Abstract
Introduction
Our aim was to estimate the difference in birthweight and in placental weight in pregnancies with type 1 diabetes, type 2 diabetes, and gestational diabetes compared with pregnancies without diabetes.
Material and methods
By using data from the Medical Birth Registry of Norway during the years 2009–2017, we included 319 076 singleton pregnancies with delivery after the 21st week of pregnancy. We used linear regression analyses to estimate the difference in birthweight and in placental weight in grams (g) in pregnancies with type 1 diabetes, type 2 diabetes, and gestational diabetes, using pregnancies without diabetes as the reference. Adjustments were made for pregnancy duration and pre-pregnancy body mass index.
Results
In pregnancies without diabetes, mean crude birthweight was 3527 g (SD 552 g). The adjusted mean birthweight was 525 g (95% CI 502–548 g) higher in pregnancies with type 1 diabetes compared with pregnancies without diabetes. In pregnancies with type 2 diabetes, and pregnancies with gestational diabetes, birthweights were 192 g (95% CI 160–223 g) and 102 g (95% CI 93–110 g) higher, respectively. Mean crude placental weight was 664 g (SD 147 g) in pregnancies without diabetes. Compared with pregnancies without diabetes, the adjusted mean placental weight was 109 g (95% CI 101–116 g) higher in pregnancies with type 1 diabetes, 50 g (95% CI 39–60 g) higher in pregnancies with type 2 diabetes, and 31 g (95% CI 28–34 g) higher in pregnancies with gestational diabetes.
Conclusions
The increase in birthweight and in placental weight associated with maternal diabetes was most pronounced for type 1 diabetes, followed by type 2 diabetes, and gestational diabetes.
1. INTRODUCTION
The prevalence of diabetes among pregnant women has increased over the past 20 years in many countries of the world.1 In particular, the prevalence of gestational diabetes has increased. However, pregnant women may also have type 1 diabetes or type 2 diabetes, and all types of diabetes are characterized by increased glucose concentrations in the blood.
Any type of diabetes during pregnancy has been associated with increased birthweight of the offspring.2, 3 High birthweight increases the risk of delivery complications, both for the mother and the child.4–6 Furthermore, babies that are heavy at birth are also more likely to be obese or have diabetes as adults.7
Placental weight is increased in diabetic pregnancies.8 High placental weight or high placental weight relative to birthweight, has been associated with several adverse outcomes such as low Apgar score,9 respiratory distress in the newborn,10 perinatal death, and cardiovascular death in adulthood.11–13
High body mass index (BMI) increases the risk of high birthweight and high placental weight, independent of maternal diabetes,14, 15 and BMI may differ between women with type 1 diabetes, women with type 2 diabetes, and those with gestational diabetes.
The duration of pregnancy is another factor that is positively associated with offspring birthweight. However, women with diabetes may have a shorter duration of pregnancy as they are more likely to suffer from complications that necessitate early delivery of the offspring.16 Thus, the observed birthweights and placental weights from diabetic pregnancies are lower than they would have been if the pregnancy had continued. If duration of pregnancy at delivery is not accounted for in the data analyses, comparisons between diabetic and non-diabetic pregnancies will be biased, and the true differences will be underestimated.
To our knowledge, no previous study has compared offspring birthweight in pregnancies with type 1 diabetes, type 2 diabetes, and gestational diabetes within the same study sample, and at the same time made adjustments for maternal BMI and pregnancy duration. Such comparisons may improve our understanding of the impact of the different types of maternal diabetes on fetal growth. Also, such comparisons may provide important information about the magnitude of the health problem associated with increased birthweight in pregnancies with maternal diabetes.
We included 319 076 singleton pregnancies in Norway, and we estimated differences in birthweight and placental weight between pregnancies with type 1 diabetes, type 2 diabetes, and gestational diabetes, using pregnancies without diabetes as the references. Differences in weight at birth were studied with and without adjustment for maternal BMI and pregnancy duration. Additionally, we studied the risk for giving birth to a large- or a small-for-gestational-age offspring according to maternal diabetes type.
2. MATERIAL AND METHODS
2.1. Study population
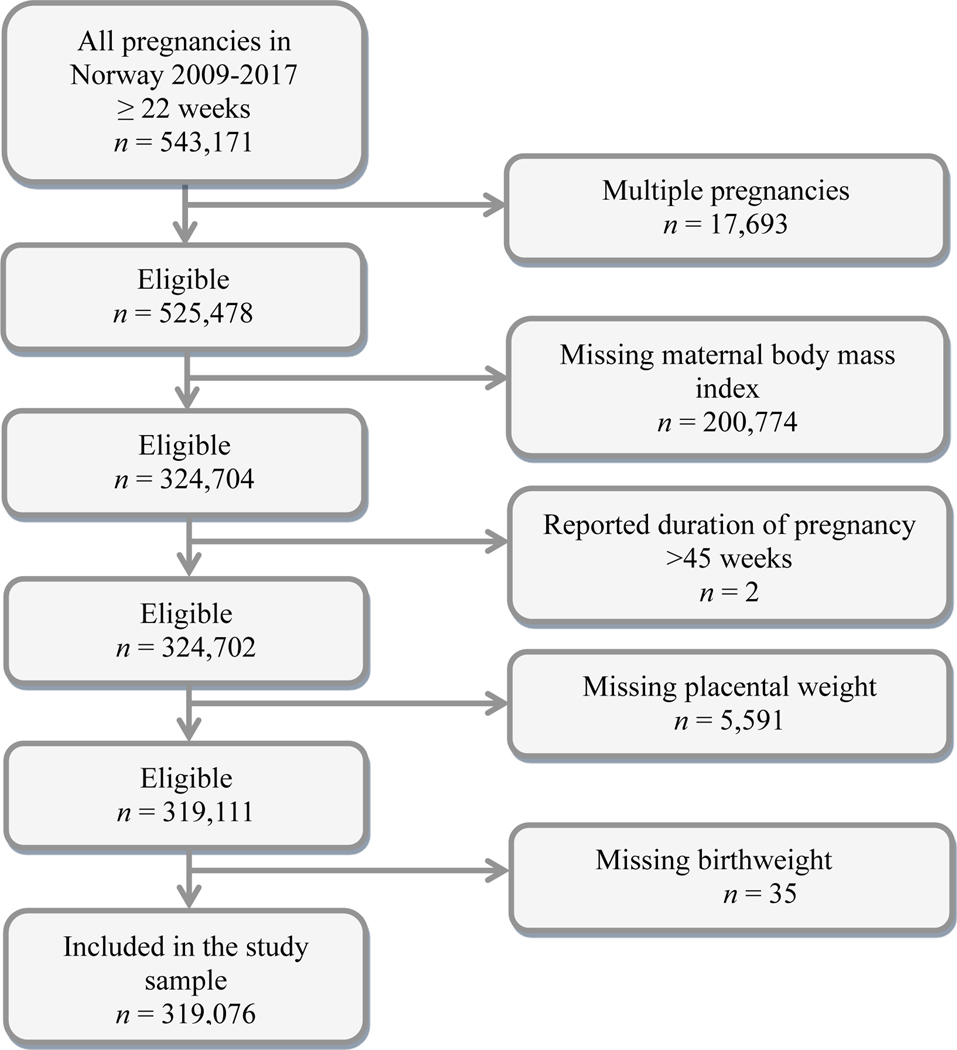
We used data from the Medical Birth Registry of Norway. All births in Norway after 16 weeks of pregnancy are compulsorily reported to this registry by the midwife or doctor who attended the delivery.17 Placental weight has been reported since 1999, maternal height and pre-pregnancy weight since 2009. We aimed to include all singleton pregnancies with delivery after the 21st week of pregnancy during the years 2009–2017, a total of 525 478 pregnancies (Figure 1). Of these, we excluded 200 774 pregnancies without information on maternal height and/or weight (Figure 1). We also excluded two pregnancies with reported delivery after 45 weeks of pregnancy, as we considered such delay in delivery improbable. Additionally, 35 pregnancies were excluded due to missing (n = 21) or outlying (n = 14) values on birthweight (birthweight <250 grams (g) or >6500 g), and 5591 pregnancies were excluded because of missing (n = 1077) or outlying (n = 4514) values on placental weight (placental weight <25 or >2500 g), leaving a total of 319 076 pregnancies for our data analyses.
Figure 1.
Flow chart of study sample
Birthweight and placental weight were measured in grams, and grams were used as a continuous variable in the data analyses. According to obstetric guidelines in Norway, the newborn infant and the placenta are weighed routinely shortly after birth. The placenta is placed in a bowl and weighed fresh with membranes and umbilical cord attached. The weight of the bowl is subtracted from the total weight.
Maternal diabetes is reported to the Medical Birth Registry as type 1 diabetes, type 2 diabetes, or gestational diabetes. Type 1 and type 2 diabetes are, in almost all cases, present before the pregnancy. Women with gestational diabetes were identified by testing for presence of glucose in the urine (with dipstick) at every antenatal clinical examination. Such testing is as part of the public antenatal health-care program in Norway.18 During our study period, an oral glucose tolerance test was offered to all patients with glucosuria, and/or previous gestational diabetes, and/or hereditary risk of diabetes, and/or immigrants from the Indian subcontinent, and/or maternal age >35 years, and/or pre-pregnancy BMI ≥27 kg/m2. Gestational diabetes was diagnosed if the fasting blood glucose concentration was <7.0 mmol/L, and if the blood glucose concentration was between ≥7.8 and <11.1 mmol/L 2 hours after intake of 75 g oral glucose in fasting (12 h) women without pre-gestational diabetes.19 These criteria are in line with the 2006 WHO criteria for impaired glucose tolerance.20
Antenatal care is offered free of charge in Norway. Almost 100% of pregnant women follow the public antenatal care program, which includes at least eight examinations by a doctor (usually a general practitioner) and/or a midwife. The frequency of the examinations increases towards the estimated date of delivery.18 Women with any type of diabetes in pregnancy are referred to clinical follow up at the hospital where the delivery is scheduled to take place. Women with diabetes type 1 and women with diabetes type 2 are most often treated with insulin in pregnancy. In the last part of our study period, some women with diabetes type 2 or gestational diabetes were treated with metformin. Women with gestational diabetes are offered dietary advice. If dietary changes do not lower the blood glucose concentrations sufficiently, insulin or metformin treatment is initiated.
Information about maternal height and pre-pregnancy weight was obtained by interview at the first antenatal clinical examination, typically at pregnancy week 8–10. BMI was calculated as weight (kg)/height (m)2 and used as a continuous variable in the data analyses.
Duration of pregnancy at delivery (in weeks) was estimated on the basis of fetal size at routine ultrasound examination 17–19 weeks after the last menstrual period. If such an examination was not performed, duration of pregnancy was based on the date of the last menstrual period (for 2.2% of the women).
2.2. Statistical analyses
2.2.1. Increase in birthweight and placental weight
We calculated crude mean offspring birthweight and mean placental weight according to maternal diabetes status (type 1 diabetes, type 2 diabetes, gestational diabetes or no diabetes). Differences in crude mean weight between groups were tested with a one-way analysis of variance with Bonferroni correction. Furthermore, we used linear regression analyses to estimate the mean difference in birthweight and in placental weight (in grams) for pregnancies with type 1 diabetes, pregnancies with type 2 diabetes, and pregnancies with gestational diabetes, compared with pregnancies without diabetes. The difference was estimated as crude and adjusted regression coefficients (B) with 95% CI. The adjusted model included duration of pregnancy at delivery and pre-pregnancy maternal BMI. In supplementary analyses, we also made adjustment for maternal age and parity. To explore the consistency of our finding, we studied separately the women with normal pre-pregnancy BMI (BMI ≥18.5 to <25 kg/m2). Additionally, we included in the analyses the women with missing BMI (Figure 1), and in these analyses, we made adjustment for duration of pregnancy at delivery.
2.3. Risk of delivering a large- or a small-for-gestational-age offspring or placenta
In separate analyses, we estimated the associations of diabetes type with delivery of a large-for-gestational-age (LGA) or a small-for-gestational-age (SGA) infant. We defined LGA as birthweight above the 90th centile, and SGA as birthweight less than the 10th centile. The definitions of LGA and SGA were based on the distribution of birthweight z-scores in the study sample as a whole.21 z-scores were calculated separately for male and female offspring. We estimated the associations of diabetes type with giving birth to an SGA (yes/no) or LGA (yes/no) infant as odds ratios (OR) with 95% CI by applying logistic regression analyses. Non-diabetic pregnancies constituted the reference group. We present crude ORs and ORs after adjustment for maternal BMI. In supplementary analyses, we also made adjustments for maternal age and parity.
Finally, we calculated z-scores of placental weight by using the same principles as for birthweight. Hence, LGA placentas had weights in the >90th centiles, and SGA placentas had weights in the <10th centiles of placental weights. We repeated the above analyses while using LGA and SGA placental weight as the outcomes.
All statistical analyses were performed by using the statistical package IBM SPSS Statistics for Windows, Version 21.0.
2.4. Ethical approval
The Norwegian Data Inspectorate has approved the use of the Medical Birth Registry of Norway for research. The data in our study are anonymous. Hence, no ethical approval was required for this specific study. We ordered the data at https://helsedata.no/, and the data were received on January 4, 2021. The Medical Birth Registry of Norway is not responsible for the data analyses or the interpretation of the results.
3. RESULTS
Of the 319 076 women in our study sample, 13 904 (4,4%) women had diabetes in pregnancy. Among the women with diabetes, 9.8% (n = 1360) had type 1 diabetes, 5.1% (n = 704) had type 2 diabetes and 85.1% (n = 11 840) had gestational diabetes (Table 1). In the study sample as a whole, mean birthweight was 3529 g (SD 555 g), and mean placental weight was 666 g (SD 148 g) (Table 1).
Table 1.
Number of pregnancies and means of study factors according to maternal diabetes status. Singleton pregnancies in Norway during the years 2009–2017.
| Diabetes status | n (%) | Pre-pregnancy BMI (kg/m2) (SD) | Duration of pregnancy (wk) (SD) | Birthweighta (g) (SD) | Placental weighta (g) (SD) |
|---|---|---|---|---|---|
| No diabetes | 304,758 (95.5) | 24.1 (4.6) | 39.4 (1.8) | 3527 (552) | 664 (147) |
| Type 1 diabetes | 1360 (0.4) | 25.8 (4.9) | 37.3 (2.2) | 3672 (725) | 730 (186) |
| Type 2 diabetes | 704 (0.2) | 30.5 (6.4) | 37.9 (2.2) | 3523 (687) | 703 (200) |
| Gestational diabetes | 11 840 (3.7) | 28.2 (6.1) | 38.9 (1.8) | 3575 (590) | 698 (161) |
| Total | 319 076 (100.0) | 24.3 (4.8) | 39.4 (1.8) | 3529 (555) | 666 (148) |
Abbreviations: BMI, maternal body mass index (kg/m2); SD, standard deviation.
The differences in means between groups of BMI, duration of pregnancy, birthweight, and placental weight are statistically significant (p < 0.05, Student’s t test).
In pregnancies without diabetes, mean crude birthweight was 3527 g (SD 552 g). Mean birthweight was highest in pregnancies with type 1 diabetes (3672 g; SD 725 g), followed by pregnancies with gestational diabetes (3575 g; SD 590 g) and pregnancies with type 2 diabetes (3523 g; SD 687 g) (Table 1).
Mean crude placental weight in pregnancies without diabetes was 664 g (SD 147 g). As for birthweight, mean placental weight was highest in pregnancies with type 1 diabetes (730 g; SD 186 g) (Table 1). Pregnancies with type 2 diabetes and pregnancies with gestational diabetes had similar mean placental weights: 703 g (SD 200 g) and 698 g (SD 161 g), respectively.
The duration of pregnancy was 2 weeks shorter in pregnancies with type 1 diabetes compared with pregnancies without diabetes (Table 1). Mean maternal BMI was highest in pregnancies with type 2 diabetes followed by pregnancies with gestational diabetes (Table 1).
After adjustments for differences between women in duration of pregnancy and maternal BMI (Table 2 Model 3), we estimated mean birthweight to be 525 g (95% CI 502−548 g) higher in pregnancies with type 1 diabetes compared with pregnancies without diabetes (Table 2 model 3). Birthweight was 192 g (95% CI 160–223 g) higher in pregnancies with type 2 diabetes, and 102 g (95% CI 93–110 g) higher in pregnancies with treated gestational diabetes coampared with pregnancies without diabetes. Table 2 presents three different models for estimation of the change in birthweight in pregnancies with different diabetes subtypes compared with pregnancies without diabetes. In model 1, crude estimates are presented. In model 2, adjustment is made for duration of pregnancy, and in model 3 adjustments are made for duration of pregnancy and maternal BMI. In all models, the increase in birthweight and in placental weight was highest in pregnancies with type 1 diabetes. The highest birthweight increase associated with type 1 diabetes was estimated after adjustment for duration of pregnancy only (model 2).
Table 2.
Crude and adjusted increase in birthweight and in placental weight in grams with 95% CI in pregnancies with type 1 diabetes, type 2 diabetes, or gestational diabetes using pregnancies without diabetes as the reference. a
| n | Crude B Model 1 b | 95% CI | Adjusted B Model 2 b | 95% CI | Adjusted B Model 3 b | 95% CI | |
|---|---|---|---|---|---|---|---|
| Increase in birthweight (grams) | |||||||
| Diabetes status | |||||||
| No diabetes (reference) | 304,758 | 0 | 0 | 0 | |||
| Type 1 diabetes | 1360 | 146*** | 116–175 | 549*** | 526–572 | 525*** | 502–548 |
| Type 2 diabetes | 704 | −5 | −46–36 | 282*** | 249–314 | 192*** | 160–223 |
| Gestational diabetes | 11,840 | 49*** | 39–59 | 159*** | 151–167 | 102*** | 93–110 |
| Duration of pregnancy (weeks) | 319,076 | 188*** | 187–189 | 191*** | 190–192 | 190*** | 190–191 |
| Maternal BMI (kg/m2) | 319,076 | 15*** | 14–15 | 14*** | 13–14 | ||
| Increase in placental weight (grams) | |||||||
| Diabetes status | |||||||
| No diabetes (reference) | 304,758 | 0 | 0 | 0 | |||
| Type 1 diabetes | 1360 | 66*** | 58–74 | 116*** | 108–123 | 109*** | 101–116 |
| Type 2 diabetes | 704 | 39*** | 28–50 | 74*** | 64–85 | 50*** | 39–60 |
| Gestational diabetes | 11,840 | 33*** | 31–36 | 48*** | 44–50 | 31*** | 28–34 |
| Duration of pregnancy (weeks) | 319,076 | 23*** | 22–23 | 23*** | 23–24 | 23*** | 23–24 |
| Maternal BMI (kg/m2) | 319,076 | 4*** | 4–4 | 4*** | 4–4 | ||
Adjustments are made for pregnancy duration at delivery (in weeks) and pre-pregnancy maternal body mass index (BMI) (in units). The increase in grams was estimated as unstandardized B-coefficients by applying linear regression analyses.
Model 1 Crude estimates. Model 2 The variables included in the analyses were maternal diabetes status and duration of pregnancy at delivery. Model 3 The variables included in the analyses were maternal diabetes status, duration of pregnancy at delivery, and maternal BMI.
Abbreviations: BMI, body mass index (kg/m2); SD, standard deviations.
p < 0.05.
p < 0.005.
p < 0.001.
The associations of diabetes types with placental weight showed similar patterns as for birthweight. Hence, after adjustment for duration of pregnancy and maternal BMI, mean placental weight was 109 g (95% CI 101–116 g) higher in pregnancies with type 1 diabetes compared with pregnancies without diabetes. The placental weight was 50 g (95% CI 39–60 g) higher in pregnancies with type 2 diabetes, and 31 g (95% CI 28–34 g) higher in pregnancies with gestational diabetes (Table 2).
In separate analyses of women with BMI >18.5 to <25 kg/m2, we estimated similar differences in birthweight and in placental weight associated with diabetes type as in the study sample as a whole (Supporting Information Table S1). We also found similar results when we included in the data analysis the pregnancies without information about maternal BMI; and in this analysis, we made adjustments for pregnancy duration (Table S2).
Women with type 1 diabetes had the highest risk of giving birth to an LGA infant (adjusted OR 6.9; 95% CI 6.2–7.8). Adjustment was made for maternal BMI, and women without diabetes were used as the reference group. For women with type 2 diabetes, the adjusted OR of giving birth to an LGA infant was 2.1 (95% CI 1.8–2.5), and for women with gestational diabetes the adjusted OR was 1.6 (95% CI 1.5–1.6) (Tables 3 and 4). All types of diabetes reduced the risk of giving birth to an SGA infant. However, only for pregnancies with type 1 diabetes was the risk significantly lower than for women without diabetes (adjusted OR 0.4; 95% CI 0.3–0.5) (Tables 3 and 4).
Table 3.
Crude and adjusted odds ratios (OR) for giving birth to a large-for-gestational-age (LGA) offspring and a small-for-gestational-age (SGA) offspring according to maternal diabetes status a
| Diabetes status | Number with LGA | Odds ratio LGA | Number with SGA | Odds ratio SGA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR | 95% CI | ORb | 95% CI | Crude OR | 95% CI | ORb | 95% CI | |||
| Without diabetes | 28 955 | 30 732 | Ref | |||||||
| Diabetes type 1 | 601 | 7.5 | 6.8–8.4 | 6.9 | 6.2–7.8 | 55 | 0.4 | 0.3–0.5 | 0.4 | 0.3–0.5 |
| Diabetes type 2 | 175 | 3.2 | 2.7–3.7 | 2.1 | 1.8–2.5 | 55 | 0.8 | 0.6–1.0 | 0.9 | 0.7–1.2 |
| Gestational diabetes | 2085 | 2.0 | 1.9–2.1 | 1.6 | 1.5–1.6 | 1008 | 0.8 | 0.8–0.9 | 0.9 | 0.9–1.0 |
Pregnancies without diabetes were used as reference group. Included were 319 076 singleton pregnancies in Norway during the period 2009–2017.
Adjustment was made for pre-pregnancy maternal body mass index.
Table 4.
Crude and adjusted odds ratios (OR) for giving birth to a large-for-gestational-age (LGA) placenta and a small-for-gestational-age (SGA) placenta according to maternal diabetes status a
| Odds ratio LGA | Odds ratio SGA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes status | Number with LGA | Crude OR | 95% CI | ORb | 95% CI | Number with SGA | Crude OR | 95% CI | ORb | 95% CI |
| Without diabetes | 29 164 | 31 008 | Ref | |||||||
| Diabetes type 1 | 440 | 4.5 | 4.0–5.1 | 4.2 | 3.7–4.7 | 65 | 0.4 | 0.4–0.6 | 0.5 | 0.4–0.6 |
| Diabetes type 2 | 166 | 2.9 | 2.5–3.4 | 2.1 | 1.8–2.5 | 51 | 0.7 | 0.5–0.9 | 0.8 | 0.6–1.1 |
| Gestational diabetes | 2013 | 1.9 | 1.8–2.0 | 1.6 | 1.5–1.6 | 829 | 0.7 | 0.6–0.7 | 0.8 | 0.7–0.8 |
Pregnancies without diabetes were used as the reference group. Included were 319 076 singleton pregnancies in Norway during the period 2009–2017.
Adjustment was made for pre-pregnancy maternal body mass index.
The adjusted ORs for delivering an LGA or SGA placenta according to diabetes type showed similar patterns as for birthweight (Tables 3 and 4). Hence, women with type 1 diabetes had the highest odds ratio of delivering an LGA placenta, and the lowest odds ratio of delivering an SGA placenta.
Additional adjustment for maternal age and parity did not notably alter any of the above associations (Table S3).
4. DISCUSSION
In this population-based study of 319 076 singleton pregnancies, we found that birthweight and placental weight were highest in pregnancies with type 1 diabetes, followed by pregnancies with type 2 diabetes and pregnancies with gestational diabetes.
It is well known that any type of diabetes in pregnancy increases the risk of delivering an LGA infant,2, 22, 23 and an LGA placenta.8 To our knowledge, no previous study has compared birthweight and placental weight between women with type 1 diabetes, type 2 diabetes, and gestational diabetes and at the same time made adjustment for pregnancy duration and maternal BMI.
Previously, a study from France found that LGA infants (birthweight above the 90th centile) were most common in pregnancies with type 1 diabetes (OR 7.7; 95% CI 6.8–8.6) followed by type 2 diabetes (OR 3.8; 95% CI 3.4– 4.2) and gestational diabetes (OR 1.8; 95% CI 1.7–1.8).3 Pregnancies without diabetes were used as the reference. Also, an Australian study found that the risk of delivering an LGA infant was highest in pregnancies with pre-gestational diabetes (type 1 and type 2 diabetes combined) (OR 4.91; 95% CI 4.28–5.63), followed by pregnancies with gestational diabetes (OR 1.65; 95% CI 1.57–1.72), using pregnancies without diabetes as the reference.24 The results from these studies support our results. However, adjustment for maternal BMI was not performed in these studies, and differences in absolute birthweight and placental weight according to diabetes type were not estimated.
Other studies did not include three different types of diabetes within the same study, but their results support that women with diabetes type 1 have the highest risk of delivering an LGA infant.23, 25 One study suggested that women with type 1 diabetes have a 12-fold increase in risk of delivering an LGA infant (OR 12.2; 95% CI 11.4–13.1) compared with women without diabetes.23 Another study reported higher risk in women with diabetes type 1 than in women with diabetes type 2.25 Women with gestational diabetes are reported to have a two-fold increase in risk of delivering an LGA infant (OR 2.19; 95% CI 1.93–2.47) compared with women without diabetes.26
All types of diabetes in pregnancy are characterized by increased maternal serum glucose concentrations. Increased maternal glucose concentrations leads to increased glucose concentrations in the fetal-placental circulation. This in turn stimulates fetal synthesis of insulin. Insulin facilitates glucose uptake into cells. It is assumed that increased glucose and insulin concentrations in the fetal-placental circulation partly explain the increased fetal growth and possibly also the increased placental growth in diabetic pregnancies.27
The relation of maternal blood glucose concentrations with giving birth to an LGA infant (>90 centile) may be dose-dependent.28 Thus, our findings may suggest that the maternal glucose concentrations in pregnancies with type 1 diabetes are higher than in pregnancies with other types of diabetes. In pregnancies with type 1 diabetes, in particular, fluctuation of maternal glucose concentrations or high glucose concentrations in early pregnancy may enhance fetal growth.24, 25, 27, 29
Insulin is a growth hormone, and pregnant women with type 1 diabetes are likely to receive higher doses and/or longer duration of insulin treatment than women with other types of diabetes in pregnancy. According to present knowledge, insulin in the mother is not transferred to the fetal-placental unit. However, our findings are compatible with enhanced fetal and placental growth as a consequence of maternal insulin treatment. Possible biological mechanisms of insulin in the maternal circulation on the placenta are being explored.30 Additionally, insulin treatment may increase the risk of maternal hypoglycemia, and hypoglycemia may trigger weight gain.31 Maternal weight gain in pregnancy is associated with increased offspring birthweight.
Other factors may enhance offspring growth and be more prevalent in pregnancies with type 1 diabetes. Vascular dysfunctions are more prevalent in type 1 diabetes than in other types of diabetes, and maternal vascular dysfunctions may cause suboptimal oxygen supply to the fetal-placental unit.32 Increased angiogenesis is a known response to hypoxia,33 and angiogenic responses are more prominent among individuals with type 1 diabetes than among individuals with other types of diabetes.34 It is conceivable that increased placental angiogenesis and vasculogenesis lead to a larger placenta and possibly also a larger fetus.
It has been suggested that the risk of delivering an LGA infant may be increased also within the upper ranges of normal blood glucose concentrations.28 The World Health Organization recommended therefore in 2013 the following diagnostic criteria for gestational diabetes; fasting maternal blood glucose concentrations between 5.1 and 6.9 mmol/L or maternal blood glucose concentration between 8.5 and 11.0 mmol/L 2 hours after oral intake of 75 g glucose.35 These WHO criteria, as well as the screening strategy for gestational diabetes, are being debated.36 Still, we lack knowledge about the effects of interventions in women diagnosed with gestational diabetes according to the 2013 WHO definition.37 The 2013 WHO criteria for gestational diabetes,35 and the International Association of Diabetes in pregnancy Study Group (IADPSG) criteria,36 include lower blood glucose concentrations than the diagnostic criteria used in our study. Hence, women with gestational diabetes in our study may, on average, have higher concentrations of blood glucose than women diagnosed according to the 2013 WHO criteria. We may therefore, have estimated a higher birthweight increase in our study than if the 2013 WHO criteria had been used to define gestational diabetes. Nevertheless, we found that the mean adjusted infant birthweight in pregnancies with treated gestational diabetes was 102 g higher than in pregnancies without diabetes. The consequences of the increase in infant birthweight associated with gestational diabetes should be further studied, and if possible separated from the effect of maternal BMI. Previously, it has been reported that infants with birthweight between the 80th and 84th centiles have the lowest perinatal mortality.38 Also, adult cardiovascular and metabolic health risks are lowest for persons with birthweight around the 90th centile.39 The magnitude of the health problem associated with gestational diabetes may vary across different parts of the world. Hence, the magnitude of the health problem and the effects of intervention should determine whether new guidelines for identification and treatment of women with gestational diabetes should replace existing guidelines.
Our large study sample allowed us to compare offspring weight and placental weight between pregnancies with different types of diabetes. Although a large proportion of the eligible pregnancies lacked information about maternal BMI (44.3%), and were excluded, we do not believe that skewed selection to the study sample has caused biased estimates. The included and the excluded pregnancies were similar in the prevalence of diabetes types, mean birthweight, placental weight, duration of pregnancy, maternal age, and parity (data not shown). Also, our results were not altered when pregnancies with missing maternal BMI information were included in the analyses.
The diagnosis of diabetes in the Medical Birth Registry of Norway has been validated.40 The specificity for diabetes (all types) was high (99%), and the specificity for type 1 diabetes was close to 100%. However, misclassification of type 2 diabetes and gestational diabetes may have occurred. Any misclassification of diabetes type may have caused underestimation rather than overestimation of the differences in offspring weight according to diabetes type.
We made adjustment for duration of pregnancy, maternal BMI, maternal age, and parity. Maternal socio-economic factors, ethnicity and weight gain in pregnancy have been associated with diabetes and offspring birthweight.41, 42 Unfortunately, we had no information about these factors.
Women with diabetes are delivered by cesarean section more often than women without diabetes. Mode of delivery could possibly influence placental weight, since the placenta is delivered quickly by cesarean section, whereas during a vaginal birth, the delivery of the placenta may be delayed. A delayed placental delivery may influence placental blood volume and thereby weight. However, in both vaginal and cesarean deliveries we found the highest adjusted mean birthweight and placental weight in pregnancies with type 1 diabetes, followed by type 2 diabetes and gestational diabetes (data not shown).
Our data hold no information about individual blood glucose concentrations or treatment during pregnancy. Women with diabetes in pregnancy were diagnosed and treated according to the Norwegian guidelines on diabetes in pregnancy (see Material and methods).43 Possible effects of the Norwegian guidelines on offspring birthweight cannot be estimated in our study.
5. CONCLUSION
In this population-based study of 319 076 singleton pregnancies, we found that offspring birthweight and placental weight were highest in pregnancies with type 1 diabetes, followed by pregnancies with type 2 diabetes and pregnancies with gestational diabetes.
Supplementary Material
Key message.
Offspring birthweight and placental weight were highest in pregnancies with type 1 diabetes, followed by pregnancies with type 2 diabetes, and pregnancies with gestational diabetes.
Funding information:
The study was funded by the South-Eastern Norway Regional Health Authority (Grant no 2013 003).
Footnotes
CONFLICT OF INTEREST
None.
References
- 1.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008; 31: 899–904. [DOI] [PubMed] [Google Scholar]
- 2.Wendland EM, Torloni MR, Falavigna M, et al. Gestational diabetes and pregnancy outcomes–a systematic review of the World Health Organization (WHO) and the international association of diabetes in pregnancy study groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billionnet C, Mitanchez D, Weill A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017; 60: 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poma PA. Correlation of birth weights with cesarean rates. Int J Gynaecol Obstet. 1999; 65: 117–123. [DOI] [PubMed] [Google Scholar]
- 5.Spydslaug A, Trogstad LI, Skrondal A, Eskild A. Recurrent risk of anal sphincter laceration among women with vaginal deliveries. Obstet Gynecol. 2005; 105: 307–313. [DOI] [PubMed] [Google Scholar]
- 6.Overland EA, Vatten LJ, Eskild A. Risk of shoulder dystocia: associations with parity and offspring birthweight. A population study of 1 914 544 deliveries. Acta Obstet Gynecol Scand. 2012; 91(4): 483–488 [DOI] [PubMed] [Google Scholar]
- 7.Hermann GM, Dallas LM, Haskell SE, Roghair RD. Neonatal macrosomia is an independent risk factor for adult metabolic syndrome. Neonatology. 2010; 98: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strom-Roum EM, Haavaldsen C, Tanbo TG, Eskild A. Placental weight relative to birthweight in pregnancies with maternal diabetes mellitus. Acta Obstet Gynecol Scand. 2013; 92: 783–789. [DOI] [PubMed] [Google Scholar]
- 9.Eskild A, Haavaldsen C, Vatten LJ. Placental weight and placental weight to birthweight ratio in relation to Apgar score at birth: a population study of 522 360 singleton pregnancies. Acta Obstet Gynecol Scand. 2014; 93: 1302–1308. [DOI] [PubMed] [Google Scholar]
- 10.Hutcheon JA, McNamara H, Platt RW, Benjamin A, Kramer MS. Placental weight for gestational age and adverse perinatal outcomes. Obstet Gynecol. 2012; 119: 1251–1258. [DOI] [PubMed] [Google Scholar]
- 11.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990; 301: 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJ. Mother’s body size and placental size predict coronary heart disease in men. Eur Heart J. 2011; 32: 2297–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risnes KR, Romundstad PR, Nilsen TI, Eskild A, Vatten LJ. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am J Epidemiol. 2009; 170: 622–631. [DOI] [PubMed] [Google Scholar]
- 14.Strom-Roum EM, Tanbo TG, Eskild A. The associations of maternal body mass index with birthweight and placental weight. Does maternal diabetes matter? A population study of 106 191 pregnancies. Acta Obstet Gynecol Scand. 2016; 95: 1162–1170. [DOI] [PubMed] [Google Scholar]
- 15.Wallace JM, Horgan GW, Bhattacharya S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta. 2012; 33: 611–618. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes in pregnancy: management from preconception to the postnatal period. https://www.nice.org.uk/guidance/ng3/resources/diabetes-in-pregnancy-management-of-diabetes-and-its-complications-from-preconception-to-the-postnatal-period-51038446021. Accessed January 2021. [PubMed]
- 17.Irgens LM. The medical birth registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000; 79: 435–439. [PubMed] [Google Scholar]
- 18.A national clinical guideline for antenatal care. Short version.: Directorate for health and social affairs, Norway. 2005; 12. [Google Scholar]
- 19.Henriksen T, Thordarson H, Clausen T. Diabetes i svangerskap. Norsk gynekologisk forening. Veileder i fødselshjelp 2008 [Diabetes in pregnancy] (Norwegian Society for Gynecology and Obstetrics, Guideline in Obstetrics). (In Norwegian) https://www.legeforeningen.no/contentassets/04d0b3c134ac4b12aa1a03c3a2666585/veileder-i-fodselshjelp-2008.pdf. Accessed June 2021.
- 20.Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation. https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf. Accessed June 2021.
- 21.Thompson JM, Irgens LM, Skjaerven R, Rasmussen S. Placenta weight percentile curves for singleton deliveries. BJOG. 2007; 114: 715–720. [DOI] [PubMed] [Google Scholar]
- 22.Casson IF, Clarke CA, Howard CV, et al. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ. 1997; 315: 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care. 2009; 32: 2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shand AW, Bell JC, McElduff A, Morris J, Roberts CL. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus; a population-based study in New South Wales, Australia, 1998–2002. Diabet Med. 2008; 25: 708–715. [DOI] [PubMed] [Google Scholar]
- 25.Ladfors L, Shaat N, Wiberg N, Katasarou A, Berntorp K, Kristensen K. Fetal overgrowth in women with type 1 and type 2 diabetes mellitus. PLoS One. 2017; 12:e0187917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012; 35: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diabetes Pedersen J. and pregnancy; blood sugar of newborn infants during fasting and glucose administration. Nord Med. 1952; 47: 1049. [PubMed] [Google Scholar]
- 28.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 29.Damm P, Mersebach H, Råstam J, et al. Poor pregnancy outcome in women with typw 1 diabetes is predicted by elevated HbA1c and spikes of high glucose values in the third trimester. J Matern Fetal Neonatal Med. 2014; 27: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiden U, Maier A, Bilban M, et al. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia. 2006; 49: 123–131. [DOI] [PubMed] [Google Scholar]
- 31.Provilus A, Abdallah M, McFarlane S. Weight gain associated with antidiabetic medications. Therapy. 2011; 8: 113–120. [Google Scholar]
- 32.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002; 287: 2570–2581. [DOI] [PubMed] [Google Scholar]
- 33.Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014; 123: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huynh J, Yamada J, Beauharnais C, et al. Type 1, type 2 and gestational diabetes mellitus differentially impact placental pathologic characteristics of uteroplacental malperfusion. Placenta. 2015; 36: 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. A world health organization guideline. Diabetes Res Clin Pract. 2014; 103: 341–363. [DOI] [PubMed] [Google Scholar]
- 36.Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrar D, Duley L, Dowswell T, Lawlor DA. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. The Cochrane Database of Systematic Reviews. 2017; 8:Cd007122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasak B, Koenen SV, Koster MP, et al. Human fetal growth is constrained below optimal for perinatal survival. Ultrasound Obstet Gynecol. 2015; 45: 162–167. [DOI] [PubMed] [Google Scholar]
- 39.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990; 301: 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engeland A, Bjorge T, Daltveit AK, Vollset SE, Furu K. Validation of disease registration in pregnant women in the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand. 2009; 88: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 41.Sletner L, Rasmussen S, Jenum AK, Nakstad B, Jensen OH, Vangen S. Ethnic differences in fetal size and growth in a multi-ethnic population. Early Hum Dev. 2015; 91: 547–554. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017; 317: 2207–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henriksen T, Thordarson H, Strøm-Roum EM, Maltby B, Kvalvik S, Holm H. Svangerskapsdiabetes Norsk gynekologisk forening. Veileder i fødselshjelp 2014[Gestational diabetes] (Norwegian Society for Gynecology and Obstetrics, Guideline in Obstetrics). (In Norwegian) http://legeforeningen.no/Fagmed/Norsk-gynekologisk-forening/Veiledere/Veileder-i-fodselshjelp-2014/Diabetes-i-svangerskapet/. 2014. Accessed June 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.