This cohort study examines a large multicenter data set over 13 influenza seasons to determine whether early use of oseltamivir is associated with improved outcomes in children hospitalized with influenza.
Key Points
Question
Among children hospitalized with influenza, is the early use of oseltamivir associated with improved outcomes?
Findings
In this cohort study, a multicenter propensity score–weighted retrospective analysis of data for 55 799 children hospitalized with influenza between 2007 and 2020 found that early (hospital day 0 or 1) oseltamivir use was associated with shorter hospital stay and lower odds of 7-day readmission, transfer to the intensive care unit, and in-hospital mortality or use of extracorporeal membrane oxygenation when adjusting for confounders.
Meaning
These data support current recommendations for early initiation of oseltamivir in children hospitalized with influenza.
Abstract
Importance
Oseltamivir is recommended for all children hospitalized with influenza, despite limited evidence supporting its use in the inpatient setting.
Objective
To determine whether early oseltamivir use is associated with improved outcomes in children hospitalized with influenza.
Design, Setting, and Participants
This multicenter retrospective study included 55 799 children younger than 18 years who were hospitalized with influenza from October 1, 2007, to March 31, 2020, in 36 tertiary care pediatric hospitals who participate in the Pediatric Health Information System database. Data were analyzed from January 2021 to March 2022.
Exposures
Early oseltamivir treatment, defined as use of oseltamivir on hospital day 0 or 1.
Main Outcomes and Measures
The primary outcome was hospital length of stay (LOS) in calendar days. Secondary outcomes included 7-day hospital readmission, late (hospital day 2 or later) intensive care unit (ICU) transfer, and a composite outcome of in-hospital death or use of extracorporeal membrane oxygenation (ECMO). Inverse probability treatment weighting (IPTW) based on propensity scoring was used to address confounding by indication. Mixed-effects models were used to compare outcomes between children who did and did not receive early oseltamivir treatment. Outcomes were also compared within high-risk subgroups based on age, presence of a complex chronic condition, early critical illness, and history of asthma.
Results
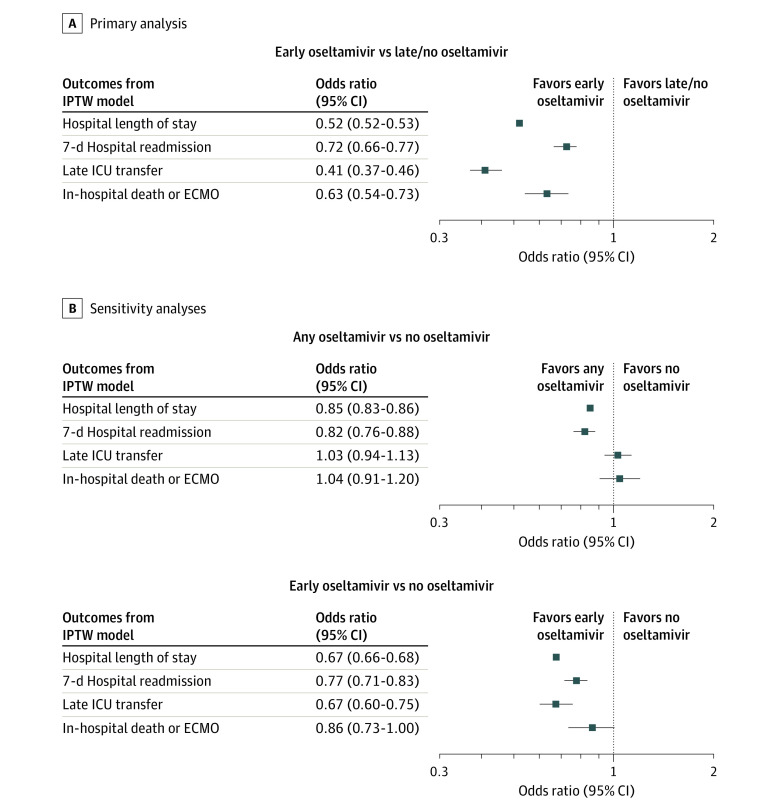
The analysis included 55 799 encounters from 36 hospitals. The median (IQR) age of the cohort was 3.61 years (1.03-8.27); 56% were male, and 44% were female. A total of 33 207 patients (59.5%) received early oseltamivir. In propensity score–weighted models, we found that children treated with early oseltamivir had shorter LOS (median 3 vs 4 days; IPTW model ratio, 0.52; 95% CI, 0.52-0.53) and lower odds of all-cause 7-day hospital readmission (3.5% vs 4.8%; adjusted odds ratio [aOR], 0.72; 95% CI, 0.66-0.77), late ICU transfer (2.4% vs 5.5%; aOR, 0.41; 95% CI, 0.37-0.46), and the composite outcome of death or ECMO use (0.9% vs 1.4%; aOR, 0.63; 95% CI, 0.54-0.73).
Conclusions and Relevance
Early use of oseltamivir in hospitalized children was associated with shorter hospital stay and lower odds of 7-day readmission, ICU transfer, ECMO use, and death. These findings support the current recommendations for oseltamivir use in children hospitalized with influenza.
Introduction
Influenza is a common illness in children, resulting in an estimated 2.8 to 10.7 million symptomatic illnesses, 1.6 to 6.1 million outpatient medical visits, 11 000 to 45 000 hospitalizations, and 110 to 600 excess deaths per year in pediatric patients.1 The antiviral drug oseltamivir remains the mainstay of treatment for influenza. Oseltamivir was first approved by the US Food and Drug Administration in 1999 and is recommended by the American Academy of Pediatrics (AAP)2 and Infectious Diseases Society of America (IDSA)3 for the treatment of all hospitalized children with influenza. These guidelines are based on findings that outpatient oseltamivir administration improves the median time to alleviation of symptoms over placebo among adults by approximately 17 hours4,5 and among children by approximately 29 hours.4,6
Despite these guidelines, there is debate as to whether oseltamivir improves patient outcomes or reduces resource use in hospitalized patients.7 One meta-analysis of observational data from the 2009 H1N1 pandemic found a mortality benefit of early oseltamivir treatment for hospitalized adults, but not in children.8 Some observational studies from the 2009 H1N1 pandemic9,10,11,12 and afterwards13,14 demonstrated improved outcomes in pediatric inpatients, though these studies were generally limited by small sample size or high risk of bias.7,15 Given these limitations and the lack of randomized clinical trial (RCT) data available, hospitalized children remain the population with the largest gap in evidence for oseltamivir treatment.16 Therefore, our objective was to use a large multicenter data set covering 13 influenza seasons to determine whether oseltamivir use was associated with improved outcomes in children hospitalized with influenza.
Methods
Data Source
We performed a multicenter retrospective cohort study of children admitted to the hospital with influenza using the Pediatric Health Information System (PHIS), an administrative database that contains inpatient, emergency department, ambulatory surgery, and observation encounter-level data from more than 50 not-for-profit, tertiary care pediatric hospitals in the United States.17 These hospitals are affiliated with the Children’s Hospital Association (Lenexa, Kansas). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. Data are deidentified at the time of data submission and are subject to reliability and validity checks before being included in the database.17 We included the 36 hospitals that reported clinical and billing data for the entire study period. The study was approved by the institutional review board at Cincinnati Children’s Hospital Medical Center with a waiver for informed consent. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients
We included all inpatients younger than 18 years who were discharged from a participating hospital between October 1, 2007, and March 31, 2020, with a primary or secondary discharge or in-hospital death diagnosis of influenza, defined as International Classification of Diseases, Ninth Revision (ICD-9), code of 487 or 488 or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), code of J09, J10, or J11.18 We used diagnostic codes for case definitions because primary clinical and laboratory data to confirm diagnoses are not available in PHIS. Diagnosis codes have previously been used to identify hospitalized children with laboratory-confirmed influenza with high specificity (99%) and positive predictive value (60%-88%).19,20
We excluded encounters resulting in transfer to another hospital, as we were unable to assess the outcomes in these encounters. For patients with repeated encounters with an influenza diagnosis, we randomly selected 1 encounter per influenza season to avoid bias toward the beginning of the influenza season where vaccine uptake and predominant strains are different than later in the season. If any selected encounter had a prior visit within 7 days, the initial visit was used for analysis because readmissions were a secondary outcome. To mitigate immortal time bias21,22 and ensure all participants were admitted for the complete exposure period, we excluded encounters with discharge, in-hospital death, or extracorporeal membrane oxygenation (ECMO) use on hospital day 0 or 1. Day 0 was the calendar day the hospital encounter began.
Exposure
Our primary exposure was early use of oseltamivir, defined as a billing charge for oseltamivir on hospital day 0 or 1. We used early oseltamivir as the drug is thought to have the highest efficacy if used early in the disease course.6,23,24 We included day 0 and 1 in the exposure period to ensure that children admitted late in the day but receiving oseltamivir within the first 24 hours of hospitalization were categorized as receiving early oseltamivir. If a patient received oseltamivir only on hospital day 2 or later, they were classified as not having received early oseltamivir.
Outcomes
Our primary outcome was hospital length of stay (LOS). We chose LOS because of the purported benefit of oseltamivir to reduce symptom duration. We calculated LOS as number of calendar days between admission date and discharge date. Secondary outcomes included 7-day hospital readmission, late intensive care unit (ICU) transfer, and a composite outcome of in-hospital death or ECMO use. This composite outcome was chosen because both death and ECMO requirement represent critical treatment failure. We defined late ICU transfer as transfer to the ICU on or after hospital day 2 after being admitted to a general ward. Use of ECMO and in-hospital mortality were identified using corresponding flags in the PHIS database.
Covariates
We selected demographic characteristics, comorbid conditions, and indicators of illness severity during the exposure period a priori as covariates in our analysis. Patient characteristics included age, sex, race, primary insurance, past diagnoses, and complex chronic conditions (CCCs). We specifically included age group (<2 years, 2-5 years, and >5 years), CCCs, and history of asthma in order to capture high-risk conditions specified in national influenza guidelines.23,24 We defined CCCs using the diagnosis code classification system reported by Feudtner et al,25 which captures diagnoses that are likely to last for at least 12 months and require subspecialty pediatric care and hospitalization. We defined history of asthma as the presence of any diagnosis of asthma (ICD-9 code of 493 or ICD-10 code of J45) in a previous encounter within PHIS, so these children were those with asthma severe enough to require emergency department care or hospitalization. To capture indicators of severity of illness at presentation, we extracted early (hospital day 0 or 1) requirement of the ICU, central venous catheter, positive pressure ventilation (defined as high-flow nasal cannula, continuous positive airway pressure, bilevel positive airway pressure, or mechanical ventilation), and systemic antibiotics.
Propensity Scoring and Weighting
To address confounding by indication (the clinical indication for treatment also affecting the outcome), we performed inverse probability treatment weighted (IPTW) analyses with weights calculated from propensity scores. We generated propensity scores for each patient using multivariable generalized linear mixed effects models with the variables above specified as covariates and using hospital-specific random intercepts and random slopes for influenza season. Because more than half of the study population received the treatment, matching based on propensity scores would result in loss of a significant portion of treated patients in the cohort. Therefore, we generated weighted samples using IPTW to estimate the average treatment effect of the treated. We evaluated the balance of baseline covariates using standardized mean differences and a love plot.
Statistical Analysis
We described patient characteristics of those who did and did not receive oseltamivir using frequencies with proportions and medians with IQRs to describe categorical and continuous variables, respectively. We evaluated the differences in outcomes between patients who did and did not receive early oseltamivir using the Wilcoxon rank sum test to compare LOS and χ2 tests to compare the secondary outcomes for unadjusted and IPTW-adjusted samples. We compared outcomes in the overall cohort, as well as within high-risk subgroups based on age, presence of CCC, history of asthma, and early ICU admission. We used generalized linear models to evaluate the association between early oseltamivir use and outcomes using the IPTW samples with early oseltamivir as the binary independent variable. For LOS, we assumed a negative binomial distribution.
We performed several sensitivity analyses. As some children only received oseltamivir late in hospitalizations (day 2 or later), we conducted 2 additional analyses to ensure we were not biasing our results through misclassification. First, we included late oseltamivir in the treated group and compared any oseltamivir treatment with none. Second, we excluded data for children receiving only late oseltamivir and compared early oseltamivir with those entirely untreated. Additionally, to ensure our models were rigorous in both adjusting for covariates and accounting for clustering of outcomes by hospital, we conducted secondary mixed models for all outcomes. In these secondary models, we used the unweighted observed cohort, adjusting for covariates and accounting for clustering by using hospital-specific random intercepts. Statistical analyses were conducted in R (version 4.0.2; R Foundation for Statistical Computing) and SAS 9.4 (SAS Institute), and a 2-sided P < .05 was considered statistically significant. Data were analyzed from January 2021 to March 2022.
Results
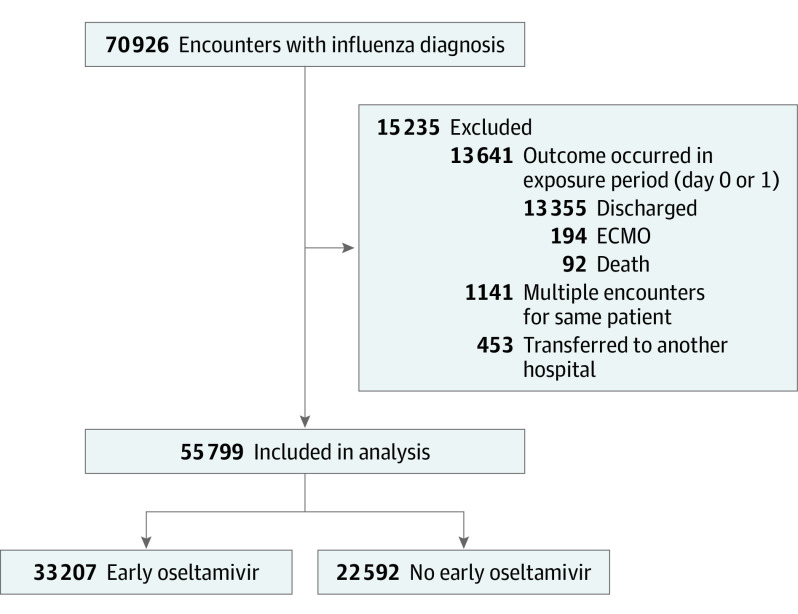
Over the 13-year study period, we identified 70 926 inpatient hospitalizations for influenza meeting our inclusion criteria, and 55 799 remained after exclusions and were included in our analysis (Figure 1). The median (IQR) age of the cohort was 3.61 years (1.03-8.27); 56% were male, and 44% were female. Over the course of the study period, 33 207 patients (59.5%) received early oseltamivir (Table 1). Of those not treated with early oseltamivir, 4098 (7%) were treated with oseltamivir on hospital day 2 or later, and 18 494 (33%) were untreated throughout the hospitalization. Median (IQR) LOS was 3 days (2-6), 2241 children (4.0%) were readmitted within 7 days, and 581 (1.1%) experienced in-hospital death or ECMO use (465 deaths, 199 ECMO recipients). Of children initially admitted to a general ward, 1486 (3.7%) required late ICU transfer.
Figure 1. Study Flow Diagram.
ECMO indicates extracorporeal membrane oxygenation.
Table 1. Unadjusted Cohort Patient Characteristics Stratified by Use of Early Oseltamivir.
| Characteristics | Early oseltamivir, No. (%) | |
|---|---|---|
| Yes | No | |
| No. of patients | 33 207 | 22 592 |
| Age categories, y | ||
| <2 | 11 624 (35.0) | 9258 (41.0) |
| 2-5 | 6806 (20.5) | 4755 (21.0) |
| >5 | 14 777 (44.5) | 8579 (38.0) |
| Gender | ||
| Female | 14 491 (43.6) | 9995 (44.2) |
| Male | 18 715 (56.4) | 12 591 (55.7) |
| Race | ||
| Black | 8336 (25.1) | 5000 (22.1) |
| White | 16 461 (49.6) | 12 245 (54.2) |
| Othera | 6782 (20.4) | 4150 (18.4) |
| Unknown | 1628 (4.9) | 1197 (5.3) |
| Insurance type | ||
| Public | 21 466 (64.6) | 13 872 (61.4) |
| Private | 10 225 (30.8) | 7279 (32.2) |
| Other | 1041 (3.1) | 1003 (4.4) |
| Unknown | 475 (1.4) | 438 (1.9) |
| Region | ||
| Midwest | 8082 (24.3) | 5271 (23.3) |
| Northeast | 3237 (9.7) | 2448 (10.8) |
| South | 12 817 (38.6) | 9570 (42.4) |
| West | 9071 (27.3) | 5303 (23.5) |
| CCC | 15 979 (48.1) | 10 581 (46.8) |
| History of asthma (without CCC) | 4170 (12.6) | 2118 (9.4) |
| Required ICU on day 0 or 1 | 9610 (28.9) | 5429 (24.0) |
Abbreviations: CCC, complex chronic condition; ICU, intensive care unit.
Other includes those identified as other, Asian, American Indian/Alaskan/Native American, or Native Hawaiian/Pacific Islander.
Compared with those not treated with early oseltamivir, children treated with early oseltamivir were more likely to be older (44.5% vs 38% age >5 years), have a CCC (48.1% vs 46.8%), and require early ICU admission (28.9% vs 24%). In this unadjusted cohort, children treated with early oseltamivir had lower median (IQR) LOS (3 days [2-5] vs 4 days [2-8]), 7-day readmissions (3.5% vs 4.8%), late ICU transfer (2.4% vs 5.4%), and in-hospital mortality or ECMO use (0.9% vs 1.3%) compared with children not treated with early oseltamivir (Table 2).
Table 2. Outcomes in Patients Treated With Early Oseltamivir vs Not Treated With Early Oseltamivir in IPTW Analysis.
| Outcome | % (95% CI) | OR (95% CI) | |||
|---|---|---|---|---|---|
| Unadjusted | IPTW model | ||||
| Early oseltamivir | No early oseltamivir | Early oseltamivir | No early oseltamivir | ||
| Hospital LOS, median (IQR), d | 3 (2-5) | 4 (2-8) | 3 (2-5) | 4 (3-9) | 0.52 (0.52-0.53) |
| 7-d Hospital readmission | 3.5 (3.3-3.7) | 4.8 (4.5-5.1) | 3.5 (3.3-3.7) | 4.8 (4.6-5.0) | 0.72 (0.66-0.77) |
| Late ICU transfer | 2.4 (2.2-2.6) | 5.4 (5.1-5.7) | 2.4 (2.2-2.6) | 5.5 (5.2-5.8) | 0.41 (0.37-0.46) |
| In-hospital death or ECMO use | 0.9 (0.8-1.0) | 1.3 (1.1-1.4) | 0.9 (0.8-1.0) | 1.4 (1.3-1.5) | 0.63 (0.54-0.73) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IPTW, inverse probability treatment weighting; LOS, length of stay; OR, odds ratio.
Results of Multivariable, Propensity Score–Weighted Analyses
Inverse probability treatment weighting using propensity scoring successfully balanced covariates between groups, with all absolute standardized mean differences in the weighted cohort less than 0.1 (eFigures 1 and 2 and eTable 1 in the Supplement). There were differences in outcomes between weighted groups for all outcomes (Table 2 and Figure 2). In the weighted analysis, we found children treated with early oseltamivir had reduced LOS (median, 3 vs 4 days; IPTW model ratio, 0.52; 95% CI, 0.52-0.53), all-cause 7-day hospital readmissions (3.5% vs 4.8%; adjusted odds ratio [aOR], 0.72; 95% CI, 0.66-0.77), late ICU transfer (2.4% vs 5.5%; aOR, 0.41; 95% CI, 0.37-0.46), and the composite outcome of death or ECMO use (0.9% vs 1.4%; aOR, 0.63; 95% CI, 0.54-0.73) when compared with children not treated with early oseltamivir. We performed 2 secondary models for each outcome in which we accounted for clustering of outcomes by hospital using random intercepts: 1 with unadjusted cohort adjusting for covariates and 1 using IPTW. The additional mixed-effects models demonstrated similar results to the primary IPTW model, and findings did not change (eFigure 3 in the Supplement).
Figure 2. Results of Weighted Analysis of Oseltamivir Use and Outcomes.
The primary analysis compares outcomes between those treated and not treated with early oseltamivir. Sensitivity analyses compared any oseltamivir vs no oseltamivir and early oseltamivir vs no oseltamivir (excluding children treated with late oseltamivir). ECMO indicates extracorporeal membrane oxygenation; ICU, intensive care unit; IPTW, inverse probability treatment weighting.
Outcomes in High-risk Subgroups in Weighted Analysis
Outcomes within subgroups based on high-risk conditions (young age, CCC, history of asthma, and early ICU admission) in the IPTW analysis are shown in Figure 3. IPTW successfully balanced distributions of covariates within each subgroup (eTables 3-6 in the Supplement). The differences in LOS, 7-day readmissions, and late ICU transfers were persistent across all subgroups. In-hospital death or ECMO use was statistically different in all subgroups except age 2 to 5 years (0.8%; 95% CI, 0.6%-1.0%, vs 1.1%; 95% CI, 0.8%-1.3%; P = .08) and among children with asthma (0.1%; 95% CI, 0.0%-0.2%, vs 0.2%; 95% CI, 0.1%-0.4%; P = .24).
Figure 3. Outcomes Within Subgroups Based on High-risk Conditions in Propensity Score–Weighted Analysis.
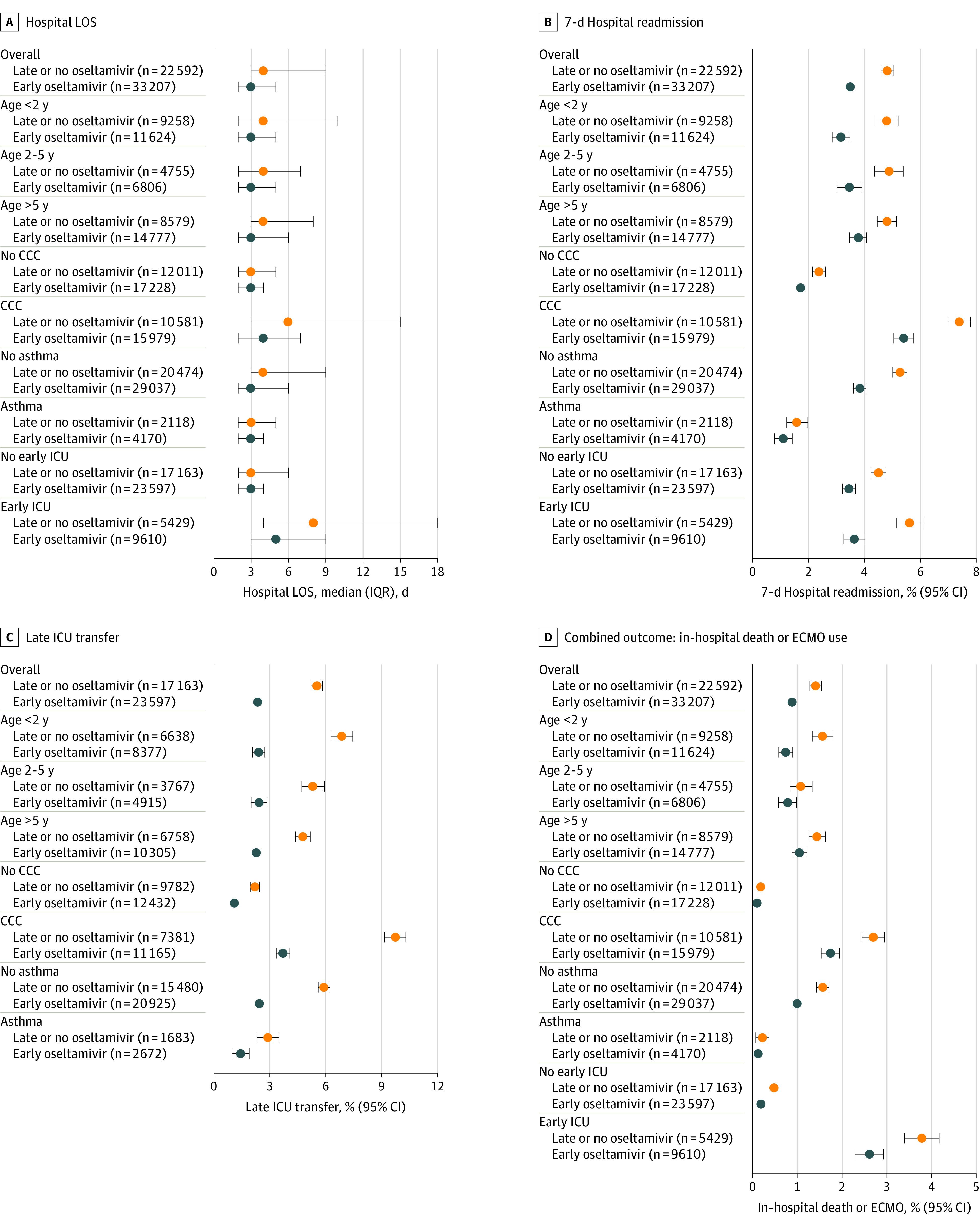
CCC indicates complex chronic condition; ICU, intensive care unit; LOS, length of stay.
Sensitivity Analyses
Results of our sensitivity analyses are shown in Figure 2B, with characteristics of children treated with late oseltamivir shown in eTable 2 in the Supplement. Children treated with late oseltamivir were older (48% were >5 years), more likely to have a CCC (68%), and less likely to have asthma history (6%). When including children treated with late oseltamivir in the treated group, oseltamivir was associated with shorter LOS and lower odds of 7-day readmission, but there was no association with late ICU transfers or death or ECMO use. When data for children treated with late oseltamivir were excluded, early oseltamivir was associated with improvement in all outcomes similar to the primary analysis.
Discussion
In this multicenter study including 55 799 children hospitalized with influenza, treatment with early oseltamivir was associated with 1-day shorter median LOS and lower odds of 7-day hospital readmissions, late transfer to the ICU, and in-hospital death or ECMO use. Results were consistent across high-risk subgroups.
The results from our large, multicenter, propensity score–weighted analysis of oseltamivir use over 13 influenza seasons in pediatric inpatients add to the current body of literature supporting oseltamivir use in hospitalized children. A study of 608 children with laboratory-confirmed influenza in 2010 to 2013 either with chronic conditions or requiring the ICU also noted a 1-day reduction in LOS with oseltamivir treatment.13 Another study demonstrated reduction in LOS among critically ill patients during the 2009 H1N1 pandemic.9 Likewise, our finding that oseltamivir treatment was associated with reduced 7-day readmissions is consistent with a prior observational study demonstrating reduced readmissions in complicated influenza.10 Others have suggested reduced mortality in critically ill children treated with oseltamivir12; however, they did not address immortal time bias (patients who died may not have had time to be treated with oseltamivir).15 Our study excluded patients who were discharged or died during the exposure period (hospital day 0 or 1) to minimize bias but still found the association.
Randomized clinical trials of oseltamivir use in children primarily exist in the outpatient setting. Our results in pediatric inpatients show interesting parallels. Our main finding of a 1-day reduction in LOS for children treated with oseltamivir dovetails with the results of meta-analyses of outpatient trials demonstrating a reduction in LOS among children by approximately 29 hours.4,6 What our findings may add to this literature is that the reduction in length of illness seems to apply to severe illness in addition to mild illness and is independent of where care is delivered. In fact, the group with the largest reduction in LOS in our study were the patients initially admitted to the ICU. Additionally, the outpatient meta-analyses did not show a reduction in symptoms among patients with asthma,4,6 which is a subgroup in our analysis with a statistically different but not clinically meaningful change in LOS (median 3 days for both groups). This may reflect that patients with a history of asthma are more likely to be hospitalized with an asthma exacerbation triggered by influenza, resulting in a more predictable and abbreviated course, as opposed to other nonwheeze etiology for severe illness or respiratory failure.
Despite AAP and IDSA guidelines recommending early oseltamivir for hospitalized children with influenza, 33% of our cohort did not receive any oseltamivir during hospitalization, and 7% were treated only later in their hospitalization. We speculate this may be due to a relative lack of quality evidence in the inpatient setting, as RCTs are likely not ethical or feasible in this population. Meta-analyses have previously demonstrated questionable benefit of oseltamivir in preventing severe influenza complications.4 Additionally, prior observational studies of oseltamivir, including those demonstrating mortality benefit, have been identified as having a high risk of bias.15 We have attempted to address many of these concerns with a large sample size, careful exclusions to address potential biases and confounding by indication, and multiple sensitivity analyses. Our data support the current recommendations by the AAP and IDSA for oseltamivir use early in influenza disease course for hospitalized children.
Limitations
This study has several limitations. The PHIS is an administrative database and therefore does not contain clinical information such as history of present illness, physical examination findings, or laboratory test results. While ICD codes have been shown to have good specificity and positive predictive value for laboratory-confirmed influenza,19,20 we cannot confirm that all included patients had laboratory-confirmed influenza. Without data about length of illness before hospital admission, some patients may have been either misclassified as not receiving oseltamivir despite having received treatment as an outpatient or not treated because they were outside the first 48 hours of influenza illness where recommendations for treatment are strongest.24 Additionally, for patients with longer period of illness before admission, the efficacy of oseltamivir may have been mitigated. We addressed confounding by indication using a weighted analysis based on propensity scores; however, there remains the possibility of residual unmeasured confounding. We attempted to minimize risk of bias by performing sensitivity analyses to examine the effect of late oseltamivir treatment and testing secondary statistical models. In the analysis of LOS, the data are limited to integer calendar days, which limits the precision of our analysis. We do not have access to encounters or revisits outside of PHIS hospitals, which may lead to underestimating 7-day readmissions. However, prior studies have shown only a small proportion (8%) of pediatric patients have revisits at a separate institution.26 For our subanalysis of patients with history of asthma, there may have been varying lead times for prior encounters with an asthma diagnosis based on patient age, year, and when the hospital joined PHIS, so it is possible some patients were misclassified. Our large sample size allows for comparison of rare outcomes and may help overcome some of our limitations. Furthermore, it would likely be unethical to perform a RCT of oseltamivir given the current recommendations, so observational studies such as this one are the most practical way to evaluate its use.
Conclusions
Early oseltamivir use in hospitalized children with influenza was associated with shorter hospital LOS and lower odds of 7-day readmission, ICU transfers, ECMO use, and death. Our findings support the current recommendations for oseltamivir use in children hospitalized with influenza.
eTable 1. Covariate distributions in unweighted and propensity score weighted samples.
eTable 2. Characteristics of children treated with early, late, and no oseltamivir during hospitalization.
eTable 3. Covariate distributions in weighted cohort by age group
eTable 4. Covariate distributions in weighted cohort by presence of a complex chronic condition
eTable 5. Covariate distributions in weighted cohort by asthma history
eTable 6. Covariate distributions in weighted cohort by early ICU requirement
eFigure 1. Love plot of standardized mean differences of covariates for unweighted and IPTW cohort in the primary analysis.
eFigure 2. Distributions of propensity scores for treatment with early oseltamivir.
eFigure 3. Results of covariate-adjusted mixed effects models of oseltamivir use and outcomes.
References
- 1.Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-137. doi: 10.1111/irv.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases . Prevention of influenza: recommendations for influenza immunization of children, 2006-2007. Pediatrics. 2007;119(4):846-851. doi: 10.1542/peds.2007-0164 [DOI] [PubMed] [Google Scholar]
- 3.Harper SA, Bradley JS, Englund JA, et al. ; Expert Panel of the Infectious Diseases Society of America . Seasonal influenza in adults and children--diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(8):1003-1032. doi: 10.1086/598513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;1:CD008965. doi: 10.1590/1516-3180.20141324T2 [DOI] [PubMed] [Google Scholar]
- 5.Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729-1737. doi: 10.1016/S0140-6736(14)62449-1 [DOI] [PubMed] [Google Scholar]
- 6.Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis. 2018;66(10):1492-1500. doi: 10.1093/cid/cix1040 [DOI] [PubMed] [Google Scholar]
- 7.Hurt AC, Kelly H. Debate regarding oseltamivir use for seasonal and pandemic influenza. Emerg Infect Dis. 2016;22(6):949-955. doi: 10.3201/eid2206.151037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthuri SG, Venkatesan S, Myles PR, et al. ; PRIDE Consortium Investigators . Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2(5):395-404. doi: 10.1016/S2213-2600(14)70041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffin SE, Leckerman K, Keren R, Hall M, Localio R, Zaoutis TE. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J. 2011;30(11):962-966. doi: 10.1097/INF.0b013e318232ede9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brogan TV, Hall M, Sills MR, et al. Hospital readmissions among children with H1N1 influenza infection. Hosp Pediatr. 2014;4(6):348-358. doi: 10.1542/hpeds.2014-0045 [DOI] [PubMed] [Google Scholar]
- 11.Eriksson CO, Graham DA, Uyeki TM, Randolph AG. Risk factors for mechanical ventilation in U.S. children hospitalized with seasonal influenza and 2009 pandemic influenza A*. Pediatr Crit Care Med. 2012;13(6):625-631. doi: 10.1097/PCC.0b013e318260114e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie JK, Yang S, Samuel MC, Uyeki TM, Schechter R. Neuraminidase inhibitors for critically ill children with influenza. Pediatrics. 2013;132(6):e1539-e1545. doi: 10.1542/peds.2013-2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell AP, Tokars JI, Reynolds S, et al. Influenza antiviral treatment and length of stay. Pediatrics. 2021;148(4):e2021050417. doi: 10.1542/peds.2021-050417 [DOI] [PubMed] [Google Scholar]
- 14.Eşki A, Öztürk GK, Gülen F, Çiçek C, Demir E. Risk factors for influenza virus related severe lower respiratory tract infection in children. Pediatr Infect Dis J. 2019;38(11):1090-1095. doi: 10.1097/INF.0000000000002447 [DOI] [PubMed] [Google Scholar]
- 15.Freemantle N, Shallcross LJ, Kyte D, Rader T, Calvert MJ. Oseltamivir: the real world data. BMJ. 2014;348:g2371. doi: 10.1136/bmj.g2371 [DOI] [PubMed] [Google Scholar]
- 16.Uyeki TM. Oseltamivir treatment of influenza in children. Clin Infect Dis. 2018;66(10):1501-1503. doi: 10.1093/cid/cix1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Children’s Hospital Association . Updated October 19, 2020. Accessed November 29, 2021. http://www.childrenshospitals.org
- 18.Fingar KR, Liang L, Stocks C. Inpatient hospital stays and emergency department visits involving influenza, 2006-2016: Statistical Brief No. 253. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2019. [PubMed] [Google Scholar]
- 19.Keren R, Wheeler A, Coffin SE, Zaoutis T, Hodinka R, Heydon K. ICD-9 codes for identifying influenza hospitalizations in children. Emerg Infect Dis. 2006;12(10):1603-1604. doi: 10.3201/eid1210.051525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feemster KA, Leckerman KH, Middleton M, et al. Use of administrative data for the identification of laboratory-confirmed influenza infection: the validity of influenza-specific ICD-9 codes. J Pediatric Infect Dis Soc. 2013;2(1):63-66. doi: 10.1093/jpids/pis052 [DOI] [PubMed] [Google Scholar]
- 21.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492-499. doi: 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 22.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- 23.Committee on Infectious Diseases . Recommendations for prevention and control of influenza in children, 2018-2019. Pediatrics. 2018;142(4):e20182367. doi: 10.1542/peds.2018-2367 [DOI] [PubMed] [Google Scholar]
- 24.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1-e47. doi: 10.1093/cid/ciy866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supat B, Brennan JJ, Vilke GM, Ishimine P, Hsia RY, Castillo EM. Characterizing pediatric high frequency users of California emergency departments. Am J Emerg Med. 2019;37(9):1699-1704. doi: 10.1016/j.ajem.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Covariate distributions in unweighted and propensity score weighted samples.
eTable 2. Characteristics of children treated with early, late, and no oseltamivir during hospitalization.
eTable 3. Covariate distributions in weighted cohort by age group
eTable 4. Covariate distributions in weighted cohort by presence of a complex chronic condition
eTable 5. Covariate distributions in weighted cohort by asthma history
eTable 6. Covariate distributions in weighted cohort by early ICU requirement
eFigure 1. Love plot of standardized mean differences of covariates for unweighted and IPTW cohort in the primary analysis.
eFigure 2. Distributions of propensity scores for treatment with early oseltamivir.
eFigure 3. Results of covariate-adjusted mixed effects models of oseltamivir use and outcomes.