Abstract
Introduction:
Surgical resection of renal cell carcinoma (RCC) with inferior vena cava (IVC) thrombus is a complex procedure with significant morbidity. Patient selection is critical to determining whether the benefits of the procedure outweigh the risks. In this study, we identified and stratified the risk factors that were associated with overall survival (OS) and recurrence-free survival (RFS) in patients undergoing surgical resection of RCC with IVC thrombus.
Methods:
We identified all patients with RCC with IVC tumor thrombus (stages cT3b and cT3c) who had undergone radical nephrectomy with tumor thrombectomy between December 1, 1993 and June 30, 2009. Kaplan-Meier method was used to estimate OS and RFS. Cox proportional hazards models were used to determine the association between risk factors and OS. Patients were stratified into 3 groups based on the number of risk factors present at diagnosis.
Results:
Two hundred twenty-four patients were included in the study. A total of 45.3% of patients had metastasis at presentation, 84.5% had cT3b, and 90.2% had clear cell RCC. cT3c, cN1, and cM1 were significantly associated with the risk of death. Group 1 patients (0 risk factors) had a median OS duration of 77.6 months (95% CI 50.5-90.4), group 2 (1 risk factor) 26.0 months (95% CI 19.5-35.2), and group 3 (≥2 risk factors) 8.9 months (95% CI 5.2-12.9; p<0.001).
Conclusions:
Stratification of patients with RCC and IVC thrombus by risk factors allowed us to predict survival duration. In patients with ≥2 risk factors, new treatment strategies with preoperative systemic therapy may improve survival.
Keywords: Renal cell carcinoma, Thrombectomy, Overall survival, Recurrence-free survival
Micro-abstract:
We assessed preoperative factors associated with overall survival in patients with renal cell carcinoma with IVC thrombus (cT2b, cT3c). We confirmed that cT3c, cN1, and cM1 were independently associated with poor OS. In patients with ≥2 risk factors, the median overall survival was 8.9 months. Stratification of patients before surgery allows us to determine whether the benefits of the procedure outweigh the risks.
INTRODUCTION
Renal cell carcinoma (RCC) with inferior vena cava (IVC) thrombus (American Joint Committee on Cancer stages cT3b and cT3c) represents 4% to 10% of all RCC cases1. Currently, the only curative treatment available is radical nephrectomy with IVC thrombectomy, a complex procedure that is associated with significant morbidity2. Because of the low incidence and complexity of the procedure, most surgeries require a multidisciplinary team and are performed at high-volume centers3-5.
Patient selection is critical to determining whether the benefits of radical nephrectomy with IVC thrombectomy outweigh the risks of the procedure. Multiple efforts have been made to identify the clinical and pathologic risk factors that are associated with recurrence and survival6-11. In this study, we identified and stratified the preoperative risk factors that were associated with recurrence and survival in patients with RCC with IVC tumor thrombus.
PATIENTS AND METHODS
After obtaining Institutional Review Board approval, we retrospectively reviewed The University of Texas MD Anderson Cancer Center (Houston, Texas) nephrectomy registry and identified 265 patients with RCC with IVC tumor thrombus (stages cT3b and cT3c) who had undergone radical nephrectomy with tumor thrombectomy between December 1, 1993 and June 30, 2009. 1
We collected patients’ demographic and clinical features: age, sex, race, performance status, and clinical stage. The pathologic features collected included histologic type, disease stage per the 2017 American Joint Committee on Cancer (8th edition) TNM standard (cT stage, cN stage, and cM stage), and the presence of sarcomatoid differentiation. Data were abstracted via a direct chart review by the authors. Patients with incomplete data were excluded.
Patient characteristics were summarized using the frequency (%) and median (interquartile range) for categorical and continuous variables. Categorical and continuous data were compared using the Fisher’s exact test and Kruskal-Wallis test, respectively. The overall survival (OS) duration was defined as the time between the date of surgery and death and was censored at the last follow-up date for patients who were still alive.
The Kaplan-Meier method was used to estimate OS and recurrence-free survival (RFS) durations from the date of radical nephrectomy and IVC thrombectomy. We compared time-to-event endpoints by subgroups using the log-rank test. X-tile software was used to determine a clinically relevant cut-point for age for OS12. A multivariate Cox proportional hazards model was then fitted for OS and RFS by including all statistically significant covariates from univariate Cox models. In addition, a landmark analysis of OS by recurrence location, starting on the recurrence day, was estimated using the Kaplan-Meier method. Patients with metastatic disease at presentation were excluded from the landmark and recurrence analyses.
The full multivariate model included all the variables from the univariate analyses with p<0.25 (Table 1). The variables with missing values ≥25%, such as operative time and performance status, were excluded from the full model; we also excluded variables with collinearity issues, such as cytoreductive nephrectomy. Backward elimination methods were used to identify the final reduced model. The backward elimination method fits the full model on all explanatory variables while identifying the least significant variable as “insignificant” (p>0.05); it removes this variable and re-estimates the model without it. These steps are repeated until the model only includes “significant” variables (p<0.05).
Table 1.
Characteristics of the study population (n=265)
| Characteristic | Result |
|---|---|
| Median age at surgery, years (IQR) | 60.3 (54.2-70.2) |
| Median hemoglobin, g/dl (IQR) | 11.90 (10.3-13.4) |
| Median corrected Ca, mg/dl (IQR) | 9.30 (9.1-9.8) |
| Median LDH (IU/L) (IQR) | 522.0 (422-693) |
| Median creatinine, mg/dl (IQR) | 1.30 (1.0-1.5) |
| Median length of stay, days (IQR) | 8.00 (6.0-13.0) |
| Sex, n (%) | |
| Male | 175 (66.0) |
| Female | 90 (34.0) |
| Race and ethnicity, n (%) | |
| White | 197 (74.3) |
| Hispanic | 55 (20.8) |
| Black | 10 (3.8) |
| Asian | 3 (1.1) |
| Clinical T stage, n (%) | |
| 3b | 224 (84.5) |
| 3c | 41 (15.5) |
| Clinical N1 | 46 (17.4) |
| Clinical M1 | 120 (45.3) |
| Pre-surgery embolization, n (%) | 50 (18.9) |
| Histologic type, n (%) | |
| Clear cell | 238 (90.2) |
| Non-clear cell | 27 (9.9) |
| Grade, n (%) | |
| 0-2 | 25 (9.5) |
| 3 | 118 (45.0) |
| 4 | 119 (45.4) |
| Sarcomatoid, n (%) | 29 (11.0) |
| Rhabdoid, n (%) | 13 (5.0) |
| Congestive heart failure, n (%) | 2 (0.8) |
| Cytoreductive nephrectomy, n (%) | 120 (45.3) |
| Side, n (%) | |
| Right | 80 (30.2) |
| Left | 184 (69.4) |
| Both | 1 (0.4) |
| Hypertension, n (%) | 135 (50.9) |
| Diabetes mellitus, n (%) | 51 (19.3) |
| Coronary artery disease, n (%) | 26 (9.8) |
| Peripheral vascular disease, n (%) | 2 (0.8) |
| Hematuria, n (%) | |
| Microhematuria | 13 (4.9) |
| Gross hematuria | 106 (40.2) |
| Flank pain, n (%) | 78 (29.6) |
| Weight loss >10 lbs, n (%) | 81 (30.7) |
| Malaise weakness fatigue, n (%) | 81 (30.7) |
| Bilateral leg edema, n (%) | 40 (15.2) |
| New-onset congestive heart failure, n (%) | 13 (4.9) |
Risk Score
OS and RFS risk scores were obtained by rounding each result to the nearest integer, for OS using the group with the lowest risk of dying, and for RFS using the group with lowest risk of recurrence as the reference. For OS, the presence of cT3c, cN1, and cM1 disease each equaled 1 point. cT3b, cN0, and cM0 equaled 0. For RFS, cN1, non-clear cell histologic type, and weight loss >10 lbs were each are worth 1 point, while cN0, clear cell histologic type, and weight loss <10 lbs were 0 points. Patients were stratified into 3 groups based on the number of risk factors: group 1, no risk factors; group 2, 1 risk factor; and group 3, ≥2 risk factors.
Nomogram
A nomogram was developed on the basis of the fitted Cox proportional hazards model for OS and assessed for calibration and discrimination. Calibration was assessed graphically using plots of the predicted probability of OS at 1 year versus the actual proportion of patients who had not died at 1 year.
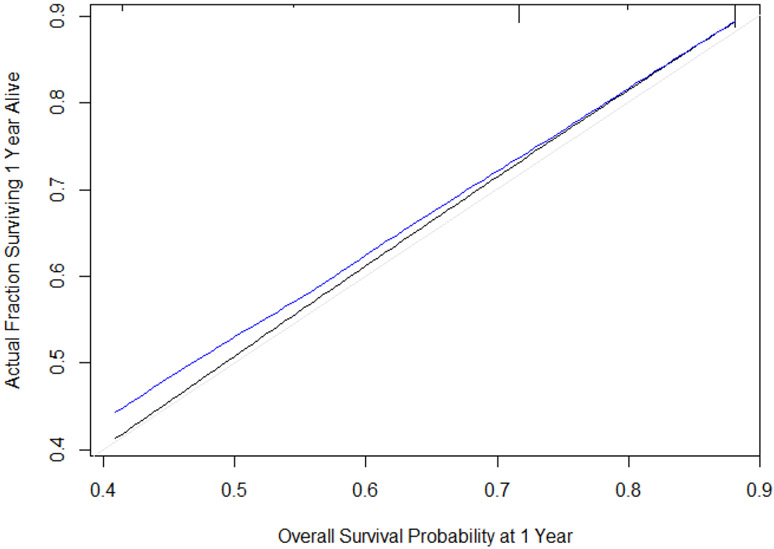
We assessed discrimination by calculating the area under the receiver operating characteristics curve for censored data. To adjust for the bias that is associated with evaluating a nomogram’s performance in the same group of patients used to build the nomogram, we repeated the calibration assessment for 500 bootstrapped samples. The bootstrapped samples’ results estimate the nomogram performance that could be expected in a separate but similar patient population. Patients were divided into 5 groups that contained a mean of 50 patients based on the predicted probability of OS. The closer the blue line is to the black line, the better the calibration.
A statistical analysis was performed using Stata/SE version 16.0 statistical software (Stata Corp. LP, College Station, TX) and R software.
RESULTS
Of the 265 patients who we identified with RCC with IVC tumor thrombus, 224 had complete data and were included in the study. Their median age was 60.3 years (IQR, 54.2-70.2 years); 66.0% were male; 84.5% had cT3b, 17.4% had cN1, and 45.3% had cM1; and 90.2% had clear cell RCC. Sarcomatoid features were present in 11.0% of patients (table 1). The median follow-up duration for all patients was 26.9 months (IQR, 10.1-64.7 months).
Overall Survival
At last follow-up, 223 patients had died, including 159 who had died of RCC. In the univariate analysis, hemoglobin, cT3b, cN1, cM1, and hypertension were all associated with OS (all p<0.05). In the multivariable analysis, cT3c (OR 1.83, 95% CI 1.21-2.76, p=0.004), cN1 (OR 2.65, 95% CI 1.83-3.84, p<0.001), and cM1 (OR 2.66, 1.96-3.61, p<0.001) were all independently associated with poor OS (Table 2).
Table 2.
Univariate and multivariable analysis for overall survival
| Univariate Analysis of OS |
Multivariable Analysis of OS | |||||
|---|---|---|---|---|---|---|
| Full Model | Reduced Model | |||||
| Characteristic | HR (95% CI for HR) |
p- value |
OR (95% Cl for OR) |
p- value |
OR (95% CI for OR) |
p- value |
| Age ≥50 (X-tile) | 1.42 (9.95-2.12) | 0.091 | ||||
| Age ≥60 (median) | 1.1 (0.85-2.12) | 0.469 | ||||
| Age ≥62 (mean) | 1.11 (0.85-1.44) | 0.44 | ||||
| Hgb | ||||||
| Below normal | 1 | . | ||||
| Normal | 0.69 (0.5-0.94) | 0.018 | 0.8 (0.58-1.11) | 0.175 | ||
| Corrected Ca++ | ||||||
| Normal | 1 | . | ||||
| Above normal | 1.13 (0.75-1.7) | 0.555 | ||||
| LDH | ||||||
| Normal | 1 | . | ||||
| Above normal | 1.31 (0.94-1.83) | 0.114 | ||||
| Sex (male vs. female) | 0.92 (0.7-1.22) | 0.572 | ||||
| Race | ||||||
| Non-Hispanic white | 1 | . | ||||
| Hispanic | 0.94 (0.68-1.32) | 0.737 | ||||
| Black | 1.28 (0.65-2.52) | 0.473 | ||||
| Asian | 2.55 (0.81-8.06) | 0.11 | ||||
| cT stage (T3c vs. T3b) | 1.59 (1.11-2.28) | 0.012 | 1.81 (1.18-2.79) | 0.007 | 1.83 (1.21-2.76) | 0.004 |
| cN1 stage | 3.08 (2.2-4.31) | <0.001 | 2.61 (1.79-3.8) | <0.001 | 2.65 (1.83-3.84) | <0.001 |
| cM1 stage | 2.51 (1.91-3.3) | <0.001 | 2.67 (1.96-3.65) | <0.001 | 2.66 (1.96-3.61) | <0.001 |
| Pre-surgical embolization Side | 0.9 (0.65-1.26) | 0.55 | ||||
| Left | 1 | . | ||||
| Right | 1.23 (0.92-1.63) | 0.164 | ||||
| Hypertension | 0.75 (0.58-0.98) | 0.033 | 0.98 (0.72-1.33) | |||
| Diabetes mellitus | 1.09 (0.79-1.51) | 0.599 | ||||
| Coronary artery disease | 1.26 (0.83-.193) | 0.28 | ||||
| Peripheral vascular disease | 1.16 (0.29-4.66) | 0.838 | ||||
| Microhematuria | 0.89 (0.48-1.64) | 0.701 | ||||
| Gross hematuria | 0.89 (0.68-1.17) | 0.405 | ||||
| Flank pain | 0.98 (0.73-1.31) | 0.868 | ||||
| Weight loss >10 lbs | 1.22 (0.92-1.63) | 0.169 | ||||
| Malaise weakness fatigue | 1.12 (0.84-1.5) | 0.427 | ||||
| New-onset congestive heart failure | 1.04 (0.56-1.9) | 0.911 | ||||
The risk score was calculated using the variables cT3c, cN1, and cM1. Patients in group 1 (0 risk factors) had a median OS duration of 77.6 months (95% CI 50.5-90.4), group 2 (1 risk factor) 26.0 months (95% CI 19.5-35.2), and group 3 (≥2 risk factors) 8.9 months (95% CI 5.2-12.9; p<0.001) (Figure 1).
Figure 1.
Kaplan-Meier curves of overall survival in patients with renal cell carcinoma and inferior vena cava thrombus, based on the preoperative score.
A nomogram was constructed using cT stage, cN stage, and cM stage to show the probability of OS at 1, 3, and 5 years (Figure 2). Figure 3 shows the plot of the predicted probability of OS at 1 year versus the actual proportion of patients who survived for 1 year without experiencing an event. The black line represents a nomogram with perfect calibration, or one in which the predicted probability is equal to the observed probability. The blue line represents the performance of our nomogram. The area under the receiver operating characteristics curve for this nomogram was 0.65.
Figure 2.

Nomogram of overall survival at 1 year, 3 years, and 5 years on the basis of the fitted Cox model.
Figure 3.
Calibration of the nomogram.
Disease Recurrence
Eighty-eight patients had recurrent disease. The 4 most common recurrence locations were the lungs (47), liver (19), bones (14), and renal fossa (10). In the univariate analysis, cN1, weight loss >10 lbs, non-clear cell histologic type, and sarcomatoid features were all associated with RFS (all p<0.05). In the multivariable analysis, cN1 (HR 2.63, 95% CI 1.51-4.6, p=0.01), weight loss >10 lbs (HR 1.76, 95% CI 1.18-2.65, p=0.006), and non-clear cell histologic type (HR 2.08, 95% CI 1.17-3.69, p=0.01) were all independently associated with poor RFS (Table 3).
Table 3.
Univariate and multivariable analysis of recurrence-free survival
| Univariate Analysis for RFS |
Multivariable Analysis for RFS |
|||
|---|---|---|---|---|
| Characteristic | HR (95% CI for HR) |
p- value |
HR (95% CI for HR) |
p- value |
| Age ≥50 (X-tile) | 1.33 (0.79-2.26) | 0.284 | ||
| Age ≥60 (median) | 0.91 (0.64-1.31) | 0.617 | ||
| Age ≥62 (mean) | 0.95 (0.64-1.36) | 0.78 | ||
| Hgb | ||||
| Below normal | 1 | . | ||
| Normal | 0.67 (0.44-1.02) | 0.065 | ||
| Corrected Ca++ | ||||
| Normal | 1 | . | ||
| Above normal | 1.25 (0.72-2.17) | 0.423 | ||
| LDH | ||||
| Normal | 1 | . | ||
| Above normal | 1.27 (0.8-2.02) | 0.318 | ||
| EGFR | ||||
| <45 | 1 | . | ||
| ≥45 | 0.76 (0.35-1.62) | 0.473 | ||
| Sex | ||||
| Male | 1 | . | ||
| Female | 0.92 (0.64-1.34) | 0.678 | ||
| Race | ||||
| White | 1 | . | ||
| Hispanic | 0.91 (0.59-1.42) | 0.686 | ||
| Black | 1 (0.43-2.33) | 0.997 | ||
| T stage | ||||
| 3b | 1 | . | ||
| 3c | 1.42 (0.87-2.3) | 0.157 | ||
| Clinical N1 | 2.67 (1.54-4.63) | <0.001 | 2.63 (1.51-4.6) | 0.01 |
| Pre-surgery embolize | 0.75 (0.46-1.2) | 0.226 | ||
| Side | ||||
| Left | 1 | . | ||
| Right | 1.06 (0.71-1.6) | 0.768 | ||
| Hypertension | 0.78 (0.54-1.12) | 0.172 | ||
| Diabetes mellitus | 0.77 (0.49-1.22) | 0.267 | ||
| Coronary artery disease | 1.27 (0.76-2.13) | 0.362 | ||
| Peripheral vascular disease | 2.67 (0.65-11) | 0.175 | ||
| Microhematuria | 1.14 (0.46-2.83) | 0.776 | ||
| Gross hematuria | 0.83 (0.56-1.21) | 0.328 | ||
| Flank pain | 0.92 (0.63-1.35) | 0.67 | ||
| Weight loss >10 lbs | 2.02 (1.36-2.99) | <0.001 | 1.76 (1.18-2.65) | 0.006 |
| Malaise, weakness, fatigue | 1.22 (0.83-1.8) | 0.3 | ||
| New-onset congestive heart failure | 1.75 | 0.134 | ||
| Non-clear cell histologic type | 2.29 (1.3-4.04) | 0.004 | 2.08 (1.17-3.69) | 0.01 |
| Grade | ||||
| 0-2 | 1 | . | ||
| 3 | 0.8 (0.44-1.46) | 0.468 | ||
| 4 | 1.42 (0.77-2.64) | 0.263 | ||
| Sarcomatoid | 2.43 (1.38-4.27) | 0.002 | 1.76 (0.98-3.15) | 0.054 |
| Rhabdoid | 1.43 (0.45-4.53) | 0.547 | ||
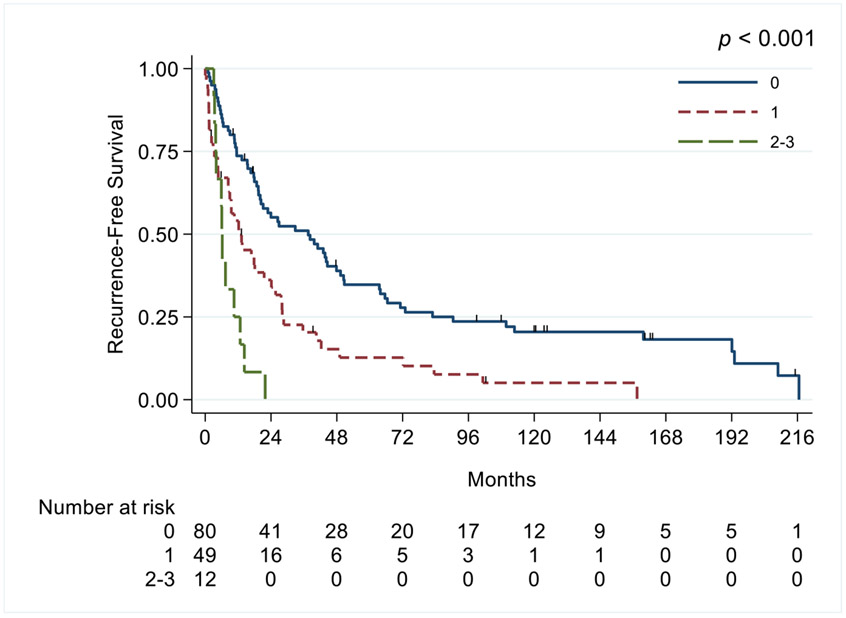
For the RFS risk score, the variables included were cN1, weight loss >10 lbs, and non-clear cell histologic type. Patients were stratified into 3 groups on the basis of their number of risk factors: group 1 (0 risk factors) had a median RFS duration of 37.5 months (95% CI 20-47.8 months), group 2 (1 risk factor) 12.2 months (95% CI 8.38-21.5), and group 3 (≥2 risk factors) 6 months (95% CI 3.41-12.7; p<0.001) (Figure 4).
Figure 4.
Kaplan-Meier curves of recurrence-free survival in patients with renal cell carcinoma and inferior vena cava thrombus, stratified by risk score.
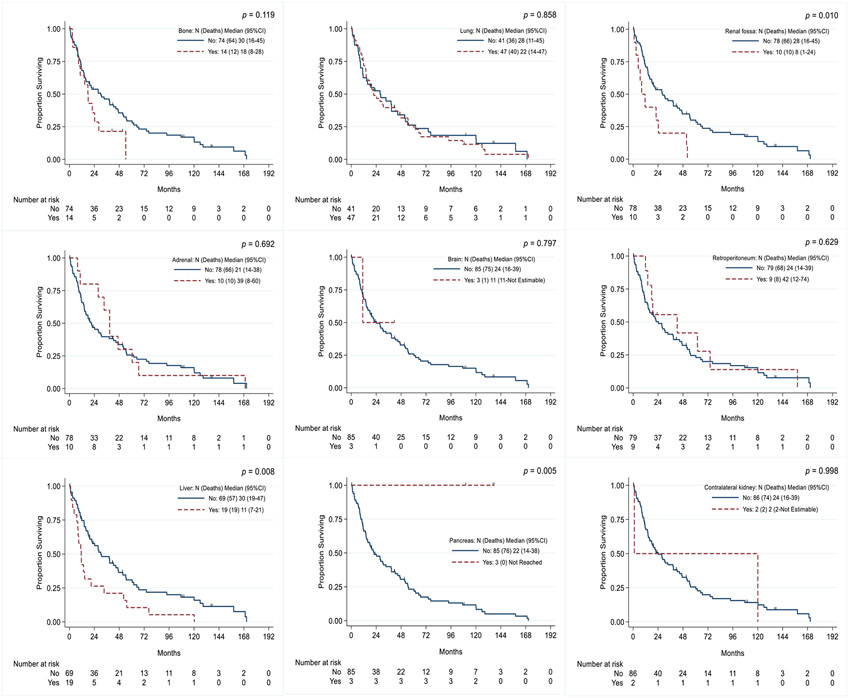
In the landmark analysis of OS by recurrence site, patients with disease recurrence in the renal fossa had a median OS duration of 8 months (95% CI 1-24 months) versus 28 months (95% CI 16-45 months) in those without recurrence in the renal fossa (p=0.01). Patients with liver metastasis had a median OS duration of 11 months (95% CI 7-21 months) versus 30 months (95% CI 19-47 months) in those without liver metastasis (p=0.008); pancreatic metastasis was also associated with a longer OS duration (p=0.005) (Figure 5).
Figure 5.
Kaplan-Meier curves of overall survival in patients with renal cell carcinoma and inferior vena cava thrombus, stratified by recurrence site.
DISCUSSION
Radical nephrectomy with tumor thrombectomy is a morbid procedure, with a 30-day mortality rate between 1.5% and 10%13. Patient selection is critical to determining whether the benefit of the procedure outweighs the risk. Our data indicate that preoperative risk factors can be used to stratify patients to predict survival and recurrence.
Multiple studies, including a meta-analysis, have identified thrombus level, tumor size, sarcomatoid differentiation, Fuhrman grade, tumor necrosis, perinephric fat invasion, cN1, and metastasis at presentation as independent predictors of OS14, 15. Our findings are consistent with those of these analyses. To improve patient selection and pre-surgical counseling, our model only included preoperative risk factors and excluded pathologic characteristics. In addition, as not all patients had metastatic disease at presentation, risk scores such as IMDC or MSKCC scores were not applicable.
Patient stratification allows surgeons to select patients objectively and mitigate potential bias. The survival model shows that the presence of ≥2 risk factors is associated with a median OS duration of 8.9 months, making the role of surgery in this group uncertain. Fortunately, in this cohort, patients with ≥2 risk factors only represent 16.5% of the study population, demonstrating that surgery results in a significant improvement in OS duration in the majority of patients with RCC and IVC tumor thrombus.
There is limited evidence regarding the benefit of neoadjuvant or preoperative systemic therapy in patients with RCC with IVC16, 17. In metastatic RCC, immunotherapy, alone or in combination with targeted molecular therapy, has led to significant improvements in OS duration18, 19. A prospective trial of adjuvant sorafenib or sunitinib in patients with non-metastatic RCC and IVC tumor thrombus showed no survival benefit16. In a small retrospective analysis of patients with metastatic RCC and IVC tumor thrombus, the combination of surgery and targeted therapy had a similar OS duration to that of targeted therapy alone17. Further prospective trials are needed to delineate the role of surgery in this group of patients with ≥2 risk factors.
The disease recurrence location differs between different histologic subtypes of RCC20. In patients with metastatic clear cell RCC, the most common metastatic sites are the lungs, lymph nodes, bones, and liver. Metastasis to the liver is consistently associated with poor survival. Metastasis to endocrine organs is less common but is associated with improved survival18-20. These findings are consistent with those in our cohort, in which liver metastasis was associated with poor survival, and pancreatic metastasis had indolent characteristics.
The presence of non-clear cell histologic type and cN1 is consistently associated with disease recurrence in patients with no evidence of metastasis at presentation21. Fifty percent of these patients have evidence of recurrence at 6 months and may benefit from clinical trials of new systemic treatments in the neoadjuvant or adjuvant setting. In addition, sarcomatoid histology has been consistently shown to be associated with worse RFS and OS22. In our study, sarcomatoid histology also emerged as a risk factor on multivariable analysis. Immune check point inhibitors (ICI) have been shown to have significant activity in patients with metastatic RCC and sarcomatoid features. A recent meta-analysis of four randomized trials showed that ICI-based systemic therapy combinations in patients with sarcomatoid RCC improved PFS, OS and objective response rate relative to targeted therapies22. In the subset of patients presenting with RCC with IVC tumor thrombus and sarcomatoid histology, the use of these agents in the neoadjuvant or adjuvant setting has the potential to improve outcomes.
The retrospective nature of our study limits our findings. Although we identified risk factors that were associated with a poor prognosis, other factors that are associated with poor prognosis in patients with metastatic RCC and IVC thrombus (systemic symptoms, hemoglobin, and a neutrophils to lymphocytes ratio >4) must be taken into consideration10. When developing our model, certain potentially prognostic clinical variables, such as performance status, had to be excluded if the data was missing in most patients.
It is possible that the outcomes of our study cannot be extrapolated to the community since MD Anderson is a high-volume center with significant experience with this type of intervention. We believe that surgeons who are experienced with this disease play a significant role in patients’ outcomes and that these patients should be referred to tertiary care centers.
Conclusions
In patients with RCC and IVC tumor thrombus, the preoperative risk factors cT3c, cN1, and cM1 were independent predictors of poor survival. Stratification of patients with RCC and IVC thrombus by risk factors allows us to predict survival duration. In patients with ≥2 risk factors, the benefit of upfront nephrectomy and IVC thrombectomy is uncertain. Our preoperative nomogram allows us to improve patient selection for surgery and to design new trials of preoperative systemic therapy to improve outcomes in these patients.
Clinical Practice Points:
Preoperative risk factors allowed us to predict survival in patients with renal cell carcinoma with inferior vena cava thrombus.
In patients with ≥2 risk factors, preoperative systemic therapy may improve survival.
After surgery, the recurrence location is associated with different overall survival, with some recurrences having an indolent behavior.
Funding:
Cancer Center Support Grant (NCI Grant P30 CA016672).
Abbreviations:
- RCC
Renal cell carcinoma
- IVC
Inferior vena cava
- OS
Overall survival
- RFS
Recurrence-free survival
- ICI
Immune Checkpoint inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
Alberto C. Pieretti: Writing - Original Draft, Project administration, Conceptualization, Methodology
Manuel Ozambela Jr: Writing - Review & Editing, Visualization
Mary E. Westerman: Investigation
Graciela M. Nogueras-Gonzalez: Formal analysis, Software
Luis A. Segarra: Data Curation
Niki M. Zacharias: Writing - Review & Editing
Ara Vaporciyan: Resources
Wayne Hofstetter: Resources
Tam Huynh: Resources
Saad Aldousari: Investigation
Surena F. Matin: Resources
Jose A. Karam: Supervision, Resources, Writing - Review & Editing
REFERENCES
- 1.Martinez-Salamanca JI, Linares E, Gonzalez J, et al. Lessons learned from the International Renal Cell Carcinoma-Venous Thrombus Consortium (IRCC-VTC). Current Urology Reports. 2014;15. [DOI] [PubMed] [Google Scholar]
- 2.Alsina AE, Wind D, Kumar A, et al. Outcomes in Renal Cell Carcinoma With IVC Thrombectomy: A Multiteam Analysis Between an NCI-Designated Cancer Center and a Quaternary Care Teaching Hospital. American Surgeon. 2020;86:1005–1009. [DOI] [PubMed] [Google Scholar]
- 3.Master VA, Ethun CG, Kooby DA, Staley CA 3rd, Maithel SK. The value of a cross-discipline team-based approach for resection of renal cell carcinoma with IVC tumor thrombus: A report of a large, contemporary, single-institution experience. Journal of Surgical Oncology. 2018;118:1219–1226. [DOI] [PubMed] [Google Scholar]
- 4.Lawindy SM, Kurian T, Kim T, et al. Important surgical considerations in the management of renal cell carcinoma (RCC) with inferior vena cava (IVC) tumour thrombus. BJU International. 2012;110:926–939. [DOI] [PubMed] [Google Scholar]
- 5.Gayed BA, Youssef R, Darwish O, et al. Multi-disciplinary surgical approach to the management of patients with renal cell carcinoma with venous tumor thrombus: 15 year experience and lessons learned. BMC Urology. 2016;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu L, Wang Z, Chen L, et al. A proposal of post-operative nomogram for overall survival in patients with renal cell carcinoma and venous tumor thrombus. Journal of Surgical Oncology. 2017;115:905–912. [DOI] [PubMed] [Google Scholar]
- 7.Abel EJ, Spiess PE, Margulis V, et al. Cytoreductive Nephrectomy for Renal Cell Carcinoma with Venous Tumor Thrombus. Journal of Urology. 2017;198:281–288. [DOI] [PubMed] [Google Scholar]
- 8.Abel EJ, Masterson TA, Karam JA, et al. Predictive Nomogram for Recurrence following Surgery for Nonmetastatic Renal Cell Cancer with Tumor Thrombus. Journal of Urology. 2017;198:810–816. [DOI] [PubMed] [Google Scholar]
- 9.Haddad AQ, Leibovich BC, Abel EJ, et al. Preoperative multivariable prognostic models for prediction of survival and major complications following surgical resection of renal cell carcinoma with suprahepatic caval tumor thrombus. Urologic Oncology. 2015;33:388.e381–389. [DOI] [PubMed] [Google Scholar]
- 10.Peyton CC, Abel EJ, Chipollini J, et al. The Value of Neutrophil to Lymphocyte Ratio in Patients Undergoing Cytoreductive Nephrectomy with Thrombectomy. European Urology Focus. 2020;6:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilki D, Hu B, Nguyen HG, et al. Impact of synchronous metastasis distribution on cancer specific survival in renal cell carcinoma after radical nephrectomy with tumor thrombectomy. Journal of Urology. 2015;193:436–442. [DOI] [PubMed] [Google Scholar]
- 12.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. [DOI] [PubMed] [Google Scholar]
- 13.Haidar GM, Hicks TD, El-Sayed HF, Davies MG. Treatment options and outcomes for caval thrombectomy and resection for renal cell carcinoma. J Vasc Surg Venous Lymphat Disord. 2017;5:430–436. [DOI] [PubMed] [Google Scholar]
- 14.Gu L, Li H, Wang Z, et al. A systematic review and meta-analysis of clinicopathologic factors linked to oncologic outcomes for renal cell carcinoma with tumor thrombus treated by radical nephrectomy with thrombectomy. Cancer Treatment Reviews. 2018;69:112–120. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Li S, Xu Z, et al. Clinical and oncological outcomes in Chinese patients with renal cell carcinoma and venous tumor thrombus extension: single-center experience. World Journal of Surgical Oncology. 2015;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu L, Li H, Chen L, et al. Postoperative Adjuvant Sorafenib or Sunitinib for Nonmetastatic Renal Cell Carcinoma with Venous Tumor Thrombus: a Prospective Cohort Study. Translational Oncology. 2017;10:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon T, Lee JL, You D, et al. Impact of surgery on the prognosis of metastatic renal cell carcinoma with IVC thrombus received TKI therapy. Journal of Surgical Oncology. 2014;110:145–150. [DOI] [PubMed] [Google Scholar]
- 18.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudani S, de Velasco G, Wells JC, et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association With Survival. JAMA Netw Open. 2021;4:e2021869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel EJ, Margulis V, Bauman TM, et al. Risk factors for recurrence after surgery in non-metastatic RCC with thrombus: a contemporary multicentre analysis. BJU International. 2016;117:E87–94. [DOI] [PubMed] [Google Scholar]
- 22.Iacovelli R, Ciccarese C, Bria E, et al. Patients with sarcomatoid renal cell carcinoma - redefining the first-line of treatment: A meta-analysis of randomised clinical trials with immune checkpoint inhibitors. Eur J Cancer. 2020. Sep;136:195–203. [DOI] [PubMed] [Google Scholar]