Key Points
Question
Is the antiamyloid antibody crenezumab safe and efficacious in people with early Alzheimer disease (AD)?
Findings
The randomized clinical trials of crenezumab CREAD (813 participants) and CREAD2 (806 participants) were discontinued early following a preplanned interim analysis indicating CREAD was unlikely to meet the primary end point. Crenezumab, 60 mg/kg delivered intravenously every 4 weeks for up to 100 weeks, was well tolerated but did not reduce clinical decline nor affect AD-relevant biomarkers.
Meaning
Crenezumab was well tolerated but did not reduce clinical decline or change disease-relevant biomarkers in participants with early AD.
These randomized clinical trials evaluate the safety and efficacy of crenezumab vs placebo in individuals with early Alzheimer disease.
Abstract
Importance
Alzheimer disease (AD), a neurodegenerative disease characterized by β-amyloid plaques and τ tangles in the brain, represents an unmet medical need with no fully approved therapeutics to modify disease progression.
Objective
To investigate the safety and efficacy of crenezumab, a humanized monoclonal immunoglobulin G4 antibody targeting β-amyloid oligomers, in participants with prodromal to mild (early) AD.
Design, Setting, and Participants
Two phase 3 multicenter randomized double-blind placebo-controlled parallel-group efficacy and safety studies of crenezumab in participants with early AD, CREAD and CREAD2, were initiated in 2016 and 2017, respectively, and were designed to evaluate the efficacy and safety of crenezumab in participants with early AD. CREAD (194 sites in 30 countries) and CREAD2 (209 sites in 27 countries) were global multicenter studies. A total of 3736 and 3664 participants were screened in CREAD and CREAD2, respectively. A total of 3736 and 3664 participants were screened in CREAD and CREAD2, respectively. Both trials enrolled individuals aged 50 to 85 years with early AD. Participants with some comorbidities and evidence of cerebral infarction or more than 4 microbleeds or areas of leptomeningeal hemosiderosis on magnetic resonance imaging were excluded. After 2923 and 2858 were excluded, respectively, 813 participants in CREAD and 806 in CREAD2 were randomly assigned in a 1:1 ratio to either placebo or crenezumab. In the final analysis, there were 409 participants in the placebo group and 404 in the crenezumab group in CREAD and 399 in the placebo group and 407 in the crenezumab group in CREAD2. Data were analyzed up until January 2019 and August 2019, respectively.
Interventions
Participants received placebo or 60 mg/kg crenezumab intravenously every 4 weeks for up to 100 weeks.
Main Outcomes and Measures
The primary outcome was change from baseline to week 105 in Clinical Dementia Rating–Sum of Boxes (CDR-SB) score.
Results
There were 813 participants in CREAD (mean [SD] age, 70.7 [8.2] years; 483 female and 330 male) and 806 in CREAD2 (mean [SD] age, 70.9 [7.7] years; 456 female and 350 male). Baseline characteristics were balanced between both groups. The between-group difference in mean change from baseline in CDR-SB score (placebo minus crenezumab) was −0.17 (95% CI, −0.86 to 0.53; P = .63) at week 105 in the CREAD study (88 placebo; 86 crenezumab). Compared with previous trials, no new safety signals were identified, and amyloid-related imaging abnormalities with edema were rare, mild, and transient. No meaningful changes in AD biomarkers were observed. Both studies were discontinued following a preplanned interim analysis indicating that CREAD was unlikely to meet the primary end point.
Conclusions and Relevance
Crenezumab was well tolerated but did not reduce clinical decline in participants with early AD.
Trial Registration
ClinicalTrials.gov Identifiers: CREAD, NCT02670083; CREAD2, NCT03114657
Introduction
Alzheimer disease (AD) is characterized by amyloid-β (Aβ) plaques and τ tangles in the brain.1 Several investigational treatments, including those targeting Aβ or τ, aim to reduce clinical decline. Aβ oligomers have been hypothesized to be the primary mediators of neurotoxicity.2 Crenezumab (RO5490245), a humanized anti-Aβ monoclonal immunoglobulin G4 antibody, binds monomeric and aggregated Aβ, with higher affinity for oligomeric Aβ. Low effector function of the immunoglobulin G4 backbone and minimal binding of crenezumab to vascular amyloid may reduce brain vasculature inflammation and risk of amyloid-related imaging abnormalities (ARIA).3,4
Crenezumab was investigated in early phase 2 clinical trials in participants with mild to moderate AD (A Study to Evaluate the Efficacy and Safety of MABT5102A in Patients With Mild to Moderate Alzheimer’s Disease [ABBY]3 and A Study to Evaluate the Impact of MABT5102A on Brain Amyloid Load and Related Biomarkers in Patients With Mild to Moderate Alzheimer's Disease [BLAZE]4). Primary end points were not met; however, exploratory analyses suggested trends toward reduced cognitive decline in mild AD subgroups and toward reduced Aβ accumulation measured by positron emission tomography (PET) at the higher of 2 doses tested (15 mg/kg delivered intravenously [IV] once every 4 weeks [Q4W]). In a phase 1b study,5 crenezumab was tolerated at doses up to 120 mg/kg IV Q4W. Two phase 3 multicenter randomized double-blind placebo-controlled parallel-group studies of crenezumab in participants with early (prodromal to mild) AD were conducted to evaluate the efficacy and safety of a dose 4 times greater (60 mg/kg of crenezumab, IV Q4W) than that tested in phase 2 in individuals with early (prodromal to mild) AD.
Methods
Study Design
CREAD and CREAD2 were randomized placebo-controlled phase 3 studies in participants with prodromal to mild AD. Study protocols were approved by the institutional review boards or ethics committees at each participating site and are available in Supplement 1. Participants were enrolled at 194 clinics across 30 countries for CREAD and 209 clinics across 27 countries for CREAD2 (eAppendix in Supplement 2). The trials were conducted in accordance with International Conference on Harmonisation E6 Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. All enrolled participants and their caregivers provided written informed consent. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Recruitment took place between March 22, 2016, and December 14, 2017, for CREAD and between March 29, 2017, and August 29, 2018, for CREAD2. Both trials enrolled individuals aged 50 to 85 years with prodromal or mild AD consistent with National Institute on Aging–Alzheimer Association criteria,6,7 increased Aβ burden (confirmed by cerebrospinal fluid [CSF] Aβ42 levels or visually read positive amyloid PET scan), a Mini-Mental State Examination score of 22 or higher, a Clinical Dementia Rating Scale–Global Score of 0.5 or 1.0, and abnormal memory function defined by a Free and Cued Selective Reminding Test cueing index score of 0.67 or lower and a free recall score of 27 or lower. Participants were excluded if their medical history included other conditions causing neurological deficit; cancer; or cardiovascular, hepatic, immune, or metabolic disorders. Other exclusion criteria included evidence of cerebral infarction or more than 4 microbleeds or areas of leptomeningeal hemosiderosis (ARIA-hemosiderosis) on magnetic resonance imaging (MRI). Participants were randomized 1:1 to crenezumab (60 mg/kg) or placebo, administered by IV infusion Q4W for up to 100 weeks. The 60 mg/kg dose was hypothesized to enhance the efficacy potential of crenezumab relative to phase 2 observations.3,4 Randomization was stratified by clinical diagnosis of AD (prodromal vs mild AD), apolipoprotein E ε4 carrier status, antidementia medication use at baseline, and geographic region. Target enrollment was 375 participants per treatment arm in each trial. Continued use and stable doses of AD medications, such as cholinesterase inhibitors, memantine, or medical food supplements, were allowed.
Outcomes
The primary efficacy measure was the Clinical Dementia Rating–Sum of Boxes (CDR-SB) score measuring decline in 6 clinical domains due to cognitive loss (score range 0 to 18, with higher scores indicating more impairment).8 Secondary efficacy measures included cognition assessed by the 13-item Alzheimer’s Disease Assessment Scale9,10 and Mini-Mental State Examination,11 function assessed by the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Inventory, its instrumental subscale,12 and other secondary efficacy measures.13,14,15,16,17,18 Biomarker assessments included CSF Aβ42, Aβ40, total τ, phosphorylated τ-181, [18F]-florbetapir amyloid PET, and [18F]–Genentech τ Probe 1 PET in subsets of participants and volumetric MRI, plasma Aβ40, and Aβ42 in all participants. Exploratory analyses were conducted using biomarkers from the current NeuroToolKit (Roche Diagnostics), a panel of robust prototype assays.19 An experimental technique was used to measure Aβ oligomers in CSF.20 Crenezumab pharmacokinetics were quantified in serum and CSF. Assessment methods and time points are described in the eMethods in Supplement 2. Safety was assessed by monitoring and recording adverse events, laboratory assessments, vital signs, electrocardiogram (ECG) findings, and brain MRI. Suicidality was assessed using the Columbia−Suicide Severity Rating Scale.21 Antidrug antibodies were measured by an enzyme-linked immunosorbent assay to detect serum anticrenezumab antibodies (eMethods in Supplement 2).
Statistical Analysis
The studies were designed to have 80% power to demonstrate a treatment effect of a 30% reduction in deterioration in CDR-SB scores with a 2-sided significance level of P < .05. Enrollment was planned for 750 participants (375 placebo; 375 crenezumab) in each study. Estimated sample sizes were based on the assumption that the mean change in CDR-SB score from baseline to week 105 was 2.6 points in the placebo group, a common standard deviation across both treatment groups for change from baseline to week 105 in mean CDR-SB score of approximately 3.07, the dose level has a true effect of a 30% relative reduction in deterioration of CDR-SB score, and 35% of randomized participants would drop out by week 105. A preplanned interim analysis for futility was conducted by the independent data monitoring committee on CREAD study data 18 months after enrollment reached 50% using SAS version 9.4 (SAS Institute); the sponsor remained blinded. The threshold for declaring futility was a relative reduction of less than 10% in CDR-SB score for crenezumab vs placebo in the 18-month mixed model for repeated measures.
Following termination of the phase 3 program in sporadic AD, efficacy analyses were conducted in the prespecified modified intent-to-treat population (defined as all randomized participants who received 1 dose or more of the study drug). Data acquired after the company press release (January 29, 2019) on futility were censored to avoid bias. A sensitivity analysis without censoring data was performed. For the primary end point, change in CDR-SB scores from baseline to week 105 (week 77 for CREAD2, since the trial was stopped before participants reached week 105) was analyzed using a mixed model for repeated measures adjusting for disease severity, apolipoprotein E ε4 status, geographic region, and symptomatic AD therapy usage. Secondary efficacy end points were also analyzed using a mixed model for repeated measures. Since expected sample sizes at week 105 were not reached due to early study termination, an exploratory analysis on CDR-SB scores using a random coefficient regression model was performed to draw further insight on the overall rate of change in both treatment arms. An exploratory mixed model for repeated measures investigated crenezumab treatment effects in participants with prodromal AD vs mild AD in CREAD/CREAD2 pooled. The 95% CIs for secondary end points were not adjusted for multiplicity or termination of hierarchical testing; thus clear inferences cannot be drawn. See the eMethods in Supplement 2 for the statistical analysis for the biomarker substudies.
Safety data included incidence, nature, and severity of adverse events. MRI safety findings were summarized, and mean change from baseline over time was assessed for clinical laboratory tests, ECG assessments, vital signs, and Columbia−Suicide Severity Rating Scale score. In CREAD, the numbers and proportion of participants with positive and negative antidrug antibody results during treatment and follow-up were summarized by treatment group (eMethods in Supplement 2). Safety analyses were conducted in the modified intent-to-treat population.
Results
Study Population Characteristics
There were 813 participants in CREAD (mean [SD] age, 70.7 [8.2] years; 483 female and 330 male; 56 [6.9%] Asian, 8 [1.0%] Black, 15 [1.8%] Native American or Alaska Native, 712 [87.6%] White, 1 [0.1%] of more than 1 race, and 21 [2.6%] of unknown race) and 806 in CREAD2 (mean [SD] age, 70.9 [7.7] years; 456 female and 350 male; 92 [11.4%] Asian, 7 [0.9%] Black, 13 [1.6%] Native American or Alaska Native, 675 [83.7%] White, 7 [0.9%] of more than 1 race, and 12 [1.5%] of unknown race). In CREAD, 409 participants were enrolled in the placebo group and 404 in the crenezumab group; in CREAD2, 399 were enrolled in the placebo group and 407 in the crenezumab group (Figure 1). Baseline characteristics in both studies were balanced between placebo and crenezumab groups regarding age, sex, ethnicity, education, and cognitive and functional scale scores (Table 1). In CREAD and CREAD2, respectively, 346 participants (42.6%) and 388 participants (48.1%) of participants had prodromal AD; 467 (57.4%) and 418 (51.9%) had mild AD.
Figure 1. Enrollment, Randomization, and Study Completion in CREAD and CREAD2.
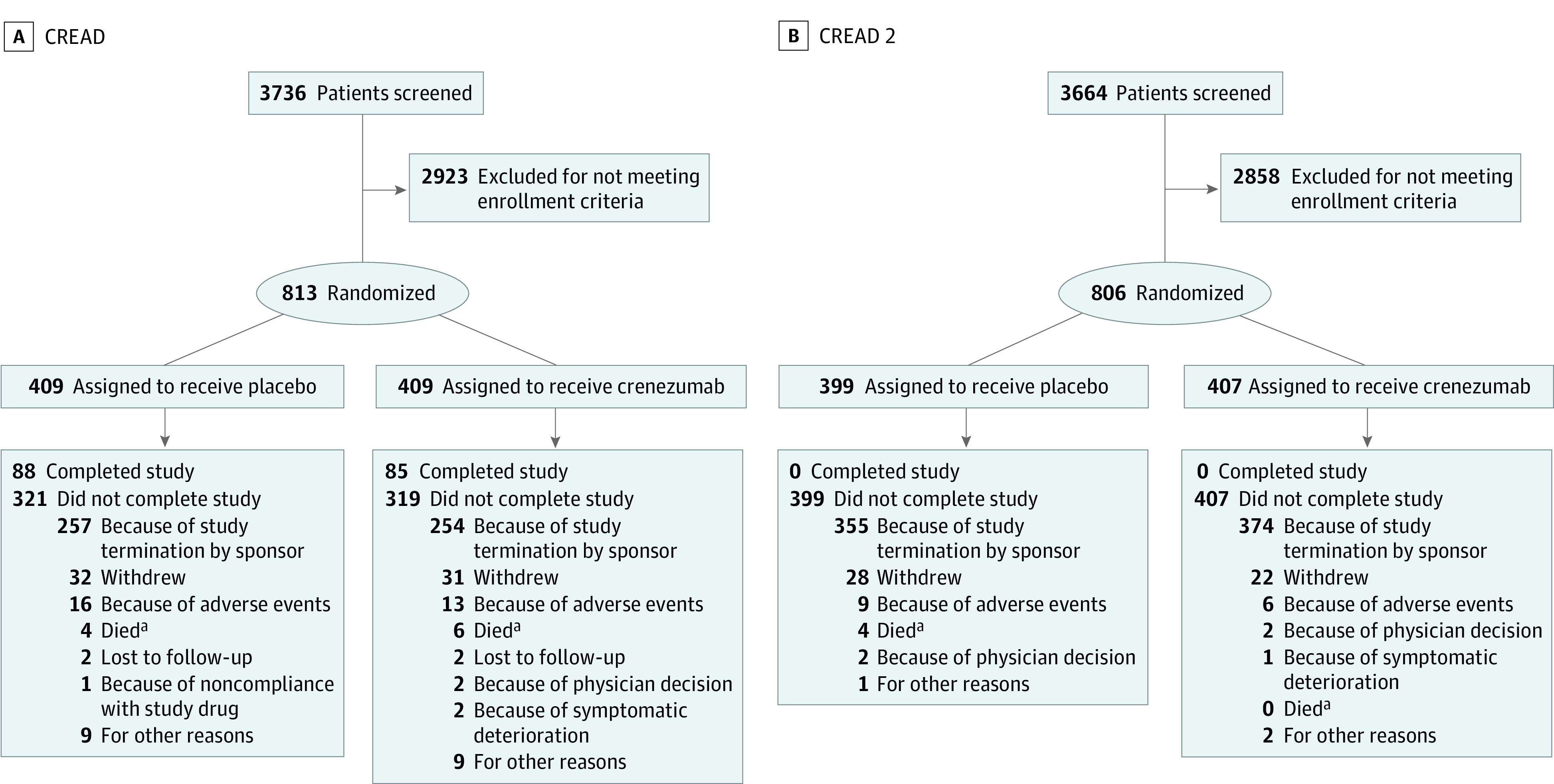
CREAD indicates A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Study of Crenezumab in Patients With Prodromal to Mild Alzheimer’s Disease.
aThe number of participant deaths does not represent the total number of deaths in the studies but only those where the death itself led to study discontinuation. A total of 13 deaths occurred (CREAD: 5 in placebo and 8 in crenezumab; CREAD2: 6 in placebo and 0 in crenezumab).
Table 1. Demographic and Baseline Characteristics for Individuals in CREAD and CREAD2a.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| CREAD | CREAD2 | |||
| Placebo (n = 409) | Crenezumab (n = 404) | Placebo (n = 399) | Crenezumab (n = 407) | |
| Age, mean (SD), y | 70.3 (8.4) | 71.0 (7.9) | 70.7 (7.9) | 71.1 (7.5) |
| Female | 247 (60.4) | 236 (58.4) | 225 (56.4) | 231 (56.8) |
| Male | 162 (39.6) | 168 (41.6) | 174 (43.6) | 176 (43.2) |
| Raceb | ||||
| Asian | 28 (6.8) | 28 (6.9) | 45 (11.3) | 47 (11.5) |
| Black | 3 (0.7) | 5 (1.2) | 4 (1.0) | 3 (0.7) |
| Native American or Alaska Native | 5 (1.2) | 10 (2.5) | 8 (2.0) | 5 (1.2) |
| White | 360 (88.0) | 352 (87.1) | 333 (83.5) | 342 (84.0) |
| >1 | 0 | 1 (0.2) | 4 (1.0) | 3 (0.7) |
| Unknown | 13 (3.2) | 8 (2.0) | 5 (1.3) | 7 (1.7) |
| Education level below undergraduate degree | 239 (58.4) | 229 (56.7) | 232 (58.1) | 229 (56.3) |
| Antidementia therapy at baseline | ||||
| Acetylcholinesterase inhibitor alone | 218 (53.3) | 212 (52.5) | 197 (49.4) | 208 (51.1) |
| Memantine alone | 20 (4.9) | 30 (7.4) | 27 (6.8) | 32 (7.9) |
| Acetylcholinesterase inhibitor and memantine | 46 (11.2) | 49 (12.1) | 55 (13.8) | 49 (12.0) |
| Prodromal disease status | 175 (42.8) | 171 (42.3) | 190 (47.6) | 198 (48.6) |
| Mild disease status | 234 (57.2) | 233 (57.7) | 209 (52.4) | 209 (51.4) |
| CDR-SB score, mean (SD) | 3.79 (1.60) | 3.88 (1.68) | 3.76 (1.55) | 3.68 (1.58) |
| MMSE score, mean (SD) | 23.4 (2.9) | 23.7 (3.0) | 23.5 (2.9) | 23.6 (2.8) |
| ADAS-Cog13 score, mean (SD) | 28.9 (7.4) | 29.4 (7.6) | 28.9 (7.3) | 28.8 (7.4) |
| ADCS-ADL Total score, mean (SD) | 67.6 (8.1) | 67.1 (8.3) | 66.8 (8.1) | 67.1 (8.5) |
| FCSRT-Free Recall score, mean (SD) | 8.0 (5.7) | 7.6 (5.0) | 7.9 (5.4) | 7.4 (5.3) |
| FCSRT-cueing index score, mean (SD) | 0.41 (0.20) | 0.39 (0.20) | 0.42 (0.20) | 0.39 (0.20) |
| APOE ε4 carrier | 292 (71.7) | 293 (72.7) | 263 (65.9) | 271 (66.9) |
| Amyloid PET SUVR,c mean (SD) | 1.37 (0.17) | 1.35 (0.16) | 1.37 (0.14) | 1.37 (0.18) |
| CSF Aβ42, mean (SD), pg/mL | 580.48 (179.83) | 568.52 (166.87) | 617.88 (191.03) | 607.10 (178.71) |
Abbreviations: Aβ, amyloid-β; ADCS-ADL, Alzheimer’s Disease Cooperative Study–Activities of Daily Living; ADAS-Cog13, Alzheimer’s Disease Assessment Scale 13; APOE ε4, apolipoprotein E ε4; CDR-SB, Clinical Dementia Rating–Sum of Boxes; CSF, cerebrospinal fluid; FCSRT, Free and Cued Selective Reminding Test; MMSE, Mini-Mental State Examination; PET, positron emission tomography; SUVR, standardized uptake value ratio.
The population presented is the intent-to-treat population with all randomized patients grouped according to their randomly assigned treatment. The between-group difference in mean change from baseline in CDR-SB score (placebo minus crenezumab) was −0.17 (95% CI, −0.86 to 0.53; P = .63)
Race data were collected per trial protocol and were self-reported using multiple choice questions. The choices for race were American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, White, and unknown. More than one choice for race could be selected.
SUVR was calculated for the composite cortical region of interest using a subcortical white matter reference region.
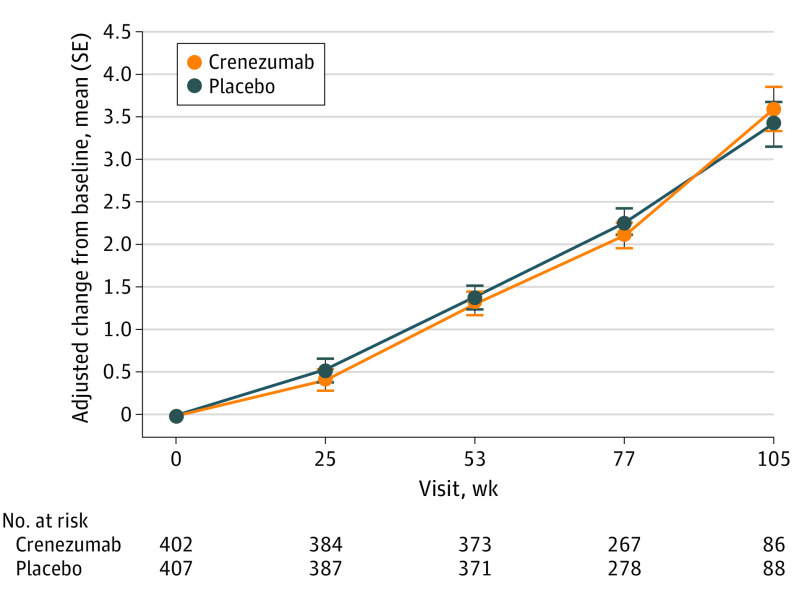
Both studies were discontinued early after a preplanned interim analysis of CREAD indicated the study was unlikely to meet the primary end point. Overall, 173 participants (21.3%) completed CREAD before discontinuation (88 [21.5%] placebo; 85 [21.0%] crenezumab); CREAD2 was discontinued before any participants completed the study (Figure 1). The mean (SD) treatment duration was 78.8 (23.3) weeks (mean [SD] cumulative dose, 1220.2 [354.0] mg/kg) in CREAD, and 41.9 (14.2) weeks (mean [SD] cumulative dose, 675.6 [214.2] mg/kg) in CREAD2. Due to the early discontinuation, sample sizes at assessment visits decreased over time and were smaller in CREAD2 than in CREAD (Table 2; Figure 2; eTable 1 in Supplement 2). Hence, this article focuses on efficacy results from CREAD and includes summarized end point data from CREAD2.
Table 2. Primary and Secondary Outcomes for CREAD.
| Variablea | Placebo | Crenezumab | Mean difference (95% CI) | ||
|---|---|---|---|---|---|
| No. | Mean change from baseline at week 105 (95% CI) | No. | Mean change from baseline at week 105 (95% CI) | ||
| CDR-SB scoreb | 88 | 3.42 (2.90 to 3.93) | 86 | 3.59 (3.07 to 4.11) | −0.17 (−0.86 to 0.53) |
| CDR-GS score | 88 | 0.55 (0.44 to 0.66) | 86 | 0.50 (0.39 to 0.61) | 0.05 (−0.10 to 0.20) |
| MMSE score | 90 | −4.63 (−5.37 to −3.89) | 87 | −4.96 (−5.72 to −4.21) | 0.33 (−0.62 to 1.29) |
| ADAS-Cog11 score | 86 | 8.43 (6.94 to 9.92) | 80 | 8.53 (7.01 to 10.05) | −0.10 (−2.08 to 1.88) |
| ADAS-Cog13 score | 86 | 9.55 (7.94 to 11.17) | 80 | 9.82 (8.16 to 11.47) | −0.26 (−2.39 to 1.87) |
| ADCS-ADL | 90 | −11.51 (−13.92 to −9.10) | 88 | −13.39 (−15.83 to −10.94) | 1.88 (−1.43 to 5.18) |
| ADCS-iADL subscore | 90 | −9.22 (−11.12 to −7.31) | 88 | −10.44 (−12.37 to −8.51) | 1.22 (−1.35 to 3.79) |
| NPI-Q total severity score | 84 | 1.02 (−0.09 to 2.12) | 87 | 1.55 (0.45 to 2.64) | −0.53 (−1.95 to 0.90) |
| Patient QoL-AD total score | 90 | −1.69 (−2.67 to –0.70) | 86 | −2.08 (−3.09 to −1.07) | 0.40 (–0.81 to 1.60) |
| ZCI-AD total score | 86 | 22.72 (12.62 to 32.82) | 87 | 24.11 (14.07 to 34.15) | −1.39 (−13.64 to 10.86) |
| Patient EQ-5D VAS score | 89 | −4.54 (–7.94 to –1.13) | 87 | −6.35 (–9.82 to −2.89) | 1.82 (−2.64 to 6.27) |
| Caregiver EQ-5D VAS total score | 89 | −3.16 (−6.53 to 0.21) | 88 | −4.09 (−7.48 to −0.71) | 0.94 (−3.45 to 5.32) |
Abbreviations: ADAS-Cog13, Alzheimer’s Disease Assessment Scale 13; ADAS-Cog11, Alzheimer’s Disease Assessment Scale 11; ADCS-ADL, Alzheimer’s Disease Cooperative Study–Activities of Daily Living; ADCS-iADL, Alzheimer’s Disease Cooperative Study–Activities of Daily Living Instrumental Subscale; CDR-GS, Clinical Dementia Rating Scale–Global score; CREAD, A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Study of Crenezumab in Patients With Prodromal to Mild Alzheimer’s Disease; MMSE, Mini-Mental State Examination; NPI-Q, Neuropsychiatric Inventory Questionnaire; QoL-AD, Quality of Life–Alzheimer Dementia; VAS, visual analogue scale; ZCI-AD, Zarit Caregiver Interview for Alzheimer Disease scale.
Analyses conducted using mixed model for repeated measures in the modified intent-to-treat analysis. Secondary efficacy measures included cognition assessed by ADAS-Cog11 (score range 0 to 70); ADAS-Cog13 (score range 0 to 85), in which higher scores indicate greater impairment9,10; MMSE (score range 0 to 30), with lower scores indicating more severe impairment11; dementia severity assessed by CDR-GS (score range 0 to 3), with higher score indicating more severe dementia13; function assessed by ADCS-ADL (score range 0 to 78); and the instrumental subscale of ADCS-ADL (score range 0 to 56),12 where lower scores indicate worse functioning. Behavioral disturbances were assessed with NPI-Q (severity score range from 0 to 36), where higher scores indicate more severe symptoms,14 The impact of crenezumab on quality of life and caregiver burden was measured using QoL-AD scale,15 ZCI-AD scale16,17 (modified from the original Zarit Burden Interview), and EQ-5D VAS score for patients and caregivers.17,18
Figure 2. Mean Change in Clinical Dementia Rating–Sum of Boxes Scores for CREAD.
CREAD indicates A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Study of Crenezumab in Patients With Prodromal to Mild Alzheimer’s Disease.
Efficacy Outcomes
In CREAD, the difference between crenezumab and placebo in mean change in CDR-SB score from baseline to week 105 was −0.17 (favored placebo; 95% CI, −0.86 to 0.53; P = .63) (Table 2; Figure 2), with the relative percentage change being −4.9%. In the smaller CREAD2 data set, the difference for crenezumab (n = 12) vs placebo (n = 15) in mean change in CDR-SB score from baseline to week 77 was 1.30 (favored crenezumab; 95% CI, 0.00 to 2.60) (eTable 1 in Supplement 2), with the relative percentage change being 40.7%. Using the random coefficient regression model approach, the differences in yearly rate of change between treatment arms (standard error [SE]), placebo minus crenezumab) in CDR-SB scores in CREAD and CREAD2 were 0.02 (0.14) and 0.46 (0.20), respectively (eMethods in Supplement 2).
In CREAD, a treatment effect was not suggested across secondary efficacy outcomes with a difference in mean change from baseline to week 105 between crenezumab treatment and placebo for 13-item Alzheimer’s Disease Assessment Scale score of −0.26 (favored placebo; 95% CI, −2.39 to 1.87) for Alzheimer’s Disease Cooperative Study–Activities of Daily Living Inventory score of 1.88 (favored placebo; 95% CI, −1.43 to 5.18), and for Mini-Mental State Examination score of 0.33 (favored placebo; 95% CI, −0.62 to 1.29). For a full list of secondary end points, see Table 2. CREAD2 results on secondary efficacy outcomes are in eTable 1 in Supplement 2. Results from a sensitivity analysis including all data (noncensored post–company press release on futility) did not change the primary or secondary outcomes.
There was no difference in change from baseline to week 105 in prodromal AD vs mild AD subgroups in the pooled data set in efficacy measures of CDR-SB, Mini-Mental State Examination, 13-item Alzheimer’s Disease Assessment Scale, and Alzheimer’s Disease Cooperative Study–Activities of Daily Living Inventory (eFigure 1 in Supplement 2; for baseline characteristics, see eTable 2 in Supplement 2).
Safety
Safety populations for CREAD and CREAD2 comprised 405 and 398 participants for the placebo groups, respectively, and 404 participants for each crenezumab group. The proportion of participants with adverse events and serious adverse events (Table 3), as well as the rates of serious and nonserious adverse events that were suspected by the investigator to be drug related were similar between groups (CREAD: placebo, 72 [17.8%] and crenezumab 78 [19.3%]; CREAD2: placebo, 52 [13.1%] and crenezumab, 52 [12.9%]). Nineteen participants died during the studies, 13 in CREAD and 6 in CREAD2 (Table 3). These deaths were not considered to be related to crenezumab treatment.
Table 3. Summary of Adverse Events During the CREAD and CREAD2 Studies.
| Event | No. (%) | |||
|---|---|---|---|---|
| CREAD | CREAD2 | |||
| Placebo (n = 405) | Crenezumab (n = 404) | Placebo (n = 398) | Crenezumab (n = 404) | |
| Participants with any adverse event | 337 (83.2) | 347 (85.9) | 291 (73.1) | 297 (73.5) |
| Participants with any serious adverse event | 63 (15.6) | 67 (16.6) | 42 (10.6) | 33 (8.2) |
| Adverse event of severe intensity | 49 (12.1) | 52 (12.9) | 24 (6.0) | 20 (5.0) |
| Participants with at least one adverse event resulting in treatment discontinuation | 15 (3.7) | 14 (3.5) | 9 (2.3) | 5 (1.2) |
| Death | 5 (1.2) | 8 (2.0) | 6 (1.5) | 0 |
| Adverse events, by PT, with incidence of ≥5% in either groupa | ||||
| Headache | 45 (11.1) | 39 (9.7) | 22 (5.5) | 25 (6.2) |
| Nasopharyngitis | 33 (8.1) | 40 (9.9) | 25 (6.3) | 24 (5.9) |
| Fall | 33 (8.1) | 43 (10.6) | 24 (6.0) | 20 (5.0) |
| Hypertension | 22 (5.4) | 27 (6.7) | 15 (3.8) | 27 (6.7) |
| Back pain | 31 (7.7) | 26 (6.4) | 15 (3.8) | 16 (4.0) |
| Upper respiratory tract infection | 29 (7.2) | 33 (8.2) | 13 (3.3) | 10 (2.5) |
| Anxiety | 21 (5.2) | 28 (6.9) | 17 (4.3) | 18 (4.5) |
| Depression | 27 (6.7) | 28 (6.9) | 14 (3.5) | 15 (3.7) |
| Diarrhea | 26 (6.4) | 25 (6.2) | 15 (3.8) | 17 (4.2) |
| Dizziness | 27 (6.7) | 23 (5.7) | 10 (2.5) | 19 (4.7) |
| Serious adverse events, by PT, with incidence of ≥0.5% in either group | ||||
| Fall | 1 (0.2) | 4 (1.0) | 6 (1.5) | 2 (0.5) |
| Pneumonia | 3 (0.7) | 5 (1.2) | 2 (0.5) | 1 (0.2) |
| Subdural hematoma | 3 (0.7) | 4 (1.0) | 0 | 3 (0.7) |
| Syncope | 4 (1.0) | 3 (0.7) | 2 (0.5) | 0 |
| Dehydration | 0 | 3 (0.7) | 0 | 1 (0.2) |
| Adverse events of interest | ||||
| New findings of amyloid-related imaging abnormalitiesb | ||||
| With edemac | 1/397 (0.3) | 1/399 (0.3) | 0 | 1/398 (0.3) |
| With hemorrhage | 31/397 (7.8) | 39/399 (9.8) | 23/388 (5.9) | 20/398 (5.0) |
| Infusion-related reactionsd | 41 (10.1) | 47 (11.6) | 31 (7.8) | 24 (5.9) |
| Pneumoniae | 7 (1.7) | 10 (2.5) | 8 (2.0) | 3 (0.7) |
Abbreviations: CREAD, A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Study of Crenezumab in Patients With Prodromal to Mild Alzheimer’s Disease; PT, preferred term.
Adverse events by PT according to Medical Dictionary for Regulatory Activities version 22.0.
Percentages are based on participants with postbaseline safety magnetic resonance imaging data.
Of 3 reported amyloid-related imaging abnormality–edema events, 2 were asymptomatic and 1 participant experienced worsening of a preexisting headache at the time of amyloid-related imaging abnormality–edema detection (crenezumab arm in the CREAD study). All were mild (Barkhof grand total score between 1 and 3).
Infusion-related reaction rates were similar between both groups (CREAD: placebo, 41 [10.1%], crenezumab, 47 [11.6%]; CREAD2: placebo, 31 [7.8%]; crenezumab, 24 [5.9%]) with the most common reactions being headache, phlebitis, and back pain. Most infusion-related reactions were nonserious and were mild to moderate in severity.
Of the 17 reported cases of pneumonia, an adverse event of interest, 5 cases were suspected to be treatment related (CREAD: placebo, 2 [0.5%], crenezumab, 2 [0.5%]; CREAD2: placebo, 1 [0.3%], crenezumab 0).
New ARIA-edema findings were reported in 3 participants (Table 3), 2 of whom experienced asymptomatic ARIA-edema and 1 of whom reported a worsening headache. All 3 cases of ARIA-edema were mild and resolved within 4 weeks. Baseline numbers of ARIA-hemosiderosis were similar between groups (CREAD: placebo, 49 [12.1%] and crenezumab, 62 [15.3%]; CREAD2: placebo, 39 [9.8%] and crenezumab, 43 [10.6%]) as were rates of newly diagnosed ARIA-hemosiderosis (Table 3). Active suicidal ideation according to the Columbia−Suicide Severity Rating Scale was rare in both treatment arms (CREAD: placebo, 9 [2.2%]; crenezumab, 8 [2.0%]; CREAD2: placebo, 6 [1.6%]; crenezumab, 5 [1.2%]), as were adverse events of suicidal ideation (CREAD: placebo, 2 [0.5%]; crenezumab, 1 [0.2%]; CREAD2: placebo, 4 [1.0%]; crenezumab, 1 [0.2%]). Other safety data are reported in Table 3. In CREAD, postbaseline incidence of antidrug antibodies was 0.5% with 2 participants having 1 transient positive ADA signal after crenezumab treatment (not tested in CREAD2 due to low ADA incidence in CREAD).
Pharmacokinetics and Biomarker Outcomes
Crenezumab reached steady state after 13 weeks of dosing in CREAD with 60 mg/kg IV Q4W and concentrations were maintained during the study. Mean (SD) steady-state serum crenezumab concentrations were 1580 (487) μg/mL and 345 (146) μg/mL for maximum concentration and minimum concentration, respectively. The mean (SD) CSF/serum ratio of crenezumab was 0.26% (0.21%). Predose total Aβ42 and Aβ40 concentrations in plasma and CSF were significantly increased following the administration of crenezumab and maintained throughout the study: mean steady-state (SD) predose Aβ42 and Aβ40 levels were 2.72 (0.55) ng/mL and 44.6 (10.0) ng/mL, respectively. Serum pharmacokinetics, CSF pharmacokinetics, and plasma pharmacodynamics in CREAD2 were consistent with those in CREAD (eTable 5 and eFigures 7 and 8 in Supplement 2). There were no significant differences in longitudinal changes between treatment groups in amyloid PET, volumetric MRI measures, CSF Aβ oligomers, total τ, phosphorylated τ-181, and other fluid biomarkers studied. From baseline to week 53, an increase in τ PET standardized uptake value ratio was measured that was higher in the crenezumab arm compared to placebo. See eTables 3-9 and eFigures 2-6 in Supplement 2 for details on biomarker outcomes.
Discussion
The randomized double-blind placebo-controlled parallel-group phase 3 studies CREAD and CREAD2 investigated the efficacy and safety of crenezumab (60 mg/kg IV Q4W) in individuals with early AD with confirmed Aβ pathology. Following early study termination, CREAD did not show a beneficial effect with crenezumab vs placebo on the primary outcome, change from baseline in CDR-SB scores at week 105. Analyses of secondary end points were consistent with the primary outcome data. Results from CREAD2, where no participants reached week 105 and few reached week 77, were consistent with CREAD. Observed small differences between treatment arms in both studies lacked consistency across end points and between studies and were consistent with random variation around the null hypothesis. Disease progression was within the hypothesized range.
These phase 3 results did not confirm the efficacy signal hypothesized in phase 2. An analysis of prodromal vs mild dementia subgroups within the pooled CREAD/CREAD2 data sets yielded no consistent treatment differences.
The safety of crenezumab was consistent with previously reported data for this molecule. ARIA-edema was rare, mild, and transient. ARIA-edema did not occur more often in the drug-treated group than in the placebo group. Incidence of new ARIA-hemosiderosis was similar between groups. Rates of treatment-emergent adverse events, including those that were serious or of severe intensity, were similar between groups. Higher rates of pneumonia and deaths reported in the crenezumab group vs placebo in the ABBY phase 2 study3 were not replicated in the CREAD studies.
Crenezumab serum exposure in CREAD was, as expected, 4 times higher than that achieved in the Study to Evaluate the Efficacy and Safety of Crenezumab in Participants With Mild to Moderate AD (ABBY)3 and the Study to Evaluate the Impact of Crenezumab on Brain Amyloid Load and Related Biomarkers in Participants With Mild to Moderate AD (BLAZE).4 The CSF/plasma ratio of 60 mg/kg of crenezumab evaluated in CREAD was consistent with observations from previous studies of lower doses3,4 and supports the notion that drug penetration into the central nervous system was not saturated at this higher dose.
Early termination and lack of efficacy observed in the CREAD trials may be due to a variety of causes. Investigational antiamyloid antibody treatments have demonstrated mixed results in late-phase clinical trials in AD.1,22 Important themes in the discussion of the reasons for this include uncertainty around the importance of Aβ as a driver of disease pathophysiology across disease stages; uncertainty around needing to target particular Aβ species, not all of which may be equally neurotoxic; uncertainty around the impact of reduced effector function of the antibody; the question of choosing a sufficiently high dose or long enough duration treatment; and the related question of evidence of sufficient target engagement.
There is still considerable interest in investigating anti-Aβ treatments; efficacy signals in symptomatic disease have been reported for anti-Aβ antibodies that target monomers (solanezumab) as well as aggregated Aβ (gantenerumab, aducanumab, lecanemab,23 donanemab24). Notably, the US Food and Drug Administration recently granted accelerated approval for the anti-Aβ antibody aducanumab for the treatment of AD after studies25 demonstrated a reduction in Aβ plaques. Clinical trials of β-secretase inhibitors, on the other hand, have been discontinued early due to futility or safety findings, including worsening in cognition in active treatment groups,1 which is not well understood.26 Crenezumab was designed to target Aβ oligomers, which have been hypothesized to be a primary mediator of neurotoxicity2; crenezumab also binds to Aβ monomers, albeit with approximately 10-fold lower affinity.27 In vivo studies in PS2APP mice appear to substantiate this preferential Aβ oligomeric binding by showing that crenezumab binds to regions surrounding the periphery of Aβ plaques and the hippocampal mossy fibers, brain regions enriched with oligomeric Aβ.2
Phase 3 studies3,4 tested crenezumab at a 60-mg/kg IV Q4W dose. Evidence of target engagement by crenezumab in clinical trial participants includes consistent and dose-dependent increases in total plasma and CSF Aβ40 and Aβ42 concentrations. Modeling of peripheral pharmacokinetics/pharmacodynamics effects of monomeric Aβ40 and Aβ42 suggested that even higher doses would yield limited additional peripheral target engagement with a dose of 120 mg/kg IV Q4W resulting in maximal effects.5,28,29 How this translates into target engagement of oligomeric Aβ in the brain remains unclear. No significant treatment effects on brain Aβ load as measured by florbetapir PET were observed. There was no evidence of treatment effects on putative downstream biomarkers in CSF and plasma. The τ PET substudy outcome should be interpreted with caution due to the limited sample size and short follow-up time. The 60-mg/kg crenezumab dose was selected as it was expected to result in increased brain exposure and target engagement relative to what was investigated in phase 2 studies while maintaining a favorable safety profile.3,4 Demonstrating target engagement in the central compartment early in development, preferably corroborated by disease-relevant downstream biomarkers, is an important consideration to help exclude lack of sufficient target engagement as a reason for lack of efficacy.
Limitations
Limitations of the studies reported here include that both studies were terminated early; hence data sets were smaller and truncated in terms of longitudinal follow-up, particularly in CREAD2, where no participants reached week 105. Analyses in prodromal vs mild dementia subgroup within the pooled CREAD/CREAD2 data sets were limited by the large overlap in terms of clinical baseline scale scores between both groups. Also, there is limited data in ethnically and racially diverse patients, given that more than 80% of participants were White in both studies.
Conclusions
CREAD and CREAD2 phase 3 crenezumab trials were terminated early due to lack of efficacy. No new safety signals were observed.
Trial protocols
eMethods
eTable 1. Primary and Secondary Outcomes for CREAD2
eTable 2. Pooled CREAD and CREAD2 Demographic and Baseline Characteristics for Individuals with Prodromal AD and Mild ADa
eTable 3. Demographic and Baseline Characteristics for Participants in the CREAD and CREAD2 Biomarker Substudies
eTable 4. Mean Changes from Baseline in Imaging and Core CSF Biomarker Concentrations
eTable 5. Unadjusted Mean Changes from Baseline in Plasma Aβ42, Aβ40, NfL, and [18F]GTP1 Tau PET
eTable 6. CREAD: CSF and PET Biomarkers, Baseline Characteristics
eTable 7. CREAD2: CSF and PET biomarkers, Baseline Characteristics
eTable 8. CREAD: CSF and PET Biomarker Outcomes
eTable 9. CREAD2 CSF and PET Biomarker Outcomes
eFigure 1. Mean Difference in Mean Change from Baseline to Week 105 vs Placebo for Exploratory Analysis Efficacy Measures in Prodromal AD vs Mild AD Dementia in Pooled CREAD and CREAD2 Population (mITT)
eFigure 2. Enrollment, Randomization, and Study Completion in Pooled CREAD Substudies
eFigure 3. Least Squares Percent Mean Change from Baseline in Whole Brain (A/D), Ventricular (B/E), and Hippocampal Volume (C/F) in CREAD/CREAD2
eFigure 4. Change from Baseline [18F]florbetapir Amyloid PET (A) at Weeks 53 and 105, and [18F]GTP1 Tau PET (B) at Week 53
eFigure 5. Mean Change from Baseline in CSF Total Aβ42 (A), Total Aβ40 (B), Aβ Oligomer (C), tTau (D), and pTau181 (E)
eFigure 6. Mean Change from Baseline for Exploratory CSF biomarkers
eFigure 7. Plasma Total Aβ42 (A), Total Aβ40 (B), and NfL (C)
eFigure 8. Relationship between crenezumab concentrations and Aβ40 and Aβ42
eFigure 9. Concordance of [18F]florbetapir Amyloid PET visual read with CSF biomarkers of Aβ42 (A), pTau181/Aβ42 ratio (B), and tTau/Aβ42 ratio (C)
eAppendix. List of study sites and principal investigators in CREAD and CREAD2
eReferences
Data sharing statement
References
- 1.Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15(2):73-88. doi: 10.1038/s41582-018-0116-6 [DOI] [PubMed] [Google Scholar]
- 2.Meilandt WJ, Maloney JA, Imperio J, et al. Characterization of the selective in vitro and in vivo binding properties of crenezumab to oligomeric Aβ. Alzheimers Res Ther. 2019;11(1):97. doi: 10.1186/s13195-019-0553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings JL, Cohen S, van Dyck CH, et al. ABBY: a phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. 2018;90(21):e1889-e1897. doi: 10.1212/WNL.0000000000005550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salloway S, Honigberg LA, Cho W, et al. Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer’s disease (BLAZE). Alzheimers Res Ther. 2018;10(1):96. doi: 10.1186/s13195-018-0424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guthrie H, Honig LS, Lin H, et al. Safety, tolerability, and pharmacokinetics of crenezumab in patients with mild-to-moderate Alzheimer’s disease treated with escalating doses for up to 133 weeks. J Alzheimers Dis. 2020;76(3):967-979. doi: 10.3233/JAD-200134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Bryant SE, Waring SC, Cullum CM, et al. ; Texas Alzheimer’s Research Consortium . Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study. Arch Neurol. 2008;65(8):1091-1095. doi: 10.1001/archneur.65.8.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S13-S21. doi: 10.1097/00002093-199700112-00003 [DOI] [PubMed] [Google Scholar]
- 10.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356-1364. doi: 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 12.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33-S39. doi: 10.1097/00002093-199700112-00005 [DOI] [PubMed] [Google Scholar]
- 13.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566-572. doi: 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- 14.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233-239. doi: 10.1176/jnp.12.2.233 [DOI] [PubMed] [Google Scholar]
- 15.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Ment Health Aging. 1999;5(1):21-32. https://psycnet.apa.org/record/1999-05045-003 [Google Scholar]
- 16.Le Scouiller S, Edgar C, Rylands A, et al. Cross-cultural adaptation of the Zarit Caregiver Interview for Alzheimer’s Disease (ZCI-AD) and the Caregiver Global Impression scales in 13 languages. Value Health. 2016;19(7):PA389. doi: 10.1016/j.jval.2016.09.244 [DOI] [Google Scholar]
- 17.Zarit SH, Orr NK, Zarit JM. The Hidden Victims of Alzheimer’s Disease: Families Under Stress. NYU Press; 1985. [Google Scholar]
- 18.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blennow K, Dage J, Johnson SC, et al. Exploring the need for robust biomarker assays in Alzheimer’s disease and other neurodegenerative diseases. Alzheimers Dement. 2019;15(75):2-231. doi: 10.1016/j.jalz.2019.06.2638 [DOI] [Google Scholar]
- 20.Yang T, Dang Y, Ostaszewski B, et al. Target engagement in an Alzheimer trial: crenezumab lowers amyloid β oligomers in cerebrospinal fluid. Ann Neurol. 2019;86(2):215-224. doi: 10.1002/ana.25513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penninkilampi R, Brothers HM, Eslick GD. Safety and efficacy of anti-amyloid-β immunotherapy in Alzheimer’s disease: a systematic review and meta-analysis. J Neuroimmune Pharmacol. 2017;12(1):194-203. doi: 10.1007/s11481-016-9722-5 [DOI] [PubMed] [Google Scholar]
- 23.Swanson C, Zhang Y, Dhadda S, et al. DT-01-07: treatment of early AD subjects with BAN2401, an anti-Aβ protofibril monoclonal antibody, significantly clears amyloid plaque and reduces clinical decline. Alzheimer’s & Dementia. 2018;14(7S):P1668. doi: 10.1016/j.jalz.2018.07.009 [DOI] [Google Scholar]
- 24.Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704. doi: 10.1056/NEJMoa2100708 [DOI] [PubMed] [Google Scholar]
- 25.Dunn B, Stein P, Cavazzoni P. Approval of aducanumab for Alzheimer disease—the FDA’s perspective. JAMA Intern Med. 2021;181(10):1276-1278. doi: 10.1001/jamainternmed.2021.4607 [DOI] [PubMed] [Google Scholar]
- 26.Wessels AM, Lines C, Stern RA, et al. Cognitive outcomes in trials of two BACE inhibitors in Alzheimer’s disease. Alzheimers Dement. 2020;16(11):1483-1492. doi: 10.1002/alz.12164 [DOI] [PubMed] [Google Scholar]
- 27.Ultsch M, Li B, Maurer T, et al. Structure of crenezumab complex with Aβ shows loss of β-hairpin. Sci Rep. 2016;6:39374. doi: 10.1038/srep39374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida K, Moein A, Bittner T, et al. Pharmacokinetics and pharmacodynamic effect of crenezumab on plasma and cerebrospinal fluid beta-amyloid in patients with mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther. 2020;12(1):16. doi: 10.1186/s13195-020-0580-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferl GZ, Fuji RN, Atwal JK, Sun T, Ramanujan S, Quartino AL. Mechanistic modeling of soluble Aβ dynamics and target engagement in the brain by anti-Aβ mAbs in Alzheimer’s disease. Curr Alzheimer Res. 2020;17(4):393-406. doi: 10.2174/1567205017666200302122307 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocols
eMethods
eTable 1. Primary and Secondary Outcomes for CREAD2
eTable 2. Pooled CREAD and CREAD2 Demographic and Baseline Characteristics for Individuals with Prodromal AD and Mild ADa
eTable 3. Demographic and Baseline Characteristics for Participants in the CREAD and CREAD2 Biomarker Substudies
eTable 4. Mean Changes from Baseline in Imaging and Core CSF Biomarker Concentrations
eTable 5. Unadjusted Mean Changes from Baseline in Plasma Aβ42, Aβ40, NfL, and [18F]GTP1 Tau PET
eTable 6. CREAD: CSF and PET Biomarkers, Baseline Characteristics
eTable 7. CREAD2: CSF and PET biomarkers, Baseline Characteristics
eTable 8. CREAD: CSF and PET Biomarker Outcomes
eTable 9. CREAD2 CSF and PET Biomarker Outcomes
eFigure 1. Mean Difference in Mean Change from Baseline to Week 105 vs Placebo for Exploratory Analysis Efficacy Measures in Prodromal AD vs Mild AD Dementia in Pooled CREAD and CREAD2 Population (mITT)
eFigure 2. Enrollment, Randomization, and Study Completion in Pooled CREAD Substudies
eFigure 3. Least Squares Percent Mean Change from Baseline in Whole Brain (A/D), Ventricular (B/E), and Hippocampal Volume (C/F) in CREAD/CREAD2
eFigure 4. Change from Baseline [18F]florbetapir Amyloid PET (A) at Weeks 53 and 105, and [18F]GTP1 Tau PET (B) at Week 53
eFigure 5. Mean Change from Baseline in CSF Total Aβ42 (A), Total Aβ40 (B), Aβ Oligomer (C), tTau (D), and pTau181 (E)
eFigure 6. Mean Change from Baseline for Exploratory CSF biomarkers
eFigure 7. Plasma Total Aβ42 (A), Total Aβ40 (B), and NfL (C)
eFigure 8. Relationship between crenezumab concentrations and Aβ40 and Aβ42
eFigure 9. Concordance of [18F]florbetapir Amyloid PET visual read with CSF biomarkers of Aβ42 (A), pTau181/Aβ42 ratio (B), and tTau/Aβ42 ratio (C)
eAppendix. List of study sites and principal investigators in CREAD and CREAD2
eReferences
Data sharing statement