Abstract
Management of respiratory failure is closely related to oxygen supplementation. Thus, its administration needed special attention according to indications to avoid the toxic effect. Oxygen supplementation in conditions of respiratory failure aims to overcome hypoxemia. Excessive oxygen exposure can cause oxygen toxicity and lead to hyperoxia. Hyperoxia is a condition in which there is an excess supply of oxygen in the tissues and organs. Clinically, respiratory failure is diagnosed if the PaO2 is less than 60 mmHg with or without an increase in carbon dioxide when the patient breathes room air. Respiratory failure is divided into acute (sudden) respiratory failure and chronic (slow) respiratory failure. The basis for managing respiratory failure consists of supportive/non-specific and causative/specific management. Oxygen should be prescribed wisely not to cause injury to organs such as the heart, lungs, eyes, nervous system, and others. Hyperoxia often occurs in managing respiratory failure, so it requires supervision, especially in administering oxygen. Oxygen should be given as needed to avoid hyperoxia. In oxygen therapy, it is necessary to pay attention to the patient's condition because each condition requires different oxygen concentrations, so dose adjustments are necessary. These conditions can be divided into critical, severe, and observation conditions. The target oxygen saturation in all these conditions is 94–98%.
Keywords: Hyperoxia, Oxygen, Respiratory failure, Toxicity
Highlights
-
•
The use of oxygen therapy should not be excessive.
-
•
Excess oxygen therapy can cause hyperoxia (oxygen toxicity).
-
•
Oxygen therapy must be adjusted to the patient's condition.
1. Introduction
Oxygen is a vital and primary gas in sustaining human life. Oxygen has been available in the atmosphere since 5 billion years ago [1]. Priestley was the first to discover oxygen and pointed out the harmful effects. Oxygen can be of good therapeutic value if used as indicated in patients with respiratory failure [[2], [3], [4]]. Respiratory failure is a syndrome in which the respiratory system cannot maintain adequate gas exchange at rest or with activity. Inability to adequately perform the essential functions of respiration, namely sending oxygen to the blood and removing carbon dioxide. Clinically respiratory failure is diagnosed when the PaO2 is less than 60 mmHg with or without increased carbon dioxide when the patient is breathing room air [[5], [6], [7]].
Respiratory failure is the primary and most common cause of illness and death. The mortality rate of respiratory failure is nearly 20% and costs about $54 million [7]. No data show the impact of respiratory failure, but in a study that observed 45 ICU patients for 2 months, the mortality rate was 34% with respiratory failure. The leading causes include pneumonia (27%), neurological disorders (19%), sepsis (12%), COPD (6%), and acute pulmonary oedema (6%). As many as 80% of patients use mechanical ventilation, and 20% use NIV (non-invasive mechanical ventilation) [8,9].
Patients with respiratory failure require oxygen as the primary therapy to treat hypoxemia. To avoid excessive oxygen, prescribing oxygen supplementation must be considered correct and according to indications. Excessive oxygen exposure can cause oxygen toxicity and hyperoxia [4,10]. Hyperoxia is a condition in which there is an excess supply of oxygen to the tissues and organs. Oxygen toxicity occurs when the alveolar partial pressure of O2 (PaO2) exceeds that inhaled under normal conditions [[10], [11], [12]].
A non-indicated administration of oxygen supplementation exceeds excessive use of antibiotics. This is often not realized and is considered good. Annually, the need for oxygen supplementation is projected to be around 800,000 individuals at the cost of $1.8 billion [11]. Prolonged exposure to hyperoxia can cause HALI (hyperoxia-induced acute lung injury). This is influenced by the increase in ROS (reactive O2 species), causing disturbances in the balance of oxidants and antioxidants, and can disrupt homeostasis [10,13].
2. Management of respiratory failure
The respiratory center that controls breathing is located below the brainstem (pons and medulla). Respiratory failure can occur if the respiratory center is disturbed. Respiratory failure can be caused by abnormalities in the lungs, heart, chest wall, respiratory muscles and the central control mechanism of ventilation in the medulla oblongata. Although not considered a direct cause, dysfunction of the heart, pulmonary circulation, systemic circulation, hemoglobin oxygen transport and systemic capillary dysfunction are essential in respiratory failure [7,14,15].
Respiratory failure is divided into acute (sudden) respiratory failure and chronic (slow) respiratory failure. Acute respiratory failure is respiratory failure that occurs in patients who have typical lung structure and function before the onset of the disease. On the other hand, chronic respiratory failure is respiratory failure that occurs in patients with chronic lung diseases such as chronic bronchitis and emphysema. Patients tolerate hypoxia and hypercapnia, which worsens gradually [7,14]. Intrapulmonary and extrapulmonary abnormalities can cause acute respiratory failure. Intrapulmonary disorders include abnormalities in the lower respiratory tract, pulmonary circulation, interstitial tissue, and alveolar capillaries. Extrapulmonary abnormalities occur in the respiratory center, neuromuscular, pleura and upper airway [7,15].
Other respiratory failure classifications include hypoxemic, hypercapnic, and mixed respiratory failure. The criteria for respiratory failure are included type I a hypoxemic respiratory failure if the PaO2 is less than 60 mmHg (SaO2 <91%) with room air or the ratio of partial pressure of oxygen/inspired oxygen fraction (P/F) <300 or a decrease in PaO2 of 10 mmHg from previous data if known; type II hypercapnic respiratory failure if PaCO2 >50 mmHg with Ph <7.35 or a PaCO2 increase of 10 mmHg from the previous data if known [10,16]. Classification based on the results of blood gas analysis is divided into 3, such as respiratory failure types I, II, and III or combined. Respiratory failure types I and II are similar as described above. Type III respiratory failure is a combination of the failure of oxygenation and ventilation characterized by hypoxemia and hypercarbia, namely a decrease in PaO2 and an increase in PaCO2 [10,17,18].
The pathophysiology of respiratory failure is fundamental in terms of its management. There are four primary mechanisms of gas exchange disturbances in the respiratory system: hypoventilation, ventilation or perfusion imbalance, right to left blood shunt, and diffusion disorders. Extrapulmonary abnormalities cause hypoventilation, whereas intrapulmonary abnormalities may encompass all these mechanisms [7,15,19]. The management of respiratory failure consists of supportive/non-specific and causative/specific management. They are generally carried out simultaneously [5,20]. Non-specific management is an indirect action aimed at improving gas exchange that includes: overcoming hypoxemia with oxygen supplementation; overcoming hypercapnia by improving ventilation, namely improving the airway, additional ventilation such as face masks, bag valve masks, mechanical ventilation; chest physiotherapy [5,21].
When the PaO2 drops acutely, immediate action is needed to raise the PaO2 to be expected. This is inversely proportional to respiratory failure due to chronic disease that becomes acute again. The patient is used to hypercarbia, so the respiratory center is not stimulated by hypercarbia drive but by hypoxemia drive. Due to the rapid rise in PaO2, the patient may become apnea [5,21]. The administration of oxygen must be considered whether the patient needs oxygen. Indications for oxygen administration should be clear. Oxygen must be administered appropriately evaluated for therapeutic benefit and to avoid toxicity [15].
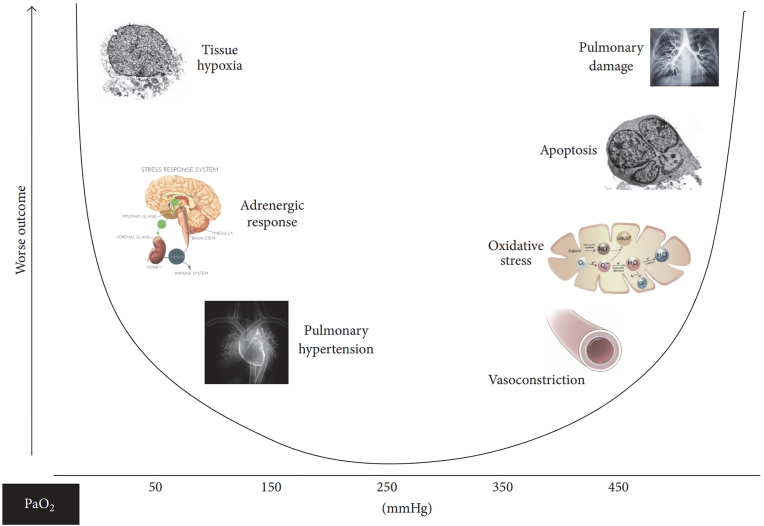
Short-term oxygen therapy is required therapy in patients with acute hypoxemia. Oxygen must be given immediately and adequately because it will cause permanent disability and death if it is not given. In this condition, oxygen should be given with a FiO2 of 60–100% in a short time, and specific therapy should be given. Furthermore, oxygen is given at a dose that can overcome hypoxemia and minimize side effects [5,21]. Oxygen should be administered by considering the concentration of PaO2 because it can affect organs such as lungs, blood vessels, cell death, and others if it is deficient or excessive (Fig. 1) [22].
Fig. 1.
Relationship of PaO2 concentration to organ [22].
In general, oxygen can be provided in two ways: low-flow systems and high-flow systems. The nasal cannula is a widely used low-flow system. A low-current nasal cannula delivers oxygen to the nasopharynx at a 1–6 L/min flow, with a FiO2 of 0.24–0.44 (24%–44%). Higher flow does not increase FiO2 significantly above 44% and can cause mucous membranes to dry. High-flow oxygen devices include venturi masks and reservoir nebulizer blenders [15]. These high-flow systems can deliver 20–40 L/min of oxygen through the mask, generally sufficient for the total respiratory requirement. Two clinical indications for the use of high-flow oxygen are patients requiring FiO2 control and hypoxic patients with abnormal ventilation [15,21].
Guidelines for oxygen administration aim to achieve normal or near-normal oxygen saturation in all acutely ill patients, patients at risk for hypercapnic respiratory failure or patients on palliative care [23]. Pulmonary oximetry should check oxygen saturation in all shortness of breath and acutely ill patients. Vital signs must be checked oxygen saturation, pulse, blood pressure, temperature and respiratory rate. A clinical assessment is recommended if saturation falls by 3% or below the target range. All critically ill patients outside the critical care area (e.g. ICU, HCU) should be assessed and monitored using the NEWS (National Early Warning Score) system [23,24].
In critically ill patients (Table 1), high-concentration oxygen should be administered immediately with a 15 L/min reservoir mask while waiting for an oximetry device. Critical conditions requiring oxygen include cardiac arrest, resuscitation, carbon monoxide poisoning, severe head injury, shock, sepsis, drowning, anaphylaxis, pulmonary hemorrhage or status epilepticus [23,25]. Administration of supplemental oxygen to improve oxygenation does not treat the underlying cause of hypoxemia. If the patient is well-circulated and the oximetry is measurable, the oxygen concentration can be reduced with a saturation target of 94–98%. If oximetry is unavailable, continue to use a reservoir mask until definitive treatment is available. Patients with COPD and other conditions are at risk of hypercapnia with respiratory acidosis, if they are in a critical condition, the initial target for oxygen saturation will be the same as for patients with other critical conditions, pending the results of blood gas analysis. If the blood gas analysis results show hypercapnia, the target oxygen saturation is 88–92% [23,24].
Table 1.
Conditions of patients requiring oxygen [23].
| Conditions of patients requiring oxygen |
|||
|---|---|---|---|
| Critical condition | Serious condition | Observation conditions | |
| Initial oxygen administration (L/min) | Reservoir mask 15 L/min | Nasal cannula 2–6 L/min or simple face mask 5–10 L/min | Nasal cannula 2–6 L/min or simple face mask 5–10 L/min |
| Oxygen targets | 94–98% | 94–98% | 94–98% |
| Examples | Heart attack Poisoning Carbon monoxide Severe head injury Shock Sepsis Drowning Anaphylaxis Lung bleeding Status epilepticus |
Acute hypoxemia acute asthma Pneumonia Lung cancer Pneumothorax Pleural effusion Pulmonary embolism Acute heart failure Severe anemia |
Stroke Myocardial infarction Acute coronary syndrome Drug overdose Bleomycin addiction Metabolic and renal disorders Nervous system disorders Emergency conditions in pregnancy |
Patients with severe conditions (Table 1) require moderate supplemental oxygen, such as (1) acute hypoxemia; (2) acute asthma; (3) pneumonia; (4) lung cancer; (5) pneumothorax; (6) pleural effusion; (7) pulmonary embolism; (8) acute heart failure; (9) severe anemia; or (10) postoperative shortness of breath. In patients with severe conditions, initial oxygen therapy involves a nasal cannula at 2–6 L/min (preferred) or a simple face mask at 5–10 L/min [23]. For patients not at risk of hypercapnic respiratory failure and who have a saturation below 85%, oxygen administration is initiated with a 15 L/min reservoir mask with an initial target oxygen saturation of 94–98%. Suppose the patients have COPD or other risk factors for hypercapnic respiratory failure, target saturation of 88–92% pending results of blood gas analysis but adjust to 94–98% if PCO2 is expected (unless there is a history of previous hypercapnic respiratory failure requiring NIV) and recheck analysis blood gas after 30–60 min later [23,24].
Observational conditions are when the patient should be closely monitored (Table 1), but oxygen therapy is not required unless the patient is hypoxemic, such as (1) stroke; (2) myocardial infarction, (3) acute coronary syndrome; (4) drug overdose; (5) bleomycin poisoning; (6) metabolic and kidney disorders; (7) nervous system disorders or (8) pregnancy emergencies. Suppose the condition that needs to be monitored is hypoxemic. In that case, initial oxygen therapy is given in a nasal cannula of 2–6 L/min or a simple face mask of 5–10 L/min unless saturation is below 85% (use a reservoir mask) or if there is a risk of hypercapnia [23]. The recommended initial target oxygen saturation is 94–98%. If oximetry is not available, administer oxygen until oximetry or blood gas analysis results are available. If the patient has COPD or other risk factors for hypercapnic respiratory failure, target oxygen saturation of 88–92% [24,26]. Oxygen should be reduced in stable patients whose oxygen saturation has reached the target. Oxygen should be discontinued once the patient can maintain saturation within or above the target range of respiratory air [23,25].
3. Hyperoxia
Oxygen has a toxic effect at high partial pressures due to increased oxygen concentration on inspiration, ambient pressure or both [1,27]. Oxygen toxicity can manifest in the central nervous system, lung and ocular, especially in premature infants. The onset and degree of toxicity vary depending on oxygen concentration and duration of oxygen administration. Prevention and early detection of oxygen toxicity require symptomatic treatment [1,28].
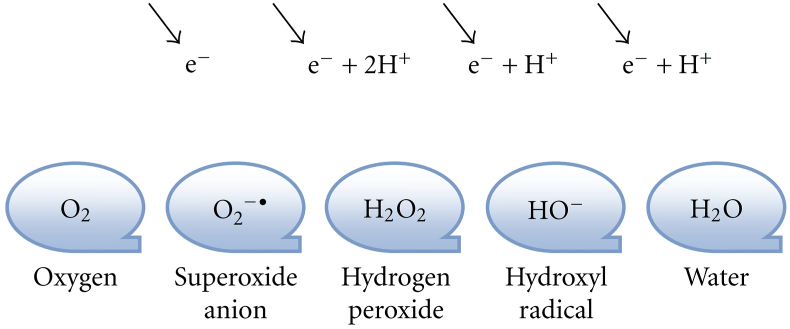
Hyperoxia is a condition in which there is an excess supply of oxygen in the tissues and organs. Oxygen toxicity occurs when the alveolar partial pressure of O2 (PaO2) exceeds that of inhaled under normal conditions. In hyperoxia's pathological condition, an influx of ROS produced in large quantities. In reducing oxygen to water (Fig. 2), four additional electrons produce three types of ROS: superoxide anion, hydrogen peroxide and hydroxyl radical. In intracellular and extracellular systems, the increase in ROS is due to overexposure to O2, thereby disrupting the balance between oxidants and antioxidants. This disruption of homeostasis can cause damage to cells and tissues. The lung response to hyperoxia involves many immune cells [10,11].
Fig. 2.
Stages of reducing oxygen to water [11].
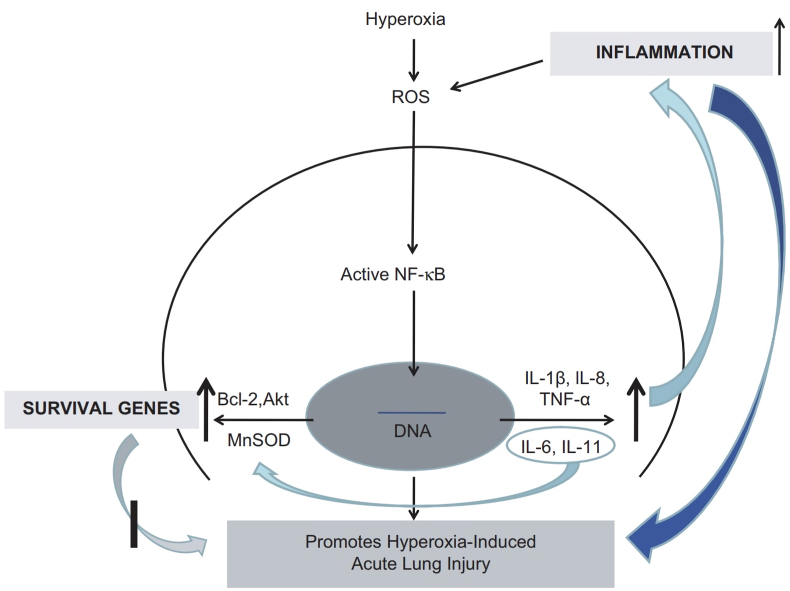
Hyperoxia induces NF-κB activation, which results in nuclear translocations that play a role in the occurrence of HALI. NF-κB has two crucial roles: (1) regulating genes involved in inflammation such as IL-8 and TNFα; (2) regulating the survival of genes such as Bcl-2, Akt and MnSOD enzyme (manganese superoxide dismutase) that protect against cell death due to hyperoxia (Fig. 3) [29,30]. The duration of exposure, atmospheric pressure and fraction of inspired oxygen (FiO2) are the basis for determining the dose of accumulated oxygen that causes toxicity. Oxygen becomes toxic to the lungs when the FiO2 is high (>0.60), administered over 24 h at standard barometric pressure (1 atmosphere absolute/ATA). Prolonged oxygen exposure of about 12 h can cause airway congestion, pulmonary oedema and atelectasis caused by damage to the lining of the bronchi and alveoli [10,22].
Fig. 3.
Schematic diagram illustrating the effects of NF-κB under hyperoxia conditions [29].
Paul Bert was the first to make an essential contribution to oxygen toxicity. In 1878, Paul Bert conducted a study that found seizures in birds exposed to air 15–20 ATA. The CNS toxic effect of oxygen is called the 'Bert effect'. In 1899, J Lorain Smith, trying to reproduce the 'Bert effect', found fatal pneumonia in mice after 4 days of exposure to 73% oxygen at 1 ATA [28]. The pulmonary toxic effect of oxygen is called the Smith effect. The partial pressure of oxygen on inspiration is about 160 mm Hg above sea level. This value can be increased by inhaling 100% oxygen, thereby increasing the inspiratory air pressure to 760 mm Hg or increasing the pressure of the breathing mixture to a theoretically no limit [1,10].
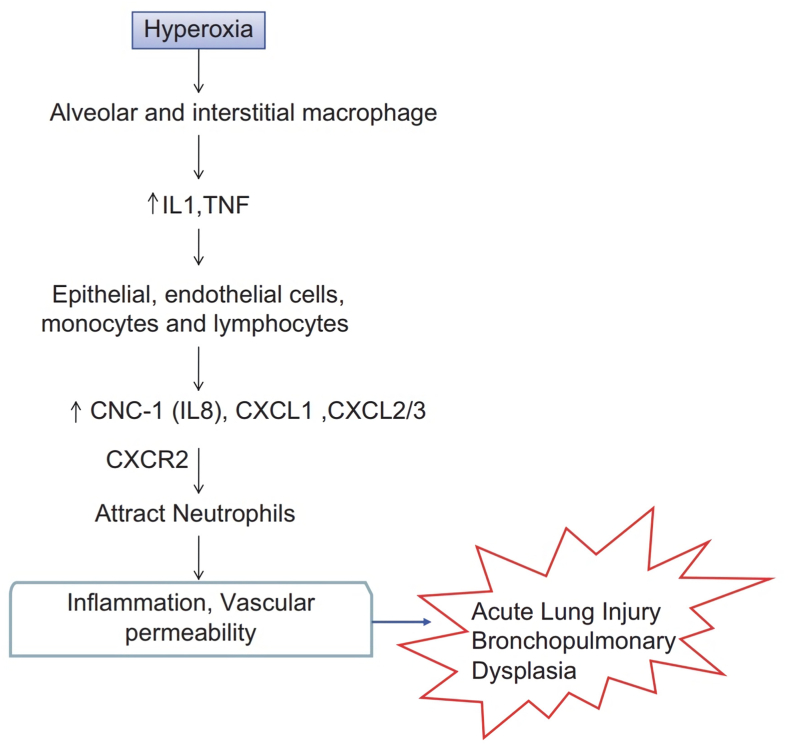
Hyperoxia causes alveolar or interstitial macrophages in the lung to release early response cytokines (IL-1 and TNF). These cytokines activate pulmonary endothelial cells, epithelial cells, monocytes and lymphocytes, which produce chemokines such as IL-8, CXCL1, and CXCL2/3. This chemokine functions by binding to the chemokine receptor CXCR2 and regulating neutrophil recruitment to sites of inflammation, leading to increased vascular permeability and inflammation in HALI and BPD (Fig. 4) [29,30].
Fig. 4.
Mechanism of hyperoxia causing ALI (Acute Lung Injury) and BPD (Bronchopulmonary Dysplasia) [29].
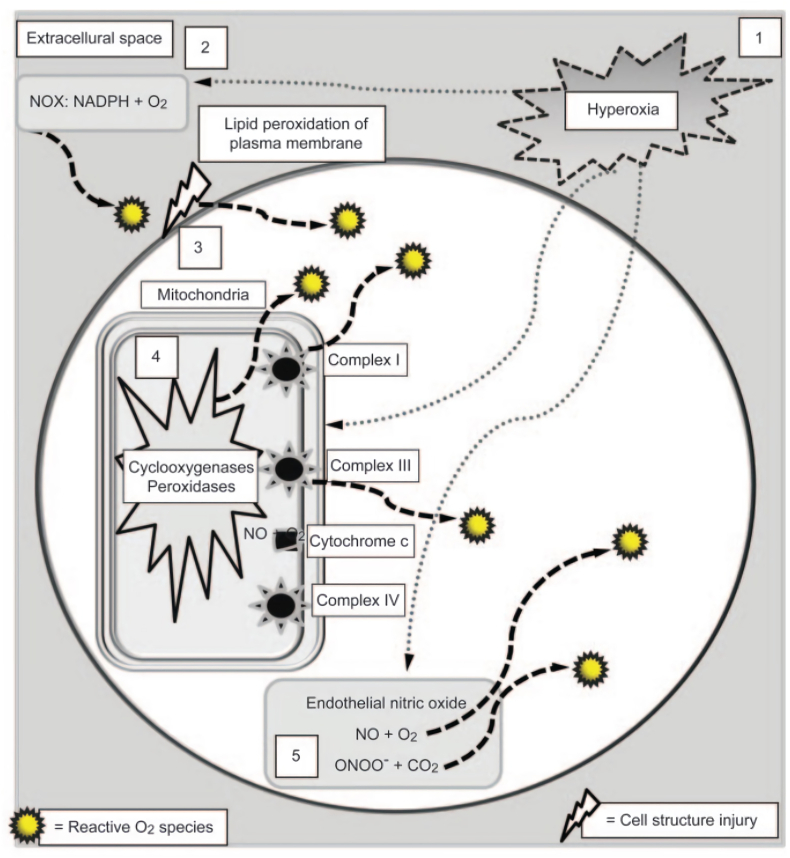
ROS spikes are regulated by the primary target cell, the pulmonary capillary endothelium (Fig. 5). There are several early mechanisms of hyperoxia, namely (1) the ROS spike in proportion to the PO2 during hyperoxia; (2) O2 molecules are reduced to ROS by NADPH (nicotinamide adenine dinucleotide phosphate hydrogen) and oxidase (NOX) in the plasma membrane; (3) damage to the plasma membrane lipid bilayer by ROS that produces additional ROS; (4) The primary source of ROS production comes from mitochondria. Accidental electron leakage usually occurs at the energy transition stage of the cytochrome chain (protein complexes I and III), but the increase is proportional to the intensity of the hyperoxia. Additional sources of ROS result from the interaction of O2 molecules with mitochondrial accretion enzymes, including cyclooxygenases, peroxidases, lipoxygenases, and cytochrome P450; (5) endothelium contains a lot of nitric oxides (NO) so that the interaction with O2 molecules and superoxide anion produces nitrogen dioxide (NO2), reactive nitrogen species and peroxynitrite anion (ONOO-). The peroxynitrite anion combines with carbon dioxide to form additional NO2 [10,17].
Fig. 5.
Initial mechanism of hyperoxia [10].
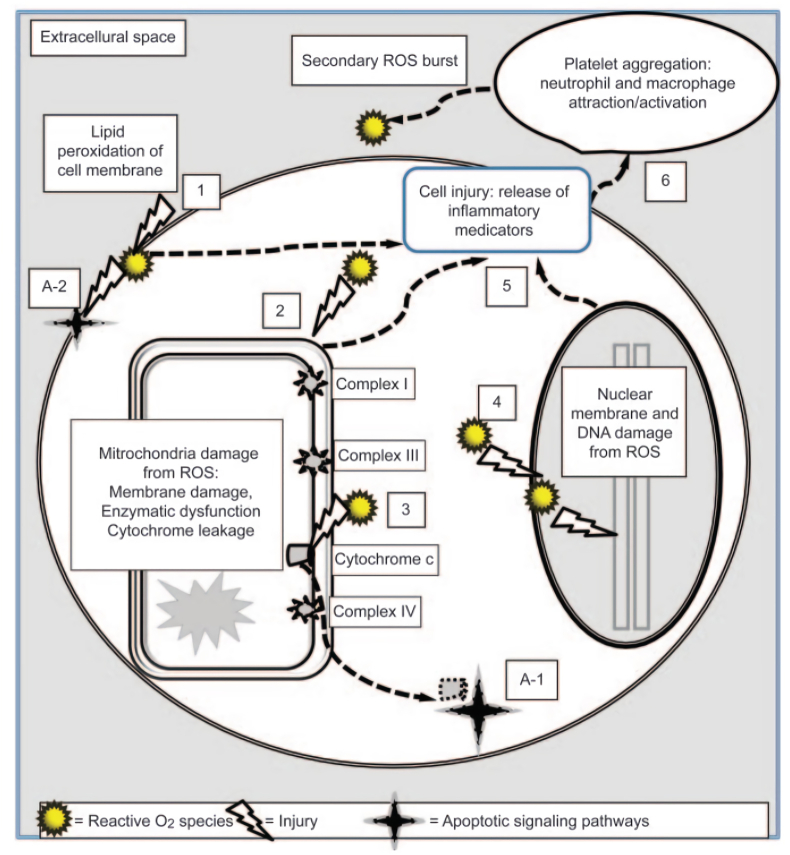
Under hyperoxia conditions, there is a ROS spike and cell death (Fig. 6). This is regulated by several mechanisms, namely: (1) Loss of plasma membrane integrity from lipid peroxidation by ROS; (2) ROS damage to mitochondrial membranes and deactivation of enzyme systems and cytochrome chains; (3) This results in the release of cytochrome C into the cytoplasm; (4) ROS damage in the nuclear membrane and DNA fragmentation; (5) Cell trauma includes steps 1, 2, and 4 that trigger the production and release of proinflammatory cytokines and chemokines into the extracellular space; (6) This activates platelets, neutrophils and macrophages, resulting in a secondary ROS spike from inflammatory cells. Direct cell trauma causes necrosis or “unplanned” cell death. In addition, the release of cytochrome C into the cytoplasm (A-1) and plasma membrane damage (A-2) triggers other cellular processes, which instruct the cell to "commit suicide" through the process of apoptosis (programmed cell death) [10,17].
Fig. 6.
Mechanisms of ROS regulation and cell death pathways due to hyperoxia [10].
Effects of hyperoxia can be seen in neonates and adults. In neonates, there is retrolental hyperplasia after exposure to high oxygen concentrations. A cardiology study found that routine oxygen supplementation in non-hypoxemic patients with acute myocardial infarction could worsen the patient's condition. In hospitalized patients who do not require supplemental oxygen, there will be an increase in PaO2 levels (generally above 120 mmHg) if given, affecting the deterioration of clinical outcomes. There are conflicting data about the dangers of hyperoxia in patients following cardiac resuscitation or mechanical ventilation. Given hyperoxia's potential harm and lack of benefit, the clinical target should not exceed the physiologic threshold [31]. Oxygen toxicity can affect several organ systems, including the central nervous system, lungs and other tissues such as the eyes [28].
4. Central nervous system (CNS) toxicity
CNS toxicity described by Bert includes various initial symptoms and signs. However, perioral twitching and small muscles of the hands, facial pallor and cogwheel breathing are common due to intense peripheral vasoconstriction due to hyperoxia and twitching of the diaphragm [28]. Vertigo, nausea, behavioral changes, confusion, and seizures may occur if exposure continues. Seizures begin with loss of consciousness and progress in three phases; (1) a tonic phase with generalized hypertonia lasting about 1 min, (2) a clonic phase with seizures lasting about 2–3 min, (3) a post-critical phase of about 10 min [1,32]. Concomitant neurogenic pulmonary oedema with seizures has also been reported. Oxygen toxicity to the CNS is influenced by increased PCO2, stress, fatigue and cold [10,11].
5. Lung toxicity
The effects of lung toxicity occur after prolonged oxygen exposure. Symptoms appear after a latency period whose duration decreases with an increase in PO2. In normal humans, initial toxic effects appear after 10 h of 1 ATA oxygen exposure. The clinical picture is divided into three phases, namely: (a) tracheobronchitis, (b) ARDS, and (c) pulmonary interstitial fibrosis [18,20,33]. Oxygen can be tolerated above sea level for about 24–48 h without severe tissue damage. Prolonged exposure causes tissue injury [1,32]. Oxygen 2 ATA can cause characteristic pulmonary signs and symptoms within 3–6 h of exposure, uncontrolled cough for about 10 h, chest pain and shortness of breath. In most patients, symptoms improve about 4 h after not being exposed to oxygen [28]. The fluid formation in the lungs causes shortness of breath and a burning feeling in the throat and chest when breathing is very painful. Oxygen is delivered to body tissues at nearly normal partial pressures of oxygen due to the hemoglobin-oxygen buffer system [1,27,28].
6. Toxic effects on other tissues
Toxic effects of oxygen on the eye include reversible narrowing of the peripheral visual field, progressive and reversible myopia, and cataracts. Ocular effects are expected when the entire eye is exposed to high-pressure oxygen, e.g. oxygen delivery via a facemask [1,27]. Neonates and premature infants exposed to high concentrations of oxygen cause retinopathy, chronic lung disease and intraventricular hemorrhage. Premature babies with a gestational age of fewer than 30 weeks or a birth weight of 1500 g have a greater risk, about 60% [1,27,28].
Hyperoxia inhibits macrophage proliferation and increases inflammation. Alveolar epithelium and alveolar capillary endothelial cells are vulnerable targets for injury by hyperoxia [6,7,14]. In Acute Lung Injury (ALI) conditions caused by hyperoxia, pulmonary microvascular hyperpermeability causes the alveoli to fill with plasma extravasation, which can cause pulmonary oedema and coagulation disorders and fibrin deposition to occur in the fibrinolysis pathways. Type II alveolar epithelial cells are injured by oxygen free radicals, disrupting surfactant production [11].
At high PaO2, the hemoglobin-oxygen buffering mechanism fails and tissue PaO2 increases by 100–1000 mm Hg. ROS's endogenous antioxidant enzyme systems are utilized in cell death at high oxygen concentrations. ROS's oxygen toxicity caused by ROS proceeds in overlapping phases based on the injury's degree of severity and recovery. These stages are initiation, inflammation, proliferation and fibrosis. Initially, there is increased ROS and antioxidant depletion, and the lung fails to clear itself of mucus. The inflammatory or exudative phase is characterized by the destruction of the lung lining and the migration of leukocytes from inflammatory mediators to the injury site. In the subacute proliferative phase, cellular hypertrophy occurs, increased surfactant secretion in removing type II alveolar cells and increased monocyte cells. The terminal phase is the fibrotic phase, where the lung has irreversible and permanent changes. There are collagen deposits and thickening of the lung interstitial spaces, and the lung becomes fibrotic [10,11].
Clinically, progressive hypoxemia, or high O2 pressure in the blood, requires increased FiO2 and assisted ventilation, which further exacerbates the pathophysiological changes associated with oxygen toxicity. The chest X-ray may show an alveolar interstitial pattern with an irregular distribution with moderate volume loss from atelectasis, but there is no clinical way to diagnose oxygen toxicity [10]. Biopsy of lung specimens may show changes in oxygen toxicity, but the biopsy excludes other causes of lung injury. Oxygen toxicity can be minimized by maintaining PaO2 <80 mmHg or FiO2 <0.40–0.5. The severity of HALI is proportional to the PO2 (significantly above 450 mm Hg, or FiO2 0.6) and the duration of exposure [8,10].
Anatomically, the surface epithelium of the lung is susceptible to a destructive inflammatory response. This inflammation damages the alveolar capillaries leading to impaired gas exchange and pulmonary oedema [10]. ROS induce the lung to secrete chemoattractant, and cytokines stimulate macrophages and monocytes, accumulating into the lungs and causing ROS. The leukocyte-ROS interaction exacerbates the injury. Studies have shown that severely reduced cell layers become increasingly oxidized, and antioxidant levels decrease, ROS-induced activation of various transduction pathways that regulate cellular responses, i.e. adaptation, repair or cell death by apoptosis, oncosis, or necrosis [11].
Increased mortality in patients with hyperoxia compared to hypoxia and normoxia (guidelines used for hyperoxia PaO2 300 mmHg) in 6326 patients with post-cardiac arrest or myocardial infarction from 120 hospitals [34]. A retrospective cohort study also found an increased mortality rate in hyperoxia in 2894 stroke patients from 84 ICUs, measuring PaO2 in the first 24 h (Hyperoxia: PaO2 300 mmHg; Normoxia: PaO2 60–300 mmHg) [35]. Another study reported an increased mortality rate in patients with hyperoxia in 83 septic patients in the ED who were given oxygen at 10 L/min [36]. In addition, an increased mortality rate was also found in conditions of severe hyperoxia compared to mild hyperoxia and normoxia (mild hyperoxia: PaO2 120–200 mmHg, severe hyperoxia: PaO2 >200 mmHg) in 14441 patients with mechanical ventilation in the ICU [37].
In patients with severe hypoxemic respiratory failure, it is advisable to reassess oxygenation goals to provide the safest support. Oxygen supplementation targets the cardiorespiratory system, including arterial PO2, arterial O2 content and cardiac output. The generally recommended targets are an arterial PO2 of 55 mmHg, a hemoglobin level of at least 7 g/dL (but may also be 9–10 g/dL), and a cardiac index above 2 L/min/m2. This target will keep O2 delivery close to normal [31].
Future study is expected to find guidelines and updates for oxygen therapy to avoid hyperoxia. Administration of oxygen therapy must be based on the patient's condition because it can have a toxicity effect if have given in excess.
7. Summary
Management of respiratory failure is closely related to the provision of oxygen supplementation, so special attention is needed in its administration, according to indications and does not have a toxic effect. Oxygen supplementation in conditions of respiratory failure aims to overcome hypoxemia. Overexposure to oxygen can cause oxygen toxicity and lead to hyperoxia. Hyperoxia is a condition in which there is an excess supply of oxygen in the tissues and organs. Clinically, respiratory failure is diagnosed if the PaO2 is less than 60 mmHg with or without an increase in carbon dioxide when the patient breathes room air. Respiratory failure is divided into acute (sudden) respiratory failure and chronic (slow) respiratory failure. The basis for managing respiratory failure consists of supportive/non-specific and causative/specific management. Oxygen should be provided wisely so as not to cause injury to organs such as the heart, lungs, eyes, nervous system, and others. Hyperoxia often occurs in managing respiratory failure, so it requires supervision, especially in administering oxygen. Oxygen should be given as needed to avoid hyperoxia. In oxygen therapy, it is necessary to pay attention to the patient's condition because each condition requires different oxygen concentrations, so dose adjustments are necessary. These conditions can be divided into critical, severe, and observation conditions. The target oxygen saturation in all these conditions is 94–98%.
Ethical approval
Not applicable.
Sources of funding
None.
Author contribution
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Consent
Not applicable.
Registration of research studies
-
1.
Name of the registry:-.
-
2.
Unique Identifying number or registration ID:-.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked):-.
Guarantor
Irmi Syafaah is the person in charge of the publication of our manuscript.
Declaration of competing interest
Elvina Elizabeth Lius and Irmi Syafaah declare that they have no conflict of interest.
Acknowledgement
We would like thank to our editor, “Fis Citra Ariyanto”.
References
- 1.Chawla A., Lavania A.K. Oxygen toxicity. Med. J. Armed Forces India. 2001;57(2):131–133. doi: 10.1016/s0377-1237(01)80133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung O.-Y., Graziano P., Smith M.L. In: Practical Pulmonary Pathology: A Diagnostic Approach. third ed. Leslie K.O., Wick M.R., editors. Elsevier; 2018. 6 - acute lung injury. 125-46.e3. [Google Scholar]
- 3.Allardet-Servent J., Sicard G., Metz V., Chiche L. Benefits and risks of oxygen therapy during acute medical illness: just a matter of dose. Rev. Med. Interne. 2019;40(10):670–676. doi: 10.1016/j.revmed.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Rahmawati Y., Maranatha D. Acute respiratory failure on varicella pneumonia in Indonesian adult with chronic hepatitis B: a case report and review article. Ann. Med. Surg. 2022;80 doi: 10.1016/j.amsu.2022.104149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slattery M., Vasques F., Srivastava S., Camporota L. Management of acute respiratory failure. Medicine. 2020;48(6):397–403. doi: 10.1016/j.mpmed.2020.03.010. [DOI] [Google Scholar]
- 6.Vo P., Kharasch V.S. Respiratory failure. Pediatr. Rev. 2014;35(11):476–484. doi: 10.1542/pir.35-11-476. quiz 85-6. [DOI] [PubMed] [Google Scholar]
- 7.Suh E.-S., Hart N. Respiratory failure. Medicine. 2012;40(6):293–297. doi: 10.1016/j.mpmed.2012.03.012. [DOI] [Google Scholar]
- 8.David-João P.G., Guedes M.H., Réa-Neto Á, Chaiben V.B.O., Baena C.P. Noninvasive ventilation in acute hypoxemic respiratory failure: a systematic review and meta-analysis. J. Crit. Care. 2019;49:84–91. doi: 10.1016/j.jcrc.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Mukhlis M., Bakhtiar A. Obstructive sleep apneu (OSA), obesitas hypoventilation syndrome (OHS) dan gagal napas. J. Respirol. 2015;1(3):94–102. doi: 10.20473/jr.v1-I.3.2015.94-102. [DOI] [Google Scholar]
- 10.Kallet R.H., Matthay M.A. Hyperoxic acute lung injury. Respir. Care. 2013;58(1):123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mach W.J., Thimmesch A.R., Pierce J.T., Pierce J.D. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs. res. pract. 2011;2011 doi: 10.1155/2011/260482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel V., Dial K., Wu J., Gauthier A.G., Wu W., Lin M., et al. Dietary antioxidants significantly attenuate hyperoxia-induced acute inflammatory lung injury by enhancing macrophage function via reducing the accumulation of airway HMGB1. Int. J. Mol. Sci. 2020;21(3) doi: 10.3390/ijms21030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Y., Lin P., Zhang W., Tan S., Zhou X., Li R., et al. DNA repair interacts with autophagy to regulate inflammatory responses to pulmonary hyperoxia. J. Immunol. 2017;198(7):2844–2853. doi: 10.4049/jimmunol.1601001. Baltimore, Md : 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad S., O'Neill S. Respiratory failure. Surg. - Oxford Int. Ed. 2021;39(10):654–659. doi: 10.1016/j.mpsur.2021.08.007. [DOI] [Google Scholar]
- 15.Roussos C., Koutsoukou A. Respiratory failure. Eur. Respir. J. Suppl. 2003;47:3s–14s. doi: 10.1183/09031936.03.00038503. [DOI] [PubMed] [Google Scholar]
- 16.Bourke S.C., Piraino T., Pisani L., Brochard L., Elliott M.W. Beyond the guidelines for non-invasive ventilation in acute respiratory failure: implications for practice. Lancet Respir. Med. 2018;6(12):935–947. doi: 10.1016/s2213-2600(18)30388-6. [DOI] [PubMed] [Google Scholar]
- 17.Dias-Freitas F., Metelo-Coimbra C., Roncon-Albuquerque R., Jr. Molecular mechanisms underlying hyperoxia acute lung injury. Respir. Med. 2016;119:23–28. doi: 10.1016/j.rmed.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Bakhtiar A., Maranatha R.A. Acute respiratory distress syndrome. J. Respirol. 2018;4(2):51–60. doi: 10.20473/jr.v4-I.2.2018.51-60. [DOI] [Google Scholar]
- 19.Syafa'ah I. Non-invasive ventilation in COVID-19 related respiratory failure. Jurnal Respirasi. 2021;7(3):139–144. doi: 10.20473/jr.v7-I.3.2021.139-144. [DOI] [Google Scholar]
- 20.Maranatha D., Rahardjo P., Lusman R. Evolution of chest CT scan manifestations in a patient recovered from COVID-19 severe pneumonia with acute respiratory distress syndrome. Respir. med. case rep. 2021;32 doi: 10.1016/j.rmcr.2021.101342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boccatonda A., Groff P. High-flow nasal cannula oxygenation utilization in respiratory failure. Eur. J. Intern. Med. 2019;64:10–14. doi: 10.1016/j.ejim.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Vincent J.L., Taccone F.S., He X. Harmful effects of hyperoxia in postcardiac arrest, sepsis, traumatic brain injury, or stroke: the importance of individualized oxygen therapy in critically ill patients. Can. Respir. J. J. Can. Thorac. Soc. 2017;2017 doi: 10.1155/2017/2834956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Driscoll B.R., Howard L.S., Earis J., Mak V. British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ open respir. res. 2017;4(1) doi: 10.1136/bmjresp-2016-000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane B., Decalmer S., Ronan O'Driscoll B. Emergency oxygen therapy: from guideline to implementation. Breathe. 2013;9(4):246–253. doi: 10.1183/20734735.025212%JBreathe. [DOI] [Google Scholar]
- 25.O'Driscoll B.R., Howard L.S., Earis J., Mak V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1) doi: 10.1136/thoraxjnl-2016-209729. ii1-ii90. [DOI] [PubMed] [Google Scholar]
- 26.Gu W.J., Zhang Z., Van Poucke S. Oxygen therapy and ventilatory support. Can. Respir. J. J. Can. Thorac. Soc. 2017;2017 doi: 10.1155/2017/2462818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson L., Paton J. Oxygen toxicity. Paediatr. Respir. Rev. 2014;15(2):120–123. doi: 10.1016/j.prrv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Reidy B.T.G., Whyte P., Neligan P.J. In: Evidence-Based Practice of Critical Care. third ed. Deutschman C.S., Neligan P.J., editors. Elsevier; 2020. 6 - is oxygen toxic? pp. 36–42.e1. [Google Scholar]
- 29.Gore A., Muralidhar M., Espey M.G., Degenhardt K., Mantell L.L. Hyperoxia sensing: from molecular mechanisms to significance in disease. J. Immunot. 2010;7(4):239–254. doi: 10.3109/1547691x.2010.492254. [DOI] [PubMed] [Google Scholar]
- 30.Roan E., Wilhelm K., Bada A., Makena P.S., Gorantla V.K., Sinclair S.E., et al. Hyperoxia alters the mechanical properties of alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;302(12):L1235–L1241. doi: 10.1152/ajplung.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntyre N.R. Supporting oxygenation in acute respiratory failure. Respir. Care. 2013;58(1):142–150. doi: 10.4187/respcare.02087. [DOI] [PubMed] [Google Scholar]
- 32.Palmer B.F., Clegg D.J. Oxygen sensing and metabolic homeostasis. Mol. Cell. Endocrinol. 2014;397(1–2):51–58. doi: 10.1016/j.mce.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Kusumawardhani N., Dewi I.P., Dharmadjati B.B. Extracorporeal membrane oxygenation used in acute respiratory distress syndrome with COVID-19: a systematic review and meta-analysis. J. Saudi Heart Assoc. 2021;33(2):177–185. doi: 10.37616/2212-5043.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilgannon J.H., Jones A.E., Shapiro N.I., Angelos M.G., Milcarek B., Hunter K., et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 35.Rincon F., Kang J., Maltenfort M., Vibbert M., Urtecho J., Athar M.K., et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit. Care Med. 2014;42(2):387–396. doi: 10.1097/CCM.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- 36.Stolmeijer R., ter Maaten J.C., Zijlstra J.G., Ligtenberg J.J. Oxygen therapy for sepsis patients in the emergency department: a little less? Eur. J. Emerg. Med. : off. j. Eur. Soc. Emerg. Med. 2014;21(3):233–235. doi: 10.1097/MEJ.0b013e328361c6c7. [DOI] [PubMed] [Google Scholar]
- 37.Helmerhorst H.J., Arts D.L., Schultz M.J., van der Voort P.H., Abu-Hanna A., de Jonge E., et al. Metrics of arterial hyperoxia and associated outcomes in critical care. Crit. Care Med. 2017;45(2):187–195. doi: 10.1097/ccm.0000000000002084. [DOI] [PubMed] [Google Scholar]