Abstract
Reptile sex determination is attracting much attention because the great diversity of sex-determination and dosage compensation mechanisms permits us to approach fundamental questions about mechanisms of sex chromosome turnover. Recent studies have made significant progress in better understanding diversity and conservation of reptile sex chromosomes, with however no reptile master sex determination genes identified. Here we describe an integrated genomics and cytogenetics pipeline, combining probes generated from the microdissected sex chromosomes with transcriptome and genome sequencing to explore the sex chromosome diversity in non-model Australian reptiles. We tested our pipeline on a turtle, two species of geckos, and a monitor lizard. Genes identified on sex chromosomes were compared to the chicken genome to identify homologous regions among the four species. We identified candidate sex determining genes within these regions, including conserved vertebrate sex-determining genes pdgfa, pdgfra amh and wt1, and demonstrated their testis or ovary-specific expression. All four species showed gene-by-gene rather than chromosome-wide dosage compensation. Our results imply that reptile sex chromosomes originated by independent acquisition of sex-determining genes on different autosomes, as well as translocations between different ancestral macro- and microchromosomes. We discuss the evolutionary drivers of the slow differentiation and turnover of reptile sex chromosomes.
Keywords: Sex determination, Reptiles, Genomics, Cytogenetics, Sex chromosome turnover
INTRODUCTION
Sex can be determined either by genes on specialized chromosomes (genotypic sex determination, GSD) or by environmental factors (environmental sex determination, ESD). Much of our knowledge on sex chromosome evolution has come from studies of model organisms such as Drosophila, chicken and mammals (principally humans and mice), in which species master sex determining genes have been identified (Bachtrog, 2013). Their heteromorphic sex chromosomes can be easily identified by cytogenetic observations because the male-specific Y chromosome, or the female-specific W chromosome is morphologically different from the X or Z chromosome. Sex chromosome differentiation occurs as the result of suppression of recombination, and is manifested by massive accumulation of transposable elements and inactivation or loss of genes (Charlesworth & Charlesworth, 2000). The sex chromosomes of many model vertebrate species have been evolutionarily stable for more than 100 million years, judging from the homology of the pair of sex chromosomes within their clade (Cortez et al., 2014; Zhou et al., 2014).
Like birds and mammals, some reptiles, amphibians and fish have stable sex chromosomes without frequent transitions (Augstenová et al., 2021; Bellott & Page, 2021; Cornejo-Páramo et al., 2020; Giovannotti et al., 2017; Kostmann et al., 2021; Kratochvíl et al., 2021b; Mezzasalma et al., 2021; Rupp et al., 2017; Stöck et al., 2021; Thépot, 2021). However, for majority of reptiles, amphibians and fishes, frequent transitions between different sex determination mechanisms have been observed (Ezaz et al., 2009b; Mank & Avise, 2009; Miura, 2017). Reptiles represent an extraordinary variety of sex determining mechanisms, including genotypic sex determination (GSD) XY and ZW systems with varying degrees of sex chromosome differentiation and temperature dependent sex determination (TSD) (Alam et al., 2018). In recent years evolution and transitions of reptiles sex chromosomes and sex determination have received renewed attention due to advances in genome sequencing and integration of cytogenetics knowledge, which revealed novel insights into the dynamic evolution and novel mechanisms in multiple reptile lineages including squamates and chelid turtles, and have been highlighted in multiple recent reviews (Bellott & Page, 2021; Kratochvíl et al., 2021b; Mezzasalma et al., 2021; Rupp et al., 2017; Thépot, 2021). However, genomic landscape of sex chromosomes and sex determining genes in many other reptiles remained uncharacterised.
The evolutionary variety of vertebrate sex determining systems has long been recognized. Cytological observations and limited gene mapping data revealed that multiple transitions between ESD and GSD, and between XY and ZW sex chromosome systems, had occurred in reptiles (Ezaz et al., 2009b), teleost fish (Mank & Avise, 2009) and anurans (Miura, 2017). However, despite this variety, extensive cytogenetic mapping of the reptile orthologues of genes that are located on sex chromosomes of model organisms (e.g., human and chicken) revealed a surprisingly frequent over-representation of particular ancestral autosomes or genomic regions (Deakin & Ezaz, 2014; Ezaz et al., 2017; O'Meally et al., 2012). However, testing the hypothesis on the existence of a conserved ancestry of amniote sex chromosomes will require in-depth comparative analysis on much wider phylogenetic context (Kratochvíl et al., 2021a).
With the development of long-read sequencing and Hi-C technologies, many genomic consortia (e.g., Vertebrate Genome Project (Rhie et al., 2021) and the Earth Biogenome Project (Lewin et al., 2018)) aim to finish the complete genomes of most vertebrate species in the next few years. However sequencing projects usually represent sex chromosomes poorly; either the homogametic sex (XX female or ZZ male) is sequenced, with the male-specific Y or female-specific W being ignored; or the heterogametic sex only is sequenced with poor representation of the X or Z, and there is great difficulty in assembling the repeat-rich Y or W (Bellott et al., 2017; Skaletsky et al., 2003).
Here, we develop a cost-effective method to identify genes borne on sex chromosomes, combining microdissection of sex chromosomes and high-throughput sequencing, followed by PCR validation and assessment as candidate sex determining genes. A similar method was pioneered to identify novel genes on the Y chromosome of marsupials (Sankovic et al., 2006), and subsequently has been applied to characterise sex chromosome sequences in a plant (Hobza & Vyskot, 2007), in a species of fish (Cocca et al., 2015), two species of anole lizards (Kichigin et al., 2016), in humans (Alvarez-Cubero et al., 2018) and in Gorrila (Tomaszkiewicz et al., 2016). We applied the method to four reptile species, revealing the great diversity of sex chromosomes, and their independent evolutionary origins.
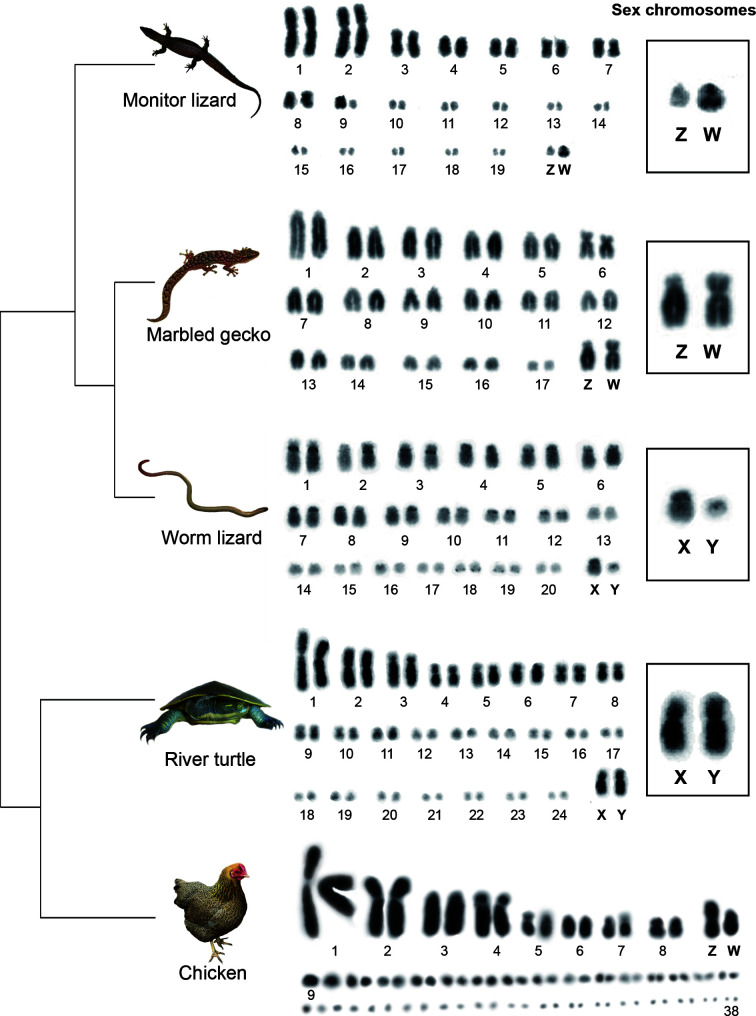
We chose four Australian reptile species, a turtle and three lizard species to represent the variety of reptile sex determining systems (Figure 1). The Murray River turtle Emydura macquarii (Martinez et al., 2008) (referred as “river turtle” hereafter) has minimally differentiated X and Y are macrochromosomes, whereas the pink-tailed worm lizard Aprasia parapulchella (Matsubara et al., 2013) (referred as “worm lizard” hereafter) has a highly differentiated XX/XY sex chromosome system in which the X and Y are microchromosomes. The marbled gecko Christinus marmoratus (Matsubara et al., 2014a) (referred as “marbled gecko” hereafter) has a pair of ZZ/ZW sex chromosomes, in which the Z and W heteromorphy involves pericentric inversion, whereas the spiny-tailed monitor lizard Varanus acanthurus (Matsubara et al., 2014b) (referred as “monitor lizard” hereafter) has ZZ/ZW heteromorphic sex chromosomes in which Z and W chromosomes are minimally differentiated microchromosomes (Figure 1).
Figure 1.
The diversity of reptile sex chromosomes
Truncated phylogeny (Pyron et al., 2013) showing karyotypes and sex chromosomes of studied reptile species, river turtle (Emydura macquarii), worm lizard (Aprasia parapulchella), Marbled gecko (Christinus marmoratus) and monitor lizard (Varanus acanthurus). Photo credit: see acknowledgements.
MATERIALS AND METHODS
Ethics Statement
Animal care and experimental procedures were performed following the guidelines of the Australian Capital Territory Animal Welfare Act 1992 (Section 40) and conducted under approval of the Committee for Ethics in Animal Experimentation at the University of Canberra (Permit Number: CEAE 11/07 and CEAE 11/12).
Chromosome preparations, sex chromosome microdissection, microdissected chromosome sequencing, probe preparations and FISH analysis
Animal collection, microdissection, preparation of sex chromosome specific probes and validation of probes were described in our previous studies (Matsubara et al., 2013, 2014a, 2014b). Briefly, we labelled sex chromosome probes by nick translation incorporating SpectrumGreen-dUTP (Abbott, USA) or SpectrumOrange-dUTP (Abbott, USA) and precipitated with 20 µg glycogen. After decantation, labeled probe pellets were resuspended in a 15 µL hybridization buffer. The resuspended probe mixture was hybridized with a drop of metaphase chromosome suspension fixed on a glass slide, covered with coverslips, and sealed with rubber cement. The slide was then denatured on a hot plate at 68.5 °C for 5 min and was hybridized overnight in a humid chamber at 37 °C for two days. The slides were then washed first with 0.4×SSC, 0.3% IGEPAL (Sigma-Aldrich, USA) at 55 °C for 2 min followed by 2×SSC, 0.1% IGEPAL for 1 min at room temperature. The slides were dehydrated by ethanol series and air-dried and then mounted with anti-fade medium Vectashield (Vector Laboratories, USA) containing 20 µg/mL DAPI (4′,6-diamidino-2-phenylindole.). We sequenced single microdisseted sex chromosome from all species except for river turtle (E. macquarii) Y chromosome, where we sequenced a pool of 5 Y chromosomes. We amplified sex chromosome DNA using GenomePlex single cell whole genome Amplification kit (Sigma, USA) following manufacturer’s instruction as described in Matsubara et al. (2013, 2014a, 2014b). Next generation sequencing of the amplified sex chromosomes DNA library was performed at the Beijing Genome Institute (BGI) using Illumina HiSeq2000 (USA). We sequenced 500 bp insert library and all reads were trimmed to 90 bp for both ends to get 2 GB clean reads.
Transcriptome assembly and annotation
RNA-Seq data from gonads and brain tissues for males and females of monitor lizard (V. acanthurus), river turtle (E. macquarii) and marbled gecko (C. marmoratus) and tail tissue from a male and female worm lizard (A. parapulchella) (Each tissue has only one sample from one individual due to the difficulty of collecting the tissues from these wild species) were used to perform de novo assembly of each species with Trinity v2.4.0 pipeline (Grabherr et al., 2011). Then we used transcoder (Grabherr et al., 2011) to do ORF prediction and cd-hit (v4.7) (Fu et al., 2012) to remove the redundant sequences with the parameters -c 1.00 -b 5 -T 8. For evaluating the quality of the assembly, we examine the number of transcripts that appear to be full-length or nearly full-length by BLAST+ (v2.6.0) (Altschul et al., 1990) with the E-value 1e-3. For worm lizard and marbled gecko, the reference species is Gekko japonicus while for river turtle, the reference species is Pelodiscus sinensis, and transcripts with a minimum 30% coverage of reference were selected.
We used the Trinotate (v2.0.1) (Grabherr et al., 2011) pipeline to annotate the transcriptome. First, we aligned the transcripts to the reference library consisting of human and chicken using blastx and the protein file using blastp with the e-value 1e-3. Also, we used HMMER (v3.1b2) to do another annotation which aligned the transcripts to the Pfam protein library according to the hidden Markov algorithm with the default parameters. Later, the transcripts and the protein, along with the alignments from blast and HMMER were fed to Trinotate to annotate the transcriptome. The transcriptomes were evaluated by assessing the number of fully reconstructed coding transcripts with their reference species, which are G. japonicus for worm lizard and marbled gecko, and P. sinensis for river turtle.
Genome assembly and annotation
We used SOAPdenovo2 (v2.0.4) pipeline (Luo et al., 2012) to assemble the Illumina DNA reads from microdissected sex chromosomes. In brief, we first tried several times with default parameters, to find the best k-mers with the longest N50. Then, we adjusted the average insertion size according to the best result and re-run the scaffold step. Afterwards, we used kgf (v1.16) with the parameters -m 5 -t 6 and Gapcloser (v1.12) to fill the gaps (Luo et al., 2012) , which finally built a de novo draft assembly for sex chromosomes of our species.
We constructed the genome assembly of monitor lizard (V. acanthurus) with the Supernova v2.1.1 pipeline (Weisenfeld et al., 2017) with the default parameters, which is a package for de novo assembly based on 10X sequencing. Briefly, the approach is to first build an assembly using read k-mers (default is 48), then resolve this assembly using read pairs (to k=200), then use barcodes to effectively resolve this assembly to k≈100 000. The final step pulls apart homologous chromosomes into phase blocks, which create diploid assemblies of large genomes.
We annotated the genome of monitor lizard (V. acanthurus) with the Braker2 v2.1.5 pipeline (Brůna et al., 2021) which combined evidence of protein homology, transcriptome and de novo prediction. First, we used RepeatMasker (v4.0.7) (Tarailo-Graovac & Chen, 2009) with parameters: -xsmall -species squamata -pa 40 -e ncbi, and the Repbase (v21.01) to annotate the repeat sequences. Then we aligned all available RNA-seq reads to the reference genome by STAR (v2.5) (Dobin et al., 2013) to construct transcriptome evidence. Later we fed the masked genome, the alignment of RNA-seq, and the reference protein sequences, which were human and chicken here, to Braker2 with parameter: --prg=exonerate, setting exonerate for protein homology prediction. Finally, the package outputs the GFF file containing the gene models, along with protein sequences and CDS sequences. Additionally, we also separately annotated the sex chromosome of monitor lizard. First, we aligned our sequences to the reference protein sequences using tblastn with parameters: -F F -p tblastn -e 1e-5. The results were then refined by GeneWise (v2.4.1) (Birney et al., 2004), and for each candidate gene, we kept the one with the best score. Within these genes, we filtered them if premature stop codons or frameshift mutations reported by GeneWise or single-exon genes with a length shorter than 100 bp, or multi-exon genes with a length shorter than 150 bp, or if the repeat content of the CDS sequence is larger than 20% exists.
Sex-linked sequences identification
For worm lizard (A. parapulchella), river turtle (E. macquarii) and marbled gecko (C. marmoratus), using an XY system as an example, we first assembled all the RNA-seq reads into a pooled transcriptome, the female reads into a XX transcriptome, and the male reads into a XY transcriptome. Then male RNA-seq reads were mapped to the XX transcriptome with bowtie2 (v2.2.9) (Langmead & Salzberg, 2012) with default parameters. Read depth was then calculated using SAMtools (v1.6) (Li, 2011), and those reads unmapped were assembled into a transcriptome, which was considered to be X reads excluded.
The pooled transcriptomes were directly mapped by Illumina DNA reads from the X and Y chromosomes, and those sequences not mapped by either X or Y reads were assigned as autosomal genes. XX transcriptome and XY transcriptome were both mapped by DNA reads from the X and Y chromosomes, the reads depth (coverage/mappable site) was calculated for each genomic regions mapped, and sequences with a depth higher than 3 and a minimum coverage of 10% with X reads, simultaneously with no alignments with Y reads were assigned as X-linked. For the transcriptome with X reads excluded, the same steps were repeated and sequences with a depth higher than 3 and a minimum coverage of 10% with Y reads and no alignments with X reads were assigned as Y-linked. Afterwards, for sequences with both reads depth (X reads and Y reads) higher than 3, along with a minimum 10% coverage with both X and Y reads, we assigned them as shared genes.
To identify the sex-linked sequences in monitor lizard (V. acanthurus), Illumina reads from both sexes were aligned to the scaffold sequences using bowtie2 with default parameters. Read depth of each sex was then calculated using SAMtools in 10 kb non-overlapping windows and normalized against the median value of depths per single base pair throughout the entire genome for the comparison between sexes. Those sequences with depth ratio of male-vs-female (M/F) ranging from 1.75 to 3, along with a read coverage ratio of male-vs-female higher than 0.8 were assigned as Z-linked sequences. For the rest of the sequences, those with M/F ratio of depth and coverage both ranging from 0.0 to 0.25 are assigned as W-linked sequences, and the remaining are assigned as autosomes. Those annotated genes in the sex-linked sequences were assigned as Z-borne or W-borne genes. Later we aligned the Z-borne genes to the W-borne genes with blastn, and those genes that have an identity higher than 0.8 and aligned length >500 bp were assigned as shared genes.
Homology comparisons
To find the orthologs of our genes with chicken, we compared the sex-linked transcripts of worm lizard (A. parapulchella), river turtle (E. macquarii) and marbled gecko (C. marmoratus), and the sex-linked genes annotated of the monitor lizard (V. acanthurus) 10× assembly to the proteins of chicken (v6, Ensembl), respectively using blastx with the e-value 1e-5. The result was filtered with the aligned AAs >30% coverage of the reference chicken protein, along with a minimum 50% identity, and returned the one-to-one best hits, with the duplications retained. Then we merged the alignment sites from the four species and calculated the total number of orthologs on the relative chicken chromosomes. With the same protocols, we found the orthologs of our sex-linked genes with chicken sex-determining genes, except for the threshold of identity which were adjusted to 40%.
Gene expression analyses
To quantify gene expression, RNA-seq reads were mapped to the transcripts of worm lizard (A. parapulchella), river turtle (E. macquarii) and marbled gecko (C. marmoratus) and the CDS of monitor lizard (V. acanthurus) by bowtie2. The raw read counts were estimated by RSEM (v1.3.1) (Li & Dewey, 2011), with both TPM expressions calculated. Those genes which have orthologs with chicken were filtered for dosage compensation analysis. Correlation within sexes for each species was tested using a Wilcoxon rank-sum test, where a significant differentiation within samples was found, with a P-value smaller than 0.05.
Gonadal biased genes were identified by calculating the fold change of gonadal to somatic expressions of the four species. For both ovary and testis, bias genes were classified into 4 categories of TPM ratio, namely <2, 2 to 3, 3 to 5, >5. For those genes that have a ratio <2 are assigned as negative, for those ≥2, are assigned as gonadal biased, and only those genes with a ratio higher than 5 were calculated in the correlation test for masculinization.
dN/dS calculation
To calculate the dN/dS values between the gametolog genes of monitor lizard and Komodo dragon, proteins sequences of Z or W gametologs from monitor lizard were aligned to the proteins of Komodo dragon, and the reciprocal-best-hit of proteins were identified by blastp with the identity level higher than 70 and the aligned amino acids higher than 70% of the protein length. Then, we aligned the coding sequence of each pair of genes using Prank (v.150803) with default parameters, and dN/dS were later calculated for each pairwise alignment using the codeml module of PAML (v4.8) (runmode=2).
INDEL calling and PCR validation of sex specific markers
Using the identified 10.81 Mb Z-borne scaffolds and 7.10 Mb W-borne scaffolds of monitor lizard (V. acanthurus), we first identified indels based on their alignment using LASTZ (v1.02)(Harris, 2007) with default parameters. On two homologous scaffolds (ChrZ_scaf_189 and Chr_W_scaf_176), we found three W-specific insertions with the lengths as 206 bp, 331 bp and 209 bp (Supplementary Table 7). At the flanking regions of these insertions, we designed two PCR primers spanning a predicted length of 1 312 bp (assay 1) and 2 479 bp (assay 2) sequence fragments for validating the sex chromosome specificity in monitor lizard (V. acanthurus). Both primer sets were amplified under the following conditions: initial denaturation at 95 °C for 5 min followed by 35 cycles of 95 °C for 30 s, 57 °C for 30 s and an extension step of 72 °C for 1 min, with final extension of 72 °C for 10 min. These two markers were validated on 60 individuals; 22 females and 38 males from five different localities distributed across the species distribution.
RESULTS
Transcriptome and genome assemblies of sex chromosomes of the four reptile species
The four reptile species have cytologically distinguishable sex chromosome pairs (Figure 1; Supplementary Figure S1); these were morphologically differentiated in the worm lizard and the monitor lizard, but more subtle for the river turtle and the marbled gecko (Matsubara et al., 2013, 2014a, 2014b). For each species, we microdissected each of their sex chromosomes, performed linear genome amplification and validated the sex chromosome specificity of the DNA products by chromosome painting (Figure 2A; Supplementary Figure S1).
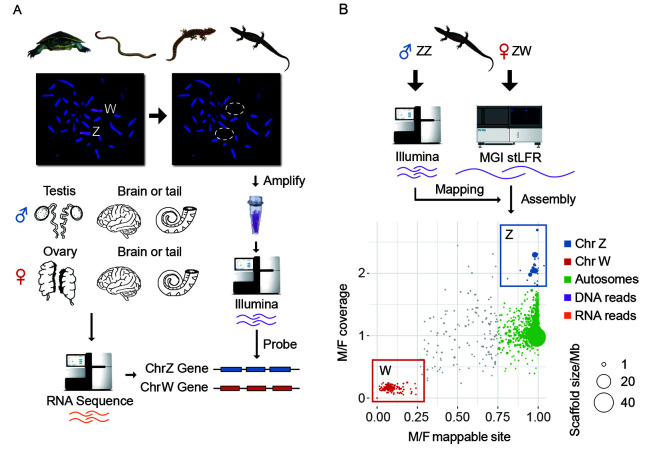
Figure 2.
Transcriptome and genome assemblies of four Australian reptile species
A: The pipeline of identifying sex-linked transcripts of river turtle, worm lizard and marbled gecko using transcriptomes of sexed tissues, and amplified probes from the microdissected sex chromosomes. The probes have been validated by chromosome painting, and Illumina reads generated from the probes were used to identify the sex chromosome genes from the de novo assembled transcripts. B: Identifying sex-linked sequences of monitor lizard based on the de novo genome assembly generated from linked (stLFR) reads. For the assembled Z-linked sequences (in blue), we found a 2-fold male vs. female (M/F) ratio of Illumina DNA sequencing coverage, but an equal mappable site between sexes. While the W-linked sequences (in red) exhibited a female-specific pattern of read coverage ratio and mappable sites. The size of each dot is scaled to its scaffold length.
For each sex chromosome, we generated up to 2 Gb clean paired-end (PE) Illumina reads from the microdissected sex chromosome DNA (Supplementary Table S1). To identify genes borne on sex chromosomes, we also produced 2 Gb transcriptomes per sample from the gonad and brain tissues for males and females of monitor lizard, river turtle and marbled gecko and somatic transcriptomes (tail tissue) from a male and female worm lizard (Figure 2B). Genomic reads derived from each sex chromosome were then used to identify sex-linked genes from de novo assembled transcript sequences of each species. We annotated a total of 11299, 15202 and 10507 non-redundant transcripts, respectively for the worm lizard, the marbled gecko, and the river turtle, using chicken genes as a reference for each.
For the monitor lizard, inferences based on transcripts and microdissected sex chromosome sequences were uncertain because its sex chromosomes were reported to have originated from translocations between fragments of multiple ancestral autosomes (also see below) (Iannucci et al., 2019), as well as the poor quality of sequences obtained from microdissected monitor lizard sex chromosomes. Therefore, we further generated 200 Gb (135× genomic coverage) single-tube long fragment linked reads (stLFR)(Wang et al., 2019) from a female individual, and 30 Gb Illumina PE reads from a male individual, so that we can identify the sex chromosomes by comparing reads between sexes. We performed de novo genome assembly and produced a female draft genome with the total length of 1.46 Gb and the scaffold N50 length of 12.8 Mb (Supplementary Table S2). The high continuity of the draft genome was evident from 94 very large scaffolds that accounted for 80% of the entire genome. Using protein sequences of human and chicken as reference, we annotated a total of 14 521 genes for the monitor lizard and identified its sex-linked sequences based on the comparisons of mapped read patterns between sexes. The putative W-linked scaffolds showed female specificity in both their mapped read number and mapped sites, whereas the Z-linked scaffolds showed a 2-fold increase of male mapped reads compared to that of female mapped reads, but an equal number of mapped sites for putative autosomal scaffolds between sexes (Figure 2B). Using this approach, we identified 10.81 Mb Z-linked scaffolds with 333 genes, and 7.10 Mb W-linked scaffolds with 87 genes.
Identification of sex-linked genes
To identify the sex chromosome-borne genes in three species other than the monitor lizard, we developed a pipeline to separately assemble transcripts of genes that are X- or Y-borne (or Z- and W-borne) using the sexed transcriptomes (Figure 3A). In brief, we considered that transcripts that were assembled using pooled RNA-seq reads of both sexes and could not be aligned using the sex chromosome DNA probes were autosomal genes. Conversely, male RNA-seq reads in XY species that could not be aligned to the female transcripts were assembled into candidate Y- borne transcript sequences. Then by comparing the mapped read numbers of Y- or X-borne probes for each candidate Y-borne transcript or each transcript assembled from female RNA-seq, we were able to categorize them into the genes that were specific to the X or to Y chromosome, or were shared between X and Y. We also conducted the same process for the ZW marbled gecko but in reverse. Those shared genes however cannot be assigned as either X/Z- or Y/W-linked.
Figure 3.
Processes involved in the identification of sex-linked genes
A: Pipeline to separately assemble the X-linked (red box), Y-linked (blue box) genes using the sexed transcriptomes (red: female; blue: male). Squares (DNA) and circles (RNA) refer to the reads from different resources. Shared genes, which can be aligned by probes from both sex chromosomes are labelled in purple colour. The autosomal genes, which cannot be aligned by the sex chromosome DNA probes are labelled in green. B: Numbers of sex-linked genes in the four reptile species. X-linked and W-linked genes are labelled in red colour, while Y-linked and Z-linked genes are labelled in blue colour. The overlapping areas refer to the genes shared between the two sex chromosomes. C: An example of PCR validation of sex-linked sequences of the monitor lizard. M refers to male individuals and F refers to female individuals; outside lane size standard 1 kb (left) and 50 bp (right) ladder.
Following our stringent filtering criteria (see Methods), we identified 193 X-borne or X-linked hemizygous genes, 1 Y-borne gene and 17 shared genes between X/Y chromosomes in the worm lizard, 21 X-borne genes, 7 Y-borne genes and 218 shared genes in the river turtle and 50 Z-borne genes 37 W-borne genes and 307 shared genes in the gecko,281 Z-borne genes 35 W-borne genes and 52 shared genes in the monitor lizard (Figure 3B; Supplementary Table S3). We considered these numbers to be conservative estimates of the sex chromosome-borne genes in these species because genes with low expression levels may not be well assembled in our transcriptome data, and there could also be a sampling bias in the sex chromosome probes captured by microdissection. We further designed primers spanning regions of insertions or deletions between the sex chromosomes and confirmed their length variations between sexes by PCR for the sex chromosome-borne genes of the monitor lizard (Figure 3C). We found no indel sequences within the coding regions of sex chromosome borne genes of the other three species, hence did not design primers for validation.
The proportion of genes that are specific for one or other sex chromosome, and the proportion that are shared, provide a good indication of the degree of genetic differentiation of the sex chromosome pair, and correlate well with our cytogenetic observations (Supplementary Figure S1). The high numbers of genes shared between the X and Y chromosomes of the river turtle suggest that its sex chromosome pair is not highly differentiated, which is consistent with the subtle difference in size and morphology of the X and Y (Figure 1). Among the three lizard species, the numbers of shared versus sex chromosome-specific genes also implied different degrees of sex chromosome differentiation. Consistent with the cytogenetic data (Figure 1; Supplementary Figure S1) (Matsubara et al., 2013), the Z and W chromosomes of the marbled gecko also shared most genes, whereas the monitor lizard showed an intermediate level of shared Z- and W-borne genes. In contrast, the worm lizard had many X-specific but very few Y-specific genes, and only a few X-Y shared genes, implying that the Y chromosome is highly degraded.
Origins of sex chromosomes of the four reptile species
By mapping the orthologues of sex-linked genes of the four reptiles to the chicken genome (GGA), we found evidence for both independent origin and convergent evolution of sex chromosomes (Figure 4; Supplementary Figure S2).
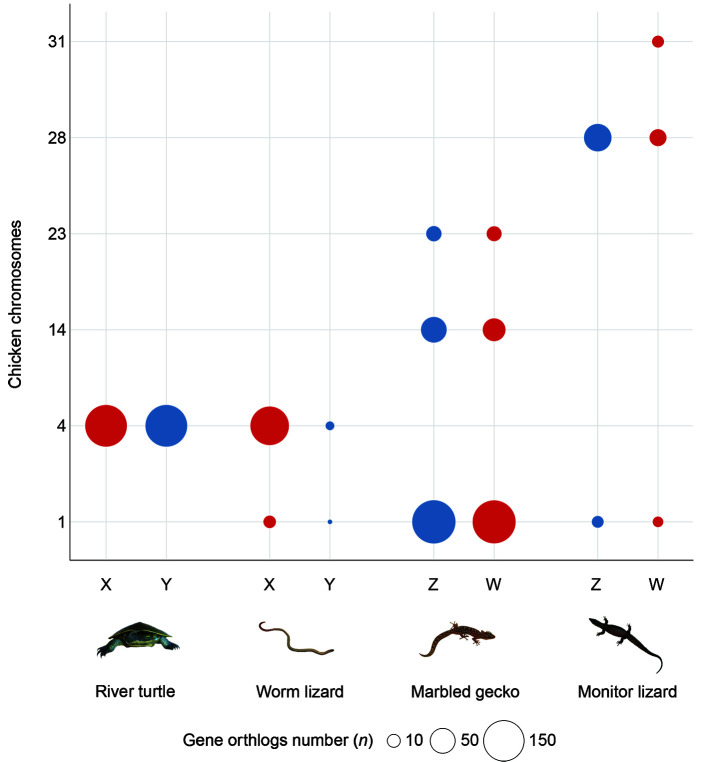
Figure 4.
Independent origin of the sex chromosomes of four reptile species
The independent origins of the sex chromosomes of four reptile species with chicken chromosomes as reference. The bubble size is scaled to the number of orthologs of sex chromosome borne genes of each reptile species within the chicken genome, and the colour indicates the type of sex chromosomes (red for X and W, and blue for Y and Z).
In each species, genes borne on the sex chromosomes clustered together predominantly on a single chicken chromosome, though in three of the species there were other minor clusters. Sex chromosomes of the three lizards were homologous to quite different regions of the chicken genome, on chromosomes GGA1, GGA4 and GGA28 respectively, implying independent origins. However, the sex chromosomes of the river turtle largely overlapped with those of the worm lizard on GGA4q, the long arm of chicken chr4. This is unlikely to represent sex chromosome identity by descent, since the turtles are more closely related to birds (in which this region is autosomal) than they are to squamates, with divergence times of ~250 million years ago (Ma) and 285 Ma respectively (Wang et al., 2013), however, in-depth analysis in multiple outgroups is warranted to test this assumption.
Secondary sites of homology between our four reptile species and the chicken genome represent fragments of sex chromosomes with a different evolutionary origin. For instance, homologs of some river turtle sex chromosome-borne genes were found on chicken microchromosome GGA32 (Supplementary Figure 2). This supports the hypothesis that the sex chromosomes of the river turtle originated by a recent translocation between an ancestral sex chromosome pair (GGA4) and a microchromosome pair (Lee et al., 2019b; Martinez et al., 2008).
The sex chromosomes of marbled gecko were mainly homologous to GGA1p, with strong secondary signals on GGA14 and GGA23 that contained very similar numbers of Z- and W-borne genes. Genes on the sex chromosomes of the monitor lizard were mainly homologous to GGA28, with strong secondary sites at GGA31, GGA33 and Z that contained similar numbers of sex chromosome-borne genes (Figure 4; Supplementary Figure 2).
Candidate sex-determining genes of the four reptile species
Novel sex chromosomes may arise when an autosomal gene acquires a sex determining function or through autosome sex chromosome fusion (Lee et al., 2019a; Pennell et al., 2015). Sex chromosome turnovers have occurred many times during reptile evolution (Bista et al., 2021; Gamble et al., 2015, 2017), possibly by a novel sex determining gene usurping the established gene (Herpin & Schartl, 2015) (e.g., sdY in rainbow trout (Yano et al., 2012), or a change to environmental sex determination and the subsequent evolution of novel genetic systems (Holleley et al., 2015). It would not, therefore, be unexpected to find different candidate sex determining genes within the genomic regions that we have identified in the four reptiles (Figures 1, 4).
To test this, we compiled a list of genes reported to be involved in the sex-determining pathways of other vertebrates (Supplementary Table S4 and Figures S3, S4) and looked for their orthologs among genes that were either identified as sex-linked or fell within the identified sex-linked region in each studied species (Figure 5A, B; Supplementary Tables S5, S6). Included in the region is GGA4q that overlaps with the homologous regions of the river turtle and the worm lizard X chromosomes and contains one candidate male-determining gene pdgfra (platelet-derived growth factor receptor alpha). Another candidate sex determining gene AR (Androgen receptor) located on GGA4p was also annotated as X-borne in these two species. This suggests an independent acquisition of AR gene because GGA4p is a microchromosome in all other birds which was fused recently in chicken. The pdgfa (platelet-derived growth factor alpha polypeptide) gene and its receptor pdgfra (platelet-derived growth factor receptor alpha) have been shown to be critical for testis development, particularly Leydig (male steroidogenic) cell development in mammals and turtles (Brennan et al., 2003; Rhen et al., 2009), whereas AR is more likely to be involved in the downstream sexual differentiation process after the gonad sex is determined (Hiort, 2013; Wilson et al., 1980). We confirmed pdgfra to be X-borne in the worm lizard using our transcriptome assembly and sex-linked probes from microdissected sex chromosomes. In the river turtle, we could not annotate pdgfra as a sex chromosome-borne gene because of a lack of mapped sex-chromosome borne probes, but it was embedded among other sex chromosome-borne genes in, so that is likely to be sex chromosome-borne also in the river turtle (Figure 5B).
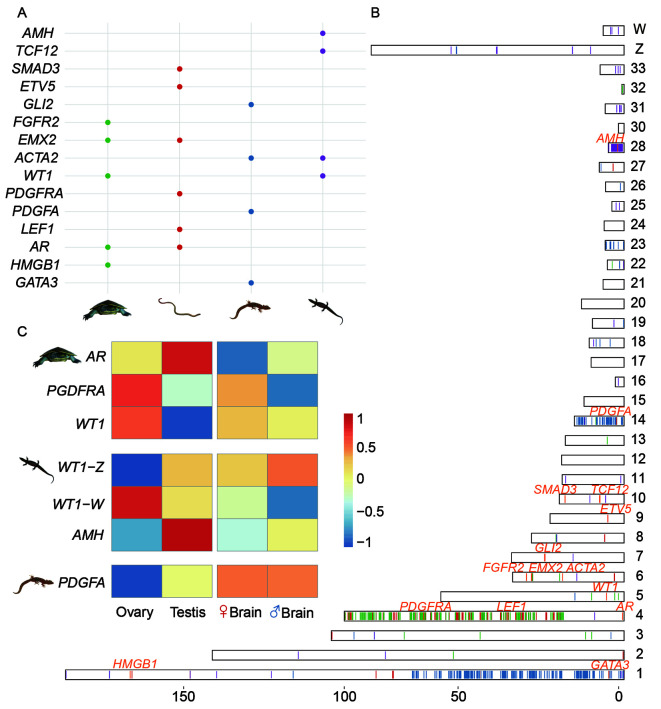
Figure 5.
Candidate sex-determining genes of the four reptile species
A: The distribution of orthologs of vertebrate sex-determining genes that were also identified as on the sex chromosomes in this study. The coloured dots correspond to such genes within each species, which were identified by blast search against the chicken genome. For panels A, B and C, the river turtle is shown by green dots or bars, the monitor lizard by purple, the worm lizard by red and the marbled gecko by blue. B: Shows the ortholog positions of the sex chromosome-borne genes of these four reptile species on chicken chromosomes, with different colours of lines for different species’ orthologs. C: Gene expression patterns in the gonad and somatic tissues of candidate sex-determining genes of the three reptile species. We did not show it for the worm lizard due to unavailability of gonad tissues.
For the marbled gecko, a promising candidate sex-determining gene is the Z-borne pdgfa (with a chicken orthologue on GGA14). For the monitor lizard, the most promising candidate upstream sex-determining gene was amh (anti Mullerian hormone), which (or the duplicated copy of which) is located on GGA28 and plays a conserved role in testis development in multiple teleost species (Herpin & Schartl, 2015), birds (Cutting et al., 2013), turtles (Zhou et al., 2019), and even the platypus (Zhou et al., 2021) and suggested to be a candidate sex determining gene in Komodo dragon (Lind et al., 2019). Intriguingly, the ortholog of wt1 (Wilms Tumour 1), an important regulator of amh and master male-determining gene Sry in human (Hossain & Saunders, 2001), was determined to be X and Y-borne in the river turtle, and Z and W-borne in the monitor lizard, and was located on a secondary chicken site of GGA5 (Figure 5B; Supplementary Figure S2).
The expression patterns of these candidate sex-determining genes within the three reptiles for which we collected the gonad transcriptomes in this study further supported their function in the sex-determination pathway of each species (Figure 5C; Supplementary Figure S5). The Z-borne pdgfa was specifically expressed in the testis of the marbled gecko; whereas its downstream receptor pdgfra, which is X-borne in the river turtle, was strongly expressed in the ovary. The X-borne wt1 of the river turtle, as well as the W-linked wt1 of the monitor lizard, were both expressed specifically in the ovary. It has to be noted that a previous study reported location of wt1 on to chromosome 1 in river turtle (Lee et al., 2019a), however our in silico analysis identified wt1 to be on X and Y chromosomes, which require further investigation. The Z-borne wt1 and amh of the monitor lizard were both specifically expressed in the testis. In summary, turnover of sex determining genes between the studied reptile species probably accounts for their sex chromosome turnovers.
Evolution of dosage compensation and sex-linked gene expression in the four reptile species
Having identified the sex chromosome-borne genes of the four distantly related reptiles, we set out to examine their diversity of dosage compensation based on comparison of gene expression levels between sexes, and between the autosomes and the sex chromosomes. Since sex chromosomes may undergo meiotic sex inactivation in germ cells, and gonads are probably not appropriate for direct comparison between sexes (Gu & Walters, 2017), we focused on comparing the expression levels between sexes in their somatic (brain, tail or blood) tissues. Genes that are shared between sex chromosomes are not expected to evolve dosage compensation. Also as mentioned earlier, we were unable to discriminate between the X- and Y-, Z- and W-borne genes (or called gametologs) based on their few divergence sites assembled from transcriptome sequences, we therefore focused on comparing the X- or Z-borne, or the hemizygous genes vs. autosomal genes for their somatic transcription level to examine the respective species’ dosage compensation pattern.
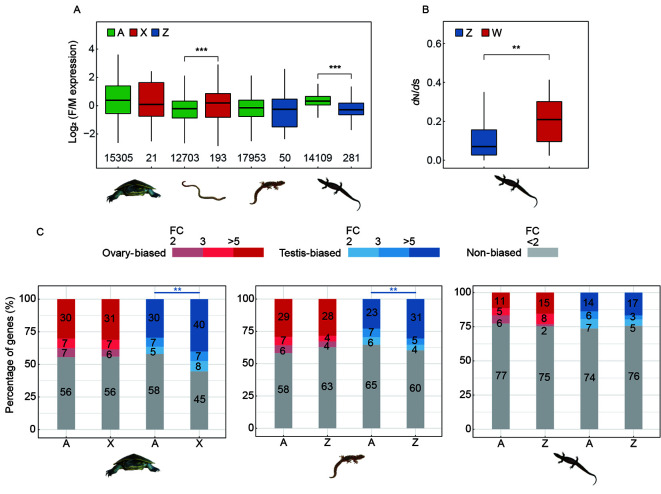
Among the four species, the worm lizard (with an XY sex system) and the monitor lizard (with a ZW sex system) have highly or moderately differentiated sex chromosomes. These two species exhibited a significantly (P<0.05, Wilcoxon test) different female vs. male expression ratio between autosomes and sex chromosomes (Figure 6A; Supplementary Figure S6). The X-borne genes were more female-biased in the worm lizard, and Z-borne genes were more male-biased in the monitor lizard, indicating incomplete dosage compensation in the two species. Similar patterns of incomplete dosage compensation have been reported before in Komodo dragon and pygopodidae lizards (Rovatsos et al., 2021). In contrast, genes on the undifferentiated sex chromosomes, as well as autosomes, of marbled gecko and river turtle showed no significant difference of their expression ratios between sexes. This could be because their Y- or W-borne genes have not degraded yet, so most genes on their X or Z chromosomes still have active partners on the Y (W) and thus there is no dosage difference between the sexes that selects for dosage compensation. In summary, all the four species examined do not seem to have evolved chromosome-wide dosage compensation mechanisms. On the other hand, for the monitor lizard with a much better assembly of its gametologs sequences, we further inspected their evolutionary rates (measured by ratios of nonsynonymous vs. synonymous substitution rates, dN/dS) using its related species Komodo dragon’s genome as a reference. Among the 52 gametologs present on both Z and W chromosomes, as expected, W gametologs exhibited a significantly (P<0.05, Wilcoxon test) faster rate of evolution than their Z-linked counterpart due to suppression of recombination (Figure 6B), indicating early signs of degeneration.
Figure 6.
Dosage compensation and sex-linked gene expression in the four reptile species
A: Comparisons of the expression levels between sexes in the somatic (brain or tail) tissues of the four species. Genes from autosomes and the sex chromosomes are labelled in different colours, (autosomes: green, chrX: red and chrZ: blue). Only genes that have orthologs in chicken are considered and the respective gene numbers are shown on the X-axis. B: Comparison of evolutionary rate measured by dN/dS ratios between Z- and W-borne gametologs in monitor lizard. A total of 52 pairs of gametologs were used. C: The comparisons of gonad specific gene expression levels between the sex chromosome against autosomes in three reptile species. Stacked bars show the proportions of biased genes with more red or blue, the higher gonad-biased. Only genes with the most gonad-biased (the bluest/reddest part) were used in the significance testing when evaluating the level of masculinization.
For the three species with gonad transcriptomes (river turtle, marbled gecko and monitor lizard), we compared gene expression levels between sex chromosome vs. autosomes in the gonad, with the expectation that gonad-specific genes may have been preferentially selected to be located or not located on the sex chromosomes due to sex chromosomes’ sex-biased selective regimes. Previous studies in Drosophila (Assis et al., 2012) and other dipteran species (Vicoso & Bachtrog, 2015) found underrepresentation of male-biased or testis-biased genes, and overrepresentation of female-biased or ovary-biased genes on the X chromosome, supporting such sex-biased selective regime. We found that testis-biased genes were overrepresented (P<0.001, Chi-square test), while ovary-biased genes were underrepresented (P<0.005, Chi-square test) on the Z chromosome of the marbled gecko (Figure 6C). However, a similar masculinization and defeminization pattern was not found on the undifferentiated Z chromosome of the monitor lizard, probably because few Z-borne genes were hemizygous (Figure 4).
The river turtle with undifferentiated XY sex chromosomes, unexpectedly showed a significant enrichment of testis-biased genes on the X chromosome relative to autosomal genes. This was probably because of cross-mapping of the reads of Y-borne genes that were not highly differentiated from those of X-borne genes. When we examined only the hemizygous X-linked genes (those without a Y-linked homolog) there was no such enrichment pattern. This suggests some Y-borne genes of the river turtle have undergone a masculinization process even though they were still retained by the Y chromosome.
DISCUSSION
Given the large genomes of many reptile species (up to 5.3 Gb), fully sequencing sex chromosomes remains costly, despite the development of long-read sequencing and Hi-C technologies. So far, in depth studies of the gene content and dosage compensation of sex chromosomes have been carried out in a handful of lizards, snake species and turtle species (Alam et al., 2018; Bista et al., 2021; Ezaz et al., 2013; Rovatsos et al., 2021; Rupp et al., 2017; Schield et al., 2019; Vicoso et al., 2013) although ZW chromosome have been sequenced and a candidate sex determining gene identified in the central bearded dragon (Deakin et al., 2016).
Here, we developed a cost-effective method to expand our knowledge of sex-linked genes and sex chromosomes in a range of non-model reptiles and applied it to four distantly related reptile species. We used it to map sex chromosome-borne genes from male and female transcriptomes that were identified by screening with DNA probes from microdissected sex chromosomes. We also applied the novel stLFR linked-read sequencing technology (Wang et al., 2019) and assembled the draft genome of monitor lizard, V. acanthurus, including the sex chromosome sequences. The newly identified gene content of the sex chromosomes of these four distantly related reptile species provided new insights into reptile sex chromosome evolution and dosage compensation.
Previous studies showed that chicken and other birds have retained a very conserved karyotype close to that of reptilian ancestor (Deakin & Ezaz, 2019; Liu et al., 2021; Waters et al., 2021a). Mapping the chicken orthologues of sex chromosome-borne genes of the monitor lizard (V. acanthurus), worm lizard (A. parapulchella) and marbled gecko (C. marmoratus) onto the chicken genome revealed examples of recruitment of different ancestral autosomes. We found that the sex chromosomes of the monitor lizard (V. acanthurus), worm lizard (A. parapulchella) and marbled gecko (C. marmoratus) have homologues on different chicken autosomes. This implies that they evolved from different autosomes in a common reptilian ancestor.
However, our finding that sex chromosomes of the distantly related pink-tailed worm lizard and river turtle (E. macquarii) both have homology to GGA4q provides a striking example of convergent recruitment of ancestral autosome regions. The long arm of the chicken chromosome 4 (GGA4q) has also been previously reported to be recruited as sex chromosomes of pygopodid gecko (Rovatsos et al., 2021). This homology may signify that the same gene (likely to be pdgfra) has independently acquired a role in sex determination in all these species. Convergent recruitment of ancestral chromosome is a region orthologous to GGA23, which we identified to be part of the sex chromosomes of marbled gecko, and the central bearded dragon (Pogona vitticeps) (Ezaz et al., 2013) and among multiple species of turtles (Montiel et al., 2017).
Several general patterns emerged from these comparative analyses of the location of the chicken orthologues of genes on reptile sex chromosomes. Firstly, sex chromosomes seemed to have frequently originated by fusion of ancestral micro- and macro-chromosomes, or between micro-chromosomes (Waters et al., 2021b). In addition to homology to the chicken microchromosome GGA28 that was reported, and also confirmed in this work as the ancestral sex chromosome of Anguimorpha species including spiny tailed monitor lizard (Lind et al., 2019; Rovatsos et al., 2019), we found that other chicken microchromosomes GGA31, 33 contained fragments homologous to genes on the sex chromosomes of spiny tailed monitor lizard. Microchromosomes also seemed to have contributed to the sex chromosomes of three other reptiles (Figures 3, 4), as well as in the previously reported green anole lizard (Alföldi et al., 2011), bearded dragon lizard (Deakin et al., 2016), soft-shell turtles (Badenhorst et al., 2013; Kawagoshi et al., 2009). The short arm of chicken chromosome 4 (which is homologous to the conserved region of the X chromosome of therian mammals, is a microchromosome in all species other than the Galliformes. These observations of homologies with chicken microchromosomes are not surprising given that half the chicken genes lie on microchromosomes.
A microchromosome origin might have contributed to the second feature of reptile sex chromosomes, most of which are less differentiated than those of birds and mammals. Homomorphic or partially differentiated sex chromosomes were found in three out of four reptiles we examined and are also described also in the giant musk turtle (Kawagoshi et al., 2014), eyelid geckos (Kawagoshi et al., 2014; Pokorná et al., 2010) and some other gecko species (Koubová et al., 2014), and skinks (Kostmann et al., 2021). The preponderance of poorly differentiated sex chromosomes in reptiles could be the result either of slow differentiation, or rapid turnover, or both. A potential cause for the generally slower rate of sex chromosome differentiation in reptiles could be the high recombination rate and gene density of the ancestral microchromosomes (International Chicken Genome Sequencing Consortium, 2004), which might prevent extensive recombination suppression and rapid differentiation between sex chromosomes in these reptiles.
Alternatively, rapid turnover of reptile sex chromosomes could explain the “ever young” partially differentiated sex chromosomes that are so common in reptiles. We have previously demonstrated (Ezaz et al., 2009b) rapid transitions between sex determination systems in agamid lizards, and our present results expand the variety and independent origins of reptile sex chromosomes. In addition, the ability to switch into an environmental sex determination mode, and then to evolve novel genetic sex determination systems, may greatly facilitate turnovers. GSD and TSD have been reported within and between closely related reptile species, e.g., in agamid lizards (Ezaz et al., 2009a), in viviparous skink (Hill et al., 2018), some turtles (Bista & Valenzuela, 2020) and eye-lid geckos (Pensabene et al., 2020). In the Australian bearded dragon, the transition from GSD to TSD was observed both in the lab and in the field (Holleley et al., 2015), despite its possession of a pair of highly differentiated sex microchromosomes (Ezaz et al., 2005).
Our identification of genes on reptile sex chromosomes enabled us to assess their transcription and assess dosage compensation. We found no evidence of global dosage compensation, even in the worm lizard A. parapulchella with highly differentiated X and Y chromosomes. This is similar to the absence of global dosage compensation in birds (Itoh et al., 2007) and reptiles (Rovatsos et al., 2021), but contrasts with the recently reported case of green anole lizard (Marin et al., 2017; Rupp et al., 2017), in which the single copy of the X chromosome is upregulated in XY males through an epigenetic mechanism similar to that in Drosophila. The absence of global dosage compensation in A. parapulchella could reflect dosage mitigation or tolerance at post-transcriptional levels, or it may be a consequence of its dosage-dependent sex-determination mechanism, similar to that in chicken, in contrast to a male-dominant XY system of the green anole.
In this work we combined cytogenetics and high-throughput sequencing to characterize the sex chromosomes of four reptile species. This greatly widened our knowledge of sex chromosome birth, death and dosage compensation in a vertebrate class that shows particular variety in modes and turnover of sex determining systems.
Thus, we used DNA from microdissected sex chromosomes to identify transcripts of genes located on the XY or ZW chromosome pairs in each species, and located their chicken orthologues on different chicken chromosomes. This revealed the diverse origins of sex chromosomes, but detected convergent evolution between distantly related reptiles (turtle and worm lizard). Our novel pipeline efficiently identified candidate sex determining genes, which differed from those of birds and mammals. We found that none of the four species showed transcription profiles expected of global chromosomal dosage compensation.
In summary, our molecular and cytogenetic characterisation of sex chromosomes in diverse taxa greatly expands our knowledge of reptile sex determination. By identifying reptile candidate sex genes and providing the means with which to identify more, we hope to realise the value of this particularly variable, but understudied, vertebrate taxon, the only one for which no master sex determining gene has yet been discovered.
The inexpensive and efficient method developed here can be applied to studying any species of eukaryote with cytologically distinct sex chromosomes, providing the basis with which to better understand the ecological and evolutionary drivers of sex chromosomes and sex determination systems.
DATA AVAILABILITY
The genomic and transcriptomic data of worm lizard (A. parapulchella), river turtle (E. macquarii), marbled gecko (C. marmoratus) and monitor lizard (V. acanthurus) have been deposited in NCBI under BioProjectID PRJNA737594. The draft genome and annotation of monitor lizard (V. acanthurus) have been deposited in Genome Warehouse (GWH) under BioProject accession No. PRJCA005583. All the data have been deposited to Science Data Bank with data CSTR: 31253.11.sciencedb.j00139.00016 and 31253.11.sciencedb.01919.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
T.E., K.M., J.A.M.G. conceived the idea. K.M., F.S., J.D. conducted lab works. Z.X.Z. and Q.Z. conducted bioinformatic analyses. Q.Z., Z.X.Z. and T.E. wrote the first draft. All co-authors contributed intellectually to writing and editing the draft multiple times. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
Authors would like to acknowledge feedback by Janine Deakin on a preliminary draft. Animal photo credit: worm lizard and marbled gecko–T.G.; monitor lizard–J.D.; river turtle–A.G. and chicken–Liesl Taylor.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32170415, 32061130208), Natural Science Foundation of Zhejiang Province (LD19C190001) and European Research Council Starting Grant (677696) to Q.Z. T.E. was supported by an ARC FT (FT110100733). This project was also partially supported by ARC DP (DP110102262) led by T.E. and University of Canberra Strategic Research Fund awarded to T.E. K.M. was supported by an ARC DP (DP110102262) led by T.E. T.G. was supported by NSF (IOS1146820). F.S. and J.D. were supported by the University of Canberra postgraduate research scholarships †Current affiliation: Department of Environmental Biology, College of Bioscience and Biotechnology, Chubu University, Kasugai, Aichi 487-8501, Japan
Contributor Information
Qi Zhou, Email: zhouqi1982@zju.edu.cn.
Tariq Ezaz, Email: Tariq.Ezaz@canberra.edu.au.
References
- 1.Alam SMI, Sarre SD, Gleeson D, Georges A, Ezaz T Did lizards follow unique pathways in sex chromosome evolution? Genes. 2018;9(5):239. doi: 10.3390/genes9050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alföldi J, Di Palma F, Grabherr M, Williams C, Kong LS, Mauceli E, et al The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature. 2011;477(7366):587–591. doi: 10.1038/nature10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Cubero MJ, Santiago O, Martínez-Labarga C, Martínez-García B, Marrero-Díaz R, Rubio-Roldan A, et al Methodology for Y Chromosome Capture: A complete genome sequence of Y chromosome using flow cytometry, laser microdissection and magnetic streptavidin-beads. Scientific Reports. 2018;8(1):9436. doi: 10.1038/s41598-018-27819-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assis R, Zhou Q, Bachtrog D Sex-biased transcriptome evolution in Drosophila. Genome Biology and Evolution. 2012;4(11):1189–1200. doi: 10.1093/gbe/evs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augstenová B, Pensabene E, Veselý M, Kratochvíl L, Rovatsos M Are geckos special in sex determination? Independently evolved differentiated ZZ/ZW sex chromosomes in carphodactylid geckos. Genome Biology and Evolution. 2021;13(7):evab119. doi: 10.1093/gbe/evab119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachtrog D Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nature Reviews Genetics. 2013;14(2):113–124. doi: 10.1038/nrg3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badenhorst D, Stanyon R, Engstrom T, Valenzuela N A ZZ/ZW microchromosome system in the spiny softshell turtle, Apalone spinifera, reveals an intriguing sex chromosome conservation in Trionychidae. Chromosome Research. 2013;21(2):137–147. doi: 10.1007/s10577-013-9343-2. [DOI] [PubMed] [Google Scholar]
- 9.Bellott DW, Page DC Dosage-sensitive functions in embryonic development drove the survival of genes on sex-specific chromosomes in snakes, birds, and mammals. Genome Research. 2021;31(2):198–210. doi: 10.1101/gr.268516.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellott DW, Skaletsky H, Cho TJ, Brown L, Locke D, Chen N, et al Avian W and mammalian Y chromosomes convergently retained dosage-sensitive regulators. Nature Genetics. 2017;49(3):387–394. doi: 10.1038/ng.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birney E, Clamp M, Durbin R GeneWise and genomewise. Genome Research. 2004;14(5):988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bista B, Valenzuela N Turtle insights into the evolution of the reptilian karyotype and the genomic architecture of sex determination. Genes. 2020;11(4):416. doi: 10.3390/genes11040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bista B, Wu ZQ, Literman R, Valenzuela N Thermosensitive sex chromosome dosage compensation in ZZ/ZW softshell turtles. Apalone spinifera. Philosophical Transactions of the Royal Society B:Biological Sciences. 2021;376(1833):20200101. doi: 10.1098/rstb.2020.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan J, Tilmann C, Capel B Pdgfr-α mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes & Development. 2003;17(6):800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brůna T, Hoff KJ, Lomsadze A, Stanke M, Borodovsky M BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genomics and Bioinformatics. 2021;3(1):lqaa108. doi: 10.1093/nargab/lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlesworth B, Charlesworth D The degeneration of Y chromosomes. Philosophical Transactions of the Royal Society B:Biological Sciences. 2000;355(1403):1563–1572. doi: 10.1098/rstb.2000.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocca E, Petraccioli A, Morescalchi MA, Odierna G, Capriglione T Laser microdissection-based analysis of the Y sex chromosome of the Antarctic fish Chionodraco hamatus (Notothenioidei, Channichthyidae) Comparative Cytogenetics. 2015;9(1):1–15. doi: 10.3897/CompCytogen.v9i1.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornejo-Páramo P, Dissanayake DSB, Lira-Noriega A, Martínez-Pacheco ML, Acosta A, Ramírez-Suástegui C, et al Viviparous reptile regarded to have temperature-dependent sex determination has old XY chromosomes. Genome Biology and Evolution. 2020;12(6):924–930. doi: 10.1093/gbe/evaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, et al Origins and functional evolution of Y chromosomes across mammals. Nature. 2014;508(7497):488–493. doi: 10.1038/nature13151. [DOI] [PubMed] [Google Scholar]
- 20.Cutting A, Chue J, Smith CA Just how conserved is vertebrate sex determination? Developmental Dynamics. 2013;242(4):380–387. doi: 10.1002/dvdy.23944. [DOI] [PubMed] [Google Scholar]
- 21.Deakin JE, Edwards MJ, Patel H, O'Meally D, Lian JM, Stenhouse R, et al Anchoring genome sequence to chromosomes of the central bearded dragon (Pogona vitticeps) enables reconstruction of ancestral squamate macrochromosomes and identifies sequence content of the Z chromosome. BMC Genomics. 2016;17:447. doi: 10.1186/s12864-016-2774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deakin JE, Ezaz T Tracing the evolution of amniote chromosomes. Chromosoma. 2014;123(3):201–216. doi: 10.1007/s00412-014-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deakin JE, Ezaz T. 2019. Understanding the evolution of reptile chromosomes through applications of combined cytogenetics and genomics approaches. Cytogenetic and Genome Research, 157(1–2): 7–20.
- 24.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ezaz T, Azad B, O'Meally D, Young MJ, Matsubara K, Edwards MJ, et al Sequence and gene content of a large fragment of a lizard sex chromosome and evaluation of candidate sex differentiating gene R-spondin 1. BMC Genomics. 2013;14:899. doi: 10.1186/1471-2164-14-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ezaz T, Quinn AE, Miura I, Sarre SD, Georges A, Marshall Graves JA The dragon lizard Pogona vitticeps has ZZ/ZW micro-sex chromosomes. Chromosome Research. 2005;13(8):763–776. doi: 10.1007/s10577-005-1010-9. [DOI] [PubMed] [Google Scholar]
- 27.Ezaz T, Quinn AE, Sarre SD, O'Meally D, Georges A, Graves JAM Molecular marker suggests rapid changes of sex-determining mechanisms in Australian dragon lizards. Chromosome Research. 2009a;17(1):91–98. doi: 10.1007/s10577-008-9019-5. [DOI] [PubMed] [Google Scholar]
- 28.Ezaz T, Sarre SD, O'Meally D, Graves JAM, Georges A. 2009b. Sex chromosome evolution in lizards: independent origins and rapid transitions. Cytogenetic and Genome Research, 127(2–4): 249–260.
- 29.Ezaz T, Srikulnath K, Graves JAM Origin of Amniote sex chromosomes: an ancestral super-sex chromosome, or common requirements? Journal of Heredity. 2017;108(1):94–105. doi: 10.1093/jhered/esw053. [DOI] [PubMed] [Google Scholar]
- 30.Fu LM, Niu BF, Zhu ZW, Wu ST, Li WZ CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamble T, Castoe TA, Nielsen SV, Banks JL, Card DC, Schield DR, et al The discovery of XY sex chromosomes in a boa and python. Current Biology. 2017;27(14):2148–2153.e4. doi: 10.1016/j.cub.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Gamble T, Coryell J, Ezaz T, Lynch J, Scantlebury DP, Zarkower D Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Molecular Biology and Evolution. 2015;32(5):1296–1309. doi: 10.1093/molbev/msv023. [DOI] [PubMed] [Google Scholar]
- 33.Giovannotti M, Trifonov VA, Paoletti A, Kichigin IG, O’Brien PCM, Kasai F, et al New insights into sex chromosome evolution in anole lizards (Reptilia, Dactyloidae) Chromosoma. 2017;126(2):245–260. doi: 10.1007/s00412-016-0585-6. [DOI] [PubMed] [Google Scholar]
- 34.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu LQ, Walters JR Evolution of sex chromosome dosage compensation in animals: a beautiful theory, undermined by facts and bedeviled by details. Genome Biology and Evolution. 2017;9(9):2461–2476. doi: 10.1093/gbe/evx154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris RS. 2007. Improved Pairwise Alignmnet of Genomic DNA. Ph. D. dissertation, The Pennsylvania State University, University Park.
- 37.Herpin A, Schartl M Plasticity of gene-regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Reports. 2015;16(10):1260–1274. doi: 10.15252/embr.201540667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill PL, Burridge CP, Ezaz T, Wapstra E Conservation of sex-linked markers among conspecific populations of a viviparous skink, Niveoscincus ocellatus, exhibiting genetic and temperature-dependent sex determination. Genome Biology and Evolution. 2018;10(4):1079–1087. doi: 10.1093/gbe/evy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiort O The differential role of androgens in early human sex development. BMC Medicine. 2013;11:152. doi: 10.1186/1741-7015-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobza R, Vyskot B Laser microdissection‐based analysis of plant sex chromosomes. Methods in Cell Biology. 2007;82:433–453. doi: 10.1016/S0091-679X(06)82015-7. [DOI] [PubMed] [Google Scholar]
- 41.Holleley CE, O'Meally D, Sarre SD, Marshall Graves JA, Ezaz T, Matsubara K, et al Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature. 2015;523(7558):79–82. doi: 10.1038/nature14574. [DOI] [PubMed] [Google Scholar]
- 42.Hossain A, Saunders GF. 2001. The human sex-determining gene SRY is a direct target of WT1. Journal of Biological Chemistry, 276(20): 16817–16823.
- 43.Iannucci A, Altmanová M, Ciofi C, Ferguson-Smith M, Milan M, Pereira JC, et al Conserved sex chromosomes and karyotype evolution in monitor lizards (Varanidae) Heredity. 2019;123(2):215–227. doi: 10.1038/s41437-018-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.International Chicken Genome Sequencing Consortium Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432(7018):695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- 45.Itoh Y, Melamed E, Yang X, Kampf K, Wang S, Yehya N, et al Dosage compensation is less effective in birds than in mammals. Journal of Biology. 2007;6(1):2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawagoshi T, Uno Y, Matsubara K, Matsuda Y, Nishida C The ZW micro-sex chromosomes of the Chinese soft-shelled turtle (Pelodiscus sinensis, Trionychidae, Testudines) have the same origin as chicken chromosome 15. Cytogenet. Cytogenetic and Genome Research. 2009;125(2):125–131. doi: 10.1159/000227837. [DOI] [PubMed] [Google Scholar]
- 47.Kawagoshi T, Uno Y, Nishida C, Matsuda Y The Staurotypus turtles and aves share the same origin of sex chromosomes but evolved different types of heterogametic sex determination. PLoS One. 2014;9(8):e105315. doi: 10.1371/journal.pone.0105315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kichigin IG, Giovannotti M, Makunin AI, Ng BL, Kabilov MR, Tupikin AE, et al Evolutionary dynamics of Anolis sex chromosomes revealed by sequencing of flow sorting-derived microchromosome-specific DNA. Molecular Genetics and Genomics. 2016;291(5):1955–1966. doi: 10.1007/s00438-016-1230-z. [DOI] [PubMed] [Google Scholar]
- 49.Kostmann A, Kratochvíl L, Rovatsos M Poorly differentiated XX/XY sex chromosomes are widely shared across skink radiation. Philosophical Transactions of the Royal Society B:Biological Sciences. 2021;288(1943):20202139. doi: 10.1098/rspb.2020.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koubová M, Pokorná MJ, Rovatsos M, Farkačová K, Altmanová M, Kratochvíl L Sex determination in Madagascar geckos of the genus Paroedura (Squamata: Gekkonidae): are differentiated sex chromosomes indeed so evolutionary stable? Chromosome Research. 2014;22(4):441–452. doi: 10.1007/s10577-014-9430-z. [DOI] [PubMed] [Google Scholar]
- 51.Kratochvíl L, Gamble T, Rovatsos M Sex chromosome evolution among amniotes: is the origin of sex chromosomes non-random? Philosophical Transactions of the Royal Society B:Biological Sciences. 2021a;376(1833):20200108. doi: 10.1098/rstb.2020.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kratochvíl L, Stöck M, Rovatsos M, Bullejos M, Herpin A, Jeffries DL, et al Expanding the classical paradigm: what we have learnt from vertebrates about sex chromosome evolution. Philosophical Transactions of the Royal Society B:Biological Sciences. 2021b;376(1833):20200097. doi: 10.1098/rstb.2020.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langmead B, Salzberg SL Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee L, Montiel EE, Navarro-Domínguez BM, Valenzuela N. 2019a. Chromosomal rearrangements during turtle evolution altered the synteny of genes involved in vertebrate sex determination. Cytogenetic and Genome Research, 157(1–2): 77–88.
- 55.Lee L, Montiel EE, Valenzuela N. 2019b. Discovery of putative XX/XY Male heterogamety in Emydura subglobosa turtles exposes a novel trajectory of sex chromosome evolution in Emydura. Cytogenetic and Genome Research, 158(3): 160–169.
- 56.Lewin HA, Robinson GE, Kress WJ, Baker WJ, Coddington J, Crandall KA, et al Earth BioGenome Project: sequencing life for the future of life. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(17):4325–4333. doi: 10.1073/pnas.1720115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li B, Dewey CN RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27(21):2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lind AL, Lai YYY, Mostovoy Y, Holloway AK, Iannucci A, Mak ACY, et al Genome of the Komodo dragon reveals adaptations in the cardiovascular and chemosensory systems of monitor lizards. Nature Ecology & Evolution. 2019;3(8):1241–1252. doi: 10.1038/s41559-019-0945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Wang ZJ, Li J, Xu LH, Liu JQ, Feng SH, et al A new emu genome illuminates the evolution of genome configuration and nuclear architecture of avian chromosomes. Genome Research. 2021;31(3):497–511. doi: 10.1101/gr.271569.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo RB, Liu BH, Xie YL, Li ZY, Huang WH, Yuan JY, et al SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1(1):18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mank JE, Avise JC. 2009. Evolutionary diversity and turn-over of sex determination in teleost fishes. Sexual Development, 3(2–3): 60–67.
- 63.Marin R, Cortez D, Lamanna F, Pradeepa MM, Leushkin E, Julien P, et al Convergent origination of a Drosophila-like dosage compensation mechanism in a reptile lineage. Genome Research. 2017;27(12):1974–1987. doi: 10.1101/gr.223727.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez PA, Ezaz T, Valenzuela N, Georges A, Marshall Graves JA An XX/XY heteromorphic sex chromosome system in the Australian chelid turtle Emydura macquarii: a new piece in the puzzle of sex chromosome evolution in turtles. Chromosome Research. 2008;16(6):815–825. doi: 10.1007/s10577-008-1228-4. [DOI] [PubMed] [Google Scholar]
- 65.Matsubara K, Gamble T, Matsuda Y, Zarkower D, Sarre SD, Georges A, et al Non-homologous sex chromosomes in two geckos (Gekkonidae: Gekkota) with female heterogamety. Cytogenetic and Genome Research. 2014a;143(4):251–258. doi: 10.1159/000366172. [DOI] [PubMed] [Google Scholar]
- 66.Matsubara K, Knopp T, Sarre SD, Georges A, Ezaz T Karyotypic analysis and FISH mapping of microsatellite motifs reveal highly differentiated XX/XY sex chromosomes in the pink-tailed worm-lizard (Aprasia parapulchella, Pygopodidae, Squamata) Molecular Cytogenetics. 2013;6(1):60. doi: 10.1186/1755-8166-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsubara K, Sarre SD, Georges A, Matsuda Y, Graves JAM, Ezaz T Highly differentiated ZW sex microchromosomes in the Australian Varanus species evolved through rapid amplification of repetitive sequences. PLoS One. 2014b;9(4):e95226. doi: 10.1371/journal.pone.0095226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mezzasalma M, Guarino FM, Odierna G Lizards as model organisms of sex chromosome evolution: what we really know from a systematic distribution of available data? Genes. 2021;12(9):1341. doi: 10.3390/genes12091341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miura I. 2017. Sex determination and sex chromosomes in amphibia. Sexual Development, 11(5–6): 298–306.
- 70.Montiel EE, Badenhorst D, Tamplin J, Burke RL, Valenzuela N Discovery of the youngest sex chromosomes reveals first case of convergent co-option of ancestral autosomes in turtles. Chromosoma. 2017;126(1):105–113. doi: 10.1007/s00412-016-0576-7. [DOI] [PubMed] [Google Scholar]
- 71.O'Meally D, Ezaz T, Georges A, Sarre SD, Graves JAM Are some chromosomes particularly good at sex? Insights from amniotes. Chromosome Research. 2012;20(1):7–19. doi: 10.1007/s10577-011-9266-8. [DOI] [PubMed] [Google Scholar]
- 72.Pennell MW, Kirkpatrick M, Otto SP, Vamosi JC, Peichel CL, Valenzuela N, et al Y fuse? Sex chromosome fusions in fishes and reptiles. PLoS Genetics. 2015;11(5):e1005237. doi: 10.1371/journal.pgen.1005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pensabene E, Kratochvíl L, Rovatsos M Independent evolution of sex chromosomes in eublepharid geckos, a lineage with environmental and genotypic sex determination. Life. 2020;10(12):342. doi: 10.3390/life10120342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pokorná M, Rábová M, Ráb P, Ferguson-Smith MA, Rens W, Kratochvíl L Differentiation of sex chromosomes and karyotypic evolution in the eye-lid geckos (Squamata: Gekkota: Eublepharidae), a group with different modes of sex determination. Chromosome Research. 2010;18(7):809–820. doi: 10.1007/s10577-010-9154-7. [DOI] [PubMed] [Google Scholar]
- 75.Pyron RA, Burbrink FT, Wiens JJ A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology. 2013;13:93. doi: 10.1186/1471-2148-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rhen T, Jangula A, Schroeder A, Woodward-Bosh R The platelet-derived growth factor signaling system in snapping turtle embryos, Chelydra serpentina: Potential role in temperature-dependent sex determination and testis development. General and Comparative Endocrinology. 2009;161(3):335–343. doi: 10.1016/j.ygcen.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rhie A, McCarthy SA, Fedrigo O, Damas J, Formenti G, Koren S, et al Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021;592(7856):737–746. doi: 10.1038/s41586-021-03451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rovatsos M, Gamble T, Nielsen SV, Georges A, Ezaz T, Kratochvíl L Do male and female heterogamety really differ in expression regulation? Lack of global dosage balance in pygopodid geckos. Philosophical Transactions of the Royal Society B:Biological Sciences. 2021;376(1833):20200102. doi: 10.1098/rstb.2020.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rovatsos M, Rehák I, Velenský P, Kratochvíl L Shared ancient sex chromosomes in varanids, beaded lizards, and alligator lizards. Molecular Biology and Evolution. 2019;36(6):1113–1120. doi: 10.1093/molbev/msz024. [DOI] [PubMed] [Google Scholar]
- 80.Rupp SM, Webster TH, Olney KC, Hutchins ED, Kusumi K, Sayres MAW Evolution of dosage compensation in Anolis carolinensis, a reptile with XX/XY chromosomal sex determination. Genome Biology and Evolution. 2017;9(1):231–240. doi: 10.1093/gbe/evw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sankovic N, Delbridge ML, Grützner F, Ferguson-Smith MA, O'Brien PCM, Graves JAM Construction of a highly enriched marsupial Y chromosome-specific BAC sub-library using isolated Y chromosomes. Chromosome Research. 2006;14(6):657–664. doi: 10.1007/s10577-006-1076-z. [DOI] [PubMed] [Google Scholar]
- 82.Schield DR, Card DC, Hales NR, Perry BW, Pasquesi GM, Blackmon H, et al The origins and evolution of chromosomes, dosage compensation, and mechanisms underlying venom regulation in snakes. Genome Research. 2019;29(4):590–601. doi: 10.1101/gr.240952.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, et al The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423(6942):825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- 84.Stöck M, Kratochvíl L, Kuhl H, Rovatsos M, Evans BJ, Suh A, et al. 2021. A brief review of vertebrate sex evolution with a pledge for integrative research: towards ‘sexomics’. Philosophical Transactions of the Royal Society B: Biological Sciences, 376(1832): 20200426.
- 85.Tarailo-Graovac M, Chen NS. 2009. Using RepeatMasker to identify repetitive elements in genomic sequences. Current Protocols in Bioinformatics, Chapter 4: Unit 4.10.
- 86.Thépot D Sex chromosomes and master sex-determining genes in turtles and other reptiles. Genes. 2021;12(11):1822. doi: 10.3390/genes12111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomaszkiewicz M, Rangavittal S, Cechova M, Sanchez RC, Fescemyer HW, Harris R, et al A time-and cost-effective strategy to sequence mammalian Y Chromosomes: an application to the de novo assembly of gorilla Y. Genome Research. 2016;26(4):530–540. doi: 10.1101/gr.199448.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vicoso B, Bachtrog D Numerous transitions of sex chromosomes in Diptera. PLoS Biology. 2015;13(4):e1002078. doi: 10.1371/journal.pbio.1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vicoso B, Emerson JJ, Zektser Y, Mahajan S, Bachtrog D Comparative sex chromosome genomics in snakes: differentiation, evolutionary strata, and lack of global dosage compensation. PLoS Biology. 2013;11(8):e1001643. doi: 10.1371/journal.pbio.1001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang O, Chin R, Cheng XF, Wu MKY, Mao Q, Tang JB, et al Efficient and unique cobarcoding of second-generation sequencing reads from long DNA molecules enabling cost-effective and accurate sequencing, haplotyping, and de novo assembly. Genome Research. 2019;29(5):798–808. doi: 10.1101/gr.245126.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Z, Pascual-Anaya J, Zadissa A, Li WQ, Niimura Y, Huang ZY, et al The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nature Genetics. 2013;45(6):701–706. doi: 10.1038/ng.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waters PD, Patel HR, Ruiz-Herrera A, Álvarez-González L, Lister NC, Simakov O, et al Microchromosomes are building blocks of bird, reptile, and mammal chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 2021a;118(45):e2112494118. doi: 10.1073/pnas.2112494118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waters PD, Patel HR, Ruiz-Herrera A, Álvarez-González L, Lister NC, Simakov O, et al. 2021b. Microchromosomes are building blocks of bird, reptile and mammal chromosomes. bioRxiv,doi: 10.1101/2021.07.06.451394.
- 94.Weisenfeld NI, Kumar V, Shah P, Church DM, Jaffe DB Direct determination of diploid genome sequences. Genome Research. 2017;27(5):757–767. doi: 10.1101/gr.214874.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson JD, Griffin JE, George FW Sexual differentiation: early hormone synthesis and action. Biology of Reproduction. 1980;22(1):9–17. doi: 10.1095/biolreprod22.1.9. [DOI] [PubMed] [Google Scholar]
- 96.Yano A, Guyomard R, Nicol B, Jouanno E, Quillet E, Klopp C, et al An immune-related gene evolved into the master sex-determining gene in rainbow trout. Oncorhynchus mykiss. Current Biology. 2012;22(15):1423–1428. doi: 10.1016/j.cub.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Q, Zhang JL, Bachtrog D, An N, Huang QF, Jarvis ED, et al Complex evolutionary trajectories of sex chromosomes across bird taxa. Science. 2014;346(6215):1246338. doi: 10.1126/science.1246338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Y, Shearwin-Whyatt L, Li J, Song ZZ, Hayakawa T, Stevens D, et al Platypus and echidna genomes reveal mammalian biology and evolution. Nature. 2021;592(7856):756–762. doi: 10.1038/s41586-020-03039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou YJ, Sun W, Cai H, Bao HS, Zhang Y, Qian GY, et al The role of anti-müllerian hormone in testis differentiation reveals the significance of the TGF-β pathway in reptilian sex determination. Genetics. 2019;213(4):1317–1327. doi: 10.1534/genetics.119.302527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
The genomic and transcriptomic data of worm lizard (A. parapulchella), river turtle (E. macquarii), marbled gecko (C. marmoratus) and monitor lizard (V. acanthurus) have been deposited in NCBI under BioProjectID PRJNA737594. The draft genome and annotation of monitor lizard (V. acanthurus) have been deposited in Genome Warehouse (GWH) under BioProject accession No. PRJCA005583. All the data have been deposited to Science Data Bank with data CSTR: 31253.11.sciencedb.j00139.00016 and 31253.11.sciencedb.01919.