Abstract
For centuries, propolis has been used to treat various diseases in traditional medicine due to its biological and pharmacological activities. It remains popular because of its potentially beneficial role in human health due to its well-known broad multispectrum properties, including antiviral, anti-inflammatory, antibacterial, anesthetic, antioxidant, anticancer, antifungal, antiprotozoal, antihepatotoxic, antimutagenic, and antiseptic activity. Numerous studies have examined the antibacterial activity of propolis and its derivatives, which include many natural antimicrobial compounds with broad spectrum activity against different bacterial types. In vitro studies have shown propolis's antibacterial activity against Gram-positive and Gram-negative bacteria. Many studies have examined propolis's effect on inhibiting bacterial growth. Several studies examining propolis's inhibition of Gram-positive and Gram-negative bacteria have shown it to be an effective antimicrobial agent. Klebsiella pneumoniae is a Gram-negative bacterium commonly associated with respiratory infections, particularly in hospital settings. Inappropriate antibiotic use may contribute to the increasing number of bacterial strains resistant to available drugs. This review summarizes the findings of previous studies on propolis and its potential mechanisms in inhibiting K. pneumoniae growth in animals.
Keywords: Propolis, Antimicrobial, Klebsiella pneumoniae, In-vivo, In-vitro
Highlights
-
•
Propolis has a potentially beneficial role in human health.
-
•
Propolis has antibacterial activity against Gram-positive and -negative bacteria.
-
•
Propolis has strong antibacterial activity against Klebsiella pneumoniae infection.
1. Introduction
Numerous studies have examined the use and effects of traditional medicines for various diseases [[1], [2], [3], [4], [5]], including the effects of herbal medicines on infectious diseases, such as candidiasis [[6], [7], [8]], typhoid fever [[9], [10], [11], [12], [13], [14]], toxoplasmosis [[15], [16], [17]], gingivitis [18,19], vaginitis [20], and the human immunodeficiency virus (HIV) [21,22], and noninfectious diseases such as diabetes [23].
Propolis is a famous herbal medicine produced by bees from a mixture of wax derived from plants to protect the beehive against infectious agents. Propolis has been used in traditional medicine for centuries due to its therapeutic effects. Its composition largely depends on the honeybee species, geographic region, and food and plant sources but generally consists of 30% wax, 50% viscose resin, pollen, and other organic materials, and 25% essential oils. Its bioactive active ingredients can make up as much as 70%, of which 58% are polyphenols and 20% are flavonoids [24].
Propolis is well-known for its antiviral, anti-inflammatory, antibacterial, anesthetic, antioxidant, anticancer, antifungal, antiprotozoal, antihepatotoxic, antimutagenic, and antiseptic activity, in addition to its cytotoxicity [24]. Many studies have examined the antibacterial activity of propolis. In vitro studies found it had antibacterial activity against various Gram-positive and Gram-negative bacteria. Among its various compounds, the flavonoids galangin and pinocembrin play important roles [24].
Klebsiella pneumoniae is a Gram-negative bacterium commonly associated with respiratory infections. It is a primary cause of nosocomial infections commonly treated with classical antibiotics [25]. However, antibiotic resistance has been occurring in these bacteria at an increasing rate [15]. Therefore, an improved understanding of the role of propolis in inhibiting the growth of the bacteria, including K. pneumoniae, may be helpful in therapeutic innovation.
Several studies have shown propolis to have no toxicity and side effects in animal models or humans. Mice are a widely used model organism for studying important aspects of human lung pathogenesis, including acute and chronic inflammatory diseases. Models of acute lung inflammation are necessary for advancing our understanding of bacterial infection in humans. Propolis contains over 200 active compounds showing clinical potential as antibacterial agents [26]. Therefore, it represents a new alternative treatment for various infections in humans. This review summarizes the findings of previous studies on propolis and its possible mechanisms in inhibiting the growth of K. pneumoniae in animals.
2. Methods
We performed a comprehensive literature search in the PubMed (US National Institutes of Health [NIH]), Scopus, EMBASE, and Google Scholar databases using the following keywords and their combinations as medical subject headings (MeSH): “propolis,” “antibacterial,” “in-vivo,” “in-vitro,” and “infectious diseases.” In addition, relevant reference lists were searched manually.
This review is reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) standards [27]. We declare that it conforms with a measurement tool to assess systematic reviews (AMSTAR) 2 guidelines and receives a high-quality score with the AMSTAR 2 checklist [28]. The review was registered at www.researchregistry.com (researchregistry8151) [29]. All relevant English language articles with any study design recorded in the above databases were included and narratively discussed.
3. Results and discussion
3.1. Propolis as an antimicrobial agent
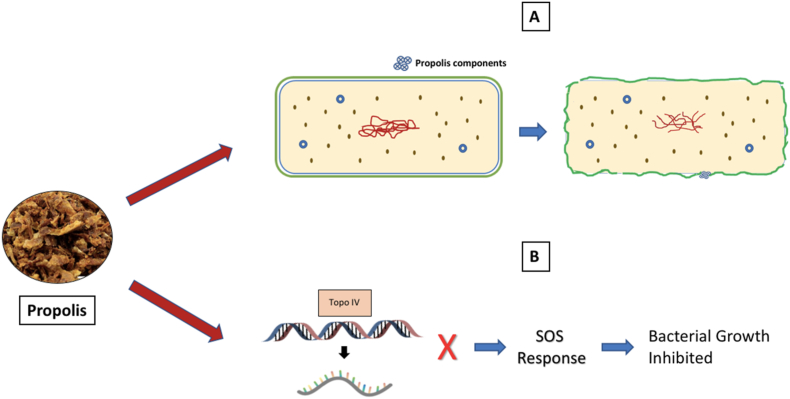
Propolis has been shown to uncouple the energy transducing cytoplasmic membrane and inhibit bacterial motility. Its components, including cinnamic and flavonoids, also have a bactericidal effect. Studies using electron microscopy showed propolis's antibacterial effect is via bacterial growth inhibition by preventing cell division, resulting in the formation of pseudo-multicellular structures formation. In addition, propolis disorganized the cytoplasm, cytoplasmic membrane, and cell wall, causing partial bacteriolysis and protein synthesis inhibition [26]. The antibacterial activity of propolis increases the organism's immunity or acts directly on bacteria via several mechanisms (Fig. 1) [26].
Fig. 1.
Antibacterial mechanism of propolis [26]. (A) The active components of propolis attach to the bacterium's cytoplasmic membrane, damaging its structural integrity and leading to its perforation, enabling the cytoplasmic contents to be expelled, causing cell death. (B) Flavonoids inhibit topoisomerase IV-dependent deactivation activity, leading to the cellular SOS response and inhibition of bacterial cell growth [26].
Interestingly, the antibacterial potential of propolis depends on its geographic location. Middle Eastern propolis shows the best activity against Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria. In contrast, German, Irish, and Korean propolis show minimal activity. Studies have found Iranian and Brazilian propolis effective against Gram-positive bacterial spores, growth, and infections but limited activity against Gram-negative bacteria [26].
There are numerous other propolis components, such as terpenoid lupeol and flavonoids, including fisetin, decanoic acids, quercetin, kaempferol, and chrysin. Similarly, another propolis component, pinocembrin, shows antibacterial activity against K. pneumoniae, Listeria monocytogenes, S. aureus, Pseudomonas aeruginosa, Streptococcus sobrinus, Enterococcus faecalis, and Streptococcus mutans [26].
However, most propolis biological activities are associated with flavonoid compounds, although many are poorly understood. Flavonoids are believed to target various bacterial structures, compromising their function, where the flavonoids’ B ring inhibits nucleic acid synthesis. It is also believed that flavonoids can reduce bacterial resistance to various antibacterial compounds via binding to the bacterial cell wall, resulting in their lysis and death [26].
3.2. Inhibition effect of propolis on K. pneumoniae growth
K. pneumoniae causes various infections, including pneumonia, urinary tract, and liver abscesses. Historically, K. pneumoniae infection in immunocompromised individuals can cause serious illness. Hypervirulent variants increase the number of individuals susceptible to infection, including those who are healthy and immunocompetent [1].
Infectious disease remains a major public health concern in low- and middle-income countries, including Indonesia, where antibiotics have been used uncontrollably and irrationally. Inappropriate antibiotic use can increase the number of bacterial strains resistant to available drugs, increasing the complexity of their treatment [1].
Several experimental animal models have been used to study the host immunological response to K. pneumoniae-induced pneumonia. Franklin et al. [25] used a mouse model to show that intraperitoneal injection of K. pneumoniae reliably and reproducibly caused peritonitis and pneumonia with a simple and easily performed procedure. Mice have often been used as the model organism to study important aspects of human lung pathogenesis. McDaniel and Allen [30] used a mouse model of acute lung inflammation induced by K. pneumoniae. Bacterial lung infections can induce some immune responses, which are determined by the level of exposure and degree of the host response. Therefore, acute lung inflammation models are necessary for understanding the role of the host immune system during bacterial infection.
The bacterial growth-inhibiting effects of propolis have been extensively studied. Several studies have shown the effectiveness of propolis as an antimicrobial agent inhibiting the growth of Gram-positive and Gram-negative bacteria. However, studies on the growth-inhibiting effects of propolis on K. pneumoniae in mice are limited.
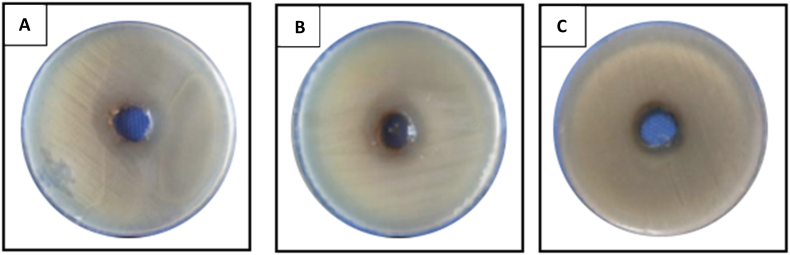
Billah [31] evaluated the antibiofilm activity of heather honey propolis and medicinal plant extracts against P. aeruginosa and S. aureus to determine the optimum biofilm growth with a 24 h time-course assay using P. aeruginosa PA14 and S. aureus NCTC 4135 strains. Two propolis extracts promoted P. aeruginosa growth but inhibited biofilm formation in S. aureus by 76.5% and 13.8%, respectively. The inhibition of biofilm formation by propolis was easier with S. aureus than with P. aeruginosa [31]. Saddiq and Danial [32] examined the antimicrobial, antioxidant, and probiotic activity of commercial Saudi Arabian propolis, performing antimicrobial activity tests on the pathogenic K. pneumoniae, P. aeruginosa, E. coli, Aspergillus niger, and Aspergillus flavus. The best antibacterial effect was observed with K. pneumoniae (32 mm; Fig. 2). Notably, the ethanol propolis extract (EEP) has high absorption activity for 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals [32].
Fig. 2.
The pathogenic bacterial growth inhibition zone on Mueller Hinton media with propolis extract treatment. (A) Pseudomonas aeroginosa ATCC9027, (B) K. pneumoniae, and (C) E. coli [32].
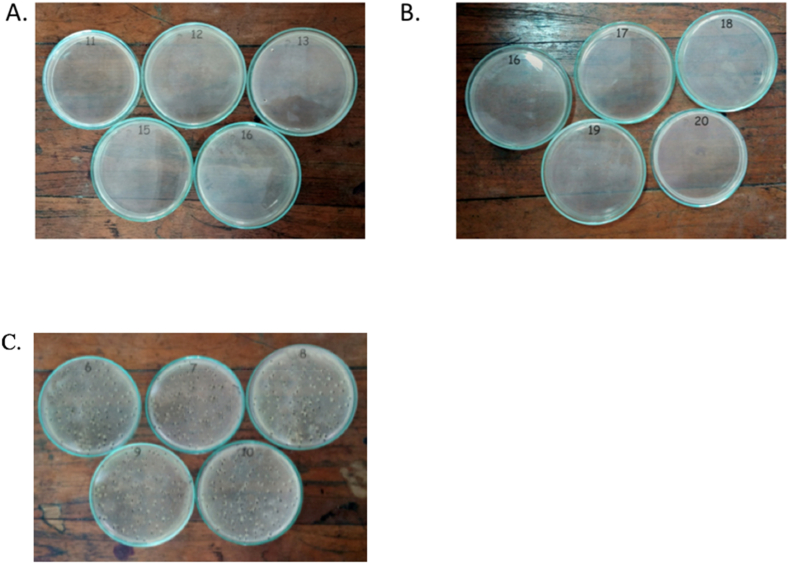
The antibacterial effect of propolis is highlighted by the results of blood cultures from mice inoculated with K. pneumoniae treated with propolis (Fig. 3A) and propolis with levofloxacin (Fig. 3B), which showed no visible bacterial growth compared to untreated K. pneumoniae (Fig. 3C).
Fig. 3.
Bacterial growth inhibition by propolis treatment. No visible K. pneumoniae growth was observed with (A) propolis or (B) propolis with levofloxacin treatments. (C) Appreciable K. pneumoniae growth was observed without treatment.
Inhibition zone diameters with propolis extract against K. pneumoniae, P. aeruginosa, E. coli, A. niger, and A. flavus are shown in Table 1. Pathogenic bacterial and fungal growth is inhibited by propolis extract. Interestingly, the growth of some probiotic bacteria is increased by propolis extract. However, further research is required to identify propolis derivatives and their effects [32].
Table 1.
Inhibition zone diameters of K. pneumoniae, P. aeruginosa, E. coli, A. niger, and A. flavus treated with propolis extract.
| Bacteria | Inhibition zone diameter (mm) |
|---|---|
| P. aeruginosa | 24 |
| K. pneumoniae | 32 |
| E. coli | 10 |
| A. flavus | 30 |
| A. niger | 20 |
Turcatto et al. [33] quantified the expression of genes encoding the antimicrobial peptides (AMPs) defensin-1, abaecin, hymenoptaecin, and apidaecin in caged workers bees fed with pollen substitute diets Megabee or Glutenose with or without propolis before injection with E. coli. Megabee is a commercial diet widely used in the USA [33,34], and Glutenose is an experimental diet developed with local ingredients in Brazil [33]. They found significant increases in the expression of defensin-1 and hymenoptaecin genes (P < 0.05) but not apidaecin and abaecin genes (P > 0.05) in bees fed a diet without propolis. However, bees injected with E. coli had significantly higher expression of all four AMPs fed a diet with propolis than without propolis (P < 0.05) [33]. However, uninfected bees showed no increase in AMP gene expression with the addition of propolis to their diet. Notably, AMP gene expression was significantly increased in bees fed on a diet with 0.1% propolis, suggesting that these genes are expressed at a low level when there is no infection but become activated in the presence of bacteria [33].
In this study, bees injected with E. coli after being fed a diet containing propolis had greatly enhanced AMP gene expression compared to uninfected bees fed the same diet without propolis. This observation provides new insights into the possible role of propolis in reducing colony susceptibility to pathogens. Applying propolis to the inside of hives decreases immune function investment by reducing AMP gene expression in uninfected adult worker bees [35]. However, it remains unknown whether the bees consumed propolis or how the bees exposed to propolis would react if infected.
Various studies have found that propolis inhibits the growth of bee pathogens in vitro. Therefore, bees can use it for self-medication, although its beneficial effects via immune system stimulation remain unknown. Drago et al. [36] investigated the in vitro activity of this substance by calculating the minimum inhibitory (MICs) and bactericidal (MBC) concentrations against various commonly isolated pathogens. The bacteriostatic or bactericidal activities of propolis were also evaluated via time-kill curves against representative respiratory pathogens, defined as a 3-log decrease in colony-forming units (CFU)/mL and <2-log decrease in CFU/mL, respectively. The time-kill kinetic data showed that propolis's antibacterial activity is mainly bacteriostatic, with a bactericidal effect only evident with selected strains at higher concentrations [36,37]. Notably, propolis at 4 × MIC was bactericidal against S. aureus, H. influenzae, and M. catarrhalis after 24 h of incubation, while at 2 × MIC, it was bacteriostatic against H. influenza. At 4 × MIC, it was also bactericidal against K. pneumoniae after 24 h of incubation and bacteriostatic after 6 h of incubation (1-log decrease) [36].
Stepanovic et al. [38] investigated the antimicrobial properties of 13 EEP samples from different regions of Serbia against 39 microorganisms, including 14 resistant or multiresistant to antibiotics, to determine synergistic activity between antimicrobials and propolis. They found that EEPs, irrespectively of microbial resistance to antibiotics, showed significant antimicrobial activities against Gram-positive bacteria (MIC = 0.078–1.25%), while Gram-negative bacteria were less susceptible (MIC = 1.25–>5%). EEPs showed synergism with selected antibiotics and an ability to enhance the activities of antifungals. These findings show the antimicrobial potential of propolis alone or in combination with specific antibiotics and antifungals, which may be of clinical interest.
4. Conclusion
Studies have shown that propolis inhibits bacterial growth through several mechanisms. It is believed that propolis can uncouple the energy transducing cytoplasmic membrane causing partial bacteriolysis and protein synthesis inhibition, limiting bacterial motility. While there are numerous propolis components, most of its biological activities are associated with flavonoid compounds that can reduce bacterial resistance to various antibacterial compounds resulting in the lysis of the bacterial cells. More research is needed to determine the antimicrobial activity of propolis on Gram-positive and Gram-negative bacteria. Clinical controlled studies are needed to determine propolis's true effectiveness. Further in vitro studies are also required to elucidate the mechanism of propolis's antimicrobial activity and determine its potential interference during early infection when the infectious agent and host's defenses interact.
Ethical approval
No require ethical approval.
Sources of funding
No funding source.
Author contributions
FFT, RN, MH, AS, IP, ARJ conceived and designed the study, conducted research, provided materials, and collected and organized data. AS, FFT, RN, MRP, ASY, ARJ and MH drafted the manuscript. RD, AS, MRP, AF, AB, MH analyzed the data and interpreted data. AS, RD, MH, FFT, IP, ASY, ARJ, AF, AB and MH wrote initial and final draft article, and provided logistic support. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Registration of research studies
Name of the registry: www.researchregistry.com
Unique Identifying number or registration ID: reviewregistry966
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Consent
No inform consent require.
Guarantor
Prof. Mochammad Hatta, MD, PhD, Clin Microbiologist (Cons).
Provenance and peer review
Not commissioned, externally peer-reviewed.
Conflict of interest
No conflicts of interest declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104388.
Contributor Information
Feni Fitriani Taufik, Email: feni_fadilla@yahoo.co.id.
Rosdiana Natzir, Email: rosdianarnatzir@yahoo.com.
Ilhamjaya Patellongi, Email: ilhamjaya28.1959@gmail.com.
Arif Santoso, Email: arifs777@gmail.com.
Mochammad Hatta, Email: hattaram@yahoo.com.
Ade Rifka Junita, Email: junitarifkade@yahoo.com.
Ahmad Syukri, Email: sukri2901@gmail.com.
Muhammad Reza Primaguna, Email: rezzprima@yahoo.com.
Ressy Dwiyanti, Email: ressy_chan@yahoo.co.id.
Andini Febrianti, Email: andinifebrianti@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wahyuni T.D., Hatta M., Bukhari A., Santoso A., Massi M.N. Increasing Natural Resistance Associated Macrophage Protein 1 serum level after Miana treatment in BALB/c induced Klebsiella pneumoniae experimental research. Ann. Med. Surg. 2021;65 doi: 10.1016/j.amsu.2021.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanto T.A., Hatta M., Bukhari A., Natzir R. Molecular and immunological mechanisms of miana leaf (coleus scutellariodes [L] benth) in infectious diseases. Biomed. Pharmacol. J. 2020;13:1607–1618. doi: 10.13005/bpj/2036. [DOI] [Google Scholar]
- 3.Syamsuri F., Hatta M., Natzir R., Alam G., Nasrum M., Bahar B., Pratiwi Rahardjo S. Expression of TLR-4 in Salmonella typhi -induced balb/c mice treated by miana leaves (coleus scutellaroides (L) benth) Indian J. Public Heal. Res. Dev. 2018;9:1449. doi: 10.5958/0976-5506.2018.02057.0. [DOI] [Google Scholar]
- 4.Syamsuri F., Hatta M., Natzir R., Alam G., Nasrum M., Dwiyanti R., Bahar B. A review: worldwide medicinal plants for typhoid fever. Indian J. Public Heal. Res. Dev. 2018;9:1461. doi: 10.5958/0976-5506.2018.00938.5. [DOI] [Google Scholar]
- 5.Syarif L.I., Junita A.R., Hatta M., Dwiyanti R., Kaelan C., Sabir M., Noviyanthi R.A., Primaguna M.R., PurnamasariI N.I. A mini review: medical plants for typhoid fever in Indonesia. Sys. Rev. Pharm. 2020;11:1171–1180. doi: 10.31838/srp.2020.6.170. [DOI] [Google Scholar]
- 6.Karo M., Hatta M., Salma W., Patellongi I., Natzir R. Effects of miana (coleus scutellariodes (L) benth) to expression of mRNA IL-37 in balb/c mice infected Candida albicans. Pharmacogn. J. 2018;10:16–19. doi: 10.5530/pj.2018.1.3. [DOI] [Google Scholar]
- 7.Br Karo M., Hatta M., Patellongi I., Natzir R., Tambaip T. IgM antibody and colony fungal load impacts of orally administered ethanol extract of Plectranthus scutellarioides on mice with systemic candidiasis. J. Pharm. Pharmacogn. Res. 2018;6:27–34. [Google Scholar]
- 8.Br Karo M., Tambaip T., Hatta M., Simanjuntak T., Irmawaty L., Rina T., Kamelia E., Rahmawati F., Bintang M. A mini review of Indonesian medicinal plants for Vulvovaginal candidiasis. Rasayan J. Chem. 2017;10:1280–1288. doi: 10.7324/RJC.2017.1041887. [DOI] [Google Scholar]
- 9.Febriza A., Natzir R., Hatta M., As’ad S., Budu, Kaelan C., Kasim V.N., Idrus H.H. The role of IL-6, TNF-α, and VDR in inhibiting the growth of Salmonella typhi: in vivo study. Open Microbiol. J. 2020;14:65–71. doi: 10.2174/1874285802014010065. [DOI] [Google Scholar]
- 10.Febriza A., Kasim V.N., Idrus H.H., Hatta M. The effects of Curcumin and vitamin D combination as inhibitor toward Salmonella typhi bacteria growth in vivo. Int. J. Appl. Pharm. 2019:116–120. doi: 10.22159/ijap.2019.v11s5.T0093. [DOI] [Google Scholar]
- 11.Febriza A., Natzir R., Hatta M., Uiterwaal C., As’ad Armyn S., Budu B., Alam G., Kasim V., Idrus H. Curcumin effects in inducing mRNA gene cathelidicin antimicrobial peptide (CAMP) in balb/c mice infected with Salmonella typhi. J. Biol. Res. - Boll. Della Soc. Ital. Di Biol. Sper. 2020 doi: 10.4081/jbr.0.8942. [DOI] [Google Scholar]
- 12.Idrus H., Hatta M., Febriza A., Novarina V. Antibacterial activities of Sapodilla fruit extract inhibiting Salmonella typhi on mice Balb/c. Int. J. Appl. Pharm. 2019;11:1–6. doi: 10.22159/ijap.2019.v11s5.T0095. [DOI] [Google Scholar]
- 13.Kasim V.N., Hatta M., Natzir R., Hadju V., Febriza A., Idrus H.H. Effects of lime (Citrus aurantifolia) peel to the expression of mRNA toll-like receptors 4 in balb/c mice-infected Salmonella typhi. "J. Adv. Pharm. Technol. Research"" (JAPTR)". 2020;11:169–173. doi: 10.4103/japtr.JAPTR_48_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasim V., Hatta M., Natzir R., Hadju V., Hala Y., Budu B., Alam G., As’ad Armyn S., Febriza A., Idrus H. Antibacterial and anti-inflammatory effects of lime (citrus aurantifolia) peel extract in mice balb/c induced salmonella typhi. J. Biol. Res. - Boll. Della Soc. Ital. Di Biol. Sper. 2020 doi: 10.4081/jbr.0.8951. [DOI] [Google Scholar]
- 15.Simanjuntak T.P., Hatta M., Tahir A.M., Sirait R.H., Karo M.B., Tambaib T., Dwiyanti R., Noviyanthi R.A., Junita A.R. Analysis of anti-toxoplasma immunoglobulin G and immunoglobulin M antibody levels after intervention with curcuma longa extract on early pregnant mice with acute toxoplasmosis. J. Global Infect. Dis. 2019;11:25–29. doi: 10.4103/jgid.jgid_28_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simanjuntak T., Hatta M., Rauf S., Prabandari S., Siagian C., Dwiyanti R. Tumor necrosis factor-alpha levels and histopathology finding after intervention with curcuma longa extract. J. Med. Sci. 2018;18:56–62. doi: 10.3923/jms.2018.56.62. [DOI] [Google Scholar]
- 17.Simanjuntak T., Hatta M., Rauf S., Irawan Y., Tahir M. Forkhead box P3 messenger-RNA expression after curcuma longa extract intervention in early pregnant mice with toxoplasmosis. Res. J. Immunol. 2018;11:1–6. doi: 10.3923/rji.2018.1.6. [DOI] [Google Scholar]
- 18.Djais A., Thahir H., Hatta M., Achmad H., Wahyuni A. Differences of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of moringa leaf extract (moringa oliefera L.) on bacteria aggregatibacter actinomycetemcomitans and porphyromonas gingivalis. Indian J. Public Heal. Res. Dev. 2019;10:896. doi: 10.5958/0976-5506.2019.02007.2. [DOI] [Google Scholar]
- 19.Khairi Amsyah U., Hatta M., Tahir H., Alam G., Asmawati A. Expression of IL-10 in A.actinomycetemcomitans induced rat treated by purple miana leaves. Biomed. Pharmacol. J. 2019;12:2099–2104. doi: 10.13005/bpj/1845. [DOI] [Google Scholar]
- 20.Sirait L., Nasrum M., Hatta M., Prihantono P. The effects of extract andaliman fruit (Zanthoxylum acanthopodium Dc) to CAMP mRNA expression and bacterial load in mice balb-C after Gardnerella vaginal Infection. Indian J. Public Heal. Res. Dev. 2018;9:607. doi: 10.5958/0976-5506.2018.01525.5. [DOI] [Google Scholar]
- 21.Tambaip T., Br Karo M., Natzir R., Bintang M., Islam A., Salma W., Hatta M. CD4 + cell impacts of orally red fruit (pandanus conoideus) oil extract in HIV patients with antiretroviral therapy. Indian J. Public Heal. Res. Dev. 2019;10:510. doi: 10.5958/0976-5506.2019.00342.5. [DOI] [Google Scholar]
- 22.Tambaip T., Br Karo M., Hatta M., Dwiyanti R., Natzir R., Nasrum Mas M., Asadul Isl A., Djawad K. Immunomodulatory effect of orally red fruit (pandanus conoideus) extract on the expression of CC chemokine receptor 5 mRNA in HIV patients with antiretroviral therapy. Res. J. Immunol. 2018;11:15–21. doi: 10.3923/rji.2018.15.21. [DOI] [Google Scholar]
- 23.Wijaya I., Taslim N.A., Natzir R., Aman A.M., Hatta M., Suhudi B., Islam A.A., Massi M.N., Patellongi I., Ichsan A.M. Molecular and immunological mechanisms of Channa striata in diabetic wound healing. Int. J. Pharmacol. Res. 2020;12:279–289. doi: 10.31838/ijpr/2020.SP2.046. [DOI] [Google Scholar]
- 24.Kurek-Górecka A., Rzepecka-Stojko A., Górecki M., Stojko J., Sosada M., Swierczek-Zieba G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules. 2013;19:78–101. doi: 10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin G.A., Scott M.J., Patel M., Hoth J.J., Peyton J.C., Cheadle W.G. A novel model of pneumonia from intraperitoneal injection of bacteria. Am. J. Surg. 2003;186:493–499. doi: 10.1016/j.amjsurg.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Almuhayawi M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020;27:3079–3086. doi: 10.1016/j.sjbs.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 28.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., Henry D.A. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syukri A., Budu, Hatta M., Amir M., Rohman M.S., Mappangara I., Kaelan C., Wahyuni S., Bukhari A., Junita A.R., Primaguna M.R., Dwiyanti R., Febrianti A. Doxorubicin induced immune abnormalities and inflammatory responses via HMGB1, HIF1-α and VEGF pathway in progressive of cardiovascular damage. Ann. Med. Surg. 2022;76 doi: 10.1016/j.amsu.2022.103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDaniel D.K., Allen I.C. Using Klebsiella pneumoniae to model acute lung inflammation in mice. Methods Mol. Biol. 2019;1960:169–180. doi: 10.1007/978-1-4939-9167-9_15. [DOI] [PubMed] [Google Scholar]
- 31.Billah Z., Attar B., Hughes L., Seidel V., Efthimiou G. A strong inhibitory effect of heather honey, propolis and medicinal plant extracts on biofilm formation by pathogenic bacteria. Access Microbiol. 2019;1 doi: 10.1099/acmi.amrmeds2019.po0008. [DOI] [Google Scholar]
- 32.Saddiq A.A., Danial E.N. Effect of Propolis as a food additive on the growth rate of the beneficial bacteria. Main Group Chem. 2014;13:223–232. doi: 10.3233/MGC-140135. [DOI] [Google Scholar]
- 33.Turcatto A.P., Lourenço A.P., De Jong D. Propolis consumption ramps up the immune response in honey bees infected with bacteria. Apidologie. 2018;49:287–296. doi: 10.1007/s13592-017-0553-z. [DOI] [Google Scholar]
- 34.Kim H., Lee M., Mustafa B., Han G., Lee S., Hwang J., Wook H. Nutritional compositional characterization on five diets for development of pollen substitute diet. J. Apic. 2021;36:63–69. doi: 10.17519/apiculture.2021.06.36.2.63. [DOI] [Google Scholar]
- 35.Borba R. UNIVERSITY OF MINNESOTA; 2015. Constitutive and Therapeutic Benefits of Plant Resins and a Propolis Envelope to Honey Bee, Apis mellifera L., Immunity and Health.https://hdl.handle.net/11299/175707 [Google Scholar]
- 36.Drago L., Mombelli B., De Vecchi E., Fassina M.C., Tocalli L., Gismondo M.R. In vitro antimicrobial activity of propolis dry extract. J. Chemother. 2000;12:390–395. doi: 10.1179/joc.2000.12.5.390. [DOI] [PubMed] [Google Scholar]
- 37.Oley M.H., Oley M.C., Wewengkang L.A.J.W., Kepel B.J., Langi F.L.F.G., Setiadi T., Aling D.M.R., Gunawan D.F., Tulong M.T., Faruk M. Bactericidal effect of hyperbaric oxygen therapy in burn injuries. Ann. Med. Surg. 2022;74 doi: 10.1016/j.amsu.2022.103314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stepanović S., Antić N., Dakić I., Svabić-Vlahović M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003;158:353–357. doi: 10.1078/0944-5013-00215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.