This cohort study examines whether perioperative gabapentin use among older adults after major surgery is associated with in-hospital adverse clinical events.
Key Points
Question
Is perioperative gabapentin use associated with in-hospital adverse clinical events among older adults after major surgery?
Findings
In this cohort study of 237 872 propensity score–matched adults aged 65 years or older, perioperative gabapentin users had significantly increased risk of delirium, new antipsychotic use, and pneumonia compared with nonusers after major surgery.
Meaning
This study suggests that careful risk-benefit assessment is needed before prescribing gabapentin for perioperative pain management to older patients.
Abstract
Importance
Gabapentin has been increasingly used as part of a multimodal analgesia regimen to reduce opioid use in perioperative pain management. However, the safety of perioperative gabapentin use among older patients remains uncertain.
Objective
To examine in-hospital adverse clinical events associated with perioperative gabapentin use among older patients undergoing major surgery.
Design, Setting, and Participants
This retrospective cohort study using data from the Premier Healthcare Database included patients aged 65 years or older who underwent major surgery at US hospitals within 7 days of hospital admission from January 1, 2009, to March 31, 2018, and did not use gabapentin before surgery. Data were analyzed from June 14, 2021, to May 23, 2022.
Exposures
Gabapentin use within 2 days after surgery.
Main Outcomes and Measures
The primary outcome was delirium, identified using diagnosis codes, and secondary outcomes were new antipsychotic use, pneumonia, and in-hospital death between postoperative day 3 and hospital discharge. To reduce confounding, 1:1 propensity score matching was performed. Risk ratios (RRs) and risk differences (RDs) with 95% CIs were estimated.
Results
Among 967 547 patients before propensity score matching (mean [SD] age, 76.2 [7.4] years; 59.6% female), the rate of perioperative gabapentin use was 12.3% (119 087 patients). After propensity score matching, 237 872 (118 936 pairs) gabapentin users and nonusers (mean [SD] age, 74.5 [6.7] years; 62.7% female) were identified. Compared with nonusers, gabapentin users had increased risk of delirium (4040 [3.4%] vs 3148 [2.6%]; RR, 1.28 [95% CI, 1.23-1.34]; RD, 0.75 [95% CI, 0.75 [0.61-0.89] per 100 persons), new antipsychotic use (944 [0.8%] vs 805 [0.7%]; RR, 1.17 [95% CI, 1.07-1.29]; RD, 0.12 [95% CI, 0.05-0.19] per 100 persons), and pneumonia (1521 [1.3%] vs 1368 [1.2%]; RR, 1.11 [95% CI, 1.03-1.20]; RD, 0.13 [95% CI, 0.04-0.22] per 100 persons), but there was no difference in in-hospital death (362 [0.3%] vs 354 [0.2%]; RR, 1.02 [95% CI, 0.88-1.18]; RD, 0.00 [95% CI, –0.04 to 0.05] per 100 persons). Risk of delirium among gabapentin users was greater in subgroups with high comorbidity burden than in those with low comorbidity burden (combined comorbidity index <4 vs ≥4: RR, 1.20 [95% CI, 1.13-1.27] vs 1.40 [95% CI, 1.30-1.51]; RD, 0.41 [95% CI, 0.28-0.53] vs 2.66 [95% CI, 2.08-3.24] per 100 persons) and chronic kidney disease (absence vs presence: RR, 1.26 [95% CI, 1.19-1.33] vs 1.38 [95% CI, 1.27-1.49]; RD, 0.56 [95% CI, 0.42-0.69] vs 1.97 [95% CI, 1.49-2.46] per 100 persons).
Conclusion and Relevance
In this cohort study, perioperative gabapentin use was associated with increased risk of delirium, new antipsychotic use, and pneumonia among older patients after major surgery. These results suggest careful risk-benefit assessment before prescribing gabapentin for perioperative pain management.
Introduction
Multimodal analgesia has been an increasingly adopted strategy in the Enhanced Recovery After Surgery pathway,1 which aims to reduce opioid use by using nonopioid analgesia, such as regional or epidural analgesia, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentinoids.2 Randomized clinical trials (RCTs) have demonstrated that multimodal analgesia improves pain control3,4,5 and decreases opioid use and its adverse effects.2,3,4,5,6,7 The American Pain Society recommends gabapentin as a component of multimodal analgesia.8 Although the contribution of perioperative gabapentin use to the overall population trend remains unknown, gabapentin use tripled from 2002 to 2015 and has been the 10th most prescribed drug in the US since 2016.9,10
Despite the widespread use of gabapentin, recent studies raised concerns about the marginal benefit and immediate harms of gabapentin use for perioperative pain management.11 Studies,12,13,14 including meta-analyses of RCTs, concluded that the evidence on gabapentin’s effectiveness is low quality owing to inconsistent and imprecise results and that its analgesic and opioid-sparing effects may be clinically insignificant. Moreover, perioperative gabapentin use was associated with dizziness and visual disturbances.13 Although there were no statistically significant differences in perioperative delirium, respiratory failure, ataxia, or falls,13 the studies did not exclude the possibility of clinically meaningful adverse events owing to small sample sizes, underrepresentation of older patients, and heterogenous surgical procedures. The American Geriatrics Society Beers Criteria15 lists gabapentin as a potentially inappropriate medication owing to its risk of sedation and respiratory depression, especially when used with opioids. Because older surgical patients are vulnerable to these adverse effects and are at increased risk of perioperative delirium, pneumonia, and death, there is a need to examine the safety of perioperative gabapentin use using a health care database of the general population that includes a large number of older adults undergoing different types of surgical procedures.
We conducted a retrospective cohort study to investigate the association of perioperative gabapentin use with in-hospital adverse clinical events using a nationwide administrative inpatient database of older adults undergoing major surgery. We hypothesized that gabapentin use would be associated with increased risk of delirium, pneumonia, and in-hospital death.
Methods
Data Source
This retrospective cohort study using the Premier Healthcare Database was approved by the institutional review board of Brigham and Women’s Hospital, and a waiver of informed consent was obtained because the data were deidentified. The Premier Healthcare Database is a deidentified, hospital-based, service-level, all-payer database containing more than 900 small-sized to medium-sized hospitals that covers approximately 25% of annual inpatient admissions in the US. The database contains information on demographic characteristics, admission status, diagnosis codes, discharge status, and date-stamped records of drugs and procedures. This nationwide database has been used to investigate the safety of medical interventions in the inpatient care setting.16,17,18 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
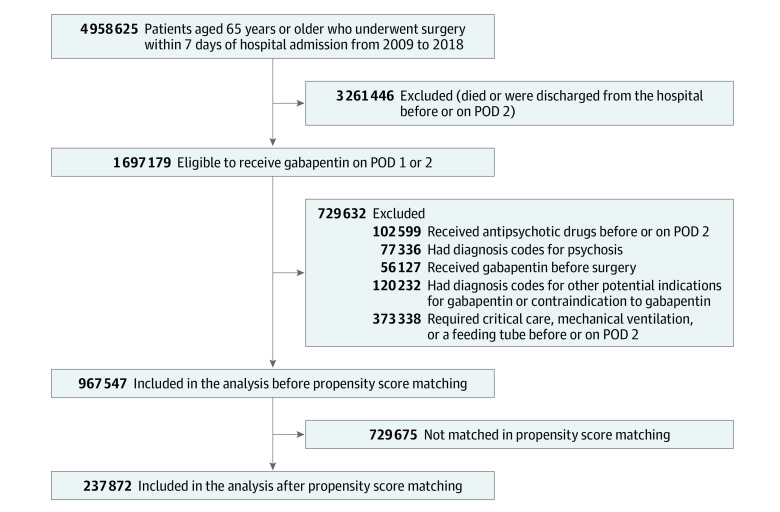
The analytic sample included adults aged 65 years or older who underwent major surgical procedures within 7 days of hospital admission from January 1, 2009, to March 31, 2018. Major surgical procedures included cardiac, gastrointestinal, genitourinary, orthopedic, neurological (excluding procedures involving the brain), thoracic, and vascular surgery, as defined by the Agency for Healthcare Research and Quality procedure classification.19,20 We excluded patients who died or were discharged before or on postoperative day 2 because we defined gabapentin use based on the exposure on the day of surgery or on postoperative days 1 or 2 (exposure-defining period). Because gabapentin is typically started before surgery and continued postoperatively, we refer to this regimen as perioperative use. We also excluded patients who had diagnosis codes for psychosis, received antipsychotics before or on postoperative day 2, received gabapentin before the day of surgery, or had diagnosis codes for other potential indications for gabapentin (alcohol use disorder, alcohol withdrawal, fibromyalgia, neuropathic pain, postherpetic neuralgia, restless legs syndrome, seizure, and social anxiety disorder) or contraindications to gabapentin (myasthenia gravis). Moreover, patients who received critical care, mechanical ventilation, or a feeding tube before or on postoperative day 2 were excluded because they had a higher acuity of illness and were unlikely to receive gabapentin orally.
Measurement of Perioperative Gabapentin Use and Covariates
We defined perioperative gabapentin use based on charge codes, which identify each service item (eg, medications and procedures) for billing and reimbursement purposes, on the day of surgery or postoperative day 1 or 2. We calculated total daily gabapentin dose (in milligrams) given during the exposure-defining period. To avoid immortal time bias,21 the group that used gabapentin and the group that did not use gabapentin were required to have survived the exposure-defining period.
The following patient-level characteristics were assessed: age, sex, race and ethnicity (Black, White, or other [American Indian, Asian/Pacific Islander, Hispanic, and other]), insurance type (commercial, Medicare, Medicaid, or other), admission type (elective, urgent, emergent, or other) and source (outpatient, emergency department, transfer, or other), surgery type (cardiac, gastrointestinal, genitourinary, orthopedic, neurological, thoracic, or vascular), combined comorbidity index (scores range from −2 to 26, with higher scores indicating greater risk of death),22 and comorbidity diagnoses. Race and ethnicity were obtained from Uniform Billing Code of 1992 billing forms. Inpatient medication use, including analgesics (acetaminophen, cyclooxygenase-2 inhibitors, nonsteroidal anti-inflammatory drugs, and opioid use and dose in morphine milligram equivalent [MME] per day), and receipt of cardiopulmonary resuscitation, dialysis, or blood transfusion before surgery and during the first 2 postoperative days were measured. We also obtained hospital bed capacity, teaching status, location (urban or rural), and geographic region. To account for changes in gabapentin-prescribing patterns and surgical outcomes over time, we recorded calendar year of the hospital admission.
Study Outcomes
The primary outcome was delirium, identified using a validated claims-based algorithm.23,24 This algorithm, which consists of explicit (ie, delirium is directly mentioned) and implicit (eg, encephalopathy) diagnosis codes of delirium (eTable 1 in the Supplement), has a positive predictive value of 80% against the Confusion Assessment Method, as validated in a previous study of 184 patients.23 As secondary outcomes, we assessed (1) new antipsychotic use, which has a positive predictive value of 92% for delirium23; (2) pneumonia, which was defined based on diagnosis codes plus intravenous antibiotic use or computed tomography of the chest; and (3) in-hospital death. Analogous to the intention-to-treat analysis in an RCT, patients were followed up from postoperative day 3 until the occurrence of the outcomes or hospital discharge regardless of the presence or duration of gabapentin therapy.
Statistical Analysis
Data were analyzed from June 14, 2021, to May 23, 2022. To reduce confounding, we performed propensity score matching.25,26 A propensity score was estimated as the probability of receiving gabapentin from a logistic regression model that included all patient- and hospital-level characteristics. We conducted 1:1 nearest-neighbor matching and assessed covariate balance based on the standardized mean difference (<0.1 was considered adequate).25 We estimated risk ratios (RRs), risk differences (RDs), and 95% CIs for delirium, new antipsychotic use, pneumonia, and in-hospital death associated with gabapentin use. We also examined the association in subgroups defined by age (<80 or ≥80 years), sex, comorbidity burden (combined comorbidity index <4 or ≥4), chronic kidney disease status (presence or absence), opioid dose (MME<15 mg per day or ≥15 mg per day), and surgery type (cardiac, gastrointestinal, genitourinary, orthopedic, neurological, thoracic, or vascular). Within each subgroup, we reestimated the propensity score, and performed 1:1 matching; we tested heterogeneity across subgroups.27 Two-sided P < .05 for heterogeneity was considered significant. We performed 3 sensitivity analyses regarding (1) gabapentin exposure present on the day of surgery, (2) dose-response relationship, and (3) unmeasured confounding. First, we repeated the analysis by defining gabapentin exposure on the day of surgery without requiring a 2-day minimum length of stay after surgery. Then, we explored a dose-response relationship using a 4-dose category of gabapentin (no use, 1 mg to <600 mg, 600 mg to <1200 mg, or ≥1200 mg). Because 4-group propensity score matching was not feasible, we used multivariable logistic regression models to adjust for covariates selected by a stepwise algorithm with a 2-sided P value threshold of 0.10 for entry and removal of a covariate from the model. In addition, because pain intensity may be an unmeasured confounder of the association between gabapentin use and delirium, we examined how the RR estimate would change under various scenarios: (1) the prevalence difference in severe pain between gabapentin users and nonusers and (2) the relative risk between severe pain and delirium from the literature (severe perioperative pain was associated with a 1.2-fold increase in delirium).28 Analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Characteristics of Study Population
After applying inclusion and exclusion criteria, 967 547 patients (mean [SD] age, 76.2 [7.4] years; 59.6% female) were eligible to initiate gabapentin for perioperative pain management (Figure 1). Of these patients, 119 087 (12.3%; mean [SD] age, 74.5 [6.7] years; 62.7% female) received gabapentin between the day of surgery (108 190 [90.8%]) and 2 days after surgery. The most common surgery type among gabapentin users was orthopedic (91 014 [76.4%]). Compared with those who did not use gabapentin, gabapentin users were younger (mean [SD] age, 74.5 [6.7] vs 76.4 [7.5] years), were more likely to be female (74 627 [62.7%] vs 501 934 [59.2%]), underwent more elective surgical procedures (93 674 [78.7%] vs 422 262 [49.8%]), and had a lower comorbidity index (mean [SD], 1.2 [2.2] vs 1.8 [2.6]). However, gabapentin users were more frequently treated with analgesics, including opioids (109 389 [91.9%] vs 686 293 [80.9%]), a higher daily opioid dose (mean [SD], 7.6 [6.1] MME vs 5.8 [5.6] MME), and psychoactive drugs, such as antidepressants (29 772 [25.0%] vs 141 942 [16.7%]) and anxiolytics (84 529 [71.0%] vs 525 736 [62.0%]) (Table 1; the complete covariate list is given in eTable 2 in the Supplement). After 1:1 propensity score matching, we identified 237 872 (118 936 pairs) gabapentin user and nonusers. Baseline characteristics were well balanced (standardized mean difference, <0.1) in the propensity score–matched cohort (Table 1).
Figure 1. Flowchart of Study Population Selection.
POD indicates postoperative day.
Table 1. Characteristics of Patients by Perioperative Gabapentin Use Before and After Propensity Score Matching.
| Characteristic | Before propensity score matching | After propensity score matchinga | ||||
|---|---|---|---|---|---|---|
| Gabapentin use (n = 119 087)b | No gabapentin use (n = 848 460)b | SMD | Gabapentin use (n = 118 936)b | No gabapentin use (n = 118 936)b | SMD | |
| Age, mean (SD), y | 74.5 (6.7) | 76.4 (7.5) | –0.27 | 74.5 (6.7) | 74.4 (6.8) | 0.01 |
| Sex | ||||||
| Female | 74 627 (62.7) | 501 934 (59.2) | 0.07 | 74 525 (62.7) | 74 682 (62.8) | <0.01 |
| Male | 44 460 (37.3) | 346 526 (40.8) | −0.07 | 44 411 (37.3) | 44 254 (37.2) | <0.01 |
| Race and ethnicity | ||||||
| Black | 9231 (7.8) | 62 406 (7.4) | 0.01 | 9215 (7.7) | 9274 (7.8) | <0.01 |
| White | 98 562 (82.8) | 697 010 (82.2) | 0.02 | 98 438 (82.8) | 98 490 (82.8) | <0.01 |
| Otherc | 11 294 (9.5) | 89 044 (10.5) | –0.03 | 11 283 (9.5) | 11 172 (9.4) | <0.01 |
| Insurance type | ||||||
| Medicare | 106 860 (89.7) | 764 277 (90.1) | –0.01 | 106 727 (89.7) | 106 931 (89.9) | –0.01 |
| Medicaid | 1704 (1.4) | 10 998 (1.3) | 0.01 | 1699 (1.4) | 1618 (1.4) | 0.01 |
| Commercial | 8115 (6.8) | 58 163 (6.9) | <0.01 | 8106 (6.8) | 8078 (6.8) | <0.01 |
| Other | 1270 (1.1) | 7806 (0.9) | 0.01 | 1269 (1.1) | 1213 (1.0) | <0.01 |
| Uninsured | 1138 (1.0) | 7216 (0.9) | 0.01 | 1135 (1) | 1096 (0.9) | <0.01 |
| Hospital admission type | ||||||
| Elective | 93 674 (78.7) | 422 262 (49.8) | 0.63 | 93 523 (78.6) | 94 096 (79.1) | –0.01 |
| Urgent | 7368 (6.2) | 85 342 (10.1) | –0.14 | 7368 (6.2) | 7258 (6.1) | <0.01 |
| Emergent | 16 780 (14.1) | 327 675 (38.6) | –0.58 | 16 780 (14.1) | 16 256 (13.7) | 0.01 |
| Other | 1265 (1.1) | 13 181 (1.6) | –0.04 | 1265 (1.1) | 1326 (1.1) | <0.01 |
| Surgery type | ||||||
| Cardiac | 624 (0.5) | 16 391 (1.9) | –0.13 | 624 (0.5) | 556 (0.5) | 0.01 |
| Gastrointestinal | 15 866 (13.3) | 195 395 (23.0) | –0.25 | 15 863 (13.3) | 15 249 (12.8) | 0.02 |
| Genitourinary | 2523 (2.1) | 48 933 (5.8) | –0.19 | 2523 (2.1) | 2461 (2.1) | <0.01 |
| Neurological | 3096 (2.6) | 12 085 (1.4) | 0.08 | 3088 (2.6) | 3217 (2.7) | –0.01 |
| Orthopedic | 91 014 (76.4) | 498 258 (58.7) | 0.39 | 90 875 (76.4) | 91 315 (76.8) | –0.01 |
| Thoracic | 1549 (1.3) | 20 288 (2.4) | –0.08 | 1549 (1.3) | 1585 (1.3) | <0.01 |
| Vascular | 4415 (3.7) | 57 110 (6.7) | –0.14 | 4414 (3.7) | 4553 (3.8) | –0.01 |
| CCI score, mean (SD)d | 1.2 (2.2) | 1.8 (2.6) | –0.24 | 1.2 (2.2) | 1.1 (2.2) | 0.01 |
| Comorbidities | ||||||
| Any tumor | 13 798 (11.6) | 137 070 (16.2) | –0.13 | 13 788 (11.6) | 13 474 (11.3) | 0.01 |
| Chronic pulmonary disease | 25 542 (21.4) | 180 276 (21.2) | <0.01 | 25 490 (21.4) | 25 196 (21.2) | 0.01 |
| Congestive heart failure | 12 625 (10.6) | 121 638 (14.3) | –0.11 | 12 607 (10.6) | 12 551 (10.6) | <0.01 |
| Dementia | 3311 (2.8) | 49 664 (5.9) | –0.15 | 3310 (2.8) | 3149 (2.6) | 0.01 |
| Kidney failure | 2846 (2.4) | 45 468 (5.4) | –0.15 | 2846 (2.4) | 2856 (2.4) | <0.01 |
| Analgesic drugs | ||||||
| Acetaminophen | 102 013 (85.7) | 598 803 (70.6) | 0.37 | 101 867 (85.6) | 102 237 (86.0) | –0.01 |
| Cyclooxygenase-2 inhibitor | 24 837 (20.9) | 65 370 (7.7) | 0.38 | 24 764 (20.8) | 24 432 (20.5) | 0.01 |
| NSAID | 26 822 (22.5) | 124 966 (14.7) | 0.20 | 26 771 (22.5) | 26 921 (22.6) | <0.01 |
| Opioid | 109 389 (91.9) | 686 293 (80.9) | 0.32 | 109 238 (91.8) | 109 561 (92.1) | –0.01 |
| Opioid dose, MME/d, mean (SD) | 7.6 (6.1) | 5.8 (5.6) | 0.31 | 7.6 (6.1) | 7.7 (6.3) | <0.01 |
| Psychoactive drugs | ||||||
| Alzheimer disease agents | 2943 (2.5) | 29 494 (3.5) | –0.06 | 2942 (2.5) | 2831 (2.4) | 0.01 |
| Antidepressants | 29 772 (25.0) | 141 942 (16.7) | 0.20 | 29 659 (24.9) | 29 720 (25.0) | <0.01 |
| Anxiolytics | 84 529 (71.0) | 525 736 (62.0) | 0.19 | 84 411 (71.0) | 84 662 (71.2) | <0.01 |
| Sedatives | 7805 (6.6) | 42 346 (5.0) | 0.07 | 7784 (6.5) | 7762 (6.5) | <0.01 |
| Hospital characteristic | ||||||
| Teaching | 55 930 (47.0) | 367 537 (43.3) | 0.07 | 55 843 (47.0) | 55 577 (46.7) | <0.01 |
| Urban | 107 083 (89.9) | 750 848 (88.5) | 0.05 | 106 944 (89.9) | 106 680 (89.7) | 0.01 |
| Geographic region | ||||||
| Northeast | 27 825 (23.4) | 182 705 (21.5) | 0.04 | 27 776 (23.4) | 27 529 (23.1) | <0.01 |
| Midwest | 18 704 (15.7) | 135 019 (15.9) | –0.01 | 18 690 (15.7) | 18 639 (15.7) | <0.01 |
| South | 50 055 (42.0) | 382 716 (45.1) | –0.06 | 50 006 (42.0) | 50 672 (42.6) | –0.01 |
| West | 22 503 (18.9) | 148 020 (17.4) | 0.04 | 22 464 (18.9) | 22 096 (18.6) | 0.01 |
Abbreviations: CCI, combined comorbidity index; MME, morphine milligram equivalent; NSAID, nonsteroidal anti-inflammatory drug; SMD, standardized mean difference.
The propensity score model included demographic information, insurance type, admission characteristics, surgery type, combined comorbidity score, comorbidities, inpatient medication use and procedures before or on postoperative day 2, hospital-level characteristics, geographic region, and calendar year.
Data are reported as number (percentage) of patients unless otherwise indicated.
Included individuals who identified as American Indian, Asian/Pacific Islander, Hispanic, or other.
Scores range from −2 to 26, with higher scores indicating greater risk of death.
Adverse Clinical Events Associated With Perioperative Gabapentin Use
Before propensity score matching, gabapentin users had lower risk of adverse clinical events than nonusers (Table 2). After propensity score matching, gabapentin users had increased risk (RR and RD) of delirium (4040 [3.4%] vs 3148 [2.6%]; RR, 1.28 [95% CI, 1.23-1.34]; RD, 0.75 [95% CI, 0.61-0.89] per 100 persons), new antipsychotic use (944 [0.8%] vs 805 [0.7%]; RR, 1.17 [95% CI, 1.07-1.29]; RD, 0.12 [95% CI, 0.05-0.19] per 100 persons), and pneumonia (1521 [1.3%] vs 1368 [1.2%]; RR, 1.11 [95% CI, 1.03-1.20]; RD, 0.13 [95% CI, 0.04-0.22] per 100 persons) compared with nonusers. In-hospital death was similar between gabapentin users and nonusers (362 [0.3%] vs 354 [0.2%]; RR, 1.02 [95% CI, 0.88-1.18]; RD, 0.00 [95% CI, –0.04 to 0.05] per 100 persons).
Table 2. Association Between Perioperative Gabapentin Use and In-Hospital Adverse Clinical Events After Major Surgery Before and After Propensity Score Matching.
| Outcome | Before propensity score matching | After propensity score matchinga | ||||||
|---|---|---|---|---|---|---|---|---|
| Gabapentin use, No. (%) (n = 119 087) | No gabapentin use, No. (%) (n = 848 460) | RR (95% CI) | RD, per 100 persons (95% CI) | Gabapentin use, No. (%) (n = 118 936) | No gabapentin use, No. (%) (n = 118 936) | RR (95% CI) | RD, per 100 persons (95% CI) | |
| Delirium diagnosis | 4051 (3.4) | 34 342 (4.0) | 0.84 (0.81 to 0.87) | –0.6 (–0.8 to –0.5) | 4040 (3.4) | 3148 (2.6) | 1.28 (1.23 to 1.34) | 0.75 (0.61 to 0.89) |
| New antipsychotic use | 945 (0.8) | 9877 (1.2) | 0.68 (0.64 to 0.73) | –0.4 (–0.4 to –0.3) | 944 (0.8) | 805 (0.7) | 1.17 (1.07 to 1.29) | 0.12 (0.05 to 0.19) |
| Pneumonia | 1522 (1.3) | 19 902 (2.3) | 0.54 (0.52 to 0.57) | –1.1 (–1.1 to –1.0) | 1521 (1.3) | 1368 (1.2) | 1.11 (1.03 to 1.20) | 0.13 (0.04 to 0.22) |
| In-hospital death | 363 (0.3) | 6360 (0.7) | 0.41 (0.37 to 0.45) | –0.4 (–0.5 to –0.4) | 362 (0.3) | 354 (0.2) | 1.02 (0.88 to 1.18) | 0.00 (–0.04 to 0.05) |
Abbreviations: RD, risk difference; RR, risk ratio.
The propensity score model included demographic information, insurance type, admission characteristics, surgery type, combined comorbidity score, comorbidities, inpatient medication use and procedures before or on postoperative day 2, hospital-level characteristics, geographic region, and calendar year.
Subgroup Analyses
Across the subgroups defined by age, sex, comorbidity burden, chronic kidney disease status, and opioid dose, the associations of gabapentin use with delirium, new antipsychotic use, and pneumonia were consistent (Figure 2 and Figure 3). The RR of delirium was greater among gabapentin users younger than 80 years than among those aged 80 years or older (1.34 [95% CI, 1.27-1.43] vs 1.21 [95% CI, 1.12-1.30]; P = .02 for heterogeneity), but the RD was similar (0.71 [95% CI, 0.57-0.86] vs 0.91 [95% CI, 0.56-1.27] per 100 persons; P = .31 for heterogeneity). The RR and RD were greater among patients with high comorbidity burden (≥4) than among those with low comorbidity burden (<4) (RR, 1.40 [95% CI, 1.30-1.51] vs 1.20 [95% CI, 1.13-1.27]; P = .001 for heterogeneity; RD, 2.66 [95% CI, 2.08-3.24] vs 0.41 [95% CI, 0.28-0.53] per 100 persons; P < .001 for heterogeneity) and among patients with chronic kidney disease (absence vs presence: RR, 1.26 [95% CI, 1.19-1.33] vs 1.38 [95% CI, 1.27-1.49]). Subgroup estimates differed only on the RD scale for risk of delirium by chronic kidney disease status (absence vs presence: RD, 0.56 [95% CI, 0.42-0.69] vs 1.97 [95% CI, 1.49-2.46] per 100 persons; P < .001 for heterogeneity), risk of new antipsychotic use by comorbidity burden (<4 vs ≥4: 0.09 [95% CI, 0.03-0.15] vs 0.65 [0.33-0.97] per 100 persons; P = .001 for heterogeneity), and risk of pneumonia by comorbidity burden (<4 vs ≥4: 0.15 [95% CI, 0.07-0.22] vs 0.66 [0.25-1.07] per 100 persons; P = .02 for heterogeneity) and chronic kidney disease status (absence vs presence: 0.14 [95% CI, 0.06-0.23] vs 0.47 [95% CI, 0.17-0.77] per 100 persons; P = .04 for heterogeneity). There was no statistically significant evidence for heterogeneity by sex or opioid dose on the RR or RD scale. Because of the small number of clinical events, certain surgery-specific estimates were imprecise, but in general, the associations were consistent with increased risk of outcomes with gabapentin use (eTable 3 in the Supplement). Perioperative gabapentin use was not associated with in-hospital death in all subgroups.
Figure 2. Subgroup Analysis of the Association Between Perioperative Gabapentin Use and Delirium Diagnosis and New Antipsychotic Use.
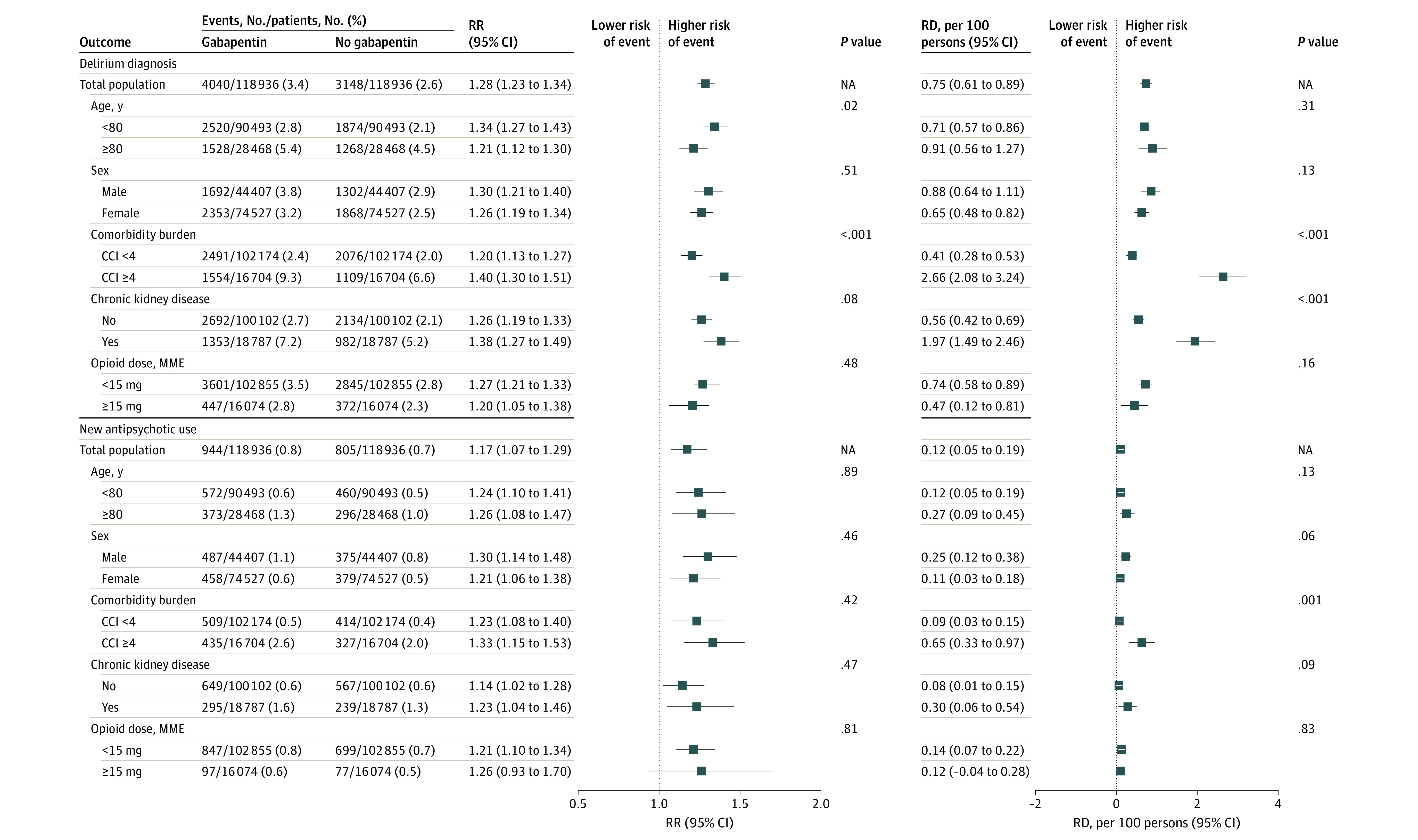
Propensity score matching was performed within each subgroup. The propensity score model included demographic information, insurance type, admission characteristics, surgery type, combined comorbidity score, comorbidities, inpatient medication use and procedures before or on postoperative day 2, hospital-level characteristics, geographic region, and calendar year. Combined comorbidity index (CCI) scores range from −2 to 26, with higher scores indicating greater risk of death. All P values are for heterogeneity. Markers indicate estimates, with horizontal lines indicating 95% CIs. MME indicates morphine milligram equivalent; RD, risk difference; and RR, risk ratio.
Figure 3. Subgroup Analysis of the Association Between Perioperative Gabapentin Use and Pneumonia and In-Hospital Death.
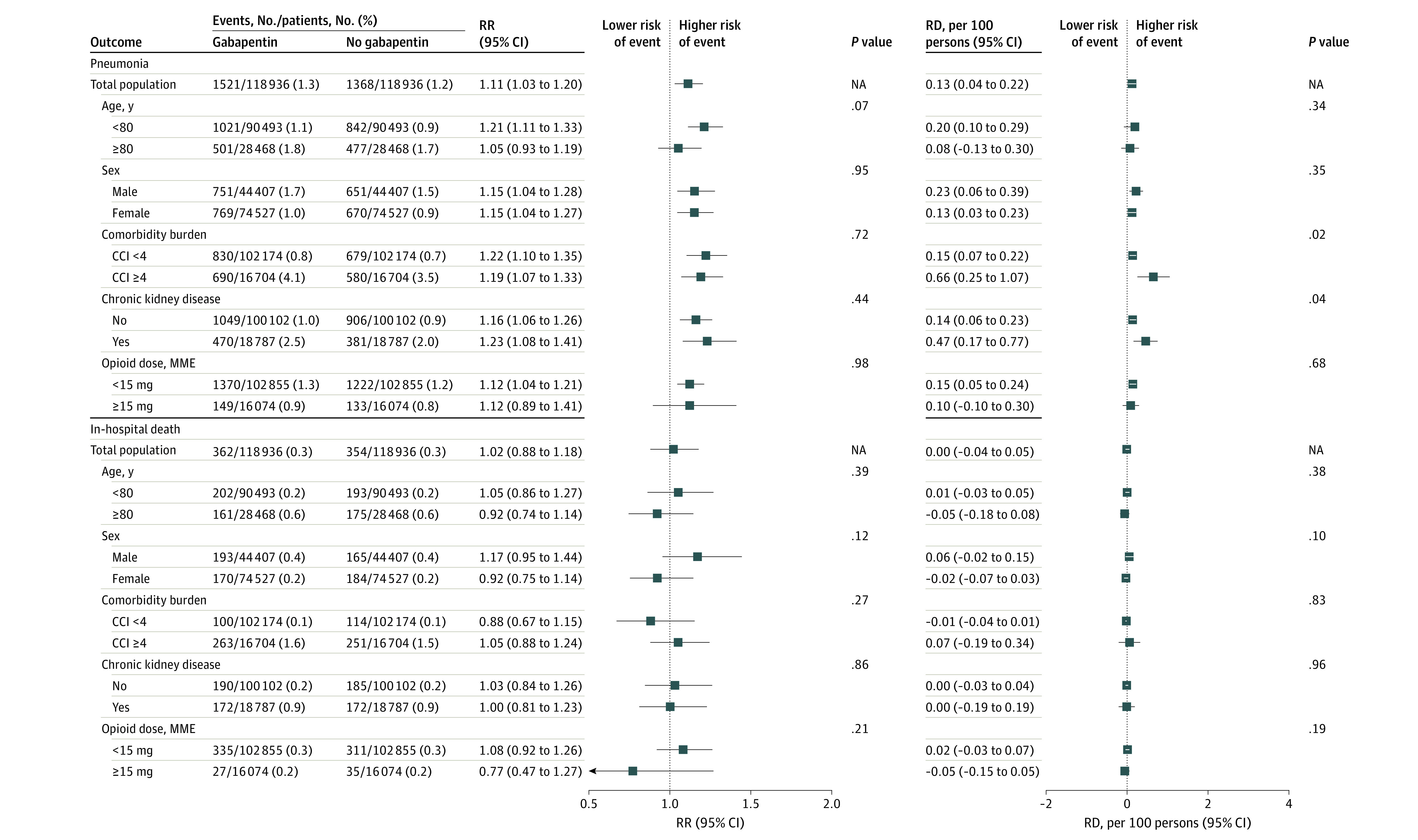
Propensity score matching was performed within each subgroup. The propensity score model included demographic information, insurance type, admission characteristics, surgery type, combined comorbidity score, comorbidities, inpatient medication use and procedures before or on postoperative day 2, hospital-level characteristics, geographic region, and calendar year. Combined comorbidity index (CCI) scores range from −2 to 26, with higher scores indicating greater risk of death. All P values are for heterogeneity. Markers indicate estimates, with horizontal lines indicating 95% CIs. MME indicates morphine milligram equivalent; RD, risk difference; and RR, risk ratio.
Sensitivity Analyses
When gabapentin exposure was defined on the day of surgery without requiring a minimum 2-day length of stay after surgery, the results were unchanged from our primary analysis (eTable 4 in the Supplement). On multivariable logistic regression analysis (eTable 5 in the Supplement), increasing gabapentin dose was associated with progressively increased risk of delirium diagnosis (1 mg to <600 mg: adjusted odds ratio [AOR], 1.25 [95% CI, 1.18-1.32]; 600 mg to <1200 mg: AOR, 1.30 [95% CI, 1.24-1.36]; ≥1200 mg: AOR, 1.43 [95% CI, 1.28-1.60]) and pneumonia (1 mg to <600 mg: AOR, 1.05 [95% CI, 0.96-1.14]; 600 mg to <1200 mg: AOR, 1.16 [95% CI, 1.07-1.25]; ≥1200 mg: AOR, 1.34 [95% CI, 1.13-1.59]) but not with new antipsychotic use and in-hospital death. In a sensitivity analysis for unmeasured confounding (eFigure in the Supplement), we found that the RR of 1.28 in our study would become null if (1) the prevalence difference of severe pain was at least 30% between the treatment groups or (2) the RR between severe pain and delirium was at least 2.0, which was greater than previously reported in the literature (ie, >2.0).28 These results suggest that unmeasured, severe pain alone is unlikely to explain our results.
Discussion
In this cohort study, we found that perioperative gabapentin use was associated with modestly increased risk of delirium, new antipsychotic use, and pneumonia but not with in-hospital death among adults aged 65 years or older after major surgery. Considering the increasing number of major surgeries performed in older adults29,30 and the negative consequences of perioperative delirium, our findings raise concern about an increasingly adopted clinical practice that involves routine use of gabapentin as part of multimodal analgesia. Our study provides evidence on the safety of perioperative gabapentin use in a representative population of older patients undergoing surgery as part of routine care.
To our knowledge, more than 200 RCTs have been conducted to evaluate the effect of gabapentin on perioperative pain control, reduction in opioid use, and adverse events. The trials varied in terms of sample size (20-697 patients),31,32 gabapentin regimen (single dose vs continued treatment with daily dose ranging from 300 mg to 1200 mg), surgery type (orthopedic, abdominal, and vascular), and study quality (low to high risk of bias). Several meta-analyses3,4,5,12,13,33,34 concluded that reductions in pain intensity 24 hours after surgery and opioid-related adverse events associated with gabapentin and placebo were inconsistent and not clinically meaningful. An RCT by Hah et al35 showed that perioperative gabapentin use had no effect on time to cessation of perioperative pain but reduced the median time to opioid cessation after surgery (25 days vs 32 days) compared with lorazepam. The rate of drug discontinuation owing to sedation or dizziness (25% in the gabapentin group and 20% in the lorazepam group) was not statistically significant, possibly because of the use of lorazepam as an active comparator. Some experts caution that the immediate harm of gabapentin may outweigh the long-term benefits of opioid cessation among older adults.11 A recent meta-analysis13 found higher rates of dizziness and visual disturbances after use of gabapentin, with no statistically significant associations with respiratory failure, ataxia, falls, or delirium. However, owing to the small sample size and underrepresentation of older adults in RCTs, the safety of perioperative gabapentin use in this population remains uncertain.
The main site of action of gabapentin is on the α2-δ subunit of calcium channels that are found in the peripheral and central nervous system,36,37 which may explain its adverse effects, such as dizziness, visual disturbance, sedation, and confusion.37 The same mechanism may also explain the increased risk of delirium, new antipsychotic use, and aspiration pneumonia. In addition, because gabapentin is solely metabolized through the kidneys, adverse effects may lead to more severe clinical consequences (eg, delirium and respiratory depression)3,38,39 among older patients with a higher prevalence of multimorbidity and chronic kidney disease. It was hypothesized that gabapentin may prevent delirium by improving pain control and reducing opioid dose, whereas gabapentin-related adverse effects may increase delirium.40 To date, several studies investigated whether gabapentin could reduce perioperative delirium. In a post hoc analysis of an RCT of 161 patients (mean age, 63 years) undergoing total knee replacement, Dighe et al41 reported that 12.0% of patients in the gabapentin group and 9% of patients in the placebo group developed delirium (P = .53). In another RCT of 697 patients (mean age, 73 years) undergoing orthopedic surgery, Leung et al32 showed that 24.0% in the gabapentin group and 20.8% in the placebo group had delirium (P = .30). However, the difference in the delirium incidence was not statistically significant. The RR estimates of delirium from these studies were consistent with our findings.
Limitations
Our study has limitations. The findings should be interpreted within the limitations of an administrative database study. First, delirium incidence in the present study population (3.4%) was lower compared with a previously reported incidence of 15% to 25% after major surgery42 owing to low sensitivity (18%) and high specificity (98%) of the present study’s delirium identification algorithm.23 This delirium identification algorithm was better at identifying hyperactive delirium, which was associated with poorer prognosis compared with hypoactive or normoactive delirium.43 For these reasons, our RD estimates may have been underestimated. Moreover, the diagnosis codes for delirium and pneumonia did not have an exact onset date in our data sets; thus, these outcomes may have been present before surgery in some patients. Second, confounding was possible. Gabapentin users were healthier and more likely to have an elective surgery compared with nonusers. After propensity score matching, the RR estimate increased from 0.84 to 1.28. If residual confounding was present in the same direction, the true RR would have been higher than the value after propensity score matching. Our sensitivity analysis suggests that unmeasured pain severity alone was unlikely to explain our results. Although the association between gabapentin and delirium did not differ by opioid dose, patients who received a higher dose had lower absolute risk of outcomes, which suggests that those patients may have been healthier than those who received a lower dose. Therefore, this subgroup analysis should be interpreted with caution. Third, outpatient medication use was unavailable in the Premier Healthcare Database. To identify patients eligible to newly receive gabapentin for perioperative pain management, we excluded those who received gabapentin before surgery, had other indications or contraindications for gabapentin, or received critical care, mechanical ventilation, or a feeding tube in the immediate perioperative period. In addition, we required at least a 2-day length of stay after surgery to define gabapentin exposure status, which may have limited the generalizability of our findings. However, the results did not change when we defined the exposure on the day of surgery without requiring a minimum length of stay after surgery.
Conclusions
In this cohort study, perioperative gabapentin use was associated with increased risk of delirium, new antipsychotic use, and pneumonia among older patients after major surgery. On the basis of these findings and those of meta-analyses of RCTs12,13,14 showing a weak opioid-sparing effect of gabapentin, clinicians should reconsider routine use of gabapentin for perioperative pain management among older adults and individualize the treatment decision after assessing the risk of immediate harms vs opioid-sparing benefits of perioperative gabapentin use. For older patients who receive gabapentin as part of multimodal analgesia, daily assessment of the appropriateness of gabapentin use may be necessary to avoid unintended harm.
eTable 1. Diagnosis Codes for Delirium
eTable 2. Characteristics of Patients by Perioperative Gabapentin Use Before and After Propensity Score Matching (Complete List of Covariates)
eTable 3. Subgroup Analysis of Perioperative Gabapentin Use and In-Hospital Adverse Clinical Events by Surgery Type
eTable 4. Sensitivity Analysis for Patients Who Received Gabapentin vs Nonusers on the Day of Surgery
eTable 5. Sensitivity Analysis for the Dose-Response Relationship Between Gabapentin and In-Hospital Adverse Clinical Events
eFigure. Sensitivity Analysis for Unmeasured Confounding by Severe Pain
References
- 1.Beverly A, Kaye AD, Ljungqvist O, Urman RD. Essential elements of multimodal analgesia in Enhanced Recovery After Surgery (ERAS) guidelines. Anesthesiol Clin. 2017;35(2):e115-e143. doi: 10.1016/j.anclin.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 2.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697. doi: 10.1001/jamasurg.2017.0898 [DOI] [PubMed] [Google Scholar]
- 3.Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: a meta-analysis. Can J Anaesth. 2006;53(5):461-469. doi: 10.1007/BF03022618 [DOI] [PubMed] [Google Scholar]
- 4.Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;126(1-3):91-101. doi: 10.1016/j.pain.2006.06.018 [DOI] [PubMed] [Google Scholar]
- 5.Hurley RW, Cohen SP, Williams KA, Rowlingson AJ, Wu CL. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med. 2006;31(3):237-247. doi: 10.1097/00115550-200605000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Talboys R, Mak M, Modi N, Fanous N, Cutts S. Enhanced recovery programme reduces opiate consumption in hip hemiarthroplasty. Eur J Orthop Surg Traumatol. 2016;26(2):177-181. doi: 10.1007/s00590-015-1722-2 [DOI] [PubMed] [Google Scholar]
- 7.Sarin A, Litonius ES, Naidu R, Yost CS, Varma MG, Chen LL. Successful implementation of an Enhanced Recovery After Surgery program shortens length of stay and improves postoperative pain, and bowel and bladder function after colorectal surgery. BMC Anesthesiol. 2016;16(1):55. doi: 10.1186/s12871-016-0223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi: 10.1016/j.jpain.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 9.Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292-294. doi: 10.1001/jamainternmed.2017.7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med. 2017;377(5):411-414. doi: 10.1056/NEJMp1704633 [DOI] [PubMed] [Google Scholar]
- 11.Kharasch ED, Clark JD, Kheterpal S. Perioperative gabapentinoids: deflating the bubble. Anesthesiology. 2020;133(2):251-254. doi: 10.1097/ALN.0000000000003394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabritius ML, Geisler A, Petersen PL, et al. Gabapentin for post-operative pain management—a systematic review with meta-analyses and trial sequential analyses. Acta Anaesthesiol Scand. 2016;60(9):1188-1208. doi: 10.1111/aas.12766 [DOI] [PubMed] [Google Scholar]
- 13.Verret M, Lauzier F, Zarychanski R, et al. ; Canadian Perioperative Anesthesia Clinical Trials (PACT) Group . Perioperative use of gabapentinoids for the management of postoperative acute pain: a systematic review and meta-analysis. Anesthesiology. 2020;133(2):265-279. doi: 10.1097/ALN.0000000000003428 [DOI] [PubMed] [Google Scholar]
- 14.Bykov K, Bateman BT, Franklin JM, Vine SM, Patorno E. Association of gabapentinoids with the risk of opioid-related adverse events in surgical patients in the United States. JAMA Netw Open. 2020;3(12):e2031647. doi: 10.1001/jamanetworkopen.2020.31647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2019 American Geriatrics Society Beers Criteria® Update Expert Panel . American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Huybrechts KF, Patorno E, et al. Adverse events associated with antipsychotic use in hospitalized older adults after cardiac surgery. J Am Geriatr Soc. 2017;65(6):1229-1237. doi: 10.1111/jgs.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patorno E, Neuman MD, Schneeweiss S, Mogun H, Bateman BT. Comparative safety of anesthetic type for hip fracture surgery in adults: retrospective cohort study. BMJ. 2014;348:g4022. doi: 10.1136/bmj.g4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358(8):771-783. doi: 10.1056/NEJMoa0707571 [DOI] [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project: procedure classes. February 18, 2016. Accessed May 23, 2019. https://www.hcup-us.ahrq.gov/toolssoftware/procedure/procedure.jsp
- 20.Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project: procedure classes refined for ICD-10-PCS. March 23, 2022. Accessed May 23, 2019. https://www.hcup-us.ahrq.gov/toolssoftware/procedureicd10/procedure_icd10.jsp
- 21.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492-499. doi: 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 22.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26(8):945-953. doi: 10.1002/pds.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bui LN, Pham VP, Shirkey BA, Swan JT. Effect of delirium motoric subtypes on administrative documentation of delirium in the surgical intensive care unit. J Clin Monit Comput. 2017;31(3):631-640. doi: 10.1007/s10877-016-9873-1 [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Pieper CF, Ahmed A, Colón-Emeric CS. Use and interpretation of propensity scores in aging research: a guide for clinical researchers. J Am Geriatr Soc. 2016;64(10):2065-2073. doi: 10.1111/jgs.14253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121-145. doi: 10.1177/096228029300200202 [DOI] [PubMed] [Google Scholar]
- 28.Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86(4):781-785. doi: 10.1097/00000539-199804000-00019 [DOI] [PubMed] [Google Scholar]
- 29.McDermott KW, Freeman WJ, Elixhauser A. Overview of operating room procedures during inpatient stays in US hospitals, 2014. HCUP statistical brief 233. Agency for Healthcare Research and Quality. December 2017. Acccessed June 8, 2022. www.hcup-us.ahrq.gov/reports/statbriefs/sb233-Operating-Room-Procedures-United-States-2014.pdf [PubMed] [Google Scholar]
- 30.McDermott KW, Liang L. Overview of operating room procedures during inpatient stays in US hospitals, 2018. HCUP statistical brief 281. Agency for Healthcare Research and Quality. August 2021. Accessed June 8, 2022. www.hcup-us.ahrq.gov/reports/statbriefs/sb281-Operating-Room-Procedures-During-Hospitalization-2018.pdf [PubMed]
- 31.Prabhakar H, Arora R, Bithal PK, Rath GP, Dash HH. The analgesic effects of preemptive gabapentin in patients undergoing surgery for brachial plexus injury—a preliminary study. J Neurosurg Anesthesiol. 2007;19(4):235-238. doi: 10.1097/ANA.0b013e3181271863 [DOI] [PubMed] [Google Scholar]
- 32.Leung JM, Sands LP, Chen N, et al. ; Perioperative Medicine Research Group . Perioperative gabapentin does not reduce postoperative delirium in older surgical patients: a randomized clinical trial. Anesthesiology. 2017;127(4):633-644. doi: 10.1097/ALN.0000000000001804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabritius ML, Strøm C, Koyuncu S, et al. Benefit and harm of pregabalin in acute pain treatment: a systematic review with meta-analyses and trial sequential analyses. Br J Anaesth. 2017;119(4):775-791. doi: 10.1093/bja/aex227 [DOI] [PubMed] [Google Scholar]
- 34.Busse JW, Bartlett SJ, Dougados M, et al. Optimal strategies for reporting pain in clinical trials and systematic reviews: recommendations from an OMERACT 12 workshop. J Rheumatol. 2015;42(10):1962-1970. doi: 10.3899/jrheum.141440 [DOI] [PubMed] [Google Scholar]
- 35.Hah J, Mackey SC, Schmidt P, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial. JAMA Surg. 2018;153(4):303-311. doi: 10.1001/jamasurg.2017.4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105(6):1805-1815. doi: 10.1213/01.ane.0000287643.13410.5e [DOI] [PubMed] [Google Scholar]
- 37.Fuzier R, Serres I, Guitton E, Lapeyre-Mestre M, Montastruc JL; French Network of Pharmacovigilance Centres . Adverse drug reactions to gabapentin and pregabalin: a review of the French pharmacovigilance database. Drug Saf. 2013;36(1):55-62. doi: 10.1007/s40264-012-0006-6 [DOI] [PubMed] [Google Scholar]
- 38.Pandey CK, Navkar DV, Giri PJ, et al. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy: a randomized, double-blind, placebo-controlled study. J Neurosurg Anesthesiol. 2005;17(2):65-68. doi: 10.1097/01.ana.0000151407.62650.51 [DOI] [PubMed] [Google Scholar]
- 39.Cavalcante AN, Sprung J, Schroeder DR, Weingarten TN. Multimodal analgesic therapy with gabapentin and its association with postoperative respiratory depression. Anesth Analg. 2017;125(1):141-146. doi: 10.1213/ANE.0000000000001719 [DOI] [PubMed] [Google Scholar]
- 40.Hughes CG, Boncyk CS, Culley DJ, et al. ; Perioperative Quality Initiative (POQI) 6 Workgroup . American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020;130(6):1572-1590. doi: 10.1213/ANE.0000000000004641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dighe K, Clarke H, McCartney CJ, Wong CL. Perioperative gabapentin and delirium following total knee arthroplasty: a post-hoc analysis of a double-blind randomized placebo-controlled trial. Can J Anaesth. 2014;61(12):1136-1137. doi: 10.1007/s12630-014-0235-5 [DOI] [PubMed] [Google Scholar]
- 42.Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMcp1605501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. J Psychosom Res. 2011;71(6):395-403. doi: 10.1016/j.jpsychores.2011.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnosis Codes for Delirium
eTable 2. Characteristics of Patients by Perioperative Gabapentin Use Before and After Propensity Score Matching (Complete List of Covariates)
eTable 3. Subgroup Analysis of Perioperative Gabapentin Use and In-Hospital Adverse Clinical Events by Surgery Type
eTable 4. Sensitivity Analysis for Patients Who Received Gabapentin vs Nonusers on the Day of Surgery
eTable 5. Sensitivity Analysis for the Dose-Response Relationship Between Gabapentin and In-Hospital Adverse Clinical Events
eFigure. Sensitivity Analysis for Unmeasured Confounding by Severe Pain