Abstract
Background
Medicinal plants have been used as traditional treatments for various human diseases for many years and they are still widely practiced throughout the world. Due to the long history of the practice, medicinal plants have become an integral part of the Ethiopian culture. This study aimed to evaluate the antibacterial activities of Vernonia auriculifera Hiern and Buddleja polystachya Fresen leaf extracts and their synergistic effect against some selected human pathogenic bacteria.
Methods
Ethanol, methanol, and n-hexane crude extracts of Vernonia auriculifera, Buddleja polystachya, and a mixture of the two-plant respective of each solvent were evaluated against tested pathogenic bacteria using the agar well diffusion method; the inhibition zones were recorded in millimeters. Gentamycin was used as a positive control, while dimethyl sulfoxide served as a negative control. The minimum inhibitory concentration of the plant extracts against test bacteria was evaluated using two-fold broth dilution methods and then Minimum bactericidal concentration was determined by sub-culturing the test dilutions from minimum inhibitory concentration tubes onto fresh Muller Hinton Agar plates incubated at 37 °C for 24 h.
Results
Maximum antibacterial inhibition zone was observed on methanol extracts of synergism against S. Typhimurium (ATCC 1333) (31.00 ± 1.73 mm) while, a minimum inhibition zone was observed on methanol extract of Buddleja polystachya, against E. coli (ATCC 35218) (5.67 ± 0.57). Minimum inhibitory concentration and minimum bactericidal concentration values of the crude extracts of Vernonia auriculifera, Buddleja polystachya, and their mixture lies between (3.125%–12.5%) and (6.25%–25%) respectively. The data were analyzed using the SPSS software package version 20 for windows.
Conclusion
The present study revealed that ethanol and methanol extracts of Vernonia auriculifera and Buddleja polystachya possess significant inhibitory effects against tested pathogens and the antibacterial activity of both plants leaf extracts was greater than the activity of currently used antibiotics (Gentamycin) against some selected organisms.
Keywords: Synergism, Antibacterial, Buddleja polystachya Fresen, Medicinal plant, Vernonia auriculifera Hiern
Abbreviations: ANOVA, Analysis of variance; ATCC, American Type Cell Culture; CFU, Colony Forming Unit; DMSO, Dimethyl Sulfoxide; MBC, Minimum Bactericidal Concentration; MDR, Multi Drug Resistance; MHA, Mueller Hinton Agar; MIC, Minimum Inhibitory Concentration; RPM, Revolution per Minute; SPSS, Statistical Package for Social Science; XDR, Extremely Drug Resistant; WHO, World Health Organization
1. Introduction
Medicinal plants have been used in almost all cultures as a source of medicine for a long period and they are still widely practiced throughout the world [1]. It has been used as a traditional treatment for various human diseases for many years in different parts of the world. Because medicinal plants have indefinite therapeutic value worldwide, researchers have recently paid attention to safer phytomedicines and biologically active compounds isolated from plant species used in herbal medicines with acceptable therapeutic keys for the development of new drugs [2]. The World Health Organization (WHO) estimates up to 80% of the world population relies on traditional medicines and the main part of traditional therapies involves the use of plant extracts [3].
Infectious diseases are major causes of death in the world, with unlimited impact in developing countries. The majority of emerging infectious diseases are caused by bacteria which can be associated with the evolution of drug-resistant strains and the devastating of the natural host defenses [4]. Antibiotics are the most important therapeutic strategies. But, only one-third of the known infectious diseases have been cured of these synthetic products.
Many plant species are reported to have pharmacological properties as they are known to possess various secondary metabolites like glycosides, saponins, flavonoids, steroids, tannins, alkaloids, terpenes which is, therefore, should be utilized to fight the disease-causing pathogens [[5], [6], [7]]. Increasing resistance bacteria against the current antibiotics resulted in numerous studies emphasizing antimicrobial agents derived from plants. Traditional medicine has become a form of complementary medicine and holds great promise as a source of effective therapy for multidrug resistance (MDR) strains of bacteria [8]. Hence, the development of drug resistance as well as the appearance of adverse side effects of certain antibiotics has led to the search for new antibacterial agents, particularly from medicinal plants.
The genus Vernonia (Compositae) contains more than 1000 species distributed throughout the world, mainly in Africa and South America (Fig. 1). V. auriculifera is a shrub, small tree, or woody herb that grows 1–7.5 m high and is easily detectable by its deep purple flowers [9]. In Ethiopia, the leaf of the plant is used for healing wounds by rubbing (as an ointment) around the wounded areas after soaking the fresh leaf with water or tying the wounded areas with fresh leaf after heating over the flame [10]. Seven triterpenoids were discovered through the phytochemical study of V. auriculifera. One triterpene of the ursane-type (-amyrin), one of the lupane-type (-lupenyl acetate), and two of the oleanane-type (-amyrin acetate and -amyrin) were discovered in leaf extracts. Oleanolic acid, the parent oleanane type triterpene, was extracted from the roots and friedlin/friedelanone and friedelin acetate from the friedelane class were present in the stem-bark, according to phytochemical analysis [11].
Fig. 1.
Morphological view of vernonia auriculifera.
B. polystachya which is known as (“Anfar”) in Amharic, belongs to the family Buddlejaceae. This plant is endemic to Ethiopia, Eritrea, Somalia, Yemen, and Saudi Arabia (Fig. 2). Using various chromatographic techniques, sixteen chemical components, including phenolic fatty acid ester, isobenzofuranone derivative, flavonoids, and triterpenic acids, were recovered from the aerial portions of B. polystachya [12]. Through various chromatography processes, sixteen bioactive compounds from the crude extract and solvent fractions of B. polystachya were extracted. These compounds were then identified using various 1D, 2D NMR, and mass spectrometry techniques. Additionally, the separated components consist of 6-O-α-L-(4″-O-trans-cinnamoyl) rhamnopyranosylcatalpol, cirsimaritin, ursolic acid, luteolin, 1′(4-hydroxyphenyl) ethanol ester of docosanoic, isobenzofuranone derivative (4-hydroxy-7-methylisobenzofuranone), sakuranetin, luteolin 7-(6″-caffeoyl)-O-β-D-glucopyranoside, kumatakenin, oleanolic acid, herbacetin 3,7,8-trimethyl ether, uvaol, 5- hydroxy-3,7,4′-trimethoxyflavone, verbascoside, linarin, luteolin 7-O-β-D-glucoside, and phenolic fatty acid ester [13]. B. polystachya used in traditional medicine as an antimicrobial, analgesic, antipyretic, hepatoprotective, hypotensive, anti-inflammatory, hypoglycemic, molluscicidal, neuroprotective, and amoebicidal remedies. B. polystachya is also used for the treatment of many skin disorders [[14], [15], [16]].
Fig. 2.
Morphological view of buddleja polystachya.
Ethno-botanical studies revealed that V. auriculifera and B. polystachya are being used in the treatment of pathogenic organisms in the traditional health care system in Ethiopia [17,18]. However, very little work has been done to evaluate their efficacy scientifically and there was no study done on the synergistic antibacterial effect of V. auriculifera and B. polystachya leaves extracts.
In Ethiopia vast knowledge on the traditional uses of medicinal plants is not fully documented and most of the information is conveyed from one generation to the other through verbally. Therefore, findings of the present study will provide scientific information to those interested to extract each plant for medicinal purposes. In addition it will also serves as a base line for the development of antibacterial drugs from these plants with further detailed study of the efficacy in preclinical trials and it will provide a clue for the isolation and identification of active ingredients responsible for the antibacterial activity. Therefore, this study aimed to evaluate the in vitro antibacterial activity of crude leaf extracts of V. auriculifera and B. polystachya and their synergetic effect against selected human pathogenic bacteria which can, in turn, provide a clue for the isolation and identification of active ingredients responsible for the antibacterial activity.
2. Materials and methods
2.1. The study area
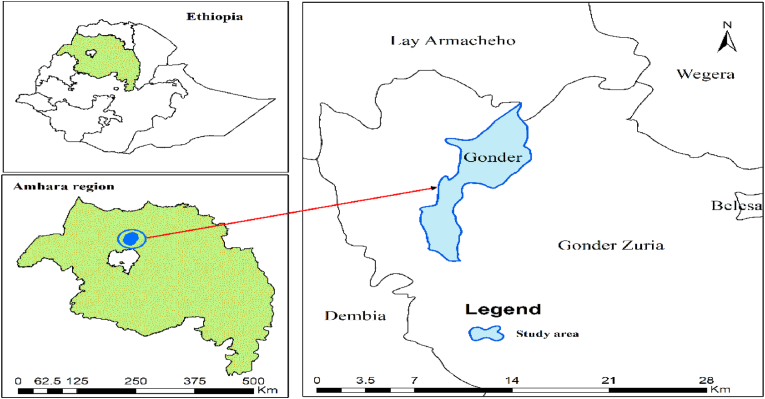
The plant leaves were collected from the area where they grow in Gondar. It is located in North West Ethiopia about 730 km away from the capital, Addis Ababa (Fig. 3).
Fig. 3.
Map of the study area [19].
2.2. Study design
The study design was Completely Randomized Design (CRD) experimental based; using appropriate methods such as determination of antibacterial activities, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC).
2.3. Sampling technique
The purposive sampling technique was used to collect leaves of V. auriculifera and B. polystachya in different areas around Gondar.
2.4. Plant collection and authentication of plant material
Fresh leaves of V. auriculifera and B. polystachya were collected from the area where they grow in Gondar, in February 2020. The plant materials were identified by Mr Abiyu Enyew, a botanist in university of Gondar, Department of Biology, College of Natural and Computational Sciences.
2.5. Preparation of plant crude extraction
The collected plant leaves were washed thoroughly in running tap water to remove debris and dust particles and then they were rinsed in distilled water. The leave samples were dried in the laboratory in open-air at room temperature and it was protected from sunlight. Once completely dry, these were grounded to a fine powder using an electronic blender, and the powder was stored in a sterile bottle at room temperature in a dark place. Ethanol, methanol, and n-hexane solvents were used for extraction. The dried and powdered leaves of each plant (100 g) were macerated separately with 600 ml of ethanol, methanol and n-hexane. This maceration process was done in a 1000 ml Erlenmeyer flask on an orbital shaker (120 rpm) at room temperature for 72 h. The extracts were filtered through a sterile Whatman No.1 filter paper and then it was concentrated in a vacuum at 40 °C using a rotary evaporator [[20], [21], [22]]. Each extract was transferred to glass vials and kept at 4 °C until further use.
2.6. Phytochemical screening of crude solvent extracts
The crude extract of the plant was used for screening phytochemicals for the presence or absence of primary and secondary metabolites such as alkaloids, flavonoids, saponins, terpenoid, glycosides, steroids, tannins, and phenols, respectively, using the standard procedure [23].
2.7. Test microorganisms
The standard American type cell culture (ATCC) Staphylococcus aureus (ATCC 43300), Escherichia coli (ATCC 35218), Pseudomonas aeruginosa (ATCC 27853) Salmonella typhimurium (ATCC 1333), and clinical isolate bacterial strains Staphylococcus aureus and Escherichia coli were used in this study. Those Bacterial strains were obtained and collected from the University of Gondar teaching hospital.
2.8. Inocula preparation
The tested bacteria were cultured separately on nutrient agar at 37 °C for 24 h. This was done by streaking the inoculating loop containing the bacteria at the top end of the agar plate moving in a zigzag horizontal pattern until 1/3 of the plate was covered. Then, two to three well-isolated overnight cultured colonies of the same morphological type were selected from an agar plate culture. The top of each colony was touched with a sterile bent wire loop and the growth was transferred into a screw-capped tube containing 5 ml of normal saline solution. The turbidity of the bacteria was adjusted with sterile saline to obtain turbidity optically comparable to that of the 0.5 McFarland turbidity standard 1.5 × 108 colony-forming units (CFU)/ml [24].
2.9. Antibacterial activity assay
Agar well diffusion method was employed to assess the antibacterial activity of V. auriculifera and B. polystachya and a mixture of both plant extract of ethanol, methanol, and n-hexane crude extract against the standard and clinically isolated human pathogenic bacteria. The antibacterial activity assay was determined according to the method described previously [25]. Sterilized Mueller Hinton Agar (MHA) medium was poured into each Petri dish and set aside to solidify under the laminar hood. After solidifying the media; the sterilized L-rode was used to spread the inoculums throughout the medium uniformly. In each Petri dish wells of 4 mm in diameter were punctured in the culture media using sterile cork borers to make four to seven uniform wells. Then wells were filled with 100 μl (use micropipette) of each extract, adjusted to the same concentration (50 mg/ml), totaled to a respective well, and allowed diffusing for 45 min. In the center, gentamycin was used as a positive control, while 50% DMSO was used as a negative control. The antibacterial activities were determined after 24 h at 37 °C incubation in the incubator. The diameter of the zone of inhibition produced by the extract was measured by a ruler and compared with the standard.
To identify synergism between the two plant extracts equal volume V. auriculifera and B. polystachya extracts were mixed and 100 μl of the mixture were totaled to a respective well in a medium. Then after 24 h of incubation at 37 °C diameters of clearing zones were measured. Each sample was used in triplicate for the determination of antibacterial activity. The work was carried out in a laminar flow hood.
2.10. Determination of the minimum inhibitory concentration (MIC)
The MIC is defined as the lowest concentration able to inhibit any visible bacterial growth on the culture plates. MIC of crude extracts of V. auriculifera and B. polystachya and their combination were performed by using two-fold broth dilution methods as the method described previously [26]. The extract solution (50 mg/ml) was serially diluted with Mueller Hinton broth as 1:1 1:2, 1:4, 1:8, 1:16, 1:32, 1:64 and 1:128 to bring 50 mg/ml, 25 mg/ml, 12.5 mg/ml, 6.25 mg/ml, 3.125 mg/ml, 1.56 mg/ml, 0.78 mg/ml, and 0.395 mg/ml concentrations, respectively and 100 μl of a standard suspension of the test organism was added to each concentration of the extract. Two test tubes containing Mueller Hinton broth without antimicrobial agent were added to each test. One of these tubes was inoculated with the test organism and served as a positive control; the other was left uninoculated and served as a control for media sterility. Then broth plates were incubated at 37 °C for 24 h. The lowest concentration, at which there was no turbidity, was recorded the MIC value of the extract.
2.11. Determination of minimum bactericidal concentration (MBC)
For MBC dilutions with no visually visible growth were taken and sub-cultured on Mueller Hinton agar media using a sterile wire loop and making a strike on the media to see bacteria growth after incubating at 37∘C for 24 h. The lowest concentration of the extract with no visible growth after incubation was taken as the minimum bactericidal concentration (MBCs) of the antimicrobial agents [27].
2.12. Statistical analysis
The data were analyzed using the SPSS software package version 20 for windows. Means and standard deviations of the triplicate data analysis were calculated via one-way analysis of variance (ANOVA) to determine the significances between the means followed by the LSD the Post Hok test (P ≤ 0.5). Microsoft Excel 2016 was employed for the analysis of MIC and MBC.
3. Result
3.1. Yields of solvent extractions
The percentage yield of crude extract in the respective solvent is shown in (Table 1). In both plant extracts the highest yield was obtained from ethanol extract of B. polystachiya (45.37%) and V. auriculifera (40.58%), respectively followed by methanol extract of B. polystachiya (30.96%) and V. auriculifera (16.52%), while the lowest yield was recorded in n-hexane extract in both plants V.auriculifera (3.52%) and B. polystachiya (2.56%).
Table 1.
The percentage yield of crude leaves extracts of V. auriculifera and B. polystachiya.
| Plant name | Parts used | Solvents | Weight of crude in(g) | Yield (%) |
|---|---|---|---|---|
| V. auriculifera | Leaf | Ethanol | 40.58 | 40.58 |
| Methanol | 16.52 | 16.52 | ||
| n-hexane | 3.52 | 3.52 | ||
| B. polystachiya | Leaf | Ethanol | 45.37 | 45.37 |
| Methanol | 30.96 | 30.96 | ||
| n-hexane | 2.56 | 2.56 |
3.2. Phytochemical screening
Phytochemical screening was carried out on ethanol and methanol solvent extract of V. auriculifera and B. polystachiya to test the presence of the most relevant bioactive compounds. The n-hexane solvent extracts of both plants were not included due to low inhibitory activity against the test organism. Ethanol extract of V. auriculifera was found almost positive except for flavonoids of ammonia test, phenols of ferric chloride test, and steroids of Salkowski tests. However, ethanol extract of B. polystachiya had positive for all tests. Similarly, methanol extracts of V. auriculifera were found positive for all tests except Salkowski tests of steroids, alkaloids, and glycosides. And methanol extract of B. polystachiya was found all positives except flavonoids of ammonia test (Table 2).
Table 2.
Phytochemical constituents of crude leaves extracts of V. auriculifera and B. polystachiya.
| Phytochemical tests | Reagent | Result of qualitative phytochemical screening of the two plant extracts |
|||
|---|---|---|---|---|---|
| V. auriculifera |
B. polystachiya |
||||
| Ethanol | Methanol | Ethanol | Methanol | ||
| Alkaloid | Mayer's reagent | + | – | + | + |
| Hager's reagent | + | – | + | + | |
| Flavonoids | Alkaline reagent | + | + | + | + |
| Lead acetate | + | + | + | + | |
| Ammonia | – | + | + | – | |
| Phenols | Alkaline reagent | + | + | + | + |
| Lead acetate | + | + | + | + | |
| Ferric chloride | – | + | + | + | |
| Tannin test | Iron salt | + | + | + | + |
| Lead acetate | + | + | + | + | |
| Ferric chloride | + | + | + | + | |
| Saponin test | Foam test | + | + | + | + |
| Sodium nitrate | + | + | + | + | |
| Steroids | Salkowski test | – | – | + | + |
| Glycosides | Liebermann's | + | + | + | + |
| Salkowski test | + | – | + | + | |
| Terpenoids | Chloroform + H2SO4 | + | + | + | + |
Note: + = presence of phytochemical and - = absence of phytochemical.
3.3. Antibacterial activity test
3.3.1. Evaluation of V. auriculifera leaf crude extracts against test bacteria
The inhibition zone of V. auriculifera leaf extracts of ethanol, methanol, and n-hexane solvents was evaluated against the standard. The mean inhibition zone (22.00 ± 2.00 mm) of ethanol extract of V. auriculifera against S.aureus (clinical isolate) was statistically significant (p = 0.02) greater than the methanol extract against this test bacterium. There was no statistically significant difference between the mean inhibition zone of ethanol and methanol extracts of V. auriculifera against S. aureus (ATCC43300). Moreover, the mean inhibition zone (9.33 ± 2.30 mm) of ethanol extract and mean inhibition zone (12.67 ± 2.88 mm) of methanol extract of V. auriculifera against E.coli (ATCC 35218) and the mean inhibition zone (9.00 ± 2.00 mm) of ethanol and mean inhibition zone (9.00 ± 2.00 mm) of methanol extract of V. auriculifera against P.aeruginosa (ATCC 27853) were not statistically significant. Whereas, the mean inhibition zone (7.67 ± 1.52 mm) of ethanol extract of V. auriculifera against S. typhimurium (ATCC1333) was statistically significant (p = 0.00) less than the methanol extracts of against this test bacterium. However, n-hexane extracts of V. auriculifera against all tested bacteria did not have an inhibitory effect (Table 3).
Table 3.
Comparison of mean inhibition zone of ethanol, methanol, and n-hexane solvent extracts of V. auriculifera against test bacteria.
| Test organism | solvents | Mean IZ(mm) ±SD | Positive and negative controls |
|
|---|---|---|---|---|
| Gentamycin | DEMO | |||
| S.aures(ATTC 43300) | Ethanol | (23.00 ± 3.00)a | (20.67 ± 3.51)a | 0.00 ± 0.00 |
| Methanol | (20.67 ± 3.05)a | (20.67 ± 3.51)a | 0.00 ± 0.00 | |
| n-hexane | (00.00 ± 0.00)b | (20.67 ± 3.51)a | 0.00 ± 0.00 | |
| S.aures(clinical isolate) | Ethanol | (22.00 ± 2.00)ab | (16.33 ± 2.51)b | 0.00 ± 0.00 |
| Methanol | (17.33 ± 2.30)bc | (16.33 ± 2.51)b | 0.00 ± 0.00 | |
| n-hexane | (00.00 ± 0.00)c | (16.33 ± 2.51)b | 0.00 ± 0.00 | |
| E.coli(ATTC 35218) | Ethanol | (09.33 ± 2.30)ac | (20.00 ± 2.00)a | 0.00 ± 0.00 |
| Methanol | (12.67 ± 2.88)ac | (20.00 ± 2.00)a | 0.00 ± 0.00 | |
| n-hexane | (00.00 ± 0.00)b | (20.00 ± 2.00)a | 0.00 ± 0.00 | |
| E.coli(clinical isolate) | Ethanol | (0.00 ± 0.00) | (16.33 ± 2.08)b | 0.00 ± 0.00 |
| Methanol | (0.00 ± 0.00) | (16.33 ± 2.08)b | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00) | (16.33 ± 2.08)b | 0.00 ± 0.00 | |
| P.auroginosa(ATTC 27853) | Ethanol | (9.00 ± 2.00)e | (20.67 ± 1.15)a | 0.00 ± 0.00 |
| Methanol | (9.00 ± 2.00)e | (20.67 ± 1.15)a | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00)b | (20.67 ± 1.15)a | 0.00 ± 0.00 | |
| S.typhimurium(ATTC 1333) | Ethanol | (7.67 ± 1.52)f | (20.67 ± 1.15)a | 0.00 ± 0.00 |
| Methanol | (28.67 ± 2.30)g | (20.67 ± 1.15)a | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00)b | (20.67 ± 1.15)a | 0.00 ± 0.00 | |
*Values were means of triplicate determinations. Values of the same column followed by different letters are significantly different at (p≤0.05). Where “IZ:Inhibition zone, SD: Standard deviation”.
The mean inhibition zone of commercial antibiotic disc Gentamycin (16.33 ± 2.51 mm) was statistically significant (p = 0.00) less than the mean inhibition zone (22.00 ± 2.00 mm) ethanol extract of V. auriculifera against S. aureus (clinical isolate). Furthermore, the mean inhibition zone (20.67 ± 1.15) of the antibiotic disc Gentamycin was statistically significant (p = 0.00) less than the mean inhibition zone (28.67 ± 2.30 mm) of methanol extract of V. auriculifera against S. Typhimurium (ATCC 1333). However, the mean inhibition zone (20.67 ± 3.51 mm) of the Gentamycin disc was not a statistically significant difference between the mean inhibition zone (23.00 ± 3.00 mm) of ethanol (20.67 ± 3.05 mm) and methanol extracts of V. auriculifera against S. aureus (ATCC43300).
The antibacterial effects of V. auriculifera crude extracts showed effective bacterial growth inhibition against almost all tested bacterial organisms except on E. coli (clinical isolate) which did not show any inhibitory activity. The plant extracts showed as low 7.67 ± 1.52 mm to as high 28.67 ± 2.30 mm diameter inhibition zones. Concerning ethanol extracts, the highest inhibition zone (23 ± 3.00 mm) was recorded against S. aureus (ATCC 43300). While the least inhibition zone was seen against S. typhimurium (ATCC1333) (7.67 ± 1.52 mm). Similarly, the highest inhibition zone for methanol extract was seen against S. typhimurium (ATCC1333) (28.67 ± 2.30 mm) while the minimum was against P.aeruginosa (ATCC 27853) 9.00 ± 2.00 mm.
3.3.2. Evaluation of B. Polystachiya leaf crude extracts against test bacteria
There was no statistically significant difference (p > 0.05) between the mean inhibition zone of ethanol, methanol, and n-hexane extracts of B. polystachiya against S. aureus (ATCC43300). Similarly, the mean inhibition zone (16.67 ± 3.05 mm) of ethanol extract and mean inhibition zone (14.67 ± 1.52 mm) of methanol extract of B. polystachiya against S. aureus (clinical isolate) were not statistically significant (P > 0.05) but n-hexane extract of B. polystachiya did not have any inhibitory effect against this test bacterium. Whereas, the mean inhibition zone (29.00 ± 2.64 mm) of methanol extract of B. polystachiya against S. typhimurium (ATCC1333) was statistically significant (P = 0.02) greater than the mean inhibition zone (18.67 ± 2.51) of ethanol extracts and the mean inhibition zone (10.00 ± 2.00) of n-hexane extracts against this test bacterium. B. polystachiya extracts of ethanol, methanol, and n-hexane did not show any inhibitory effect against E.coli (clinical isolate), P.aeruginosa (ATCC27853), and E.coli (ATCC35218) except methanol extract (5.67 ± 0.57 mm) against E.coli (ATCC35218). The plant extracts showed as low (5.67 ± 0.57 mm) to as high (29.00 ± 2.64 mm) diameter inhibition zones. Regarding ethanol extracts, the highest inhibition zone (20.33 ± 1.52 mm) was recorded against S. aureus (ATCC 43300). While the least inhibition zone was seen against S. aureus (clinical isolate) (16.67 ± 3.05 mm). Similarly, the highest inhibition zone for methanol extract was seen against S. typhimurium (ATCC1333) (29.00 ± 2.64 mm) while, the minimum was against E. coli (ATCC 35218) (5.67 ± 0.57 mm). And the highest inhibition zone (18.00 ± 2.64 mm) of n-hexane extract was recorded against S. aureus (ATCC 43300). While the least inhibition zone was seen against S. typhimurium (ATCC1333) (10.00 ± 2.00 mm) (Table 4).
Table 4.
Comparison of mean inhibition zone of ethanol, methanol, and n-hexane solvent extracts of B. polystachiya against test bacteria.
| Test organism | solvents | Mean IZ(mm) ±SD | Positive and negative controls |
|
|---|---|---|---|---|
| Gentamycin | DEMO | |||
| S.aureus(ATTC 43300) | Ethanol | (20.33 ± 1.52)a | (20.67 ± 3.51)a | 0.00 ± 0.00 |
| Methanol | (20.67 ± 2.08)a | (20.67 ± 3.51)a | 0.00 ± 0.00 | |
| n-hexane | (18.00 ± 2.64)a | (20.67 ± 3.51)a | 0.00 ± 0.00 | |
| S.aureus(clinical isolate) | Ethanol | (16.67 ± 3.05)b | (16.33 ± 1.52)b | 0.00 ± 0.00 |
| Methanol | (14.67 ± 1.52)b | (16.33 ± 1.52)b | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00)c | (16.33 ± 1.52)b | 0.00 ± 0.00 | |
| E.coli(ATTC 35218) | Ethanol | (0.00 ± 0.00)c | (18.67 ± 3.05)a | 0.00 ± 0.00 |
| Methanol | (5.67 ± 0.57)bc | (18.67 ± 3.05)a | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00)c | (18.67 ± 3.05)a | 0.00 ± 0.00 | |
| E.coli(clinical isolate) | Ethanol | (0.00 ± 0.00) | (15.00 ± 1.73)b | 0.00 ± 0.00 |
| Methanol | (0.00 ± 0.00) | (15.00 ± 1.73)b | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00) | (15.00 ± 1.73)b | 0.00 ± 0.00 | |
| P.aeruginosa(ATTC 27853) | Ethanol | (0.00 ± 0.00) | (20.67 ± 1.52)a | 0.00 ± 0.00 |
| Methanol | (0.00 ± 0.00) | (20.67 ± 1.52)a | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00) | (20.67 ± 1.52)a | 0.00 ± 0.00 | |
| S.typhimurium(ATTC 1333) | Ethanol | (18.67 ± 2.52)d | (22.33 ± 0.57)a | 0.00 ± 0.00 |
| Methanol | (29.00 ± 2.64)e | (22.33 ± 0.57)a | 0.00 ± 0.00 | |
| n-hexane | (10.00 ± 2.00)f | (22.33 ± 0.57)a | 0.00 ± 0.00 | |
*Values were means of triplicate determinations. Values of the same column followed by different letters are significantly different at (p ≤ 0.05). Where “IZ: Inhibition zone, SD: Standard deviation”.
The mean inhibition zone (22.33 ± 0.57 mm) of Gentamycin disc was statistically significant (p = 0.00) less than the mean inhibition zone (29.00 ± 2.64 mm) of methanol extracts of B. polystachiya against S. typhimurium (ATCC1333).
3.3.3. Evaluation of synergetic antibacterial activity
There was no statistically significant difference (p > 0.05) between the mean inhibition zone of ethanol and methanol mixed plant extracts against S. aureus (ATCC43300). However, the mean inhibition zone of n-hexane of mixed plant extract (14.67 ± 1.15) was significantly (p = 0.00) less than the mean inhibition zone of ethanol and methanol mixed plant crude extracts against this test bacterium. The mean inhibition zone of mixed plant crude extracts of ethanol and methanol on S. aureus (clinical isolate) did not show any significant difference. Whereas, the mean inhibition zone (31.00 ± 1.73 mm) of mixed plant methanol crude extracts was statistically significant (p = 0.00) greater than the mean inhibition zone (13.33 ± 2.51 mm) of ethanol and mean inhibition zone (8.00 ± 2.64 mm) of n-hexane mixed plant crude extracts against S. typhimurium (ATCC1333) (Table 5).
Table 5.
Comparison of mean inhibition zone of ethanol, methanol, and n-hexane solvent extracts of synergism against test bacteria.
| Test organism | solvents | Mean IZ(mm) ±SD | Positive and negative controls |
|
|---|---|---|---|---|
| Gentamycin | DEMO | |||
| S.aureus(ATTC 43300) | Ethanol | (25.67 ± 2.51)a | (19.23 ± 1.51)a | 0.00 ± 0.00 |
| Methanol | (22.67 ± 2.08)a | (19.23 ± 1.51)a | 0.00 ± 0.00 | |
| n-hexane | (14.67 ± 1.15)b | (19.23 ± 1.51)a | 0.00 ± 0.00 | |
| S.aureus(clinical isolate) | Ethanol | (19.00 ± 7.55)ab | (17.63 ± 2.35)b | 0.00 ± 0.00 |
| Methanol | (17.00 ± 2.64)ab | (17.63 ± 2.35)b | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00)c | (17.63 ± 2.35)b | 0.00 ± 0.00 | |
| E.coli(ATTC 35218) | Ethanol | (7.67 ± 0.57)bc | (19.84 ± 2.16)a | 0.00 ± 0.00 |
| Methanol | (8.67 ± 3.05)bc | (19.84 ± 2.16)a | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00)c | (19.84 ± 2.16)a | 0.00 ± 0.00 | |
| E.coli(clinical isolate) | Ethanol | (0.00 ± 0.00) | (15.67 ± 2.30)b | 0.00 ± 0.00 |
| Methanol | (0.00 ± 0.00) | (15.67 ± 2.30)b | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00) | (15.67 ± 2.30)b | 0.00 ± 0.00 | |
| P.auroginosa(ATTC 27853) | Ethanol | (0.00 ± 0.00)c | (21.18 ± 1.64)a | 0.00 ± 0.00 |
| Methanol | (9.33 ± 2.08)d | (21.18 ± 1.64)a | 0.00 ± 0.00 | |
| n-hexane | (0.00 ± 0.00)c | (21.18 ± 1.64)a | 0.00 ± 0.00 | |
| S.typhimurium(ATTC 1333) | Ethanol | (13.33 ± 2.51)e | (21.67 ± 0.57)a | 0.00 ± 0.00 |
| Methanol | (31.00 ± 1.73)f | (21.67 ± 0.57)a | 0.00 ± 0.00 | |
| n-hexane | (8.00 ± 2.64)g | (21.67 ± 0.57)a | 0.00 ± 0.00 | |
*Values were means of triplicate determinations. Values of the same column followed by different letters are significantly different at (p ≤ 0.05). Where “IZ: Inhibition zone, SD: Standard deviation”.
When comparing the mean inhibition zone of the commercial antibiotic disc Gentamycin with mixed plant crude extracts, there was no statistically significant difference (p > 0.05) between the mean inhibition zone of ethanol and methanol mixed plant crude extracts against S.aureus (clinical isolate) While, the mean inhibition zone (25.67 ± 2.51) of ethanol extract of the mixture against S. aureus (ATCC 43300) and methanol extracts of the mixture against S.typhimurium (ATCC 1333) was statically significant (p > 0.05) greater than Gentamycin disc. The highest inhibition zone (31.00 ± 1.73 mm) of methanol extracts of mixed plant was recorded against S. typhimurium (ATCC1333) while the lowest inhibition zone (7.67 ± 0.57 mm) of ethanol extracts of the mixed plant was recoded against E. coli (ATTC35218).
3.4. Determination of the minimum inhibitory concentration (MIC)
3.4.1. Minimum inhibitory concentration (MIC) of V. auriculifera
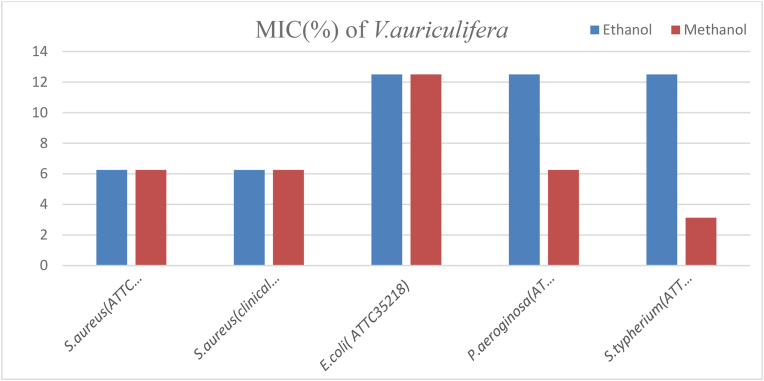
The MIC value of ethanol and methanol crude extracts of V. auriculifera against S. aureus (ATCC43300), S. aureus (clinical isolate), and methanol crude extract of the plant against P. aeruginosa (ATCC27853) was 6.25%. Whereas, the MIC value of ethanol and methanol crude extracts of the plant against E. coli (ATCC35218), ethanol crude extract of the plant against P. aeruginosa (ATCC27853), and ethanol crude extract of the plant against S. typhimurium (ATCC1333) was 12.5%. Moreover, the MIC value of methanol crude extract of the plant against S. typhimurium (ATCC1333) was 3.125% which was the lowest MIC value of the plant crude extracts against the test organism (Fig. 4).
Fig. 4.
MIC of V. auriculifera against the test organisms.
3.4.2. Minimum inhibitory concentration (MIC) of B. Polystachiya
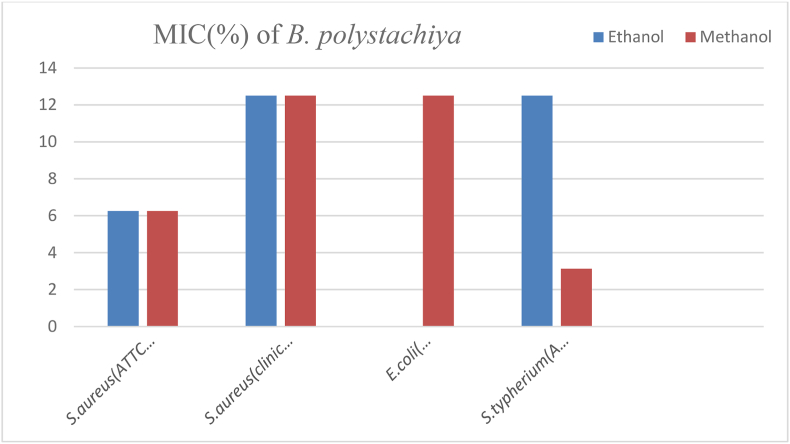
The MIC value of ethanol and methanol crude extracts of the plant against S.aureus (ATCC43300) was 6.25%. While, the MIC value of ethanol and methanol crude extract s of the plant against S.aureus (clinical isolate), methanol crude extracts of the plant against E.coli (ATCC35218), and ethanol crude extracts of the plant against S. typhimurium (ATCC1333) were 12.5% and which were the highest MIC value of the plant extracts against the test bacteria. The least MIC value of 3.125% was recorded by methanol crude extracts of the plant against S. typhimurium (ATCC1333) (Fig. 5).
Fig. 5.
MIC of B. polystachiya against the test organisms.
3.4.3. Synergetic minimum inhibitory concentration (SMIC)
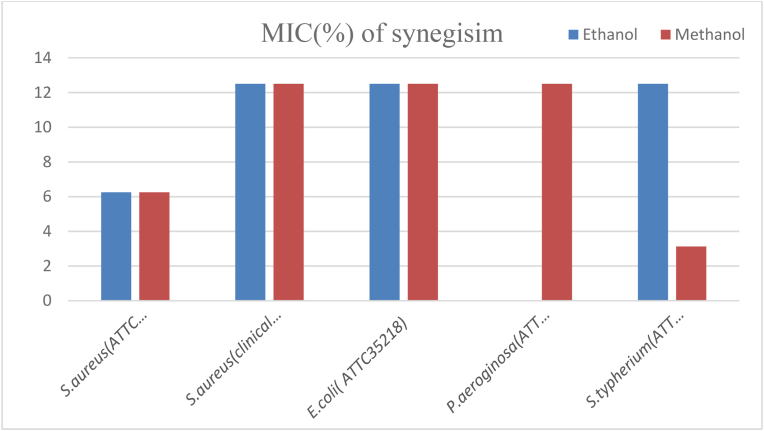
The MIC value of the mixture of V. auriculifera and B. polystachiya was indicated. The MIC value of ethanol and methanol crude extracts of the two plant mixture against S. aureus (ATCC43300) was 6.25%. However, ethanol and methanol crude extracts of the two plant mixture against S. aureus (clinical isolate) and E. coli (ATCC35218) had equal MIC values (12.5%). Moreover, methanol crude extract of the two plant mixture against P. aeruginosa (ATCC27853) and ethanol crude extract of the two plant mixture against S. typhimurium (ATCC1333) were also 12.5%. While the MIC value of methanol crude extract of the two-plant mixture against S. typhimurium (ATCC1333) was 3.125% and which was the least MIC value of the two-plant mixture against the test organism (Fig. 5, Fig. 6).
Fig. 6.
MIC value of synergism against the test organisms.
3.5. Determination of the minimum bactericidal concentration (MBC)
3.5.1. Minimum bactericidal concentration (MBC) of V. auriculifera
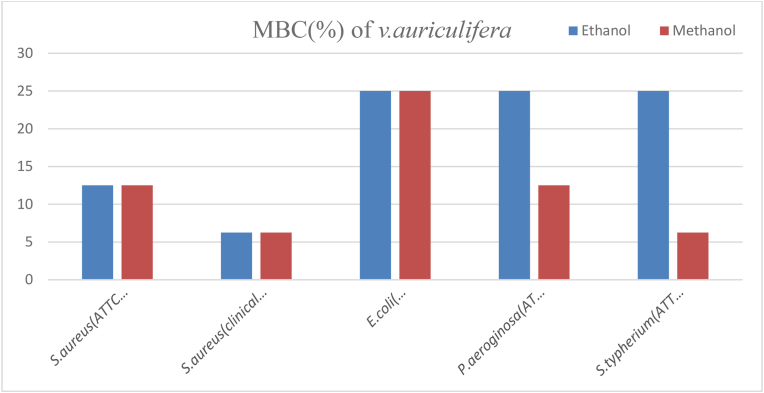
The MBC value of ethanol and methanol crude extract of V. auriculifera against S. aureus (ATCC43300) and the methanol crude extract of the plant against P. aeruginosa (ATCC27853) had equal MBC values (12.5%). However, the MBC value of ethanol and methanol crude extract of V. auriculifera against E. coli (ATCC35218), ethanol crude extract of V. auriculifera against P. aeruginosa (ATCC27853), and S. typhimurium (ATCC1333) had the same MBC value (25%). The lowest MBC value (6.25%) were recorded by ethanol and methanol crude extracts of V. auriculifera against S. aureus (clinical isolate) and methanol crude extract of V. auriculifera against S. typhimurium (ATCC1333) (Fig. 7).
Fig. 7.
MBC value of V. auriculifera against the test organisms.
3.5.2. Minimum bactericidal concentration (MBC) of B. Polystachiya
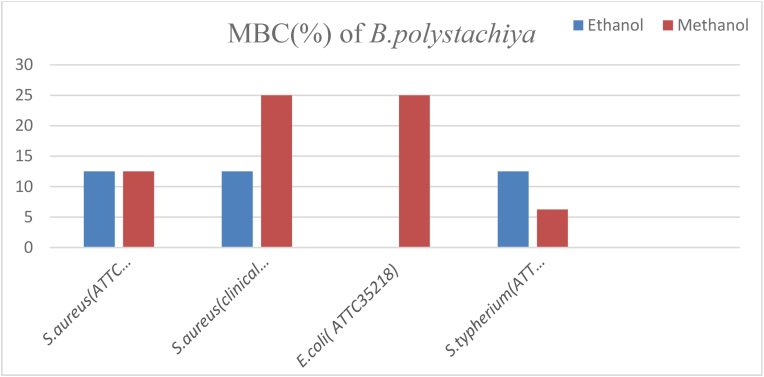
Ethanol and methanol crude extracts of B. polystachiya against S. aureus (ATCC43300) and ethanol crude extract of B. polystachiya against S. aureus (clinical isolate) and S. typhimurium (ATCC1333) had equal MBC values (12.5%). The maximum MBC value of 25% was observed on methanol crude extract of B. polystachiya against S. aureus (clinical isolate) and E. coli (ATCC35218). While the minimum MBC value of 6.25% was observed on methanol crude extract of B.polystachiya against S. typhimurium (ATCC1333) (Fig. 8).
Fig. 8.
MBC values of B. polystachiya against the test organisms.
3.5.3. Synergetic minimum bactericidal concentration (SMBC)
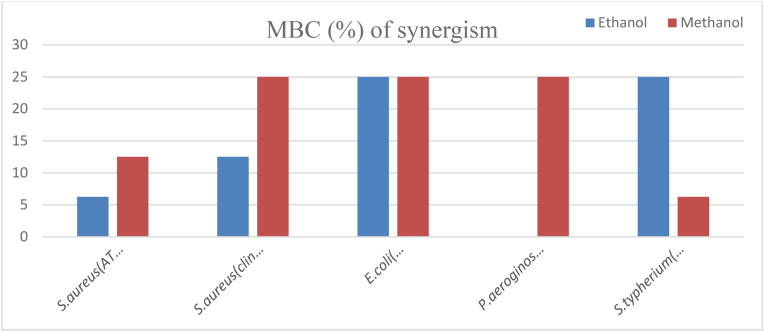
The MBC value of methanol crude extract of the two plant mixture against S. aureus (ATCC43300) and ethanol crude extract against S. aureus (clinical isolate) was 12.5%.
Methanol crude extract of the mixture against S. aureus (clinical isolate), E. coli (ATCC35218), and P. aeruginosa (ATCC27853), and ethanol extract of the mixture against E. coli (ATCC) and S. typhimurium (ATCC 1333) had equal MBC values (25%) and which were the maximum MBC values of the extracts against the test organism. But the minimum MBC value (6.25%) were observed on ethanol crude extract of the two plant mixture against S. aureus (ATCC43300) and methanol crude extract of the mixture against S. typhimurium (ATCC1333) (Fig. 9).
Fig. 9.
MBC value of synergism of the two plants against the test organism.
4. Discussion
The result of this study revealed that the ethanol and methanol extracts of V. auriculifera showed the presence of most of the bioactive compounds. Thus, the finding agreed with the previous result that methanol extracts of V. auriculifera steroids and alkaloids were absent [17]. It also showed that the ethanol and methanol crude extracts of B. polystachiya were rich sources of most bioactive compounds. This result was in line with the study done by previously [28].
In this study three extraction solvents namely, ethanol, methanol, and n-hexane were used for the extraction of both plants. Among these solvents n-hexane, crude extracts of V. auriculifera did not show any inhibitory activity against all the tested microorganisms except little effect on the extract of B. polystachiya against S.aureus (ATCC43300) and S. typhimurium (ATCC1333). This could be the plant material contains a high level of polar compounds that are soluble in solvents with high polarity such as ethanol and methanol and these differences in the polarity of the extraction solvents could cause a wide variation in the level of bioactive compounds in the extract [29].
The result of this study indicates that ethanol and methanol leaf crude extracts of V. auriculifera showed excellent inhibitory activity against S. aureus (ATCC43300), S. aureus (clinical isolate), and S. typhimurium (ATCC1333) and it was showed moderate inhibitory activity against E. coli (ATCC 35218) and P. aeruginosa (ATCC 27853). This result was supported by previous reports, triterpenoids isolated from V. auriculifera possess antibacterial activity [30], and methanol leaf extract was promising antibacterial activity against S. aureus [17].
In this study ethanol and methanol leaf crude extract of B. polystachiya also showed excellent antibacterial activity against S. typhimurium (ATCC1333) followed by S. aureus (ATCC43300) and S. aureus (clinical isolate). But it had not any inhibitory activity against E. coli (ATCC 35218), E. coli (clinical isolate), and P. aeruginosa (ATCC 27853) except methanol extract of the plant that showed very weak and negligible antibacterial potential against E. coli (ATCC 35218). A similar result was reported earlier, methanol extracts of B. polystachiya showed good antibacterial activity against S. aureus (ATCC 2923). However, the mean inhibition zone of methanol extract of B. polystachiya against E. coli (ATCC 35218) was slightly lower than the result obtained methanol extract of B. polystachiya against E. coli (ATCC 2592) [31].
Interestingly, the mean inhibition zone of methanol crude extract of both plants was surprising that a higher inhibition zone was recorded against gram-negative bacteria S. typhimurium (ATCC 1333) but the rest of gram-negative bacteria showed a lower inhibition zone than gram-positive bacteria by the two plant extracts. This indicates that the susceptible nature of these bacterial species could be due to the differences in the resistance mechanisms to the bioactive compounds perceived in each crude solvent among the test bacteria. For example, P. aeruginosa and E. coli have the inherent ability to produce different resistance mechanisms like efflux pumps [32].
In the study, the ethanol and methanol leaf crude extracts of a mixture of V. auriculifera and B. polystachiya mean inhibition zone of the synergetic antibacterial effect against S.aureus (ATCC 43300) was significantly (p < 0.05) greater than the mean inhibition zone of the individual plant extract alone. Likewise, the methanol leaf crude extract of V. auriculifera and B. polystachiya mean inhibition zone against S. typhimurium (ATCC1333) was greater than the mean inhibition zone of the individual plant alone. However, the mean inhibition zone of ethanol extract of V. auriculifera against S. aureus (clinical isolate) was significantly (p < 0.05) greater than the mean inhibition zone of B. polystachiya and the synergism. Similarly, the mean inhibition zone of methanol extracts of V. auriculifera against E. coli (ATCC 35218) was also significantly greater than the mean inhibition zone of B. polystachiya and the synergism. The possible reason for the enhanced antibacterial activity of the crude extracts may be due to the synergetic or additive effect of the plant secondary metabolites.
The MIC and the MBC values of the crude extracts of V. auriculifera, B. polystachiya, and a mixture of them lie between (3.125%–12.5%) and (6.25%–25%) respectively. This result indicates that the most susceptible bacteria S. typhimurium (ATCC1333) was inhibited by the lowest concentration of the antibacterial agent. These MIC values were the least compared to the earlier report [31], and documented MBC values of 50% and above for many of the tested pathogenic bacterial organisms [33]. Despite there being no previous reports available for better comparison, V. auriculifera and B. polystachiya extracts and a mixture of them showed reasonably outstanding antibacterial activity to all tested pathogenic bacteria except E. coli (clinical isolate). This variation might be the nature of the resistance mechanism of the bacteria or the extract bioactive compounds against the test bacteria.
As a limitation, this study didn't isolate and identify the active compounds involved in the antibacterial activity of the plant's. For the current antibacterial activity, a small number of test microorganisms were used.
5. Conclusion
V. auriculifera and B. polystachiya contain potential antibacterial components that may be of great use for the development of pharmaceutical industries as a therapy against the disease especially caused by S. aureus and S. typhimurium. The ethanol and methanol extracts of V. auriculifera and B. polystachiya possess significant inhibitory effect against tested pathogens and the antibacterial activity of both plant leaf extracts were greater than the activity of currently used antibiotics (Gentamycin) against some selected organisms. Therefore, this study provides scientific support for the traditional use of the two plants in the treatment of bacterial infections.
CRediT author statement
FH: contributed to designing the study, manuscript preparation, and finalization, contributed to data analysis, and data interpretation, participated in the supervision of the study. NB: contributed to designing the study, manuscript preparation, and finalization, contributed to data analysis, and data interpretation, participated in the supervision of the study. MTG: contributed to data analysis, and data interpretation, participated in the supervision of the study. ZDK: contributed to data analysis, and data interpretation, participated in the supervision of the study. TAD: contributed to data analysis, and data interpretation, participated in the supervision of the study. All authors read and approved the final manuscript.
Ethical consideration
Ethical clearance was obtained from the Ethical Review Committee of Institute of biotechnology, University of Gondar, Ethiopia.
Consent for publication
Not applicable.
Availability of data and materials
All the necessary materials can be found in the text. Due to the privacy policy, the confidential data materials could only be obtained with the permission of the corresponding authors.
Funding
There was no funding source for this study.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgment
We would like to thank the University of Gondar, Institute of Biotechnology, Department of Medical Biotechnology for supporting chemicals, reagents, and materials.
References
- 1.Asressu K.H. 2013. Antimicrobial activity and phytochemical screening of crude extracts of medicinal plants grown in eastern Ethiopia. [Google Scholar]
- 2.Pavithra P., et al. Antibacterial activity of plants used in Indian herbal medicine. Int J Green Pharm. 2010;4(1) [Google Scholar]
- 3.Organization W.H. World Health Organization; 2019. WHO global report on traditional and complementary medicine 2019. [Google Scholar]
- 4.Mulder N.J., et al. Using biological networks to improve our understanding of infectious diseases. Comput Struct Biotechnol J. 2014;11(18):1–10. doi: 10.1016/j.csbj.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EL-Kamali H.H., EL-Amir M.Y. Antibacterial activity and phytochemical screening of ethanolic extracts obtained from selected Sudanese medicinal plants. Curr Res J Biol Sci. 2010;2(2):143–146. [Google Scholar]
- 6.Hussain H., et al. Chemical constituents and antimicrobial activity of Salix subserrata. Record Nat Prod. 2011;5(2):133. [Google Scholar]
- 7.Lalitha P., et al. Antimicrobial activity and phytochemical screening of an ornamental foliage plant, Pothos aurea (Linden ex Andre) Int J Chem. 2010;1(2):63–71. [Google Scholar]
- 8.Esimone C., et al. In vitro antimicrobial evaluation of lozenges containing extract of garlic and ginger. Int. J. Health Res. 2010;3(2):105–110. [Google Scholar]
- 9.Beentje H., Adamson J., Bhanderi D. National Museums of Kenya; 1994. Kenya trees, shrubs, and lianas. [Google Scholar]
- 10.Enyew A., Asfaw Z., Kelbessa E., Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Curr Res J Biol Sci. 2014 Jul 20;6(4):154–167. [Google Scholar]
- 11.Kiplimo J.J., Koorbanally N.A., Chenia H. Triterpenoids from Vernonia auriculifera Hiern exhibit antimicrobial activity. Afr. J. Pharm. Pharmacol. 2011;5(8):1150–1156. [Google Scholar]
- 12.Al Ati H.Y., Fawzy G.A., El Gamal A.A., Khalil A.T., El Din El Tahir K., Abdel-Kader M.S., Gilani A.H. Phytochemical and biological evaluation of Buddleja polystachya growing in Saudi Arabia. Pak J Pharm Sci. 2015 Jul 1;28(4):1533–1540. [PubMed] [Google Scholar]
- 13.Al Ati H.Y., Fawzy G.A., El Gamal A.A., Khalil A.T., El Din El Tahir K., Abdel-Kader M.S., Gilani A.H. Phytochemical and biological evaluation of Buddleja polystachya growing in Saudi Arabia. Pak J Pharm Sci. 2015 Jul 1;28(4):1533–1540. [PubMed] [Google Scholar]
- 14.El-Gamal A., et al. Chemical composition of buddleja polystachya aerial parts and its bioactivity against Aedes aegypti. Nat Prod Res. 2017:1. doi: 10.1080/14786419.2017.1378213. 1540. [DOI] [PubMed] [Google Scholar]
- 15.Tuasha N., Petros B., Asfaw Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J Ethnobiol Ethnomed. 2018;14(1):1–21. doi: 10.1186/s13002-018-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Getahun A., et al. In vivo evaluation of 80% methanolic leaves crude extract and solvent fractions of buddleja polystachya fresen (buddlejaceae) for wound healing activity in normal and diabetic mice. Metabol. Open. 2021;11 doi: 10.1016/j.metop.2021.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albejo B., et al. Phytochemical investigation and antimicrobial activity of leaves extract of Vernonia auriculifera Hiern. J. Pharm. Pharmacogn. Res. 2015;3(6):141–147. [Google Scholar]
- 18.Moges A., Moges Y. Plant science-structure, anatomy and physiology in plants cultured in vivo and in vitro. 2019. Ethiopian common medicinal plants: their parts and uses in traditional medicine-ecology and quality control; p. 21. [Google Scholar]
- 19.Authority E.M. Addis abeba: Ethiopian mapping authority. 2008. National atlas of Ethiopia. [Google Scholar]
- 20.Anas K, Jayasree PR, Vijayakumar T, Kumar PR. In vitro antibacterial activity of Psidium guajava Linn. Leaf extract on clinical isolates of multidrug resistant Staphylococcus aureus. [PubMed]
- 21.Kifle Z.D., Woldeyohanin A.E., Sema F.D., Debeb S.G., Kasahun A.E., Demeke C.A., Belayneh Y.M. In vivo hypoglycemic, antihyperglycemic and antidyslipidemic effects of the solvent fractions of Hagenia abyssinica leaves in mice. Metabol. Open. 2021 Dec 1;12 doi: 10.1016/j.metop.2021.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.B Belayneh Y.M., Amare G.G., Meharie B.G., Kifle Z.D. Evaluation of the antiulcerogenic activity of hydromethanol extracts of Solanum incanum L.(Solanaceae) leaves and roots in mice; single and repeated dose study. Metabol. Open. 2021 Sep 1;11 doi: 10.1016/j.metop.2021.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harborne A. springer science & business media; 1998. Phytochemical methods a guide to modern techniques of plant analysis. [Google Scholar]
- 24.Murray P., et al. Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteriav. Manual Clin. Microbiol. 2015;22(4):340–350. [Google Scholar]
- 25.Rahman K., et al. Antibacterial activity of important medicinal plants on human pathogenic bacteria. Int J Appl Agric Res. 2015;6(6):106–111. [Google Scholar]
- 26.Abew B., Sahile S., Moges F. In vitro antibacterial activity of leaf extracts of Zehneria scabra and Ricinus communis against Escherichia coli and methicillin resistance Staphylococcus aureus. Asian Pac J Trop Biomed. 2014;4(10):816–820. [Google Scholar]
- 27.Patel R.V., Thaker V.T., Patel V. Antimicrobial activity of ginger and honey on isolates of extracted carious teeth during orthodontic treatment. Asian Pac J Trop Biomed. 2011;1(1):S58–S61. [Google Scholar]
- 28.Atsbeha B., Mammo F., Kibret B. Phytochemical investigation on the leaves of Buddleja Polystachya (ethanol extract) Int J Integrat Sci Innov Technol. 2014;3 07-10. [Google Scholar]
- 29.Turkmen N., Sari F., Velioglu Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006;99(4):835–841. [Google Scholar]
- 30.Joyce J.K., Neil A.K., Hafizah C. Triterpenoids from Vernonia auriculifera Hiern exhibit antimicrobial activity. Afr. J. Pharm. Pharmacol. 2011;5(8):1150–1156. [Google Scholar]
- 31.Alemu F., Andualem B. Antimicrobial efficacy of different solvent crude extracts of antibiotics from Buddleja polystachya against standard and drug resistant bacteria and Candida albicans. World Appl Sci J. 2014;32:1621–1630. [Google Scholar]
- 32.Iyer R., Erwin A.L. Direct measurement of efflux in Pseudomonas aeruginosa using an environment-sensitive fluorescent dye. Res Microbiol. 2015;166(6):516–524. doi: 10.1016/j.resmic.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Kadaikunnan S., Rejiniemon T.S., Khaled J.M., Alharbi N.S., Mothana R. In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann Clin Microbiol Antimicrob. 2015 Dec;14(1):1. doi: 10.1186/s12941-015-0069-1. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the necessary materials can be found in the text. Due to the privacy policy, the confidential data materials could only be obtained with the permission of the corresponding authors.