Abstract
Background
Right-sided colonic diverticulitis (RCD) and left-sided colonic diverticulitis (LCD) are considered distinct diseases. However, separate guidelines for RCD do not exist. Since the establishment of RCD management would first require evaluation of disease characteristics and recurrence patterns, this study has aimed to investigate the differences in the clinical characteristics between RCD and LCD and the recurrence patterns of RCD.
Methods
Patients admitted for colonic diverticulitis between January 2012 and August 2020 were retrospectively reviewed. Clinical characteristics and recurrence rates in RCD and LCD patients, and predictors for recurrence and the recurrence patterns of RCD were analyzed.
Results
In total, 446 colonic diverticulitis patients (343 RCD, 103 LCD) were included in this study. RCD patients were more likely to be male, younger, taller, heavier, smoke, drink alcohol, have better physical performance scores, lower modified Hinchey stages and better initial laboratory findings. LCD patients were more likely to receive invasive treatments, have longer fasting and hospital days, higher mortality and cumulative recurrence rates (20.5% vs. 30.4%, P = 0.007). Recurrences in most RCD patients were of similar disease severity and received the same treatments for initial attacks, with rates of recurrence increasing after each recurrence. Predictors of the recurrence of RCD were complicated diverticulitis (hazard ratio[HR] 2.512, 95% confidence interval[CI] 0.127–5.599, p = 0.024) and percutaneous drainage (HR 6.549, 95% CI 1.535–27.930, p = 0.011).
Conclusion
RCD is less severe and has a lower recurrence rate than LCD, suggesting that RCD should be treated conservatively. Patients with complicated diseases and those requiring percutaneous drainage are more likely to experience a disease recurrence, suggesting nonsurgical management may be insufficient.
Keywords: Conservative treatment, Percutaneous drainage, Modified Hinchey classification, Right-sided colonic diverticulitis, Recurrence
Abbreviations: CI, Confidence interval; CRP, C-reactive protein; HR, Hazard ratio; LCD, Left-sided colonic diverticulitis; RCD, Right-sided colonic diverticulitis; PCD, percutaneous drainage
Highlights
-
•
Right-sided colonic diverticulitis patients were more likely to be male, younger, taller, heavier, smoke and drink alcohol.
-
•
Right-sided colonic diverticulitis is less severe and has a lower recurrence rate than left-sided colonic diverticulitis.
-
•
Patients with complicated disease and those requiring percutaneous drainage are more likely to experience disease recurrence.
1. Introduction
The overall incidence of colonic diverticulitis is increasing worldwide [1,2]. This disease has been associated with a significant economic burden, including health-care resource utilization and costs [3,4]. Additionally, colonic diverticulitis recurs frequently, with the overall disease recurrence during a 10-year follow-up period being as high as 40% [5], and the burden of disease increasing with each recurrent attack. In Western countries, diverticulitis predominantly presents as left-sided colonic diverticulitis (LCD), whereas right-sided colonic diverticulitis (RCD) is highly prevalent in Asian populations [6]. In addition to differing in anatomic locations and geographic distributions, RCD and LCD differ in embryonic origins and clinical characteristics [7,8]. RCD is characterized by both true and false diverticula, while LCD is characterized almost entirely by false diverticula [9]. Taken together, these findings suggest that RCD and LCD are separate disease entities differing in etiology and pathology. These differences indicate a need for clinical practice guidelines to distinguish between the management of RCD and LCD.
Although the natural history and management of LCD have been well described [10], the disease characteristics and long-term outcomes of RCD have not been well defined. To date, guidelines for the optimal management of RCD have not been fully established. Existing guidelines for LCD have limited applicability to patients with RCD [11], suggesting the need for a more suitable management approach specific to RCD. Although surgery may improve the quality of life and relieve long-term problems caused by recurrent diverticulitis [12], colorectal surgery itself is associated with risks, thus highlighting the importance of knowing the relative efficacy of surgical management of diverticulitis [13]. Identifying the risk factors that predict recurrence of diverticulitis, choosing the correct therapeutic approach to minimize complications and determining the indications and timing of surgical management have therefore become increasingly important.
This study was designed to compare the clinical characteristics of patients with RCD to those with LCD, to evaluate RCD recurrence rates and patterns by severity and treatment, and to identify any predictors associated with the recurrence of RCD.
2. Patients and methods
2.1. Study population and data collection
Electronic medical records of patients admitted to Seoul National University Hospital and Seoul Metropolitan Government Seoul National University Boramae Medical Center for treatment of colonic diverticulosis between January 2012 and August 2020 were retrospectively reviewed. Patients treated in the outpatient clinic for diverticulosis were not included. A total of 777 patients diagnosed with colonic diverticulosis were admitted to these hospitals. Patients with colonic diverticular disease, such as diverticular bleeding without definite inflammation or incidentally found diverticulosis during work up and treatment for a different disease, were excluded. After exclusion of these patients, 446 patients with colonic diverticulitis were included in the study. The study protocol was approved by the Institutional Review Boards of Seoul National University Hospital (IRB 30-2020-231) and Seoul Metropolitan Government Seoul National University Boramae Medical Center (IRB 2105-110-1219), and the need for individual consent was waived by the IRB. Our work has been reported in line with the STROCSS2021 criteria [14]. This study was registered on ResearchRegistry.com (registration number: researchregistry8137).
2.2. Outcomes of interest and definitions
RCD was defined as diverticulitis from the cecum to the splenic flexure, whereas LCD was defined as diverticulitis from the descending colon to the sigmoid colon. Diverticulitis severity was assessed according to CT-based modified Hinchey classification [15], with stage 0 characterized by diverticulosis with swelling of the colon wall; stage 1a showing confined peridiverticular or pericolic fat stranding with colonic wall thickening; stage 1b showing the same image findings as stage 1a plus pericolic or mesocolic abscess; stage 2 shows the same image findings as stage 1a plus pelvic, distant intraabdominal or retroperitoneal abscess; stage 3 defined as free air with localized or generalized ascites and possible peritoneal wall thickening; and stage 4 referring to fecal spillage with open communication between the bowel lumen and the peritoneal cavity through a diverticular lesion [16]. Stages 0 and 1a were categorized as uncomplicated diverticulitis, whereas stages 1b, 2, 3, or 4 were categorized as complicated diverticulitis.
2.3. Statistical analysis
Continuous and categorical variables were compared using Student's t-tests and Chi-square tests, respectively. The cumulative incidence of recurrence was analyzed using the Kaplan-Meier method and compared using the log-rank test. Predictors of the recurrence of RCD were determined using Cox proportional regression analysis. Predictors with P-values <0.05 were included in a multivariable analysis. All statistical analyses were performed using SPSS 25.0 software for Windows (IBM, Armonk, New York, USA), with two tailed P values < 0.05 considered statistically significant.
3. Results
3.1. Patient and disease characteristics
The demographic and clinical characteristics of the patients are summarized in Table 1. The mean follow-up period of all the patients was 26.6 ± 30.0 months, with no significant difference between the RCD and LCD groups. The male-to-female ratio was higher in the RCD (205:138) than in the LCD (49:54) group. RCD patients were also significantly younger (43.5 ± 16.2 years vs. 65.1 ± 15.0 years, p < 0.001), taller (165.2 ± 10.8 cm vs. 160.7 ± 9.9 cm, p < 0.001), and heavier (66.7 ± 14.9 kg vs. 62.5 ± 12.8 kg, p < 0.009) than LCD patients. Moreover, higher percentages of RCD patients smoked (p < 0.001) and drank alcohol (p < 0.001). LCD patients had a longer prehospital duration of symptoms (p < 0.001) and presented with more diverse chief complaints aside from abdominal pain (p < 0.001). Laboratory findings showed that more RCD patients had hemoglobin levels of >12 g/dL (86.0% vs. 67.0, p < 0.001), creatinine levels of ≤1.2 mg/dL (93.0% vs. 78.6%, p < 0.001) concentrations, and albumin level >3.4 g/dL (93.0% vs. 67.0%). However, the percentage of patients with white blood cell count of >4 103/μL and ≤11 mg/dL (45.2 vs.43.7, p = 0.788) and C-reactive protein (CRP) levels of >1 mg/dL (82.8 vs. 81.6, p = 0.770) were similar between RCD and LCD patients. Disease severity was also higher in the LCD group (p < 0.001). Whereas 54.3% of the LCD patients had complicated disease (stages 1b and above), 84.3% of the RCD patients had uncomplicated disease (stages 0 and 1) (Table 1).
Table 1.
Demographic and clinical characteristics of patients with right-sided and left-sided diverticulitis.
| Variables | Total (n = 446) | Right-sided (n = 343) | Left-sided (n = 103) | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 254 (57.0) | 205 (59.8) | 49 (47.6) | 0.028 |
| Female | 192 (43.0) | 138 (40.2) | 54 (52.4) | |
| Age (years) | 48.5 g ± 18.3 | 43.5 ± 16.2 | 65.1 ± 15.0 | <0.001 |
| Height (cm) | 164.1 ± 10.7 | 165.2 ± 10.8 | 160.7 ± 9.9 | <0.001 |
| Weight (kg) | 65.8 ± 14.5 | 66.7 ± 14.9 | 62.5 ± 12.8 | 0.009 |
| Body mass index (kg/m2) | 25.3 ± 26.0 | 25.7 ± 29.6 | 24.1 ± 3.9 | 0.586 |
| Smoking | 149 (33.4) | 130 (37.9) | 19 (18.4) | <0.001 |
| Alcohol | 194 (43.5) | 177 (51.6) | 17 (16.5) | <0.001 |
| ASA physical status scores | ||||
| 1 | 274 (61.4) | 241 (70.3) | 33 (32.0) | <0.001 |
| 2 | 145 (32.5) | 89 (25.9) | 56 (54.4) | |
| 3 | 27 (6.1) | 13 (3.8) | 14 (13.6) | |
| Prehospital duration of symptoms (days) | 5.2 ± 13.4 | 4.4 ± 13.2 | 8.2 ± 13.8 | 0.013 |
| Chief complaint | ||||
| Abdominal pain | 424 (95.1) | 334 (97.4) | 90 (87.4) | <0.001 |
| Fever | 11 (2.5) | 3 (0.9) | 8 (7.8) | |
| Change in bowel habits | 5 (1.1) | 3 (0.9) | 2 (1.9) | |
| Others | 6 (1.3) | 3 (0.9) | 3 (2.9) | |
| Body temperature (oC) | 36.9 ± 0.6 | 36.9 ± 0.6 | 36.9 ± 0.7 | 0.927 |
| Laboratory findings | ||||
| Hemoglobin (g/dL) | ||||
| ≤12 | 82 (18.4) | 48 (14.0) | 34 (33.0) | <0.001 |
| >12 | 364 (81.6) | 295 (86.0) | 69 (67.0) | |
| White blood cell count (103/μL) | ||||
| >4 and ≤11 | 200 (44.8) | 155 (45.2) | 45 (43.7) | 0.788 |
| ≤4 or > 11 | 246 (55.2) | 188 (54.8) | 58 (56.3) | |
| C-reactive protein (mg/dL) | ||||
| ≤1 | 78 (17.5) | 59 (17.2) | 19 (18.4) | 0.770 |
| >1 | 368 (82.5) | 284 (82.8) | 84 (81.6) | |
| Creatinine (mg/dL) | ||||
| ≤1.2 | 400 (89.7) | 319 (93.0) | 81 (78.6) | <0.001 |
| >1.2 | 46 (10.3) | 24 (7.0) | 22 (21.4) | |
| Albumin (g/dL) | ||||
| ≤3.4 | 58 (13.0) | 24 (7.0) | 34 (33.0) | <0.001 |
| >3.4 | 388 (87.0) | 319 (93.0) | 69 (67.0) | |
| Modified Hinchey stage | ||||
| Uncomplicated (Stages 0-1a) | ||||
| Stage 0 | 45 (10.1) | 39 (11.4) | 6 (5.8) | |
| Stage Ia | 291 (65.2) | 250 (72.9) | 41 (39.8) | |
| Complicated (Stages 1b and above) | ||||
| Stage Ib | 74 (16.6) | 50 (14.6) | 24 (23.3) | <0.001 |
| Stage II | 19 (4.3) | 3 (0.9) | 16 (15.5) | |
| Stage III | 15 (3.4) | 1 (0.3) | 14 (13.6) | |
| Stage IV | 2 (0.4) | 0 (0.0) | 2 (1.9) | |
| Follow-up period (months) | 26.6 ± 30.0 | 26.6 ± 30.4 | 26.9 ± 28.7 | 0.924 |
ASA; American Society of Anesthesiologists.
3.2. Types of treatment
The types of treatment differed in the RCD and LCD groups (Table 2). Higher percentages of LCD than of RCD patients underwent percutaneous drainage (PCD) interventions (6.8% vs. 1.7%) and surgical treatments (32.1% and 5.2%) than RCD patients (p < 0.001). Emergency operations were performed on 0.9% (3/18) RCD and 21.4% (22/33) of LCD patients. None of the RCD patients required stoma formation, compared with 19 (57.6%) LCD patients. An antibiotic combination of ceftriaxone and metronidazole was mainly used in both groups, but the proportion of patients treated with broad-spectrum antibiotics, such as piperacillin-tazobactam, vancomycin, and meropenem, was higher in the LCD group (p < 0.001).
Table 2.
Treatments administered to patients with right-sided and left-sided diverticulitis.
| Variables | Total (n = 446) | Right-sided (n = 343) | Left-sided (n = 103) | p-value |
|---|---|---|---|---|
| Treatment | ||||
| Antibiotics | 382 (85.7) | 319 (93.0) | 63 (61.2) | <0.001 |
| Percutaneous drainage | 13 (2.9) | 6 (1.7) | 7 (6.8) | |
| Emergency operation | 25 (5.6) | 3 (0.9) | 22 (21.4) | |
| Elective operation | 26 (5.8) | 15 (4.4) | 11 (10.7) | |
| Antibiotics | ||||
| Cephalosporins | 33 (7.4) | 25 (7.3) | 8 (7.8) | <0.001 |
| Cephalosporins and metronidazole | 347 (77.8) | 277 (80.8) | 70 (68.0) | |
| Quinolone | 22 (4.9) | 16 (4.7) | 6 (5.8) | |
| Quinolone and metronidazole | 18 (4.0) | 15 (4.4) | 3 (2.9) | |
| Broad-spectrum antibiotics | 26 (5.8) | 10 (2.9) | 16 (15.5) | |
| Operation | n = 51 (11.4) | n = 18 (5.2) | n = 33 (32.0) | |
| Resection and anastomosis | 32 (62.7) | 18 (100.0) | 14 (42.4) | <0.001 |
| Resection with stoma formation | 19 (37.3) | 0 (0.0) | 19 (57.6) | |
| NPO duration (days) | 4.0 ± 4.1 | 3.3 ± 2.8 | 6.4 ± 6.3 | <0.001 |
| Hospital days | 7.4 ± 8.1 | 5.7 ± 4.8 | 13.1 ± 12.8 | <0.001 |
| Mortality during admission period | 2 (0.4) | 0 (0.0) | 2 (1.9) | 0.010 |
| Recurrence | 46 (10.3) | 33 (9.6) | 13 (12.6) | 0.380 |
| Colonoscopy within 6 months | 99 (22.2) | 87 (25.4) | 12 (11.7) | 0.003 |
| Colorectal cancer incidence after event | 4 (0.9) | 2 (0.6) | 2 (1.9) | 0.200 |
NPO; nil per os.
The mean NPO duration (3.3 days vs. 6.4 days, p < 0.001) and hospital stay (5.7 days vs. 13.1 days, p < 0.001) were both significantly shorter in the RCD than in the LCD group. Two LCD patients (1.9%) died in hospital, compared with none of the RCD patients. Two patients in each group were diagnosed with colorectal cancer during the follow-up period (Table 2).
3.3. RCD recurrences
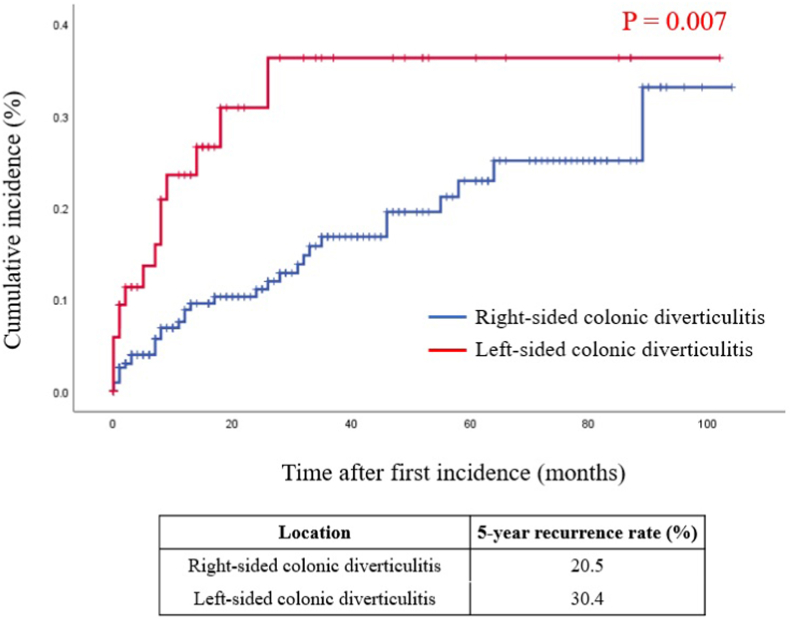
The cumulative incidence of recurrence was significantly higher in the LCD than in the RCD group, with 5-year recurrence rates of 30.4% and 20.5%, respectively (p = 0.007) (Fig. 1).
Fig. 1.
Kaplan-Meier analysis of the cumulative incidence of recurrence in patients with right-sided and left-sided diverticulitis.
The cumulative recurrence rate of left-sided diverticulitis was significantly higher than that of right-sided diverticulitis (39.4% vs. 20.5%, p = 0.007 by log-rank test).
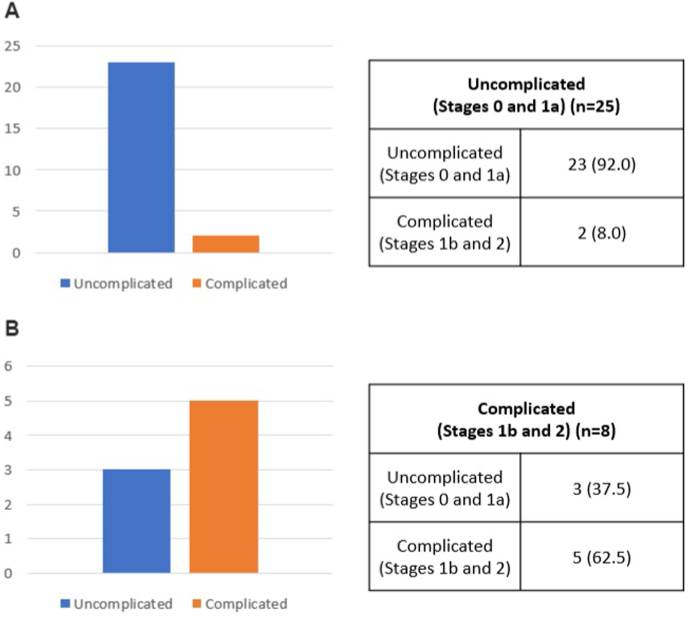
Most recurrences were of the similar or milder severity to the initial episode. For example, of the 25 patients with initially uncomplicated RCD, 23 (92.0%) had recurrences of uncomplicated disease, whereas two (8.0%) had recurrences of complicated disease. Of the 8 patients with initially complicated RCD, 3 (37.5%) had recurrences of uncomplicated disease, whereas 5 (62.5%) had recurrences of complicated disease (Fig. 2).
Fig. 2.
Recurrence patterns of right-sided colonic diverticulitis as a function of modified Hinchey stage.
(a) Of the 25 patients with initially uncomplicated (modified Hinchey stage 0 and 1a) right-sided diverticulitis, 23 (92%) experienced recurrence as uncomplicated disease, whereas two (8%) experienced recurrence as complicated disease.
(b) Of the eight patients with initially complicated (modified Hinchey stage 1b and 2) right-sided diverticulitis, three (37.5%) experienced recurrence as uncomplicated disease and five (62.5%) experienced recurrence as complicated disease.
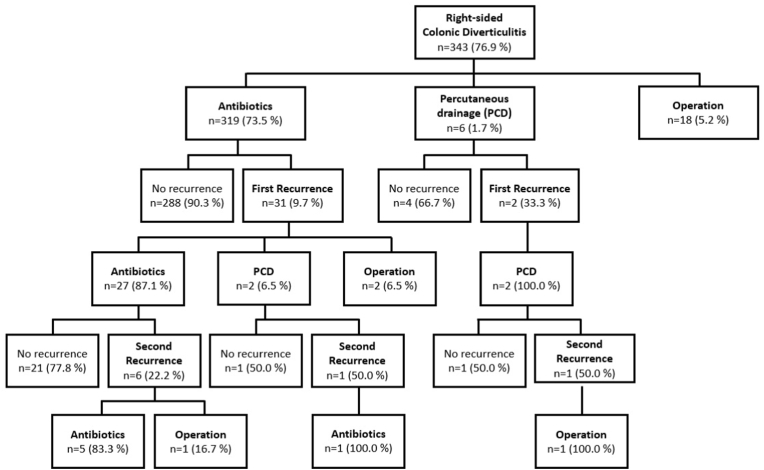
Fig. 3 shows a flow diagram of the recurrence pattern of RCD by treatment. RCD recurred in only 9.7% of patients initially receiving conservative treatment alone, compared with 33.3% of patients initially treated with PCD interventions. All patients with recurrences who were treated initially with PCD interventions underwent PCD interventions again on first recurrence. The recurrence rate increased after each recurrence in both groups, with second recurrences observed in 22.2% of patients treated with antibiotics and 50% of patients treated with PCD interventions. Up to 50.0% of patients treated with PCD interventions experienced recurrences, regardless of previous treatment history. Three of the 319 (0.9%) patients treated with antibiotics and one of the six (16.7%) patients treated with PCD interventions eventually underwent surgery on either the first or second recurrence (Fig. 3).
Fig. 3.
Recurrence patterns of right-sided colonic diverticulitis by treatment.
Recurrence rates increased after each recurrence, regardless of treatment. Recurrences occurred in 33.3–50.0% of patients after treatment with PCD interventions. In most cases, patients who experienced recurrences received the same treatment as at the initial episode.
Univariate analysis showed that variables associated with a high recurrence rate were modified Hinchey classification and PCD intervention. Complicated disease (HR = 2.51, 95% CI: 0.13–5.60, p < 0.024) and PCD interventions (HR = 6.55, 95% CI: 1.54–27.93, p < 0.011) were associated with a high risk of diverticulitis recurrence. Multivariate analysis, however, showed that both complicated disease (HR = 2.07, 95% CI: 0.85–5.06, p = 0.111) and PCD interventions (HR = 3.56, 95% CI: 0.71–17.83, p = 0.123) were not significant independent predictors of recurrence rate (Table 3).
Table 3.
Predictors of recurrence of right-sided colonic diverticulitis.
| Variable | Patients (n) | Univariate analysis |
||
|---|---|---|---|---|
| HR | 95% CI | p-value | ||
| Sex | ||||
| male/female | 192/133 | 1.030 | 0.516–2.056 | 0.933 |
| Age (yr) | ||||
| ≤60/> 60 | 273/52 | 0.982 | 0.405–2.381 | 0.968 |
| Body mass index (kg/m2) | ||||
| ≤25/> 25 | 208/116 | 0.587 | 0.273–1.265 | 0.174 |
| Smoking | ||||
| no/yes | 206/119 | 1.435 | 0.719–2.864 | 0.306 |
| Alcohol | ||||
| no/yes | 158/167 | 1.308 | 0.658–2.598 | 0.443 |
| ASA physical status scores | ||||
| 1 | 230 | 0.575 | 0.247–1.338 | 0.199 |
| 2 | 83 | 1.046 | 0.245–4.476 | 0.951 |
| 3 | 12 | |||
| Prehospital duration of symptoms (days) | ||||
| ≤2/>2 | 208/117 | 0.962 | 0.473–1.956 | 0.914 |
| Previous attack history | ||||
| no/yes | 291/34 | 0.575 | 0.175–1.892 | 0.362 |
| Modified Hinchey stage | ||||
| 0, 1a/1b, 2 | 278/47 | 2.512 | 0.127–5.599 | 0.024 |
| Treatment | ||||
| Antibiotics/PCD | 319/6 | 6.549 | 1.535–27.930 | 0.011 |
| Hemoglobin (g/dL) | ||||
| ≤12/>12 | 43/282 | 1.822 | 0.554–5.987 | 0.323 |
| White blood cell count (1000/μL) | ||||
| ≤11/> 11 | 145/180 | 1.315 | 0.658–2.628 | 0.438 |
| C-reactive protein (mg/dL) | ||||
| ≤3/> 3 | 97/226 | 0.700 | 0.344–1.425 | 0.326 |
| Creatinine (mg/dL) | ||||
| ≤1.2/> 1.2 | 302/22 | 0.327 | 0.045–2.392 | 0.271 |
| Albumin (g/dL) | ||||
| ≤3.4/> 3.4 | 19/305 | 0.689 | 0.206–2.306 | 0.545 |
| Aspirin | ||||
| no/yes | 305/20 | 1.970 | 0.692–5.610 | 0.204 |
| NSAIDs | ||||
| no/yes | 308/17 | 1.165 | 0.279–4.872 | 0.834 |
| Opioids | ||||
| no/yes | 317/8 | 2.613 | 0.624–10.954 | 0.189 |
| Corticosteroids | ||||
| no/yes | 318/7 | 1.574 | 0.365–6.784 | 0.543 |
| Statin | ||||
| no/yes | 284/41 | 0.969 | 0.398–2.360 | 0.945 |
ASA; American Society of Anesthesiologists, HR; hazard ratio, CI; confidence interval, PCD; percutaneous drainage, NSAIDs, non-steroidal anti-inflammatory drugs.
4. Discussion
Patients with RCD were more likely to be younger and male, with less severe disease and a lower recurrence rate. In contrast, patients with LCD had more severe disease with worse laboratory findings, such as higher CRP and creatinine concentrations and lower hemoglobin and albumin concentrations on presentation. Due to the evident and significant differences in patient and disease characteristics between RCD and LCD, the two groups were managed differently, with RCD managed more conservatively and LCD more frequently managed with invasive procedures. Those who underwent PCD interventions had a high percentage of recurrence and were more likely to undergo surgery upon recurrence.
Conservative treatment, including hospital admission, restriction of oral intake, IV fluids, and antibiotics, has been shown to be adequate for patients with uncomplicated diverticulitis both in the right and left colon [[17], [18], [19], [20], [21]]. The present study found that 93% of patients with RCD, regardless of disease severity, were treated with antibiotics alone. The disease recurred in only 9.7% of patients treated conservatively, with 87.1% of patients who experienced recurrence again treated conservatively. These findings indicate that conservative treatment alone, without invasive procedures, may be sufficient for most patients with RCD. Several recent studies have even questioned the need for antibiotics in patients with uncomplicated diverticulitis, reporting the non-inferiority in outcomes of those who were observed without antibiotics compared with patients who received antibiotics [[22], [23], [24]]. The patients included in this present study were treated between 2012 and 2020, during which antibiotics were routinely used for diverticulitis, regardless of severity. Moreover, our cohort consisted of only admitted patients, who are more likely to have more severe symptoms compared to those managed in the outpatient setting, and so antibiotics were considered necessary upon admission. However, although antibiotics were administered to all admitted patients at first, they were discontinued if the patients showed improved symptoms and laboratory findings after the they were clinically re-evaluated during their admission period. Nevertheless, because more and more recent evidences point to antibiotic avoidance for uncomplicated diverticulitis, antibiotics may not be necessary for uncomplicated disease.
Surgical management is required for certain cases of diverticulitis complicated by abscess formation, perforation, intestinal obstruction, or fistula formation [25]. Similarly, all of the RCD patients in the present study who initially presented with diverticulitis of modified Hinchey stages 3 and above underwent surgery in response to their first attack. Although the decision to perform urgent surgery in patients with severe or complicated diverticulitis may not be difficult, the decision to recommend elective surgery to initially unoperated patients after recovery from acute diverticulitis remains unclear [26]. Reaching consensus on the need for delayed elective surgery and the optimal timing of surgery for the prevention or management of recurrent diverticulitis is important, especially because of the high rate of recurrence and the economic burden that follows each attack [27]. Historically, surgical treatment was recommended after two attacks of uncomplicated diverticulitis or after one attack in patients aged under 40 years [28]. More recently, treatment has been individualized based on each patient's immune status, development of chronic pain, frequency of attacks, and complications [13]. In the present study, the rate of recurrence was significantly lower in patients with RCD than with LCD. Because both the recurrence rate and disease severity are lower in RCD patients, these patients should preferably be treated conservatively, thereby avoiding unnecessary aggressive treatment. Additionally, recurrences in most patients with initially complicated disease were also complicated, with patients who initially received PCD interventions undergoing PCD interventions upon recurrence. Therefore, while RCD patients may not require surgical treatment initially, because the risk of a new recurrence increases after each recurrence [29,30], those with higher modified Hinchey stage disease requiring PCD interventions should be considered for delayed elective surgery.
Risk factors for the recurrence of RCD reported in previous studies have included the presence of multiple diverticula, intraperitoneal diverticulitis, smoking, longer symptom duration before hospitalization, longer hospitalization period, and age under 40 years [8,[31], [32], [33], [34]]. The present study found that complicated RCD and treatment with PCD intervention were predictors of recurrence of RCD in univariate analysis, which has not been noted in previous studies. The lack of consensus on risk factors and predictors of RCD recurrence indicates the need for additional studies of larger populations.
Recently, more studies on the differences in the clinical outcomes of RCD and LCD are being reported. Previous studies have reported similar results on the differences in the clinical characteristics of RCD and LCD, showing that RCD affects younger male patients with a less severe disease [8,[35], [36], [37]]. However, as mentioned earlier, the risk factors for RCD recurrences are reported differently from study to study [8,[31], [32], [33], [34]]. In our study, we showed more severe disease, defined as disease with higher Hinchey stages and those that require PCD interventions, were predictors of RCD recurrences. Furthermore, whereas previous studies have analyzed the recurrence rates of RCD, our study provides a detailed flow diagram and description on the pattern of recurrence of RCD. We showed that all patients with recurrences who were treated initially with PCD interventions underwent PCD interventions again on first recurrence, and that the recurrence rate increased after each recurrence in both patients treated with antibiotics and PCD interventions. This study included a large patient cohort from two large centers in a country with one of the highest RCD incidence rates in the world. The results of this study may be a foundation for future studies and assist in designing guidelines for the management of RCD.
This study had several limitations. First, because of the study's retrospective design, the follow-up period ranged widely and was not uniform. Also, we were not able to evaluate recurrences in patients who did not return to the hospital. Second, only patients admitted to hospital during the study period were included, indicating that patients with less severe diseases who were not hospitalized were not included in this study. Furthermore, despite the inclusion of only patients with more severe symptoms on their first attack, most of the patients could be managed with conservative treatment alone. Finally, because only 33 patients experienced RCD recurrence during the follow-up period, the recurrence patterns reported were descriptive. Additional prospective studies and clinical trials with longer follow-up periods are warranted to fully determine the patterns of recurrence and to establish the optimal treatment for RCD.
5. Conclusions
Differences between RCD and LCD in patient and disease characteristics indicate that these two conditions should be treated as separate disease entities, with different guidelines for optimal management. Because the severity of RCD is lower at initial presentation and because RCD can be effectively managed with antibiotics alone, with a lower cumulative recurrence rate, patients with RCD should initially receive conservative treatment. However, because patients with complicated RCD requiring PCD interventions have a higher recurrence along with more severe disease, nonsurgical management may not be sufficient.
Ethical approval
The study protocol was approved by the Institutional Review Boards of Seoul National University Hospital (IRB 30-2020-231) and Seoul Metropolitan Government Seoul National University Boramae Medical Center (IRB 2105-110-1219), and the need for individual consent was waived by the IRB.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Rumi Shin were involved in the conceptualization and supervision of the study. Moon Young Oh was involved in the data collection, data analysis, and drafting of the manuscript. All authors provided critical feedback and approved the final version of the manuscript.
Consent
The need for individual consent was waived by the IRB.
Registration of research studies
The study protocol was approved by the Institutional Review Boards of Seoul National University Hospital (IRB 30-2020-231) and Seoul Metropolitan Government Seoul National University Boramae Medical Center (IRB 2105-110-1219).
Guarantor
Dr. Rumi Shin, Department of Surgery. Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, 07061, Korea E-mail: roomie79@gmail.com.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank all our personnel who assisted in serving our patients.
References
- 1.Masoomi H., Buchberg B.S., Magno C., Mills S.D., Stamos M.J. Trends in diverticulitis management in the United States from 2002 to 2007. Arch. Surg. 2011;146:400–406. doi: 10.1001/archsurg.2010.276. [DOI] [PubMed] [Google Scholar]
- 2.Mizuki A., Tatemichi M., Nakazawa A., Tsukada N., Nagata H., Kanai T. Changes in the clinical features and long-term outcomes of colonic diverticulitis in Japanese patients. Intern. Med. 2017;56:2971–2977. doi: 10.2169/internalmedicine.7710-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy V.B., Longo W.E. The burden of diverticular disease on patients and healthcare systems. Gastroenterol. Hepatol. 2013;9:21–27. [PMC free article] [PubMed] [Google Scholar]
- 4.Sandler R.S., Everhart J.E., Donowitz M., Adams E., Cronin K., Goodman C., Gemmen E., Shah S., Avdic A., Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 5.Lahat A., Avidan B., Sakhnini E., Katz L., Fidder H.H., Meir S.B. Acute diverticulitis: a decade of prospective follow-up. J. Clin. Gastroenterol. 2013;47:415–419. doi: 10.1097/MCG.0b013e3182694dbb. [DOI] [PubMed] [Google Scholar]
- 6.Law W.L., Lo C.Y., Chu K.W. Emergency surgery for colonic diverticulitis: differences between right-sided and left-sided lesions. Int. J. Colorectal Dis. 2001;16:280–284. doi: 10.1007/s003840100339. [DOI] [PubMed] [Google Scholar]
- 7.Hajibandeh S., Smart N.J., Maw A. Meta-analysis of the demographic and prognostic significance of right-sided versus left-sided acute diverticulitis. Colorectal Dis. 2020;22:1908–1923. doi: 10.1111/codi.15328. [DOI] [PubMed] [Google Scholar]
- 8.Lee K.Y., Lee J., Park Y.Y., Kim Y., Oh S.T. Difference in clinical features between right- and left-sided acute colonic diverticulitis. Sci. Rep. 2020;10:3754. doi: 10.1038/s41598-020-60397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore T., Jordan C., Edelstein E. Right-sided diverticulitis mimics appendicitis. J. Emerg. Med. 2013;44:29–32. doi: 10.1016/j.jemermed.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Feingold D., Steele S., Lee S., Kaiser A., Boushey R., Buie W.D., Rafferty J.F. Practice parameters for the treatment of sigmoid diverticulitis. Dis. Colon Rectum. 2014;57:284–294. doi: 10.1097/DCR.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 11.Strate L.L., Morris A.M. Epidemiology, pathophysiology, and treatment of diverticulitis. Gastroenterology. 2019;156:1282–1298. doi: 10.1053/j.gastro.2018.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel S.V., Hendren S., Zaborowski A., Winter D. Evidence-based reviews in surgery long-term outcome of surgery versus conservative management for recurrent and ongoing complaints after an episode of diverticulitis: five-year follow-up results of a multicenter randomized controlled trial (DIRECT-Trial) Ann. Surg. 2020;272:284–287. doi: 10.1097/SLA.0000000000003920. [DOI] [PubMed] [Google Scholar]
- 13.Al Harakeh H., Paily A.J., Doughan S., Shaikh I. Recurrent acute diverticulitis: when to operate? Inflamm. Intest. Dis. 2018;3:91–99. doi: 10.1159/000494973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathew G., Agha R. For the STROCSS Group, STROCSS 2021: strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. Article 106165. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser A.M., Jiang J.K., Lake J.P., Ault G., Artinyan A., Gonzalez-Ruiz C., Essani R., Beart R.W. The management of complicated diverticulitis and the role of computed tomography. Am. J. Gastroenterol. 2005;100:910–917. doi: 10.1111/j.1572-0241.2005.41154.x. [DOI] [PubMed] [Google Scholar]
- 16.Klarenbeek B.R., de Korte N., van der Peet D.L., Cuesta M.A. Review of current classifications for diverticular disease and a translation into clinical practice. Int. J. Colorectal Dis. 2012;27:207–214. doi: 10.1007/s00384-011-1314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso S., Pera M., Pares D., Pascual M., Gil M.J., Courtier R., Grande L. Outpatient treatment of patients with uncomplicated acute diverticulitis. Colorectal Dis. 2010;12:278–282. doi: 10.1111/j.1463-1318.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 18.Etzioni D.A., Chiu V.Y., Cannom R.R., Burchette R.J., Haigh P.I., Abbas M.A. Outpatient treatment of acute diverticulitis: rates and predictors of failure. Dis. Colon Rectum. 2010;53:861–865. doi: 10.1007/DCR.0b013e3181cdb243. [DOI] [PubMed] [Google Scholar]
- 19.Ha G.W., Lee M.R., Kim J.H. Efficacy of conservative management in patients with right colonic diverticulitis. ANZ J. Surg. 2017;87:467–470. doi: 10.1111/ans.13028. [DOI] [PubMed] [Google Scholar]
- 20.Tan K.K., Wong J., Sim R. Non-operative treatment of right-sided colonic diverticulitis has good long-term outcome: a review of 226 patients. Int. J. Colorectal Dis. 2013;28:849–854. doi: 10.1007/s00384-012-1595-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.H., Ahn B.K., Lee K.H. Conservative treatment of uncomplicated right-sided diverticulitis: a systematic review and meta-analysis. Int. J. Colorectal Dis. 2021;36:1791–1799. doi: 10.1007/s00384-021-03913-x. [DOI] [PubMed] [Google Scholar]
- 22.Au S., Aly E.H. Treatment of uncomplicated acute diverticulitis without antibiotics: a systematic review and meta-analysis. Dis. Colon Rectum. 2019;62:1533–1547. doi: 10.1097/DCR.0000000000001330. [DOI] [PubMed] [Google Scholar]
- 23.Desai M., Fathallah J., Nutalapati V., Saligram S. Antibiotics versus No antibiotics for acute uncomplicated diverticulitis: a systematic review and meta-analysis. Dis. Colon Rectum. 2019;62:1005–1012. doi: 10.1097/DCR.0000000000001324. [DOI] [PubMed] [Google Scholar]
- 24.Tandon A., Fretwell V.L., Nunes Q.M., Rooney P.S. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20:179–188. doi: 10.1111/codi.14013. [DOI] [PubMed] [Google Scholar]
- 25.Oh H.K., Han E.C., Ha H.K., Choe E.K., Moon S.H., Ryoo S.B., Jeong S.Y., Park K.J. Surgical management of colonic diverticular disease: discrepancy between right- and left-sided diseases. World J. Gastroenterol. 2014;20:10115–10120. doi: 10.3748/wjg.v20.i29.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolin D.A. Timing of elective surgery for diverticular disease. Clin. Colon Rectal Surg. 2009;22:169–172. doi: 10.1055/s-0029-1236161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salem L., Veenstra D.L., Sullivan S.D., Flum D.R. The timing of elective colectomy in diverticulitis: a decision analysis. J. Am. Coll. Surg. 2004;199:904–912. doi: 10.1016/j.jamcollsurg.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Rafferty J., Shellito P., Hyman N.H., Buie W.D. Practice parameters for sigmoid diverticulitis. Dis. Colon Rectum. 2006;49:939–944. doi: 10.1007/s10350-006-0578-2. [DOI] [PubMed] [Google Scholar]
- 29.Hupfeld L., Burcharth J., Pommergaard H.C., Rosenberg J. Risk factors for recurrence after acute colonic diverticulitis: a systematic review. Int. J. Colorectal Dis. 2017;32:611–622. doi: 10.1007/s00384-017-2766-z. [DOI] [PubMed] [Google Scholar]
- 30.Justin V., Uranues S., Rabl H., Fingerhut A. Quality of life in uncomplicated recurrent diverticulitis: surgical vs. conservative treatment. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-67094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M.R., Kye B.H., Kim H.J., Cho H.M., Oh S.T., Kim J.G. Treatment of right colonic diverticulitis: the role of nonoperative treatment. J. Korean. Soc. Coloproctol. 2010;26:402–406. doi: 10.3393/jksc.2010.26.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y.C., Chung J.W., Baek H.J., Lee W.S., Kim D., Park Y.H., Yang J.Y., Lee W.K. Risk factors for recurrence of right colonic diverticulitis. Dig. Surg. 2019;36:509–513. doi: 10.1159/000494297. [DOI] [PubMed] [Google Scholar]
- 33.Park H.C., Kim B.S., Lee K., Kim M.J., Lee B.H. Risk factors for recurrence of right colonic uncomplicated diverticulitis after first attack. Int. J. Colorectal Dis. 2014;29:1217–1222. doi: 10.1007/s00384-014-1941-8. [DOI] [PubMed] [Google Scholar]
- 34.Park S.M., Kwon T.S., Kim D.J., Lee Y.S., Cheung D.Y., Oh S.T., Kim J.G., Lee I.K. Prediction and management of recurrent right colon diverticulitis. Int. J. Colorectal Dis. 2014;29:1355–1360. doi: 10.1007/s00384-014-1938-3. [DOI] [PubMed] [Google Scholar]
- 35.Manabe N., Haruma K., Nakajima A., Yamada M., Maruyama Y., Gushimiyagi M., Yamamoto T. Characteristics of colonic diverticulitis and factors associated with complications: a Japanese multicenter, retrospective, cross-sectional study. Dis. Colon Rectum. 2015;58:1174–1181. doi: 10.1097/DCR.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z., Zhang B., Wu D., Jin Y. Characteristics of predominantly right-sided colonic diverticulitis without need for colectomy. BMC Surg. 2020;20:202. doi: 10.1186/s12893-020-00863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajibandeh S., Hajibandeh S., Smart N.J., Maw A. Meta-analysis of the demographic and prognostic significance of right-sided versus left-sided acute diverticulitis. Colorectal Dis. 2020;22:1908–1923. doi: 10.1111/codi.15328. [DOI] [PubMed] [Google Scholar]