M, et al Kindlin-2 inhibits the Hippo signaling pathway by promoting degradation of MOB1. Cell Reports. 2019;29(11):3664–3677.e5. doi: 10.1016/j.celrep.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 110.Sugaya K, Ogawa Y, Hatano T, Koyama Y, Miyazato T, Naito A, et al Compensatory renal hypertrophy and changes of renal function following nephrectomy. Hinyokika Kiyo. 2000;46(4):235–240. [PubMed] [Google Scholar]
- 111.Sun Y, Fujigaki Y, Sakakima M, Fujikura T, Togawa A, Huang YJ, et al Acquired resistance to rechallenge injury in rats that recovered from mild renal damage induced by uranyl acetate: accelerated proliferation and hepatocyte growth factor/c-Met axis. Clinical and Experimental Nephrology. 2011;15(5):666–675. doi: 10.1007/s10157-011-0453-x. [DOI] [PubMed] [Google Scholar]
- 112.Szeto SG, Narimatsu M, Lu ML, He XL, Sidiqi AM, Tolosa MF, et al YAP/TAZ are mechanoregulators of TGF-β-smad signaling and renal fibrogenesis. Journal of the American Society of Nephrology. 2016;27(10):3117–3128. doi: 10.1681/ASN.2015050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M, et al Severity and frequency of proximal tubule injury determines renal prognosis. Journal of the American Society of Nephrology. 2016;27(8):2393–2406. doi: 10.1681/ASN.2015060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang XM, Sun YX, Wan GL, Sun JQ, Sun JW, Pan CC Knockdown of YAP inhibits growth in Hep-2 laryngeal cancer cells via epithelial-mesenchymal transition and the Wnt/β-catenin pathway. BMC Cancer. 2019;19(1):654. doi: 10.1186/s12885-019-5832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, Haber DA, et al Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 116.Totaro A, Castellan M, Di Biagio D, Piccolo S Crosstalk between YAP/TAZ and Notch Signaling. Trends in Cell Biology. 2018;28(7):560–573. doi: 10.1016/j.tcb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK Failed tubule recovery, AKI-CKD transition, and kidney disease progression. Journal of the American Society of Nephrology. 2015;26(8):1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wada KI, Itoga K, Okano T, Yonemura S, Sasaki H Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138(18):3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 119.Wang H, Liu C, Zhao YX, Gao G Mitochondria regulation in ferroptosis. European Journal of Cell Biology. 2020a;99(1):151058. doi: 10.1016/j.ejcb.2019.151058. [DOI] [PubMed] [Google Scholar]
- 120.Wang J, Zhu PJ, Li RB, Ren J, Zhou H Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biology. 2020b;30:101415. doi: 10.1016/j.redox.2019.101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang JY, Liu Y, Wang YQ, Sun L The cross-link between ferroptosis and kidney diseases. Oxidative Medicine and Cellular Longevity. 2021;2021:6654887. doi: 10.1155/2021/6654887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu CL, Chang CC, Yang TH, Tsai ACD, Wang JL, Chang CH, et al Tubular transcriptional co-activator with PDZ-binding motif protects against ischemic acute kidney injury. Clinical Science. 2020;134(13):1593–1612. doi: 10.1042/CS20200223. [DOI] [PubMed] [Google Scholar]
- 123.Wu SA, Huang JB, Dong JX, Pan DJ. 2003. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell, 114(4): 445–456.
- 124.Xiang LS, Gilkes DM, Hu HX, Takano N, Luo WB, Lu HQ, et al Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5(24):12509–12527. doi: 10.18632/oncotarget.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiao L, Xu XX, Zhang F, Wang M, Xu Y, Tang D, et al The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biology. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al Ferroptosis: process and function. Cell Death & Differentiation. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xing J, He YC, Wang KY, Wan PZ, Zhai XY Involvement of YTHDF1 in renal fibrosis progression via up-regulating YAP. The FASEB Journal. 2022;36(2):e22144. doi: 10.1096/fj.202100172RR. [DOI] [PubMed] [Google Scholar]
- 128.Xu CH, Wang L, Zhang Y, Li WL, Li JH, Wang Y, et al Tubule-specific Mst1/2 deficiency induces CKD via YAP and non-YAP mechanisms. Journal of the American Society of Nephrology. 2020a;31(5):946–961. doi: 10.1681/ASN.2019101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu D, Chen PP, Zheng PQ, Yin F, Cheng Q, Zhou ZL, et al KLF4 initiates sustained YAP activation to promote renal fibrosis in mice after ischemia-reperfusion kidney injury. Acta Pharmacologica Sinica. 2021;42(3):436–450. doi: 10.1038/s41401-020-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu J, Li PX, Wu J, Gao YJ, Yin MX, Lin Y, et al Involvement of the Hippo pathway in regeneration and fibrogenesis after ischaemic acute kidney injury: YAP is the key effector. Clinical Science. 2016;130(5):349–363. doi: 10.1042/CS20150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu Y, Yuan XD, Wu JJ, Chen RY, Xia L, Zhang M, et al The N6-methyladenosine mRNA methylase METTL14 promotes renal ischemic reperfusion injury via suppressing YAP1. Journal of Cellular Biochemistry. 2020b;121(1):524–533. doi: 10.1002/jcb.29258. [DOI] [PubMed] [Google Scholar]
- 132.Yang L, Humphreys BD, Bonventre JV Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contributions to Nephrology. 2011;174:149–155. doi: 10.1159/000329385. [DOI] [PubMed] [Google Scholar]
- 133.Yang WH, Ding CKC, Sun TA, Rupprecht G, Lin CC, Hsu D, et al The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Reports. 2019;28(10):2501–2508.e4. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang WH, Chi JT Hippo pathway effectors YAP/TAZ as novel determinants of ferroptosis. Molecular & Cellular Oncology. 2020;7(1):1699375. doi: 10.1080/23723556.2019.1699375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang WH, Huang ZQ, Wu JL, Ding CKC, Murphy SK, Chi JT A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Molecular Cancer Research. 2020;18(1):79–90. doi: 10.1158/1541-7786.MCR-19-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang WH, Lin CC, Wu JL, Chao PY, Chen K, Chen PH, et al The Hippo pathway effector YAP promotes ferroptosis via the E3 ligase SKP2. Molecular Cancer Research. 2021;19(6):1005–1014. doi: 10.1158/1541-7786.MCR-20-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yin F, Yu JZ, Zheng YG, Chen Q, Zhang NL, Pan DJ Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154(6):1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, et al Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu FX, Zhang YF, Park HW, Jewell JL, Chen Q, Deng YT, et al Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes & Development. 2013;27(11):1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yu FX, Zhao B, Guan KL Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yu SMW, Bonventre JV Acute kidney injury and maladaptive tubular repair leading to renal fibrosis. Current Opinion in Nephrology and Hypertension. 2020;29(3):310–318. doi: 10.1097/MNH.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yuan Q, Tan RJ, Liu YH Myofibroblast in kidney fibrosis: origin, activation, and regulation. Advances in Experimental Medicine and Biology. 2019;1165:253–283. doi: 10.1007/978-981-13-8871-2_12. [DOI] [PubMed] [Google Scholar]
- 143.Zager RA 'Biologic memory' in response to acute kidney injury: cytoresistance, toll-like receptor hyper-responsiveness and the onset of progressive renal disease. Nephrology Dialysis Transplantation. 2013;28(8):1985–1993. doi: 10.1093/ndt/gft101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang J, Bi JB, Ren YF, Du ZQ, Li T, Wang T, et al Involvement of GPX4 in irisin's protection against ischemia reperfusion-induced acute kidney injury. Journal of Cellular Physiology. 2021;236(2):931–945. doi: 10.1002/jcp.29903. [DOI] [PubMed] [Google Scholar]
- 145.Zhang NL, Bai HB, David KK, Dong JX, Zheng YG, Cai J, et al The merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Developmental Cell. 2010;19(1):27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang WJ, Gao YJ, Li PX, Shi ZB, Guo T, Li F, et al VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Research. 2014;24(3):331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei QY, et al Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes & Development. 2011a;25(1):51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ-TRCP. Genes & Development. 2010;24(1):72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao B, Li L, Wang L, Wang CY, Yu JD, Guan KL Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes & Development. 2012;26(1):54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao B, Tumaneng K, Guan KL The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nature Cell Biology. 2011b;13(8):877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao B, Wei XM, Li WQ, Udan RS, Yang Q, Kim J, et al Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & Development. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao B, Ye X, Yu JD, Li L, Li WQ, Li SM, et al TEAD mediates YAP-dependent gene induction and growth control. Genes & Development. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng ZH, Li CL, Shao GZ, Li JQ, Xu KX, Zhao ZH, et al Hippo-YAP/MCP-1 mediated tubular maladaptive repair promote inflammation in renal failed recovery after ischemic AKI. Cell Death & Disease. 2021;12(8):754. doi: 10.1038/s41419-021-04041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou WR, Zhao S, Xu SJ, Sun ZX, Liang YR, Ding XQ RacGAP1 ameliorates acute kidney injury by promoting proliferation and suppressing apoptosis of renal tubular cells. Biochemical and Biophysical Research Communications. 2020;527(3):624–630. doi: 10.1016/j.bbrc.2020.04.140. [DOI] [PubMed] [Google Scholar]
- 155.Zhou X, Wang Z, Huang W, Lei QY G protein-coupled receptors: bridging the gap from the extracellular signals to the Hippo pathway. Acta Biochimica et Biophysica Sinica. 2015;47(1):10–15. doi: 10.1093/abbs/gmu108. [DOI] [PubMed] [Google Scholar]
- 156.Zhou XN, Xiao F, Sugimoto H, Li BR, Mcandrews KM, Kalluri R Acute kidney injury instigates malignant renal cell carcinoma via CXCR2 in mice with inactivated Trp53 and Pten in proximal tubular kidney epithelial cells. Cancer Research. 2021;81(10):2690–2702. doi: 10.1158/0008-5472.CAN-20-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, et al On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Central Science. 2017;3(3):232–243. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zuk A, Bonventre JV Recent advances in acute kidney injury and its consequences and impact on chronic kidney disease. Current Opinion in Nephrology and Hypertension. 2019;28(4):397–405. doi: 10.1097/MNH.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]