Abstract
Objectives:
Carbapenem-resistant Enterobacterales (CRE) remain an urgent public health priority in the United States. CRE poses a major threat to patients in healthcare and a potential risk to the community. This study examined the epidemiological trends, clinical, and microbiological data of CRE in the Greater Houston region of Texas.
Methods:
A multi-institutional retrospective observational study was conducted using surveillance data collected from 2015 to 2020. Predictors of incidence rates of CRE were determined by a negative binomial regression fit using a generalized estimation equation.
Results:
Over a 6-year period, 4236 CRE cases were reported, of which Klebsiella pneumoniae accounted for 84.8%. The results show a steady increase in CRE cases, with a sharp rise since 2018. The majority of carbapenemase-producing Enterobacterales were Klebsiella pneumoniae carbapenemase (KPC)-producing (77.2%), followed by other rare carbapenemases, which includes OXA-48, NDM, IMP, VIM, coproduction of KPC with OXA-48, KPC with NDM, and NDM with OXA-48. Acute care hospitals (ACH) accounted for 68.5% of the source of CRE cases. The incidence rate of CRE cases reported from ACH and long-term acute care (LTAC) facilities was 1.16 times that of long-term care facilities (adjusted rate ratio [ARR] = 1.16, 95% confidence interval [CI]:1.04–1.30). The incidence rate of CRE among patients with indwelling devices was 15% (ARR = 0.85, 95% CI: 0.79–0.92) lower than that of patients without indwelling devices.
Conclusion:
The rise in the rate of CRE cases despite aggressive infection prevention and control strategies in the region is alarming. Evaluating and improving the current infection control strategies may be warranted.
Keywords: CRE, Surveillance, Carbapenemase, KPC, Enterobacterales, Healthcare-associated infections, Antibiotics, Antimicrobial-resistance
1. Introduction
Carbapenem-resistant Enterobacterales (CRE) continue to pose a significant public health challenge, and slowing the spread of CRE has become a top public health priority [1–4]. CRE infections are associated with high mortality rates of up to 50% [5]. High-risk patients such as those with comorbid conditions in healthcare settings exhibit higher mortality rates [6]. The emergence and clonal spread of carbapenemaseshave largely driven the spread of CRE in healthcare settings. These enzymes destroy the action of β-lactam antibiotics such as carbapenems, which are considered highly effective against Gram-negative bacterial pathogens such as Enterobacterales [7, 8]. Carbapenemase-producing Enterobacterales (CPE) are of a particular public health concern due to their potential to spread to other Gram-negative organisms via mobile genetic elements [9, 10]. Previous reports in the United States (U.S.) estimated that about 32% of all CRE are carbapenemase-producers [1, 11].
CPE can also spread easily in healthcare settings, and it is frequently associated with healthcare outbreaks with the potential to cause regional outbreaks [4, 12]. Furthermore, due to the high mobility of their genetic material encoding resistance, new antibiotic resistance can emerge rapidly at any time [4, 13, 14]. The most frequently encountered carbapenemase-encoding genes include Klebsiella pneumoniae carbapenemase (KPC), New Delhi metallo-β-lactase (NDM), and oxacillinase-48 (OXA-48); however, KPC remains the most frequently identified in the U.S. [4, 13].
The extent of CRE transmission in healthcare facilities varies depending on the type and services provided. CRE infections are most commonly reported in long-term acute-care hospitals [15]. Additionally, patients admitted from high-acuity long-term care facilities to acute care facilities are more likely to be colonized with CPE [16]. Also, up to 50% of nursing home residents are colonized with multidrug-resistant organisms (MDROs) [17]. Horizontal transmission may be the culprit if an infection occurs within a hospital three days after patient admission or importation from other facilities during patient transfer between healthcare facilities if the infection was identified less than three days after admission [18].
Cases of CRE and CPE have reportedly increased in the U.S. over the last decade [4, 19, 20]. However, trends in clinical and molecular epidemiology of CRE vary by state or geographic area [21]. In some regions of the U.S., there has been a significant increase in carbapenem-resistant K. pneumoniae from clinical isolates [11]. Widespread hospital outbreaks, including a persistent and hyper-epidemic clone, were also reported across many states [22, 23]. While a recent report indicated that CRE has shown a modest reduction in trends [1] due to aggressive infection prevention measures, other studies have shown that the rate of CRE cases per hospital admission have increased over time [24]. We sought to examine the clinical epidemiology and trends of CRE in Texas, particularly in the Greater Houston region, due to the lack of current epidemiology of this major public health problem. Houston is the largest city in the Southern U.S. region and has a racially/ethnic diverse population.
2. Materials and methods
2.1. Study design and setting
A multi-institutional retrospective observational study was conducted using data from the Greater Houston metropolitan area, the fifth-most populous in the U.S., with an estimated population exceeding 7 million in 2020 [25]. Houston houses the largest medical centre in the nation and has the largest number of healthcare facilities that included multiple acute care facilities, long-term care facilities, and nursing homes facilities. In this study, CRE cases reported from multiple healthcare institutions across the region were included.
2.2. Data source and collection procedure
This study used CRE surveillance data obtained from the Houston Health Department and the neighbouring Fort Bend County Health and Human Services collected from 2015 to 2020. Since spring of 2014, CRE has been a reportable condition for surveillance in the state of Texas, with the data coming from a variety of healthcare facilities throughout the region. The CRE surveillance database contains patient demographic information, electronic laboratory reports, clinical data, and risk history. To reduce missing data and improve data quality, available medical records, clinical laboratory reports, molecular test results from referral public health laboratories, and surveillance documentation forms from approximately 2000 cases were abstracted.
Cases were ascertained based on clinical laboratory tests and antibiotic susceptibility results. Clinical laboratories conducted culture for organism identification followed by antibiotic susceptibility tests. To classify isolates as susceptible or resistant, the results of the primary antibiotic susceptibility results were used. Electronic laboratory reports from clinical laboratories, public health laboratories, and antibiotics resistant laboratory networks (ARLN) were also used. The Houston Health Department public health laboratory and the ARLN performs molecular testing on specimens submitted by the local healthcare facilities when unusual susceptibility tests are identified or outbreak and cluster transmission are suspected. Further, the presence of carbapenemase enzyme and carbapenemase genes was determined based on molecular tests performed at the clinical laboratories, public health laboratories, and referral laboratories. Methods for enzyme detection for these laboratories included phenotypic and genotypic methods, including modified carbapenem inactivation method (mCIM), polymerase chain reaction (PCR), and whole-genome sequencing.
2.3. Study population and variables of the study
The study population included all laboratory-confirmed CRE cases, based on the culture of specimen collected from all anatomical sites, including infection and colonization. CRE was defined as Enterobacterales species such as K. pneumoniae, Escherichia coli, Klebsiella oxytoca, E. cloacae, and Enterobacter aerogenes that are resistant to at least one carbapenem antibiotic or produce a carbapenemase enzyme. Unique patients were included if CRE was identified at least 6 months after the index case. Cases were excluded if they did not meet the case definition or had incomplete records or documentation of the type of organisms, and if the residential address or the reporting healthcare facility was not in the Greater Houston region.
The primary outcome variable of the study was CRE case rate over the past 6 years, as measured by case counts. The secondary outcome variable included the prevalence of CPE, the type of carbapenemase genes identified, and the source of infection. The source of infection was operationally classified as hospital-onset based on the recommendation of CDC and the Society for Healthcare Epidemiology of America, if the CRE infection was identified 3 days after admission, specimen collection date after 48 hours of admission into the facility, or the patient was already a resident of a healthcare facility for more than 3 days.
Among the CRE cases identified, those with specimen collection 48 hours after admission to the facility or who were residents of a long-term care facility were considered horizontal transmission or nosocomial infection. If the specimen was collected within 48 hours of admission, but the patient was a resident of another healthcare facility or directly transferred from another facility, or the patient had been admitted to a healthcare facility in the past 6 months, it was classified as community-onset healthcare-associated. If the specimen was collected within 48 hours of admission into the hospital and the patient had never been admitted to a healthcare facility in the past 6 months and was admitted from home, the CRE case was classified as a community-acquired infection.
The independent variables included demographics such as age, sex, and race/ethnicity, microbiology such as specimen collection date, types of organism, carbapenemase production, and type of carbapenemase enzyme, presence of indwelling medical devices, clinical syndrome, healthcare exposure risk history such as residence in a healthcare facility, source of patient transfer, and type of facility reporting the case, which included acute care hospitals (ACH), long-term acute care hospitals (LTAC), and long-term care facilities (LTCF).
2.4. Statistical analysis
All statistical analyses conducted were two-tailed at a 5% level of significance using SAS 9.4 (SAS Institute, Cary, NC, USA). The distribution of cases was described using summary statistics. The Wilcoxon rank-sum test was used to compare the order in the observed case counts across two groups, and the Kruskal-Wallis test was used for comparison across more than two groups. The Pearson χ2 test was used to assess the association between categorical variables. The Spearman rank correlation test was used for ordinal trend tests. The incidence rate of CRE cases for the surveillance period was the primary outcome. Negative binomial regression analysis under a generalized estimating equations (GEE) framework with an exchangeable working correlation structure was performed to examine both univariate and adjusted (multivariable) associations between CRE rates and independent variables: age, race/ethnicity, healthcare facility residence, carbapenemase production, specimen source, type of case reporting facility, source of case transfer, presence of indwelling medical devices, and underlying medical conditions. This allows for estimation of coefficients of predictors of the incidence rate of CRE accommodating potential correlation between consecutive case counts. Variables with a P-value less than 0.1 upon univariate analysis were considered for inclusion in a multivariable model to examine predictors of the rate of CRE. A stepwise variable selection method was used to build the multivariable model.
3. Results
A total of 4236 CRE cases were reported between 2015 and 2020 (Fig. 1), and the number of cases reported per year was significantly different (P < 0.01). A sharp increase in cases was reported in 2018 and 2019, but a decline of nearly 50% was observed in 2020 compared to the preceding years. Generally, the cumulative CRE cases reported per month within the past 3 years has been alarming (Fig. 1).
Fig. 1.
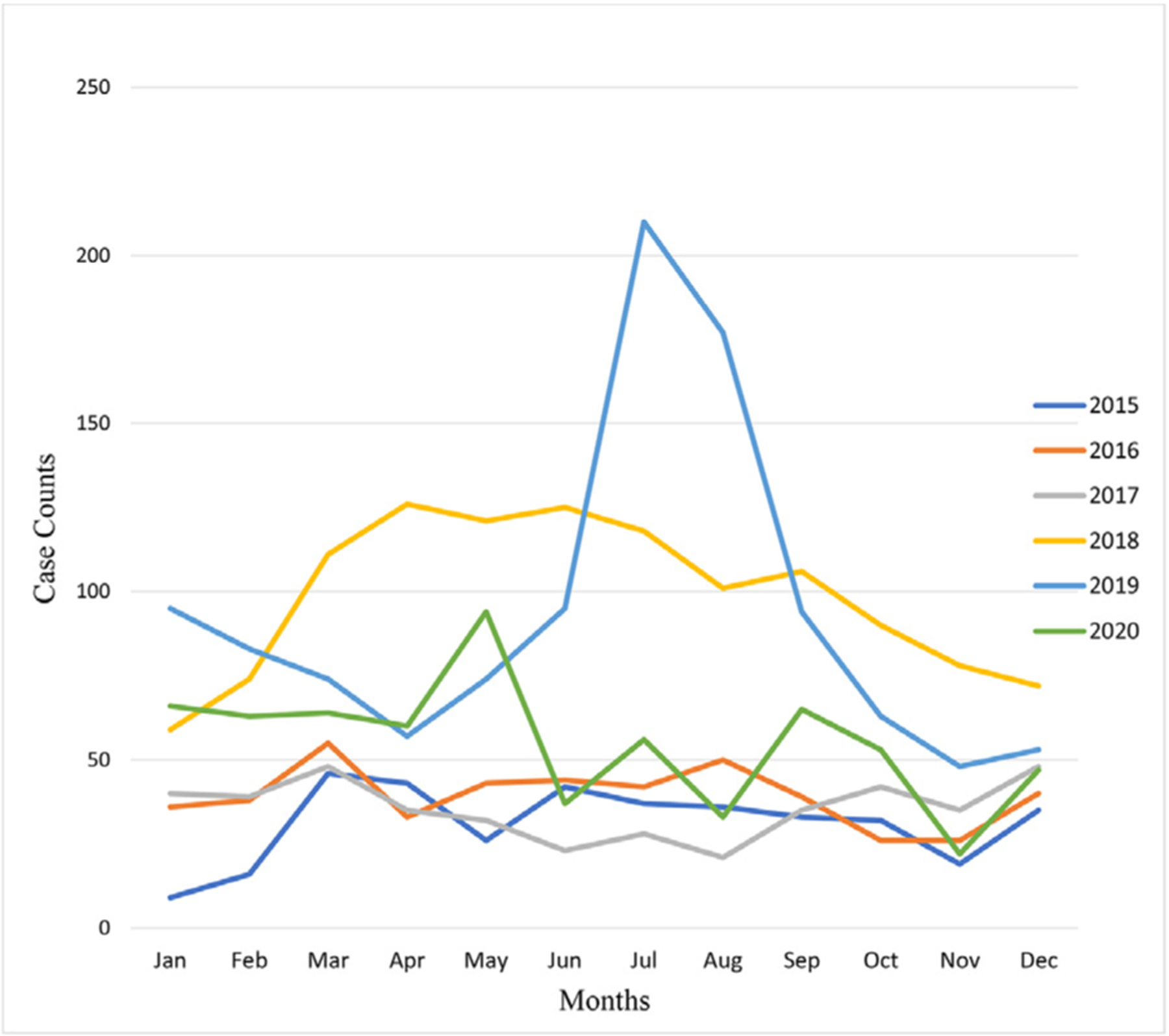
Trends in CRE cases reported in the Greater Houston region, Texas, 2015–2020.
Patients identified with CRE were more likely to be older, with a median and interquartile age range of 62 (52,75) years, and either Black/African American or White race (Table 1). The reported CRE cases were also significantly different based on race/ethnicity (P < 0.001), source of case report (P < 0.001), and healthcare facility residence (P < 0.001), but not by gender. The majority (68.5%) of the cases were reported from ACH, followed by LTACs, and about 18% were from residents of a healthcare facility. However, most of the cases were transferred or admitted from either LTCFs (38.1%) or nursing homes (39.6%) (Table 2).
Table 1.
Demographic and baseline characteristics of patients identified with CRE in the Greater Houston region, Texas, 2015–2020
| Characteristics | N | % | P value |
|---|---|---|---|
| Age in years, median [IQR] | 62[52,75] | 0.001 | |
| Gender | 0.070 | ||
| Female | 2142 | 50.6 | |
| Male | 2091 | 49.4 | |
| Race/Ethnicity | < 0.001 | ||
| Asian | 208 | 5.6 | |
| Black | 1174 | 31.7 | |
| White | 1199 | 32.4 | |
| Hispanic | 660 | 17.8 | |
| Others/multiracial | 123 | 3.3 | |
| Unknown | 872 | 20.6 | |
| Healthcare facility resident | < 0.001 | ||
| Yes | 798 | 18.8 | |
| No | 3438 | 81.2 | |
| Case report year | < 0.001 | ||
| 2015 | 374 | 8.8 | |
| 2016 | 472 | 11.1 | |
| 2017 | 426 | 10.1 | |
| 2018 | 1181 | 27.9 | |
| 2019 | 1123 | 26.5 | |
| 2020 | 660 | 15.6 | |
| Source of case report | < 0.001 | ||
| Outpatient clinic | 335 | 7.9 | |
| Acute care hospital | 2903 | 68.5 | |
| Long-term acute care | 897 | 21.2 | |
| Long-term care facility | 52 | 1.2 | |
| Other source | 49 | 1.2 |
NOTE: P value is comparing distributions and were from Wilcoxon rank-sum test or Kruskal-Wallis test as appropriate, and from t-test for age.
IQR, interquartile range.
Table 2.
Microbiology and clinical characteristics of the CRE cases in the Greater Houston region, Texas, 2015–2020
| Characteristics | N | % | P value |
|---|---|---|---|
| Organisms | 0.003 | ||
| K. pneumoniae | 3595 | 84.8 | |
| E. coli | 468 | 11.1 | |
| Enterobacter cloacae a | 61 | 1.5 | |
| Enterobacter aerogenes | 55 | 1.4 | |
| Klebsiella oxytoca | 24 | 0.6 | |
| Proteus mirabilis b | 16 | 0.4 | |
| Serratia marcescens c | 8 | 0.2 | |
| Specimen source | < 0.001 | ||
| Blood | 319 | 7.5 | |
| Respiratory | 566 | 13.4 | |
| Wound/tissue | 598 | 14.1 | |
| Urine | 2,038 | 48.1 | |
| Body fluid | 108 | 2.6 | |
| Medical devices | 11 | 0.3 | |
| Swabs | 48 | 1.1 | |
| Other sources | 548 | 12.9 | |
| Source of infection onset known (n = 1959)d | 1959 | 46.3 | 0.782 |
| Hospital-acquired infection | 798 | 40.7 | |
| Healthcare-associated-community onset | 687 | 35.1 | |
| Community-associated infection | 474 | 24.2 | |
| Source of infection onset unknown | 2,277 | 53.7 | |
| Clinical syndrome | < 0.001 | ||
| Urinary tract infections | 1,997 | 47.2 | |
| Pneumonia or respiratory illnesses | 589 | 13.9 | |
| Bloodstream infection | 321 | 7.6 | |
| Wound or surgical site infections | 601 | 14.2 | |
| Other infections | 655 | 15.5 | |
| Colonization | 69 | 1.6 | |
| Carbapenemase production | < 0.001 | ||
| Carbapenemase positive (CPE) | 680 | 16.1 | |
| Carbapenemase negative | 184 | 4.3 | |
| Carbapenemase status unknown | 3,372 | 79.6 | |
| Types of carbapenemase (of the 680 CPE cases) | |||
| KPC | 525 | 77.2 | |
| NDM | 45 | 6.7 | |
| OXA-48 | 7 | 1.0 | |
| IMP | 1 | 0.1 | |
| VIM | 1 | 0.1 | |
| KPC and OXA-48 | 1 | 0.1 | |
| KPC and NDM | 1 | 0.1 | |
| NDM and OXA-48 | 1 | 0.1 | |
| Extended spectrum-β-lactamase (ESBL) positivee | 1624 | 38.4 | < 0.001 |
| ESBL result unknown | 2612 | 61.7 | |
| Admitted to healthcare | < 0.001 | ||
| Yes | 3425 | 81.7 | |
| No | 768 | 18.3 | |
| Admitting facility type | 0.609 | ||
| Acute care hospital | 2889 | 85.6 | |
| Long-term acute hospital | 438 | 13.0 | |
| Long-term care facility | 47 | 1.4 | |
| Case admitted from | 0.012 | ||
| Home or home healthcare | 733 | 39.6 | |
| Long-term care or nursing home | 706 | 38.1 | |
| Long-term acute care | 125 | 6.7 | |
| Acute care hospital | 288 | 15.6 | |
| Indwelling medical devices | < 0.001 | ||
| Yes | 1331 | 32.4 | |
| No | 2778 | 67.6 | |
| Underlying disease or chronic medical condition | < 0.001 | ||
| Yes | 1631 | 38.5 | |
| No or unknown | 2605 | 61.5 |
Five were NDM-producing, five KPC-producing, and one case was VIM-producing E. cloacae.
Two cases were KPC-producing P. mirabilis.
Two cases were NDM-producing S. marcescens cases and were identified from blood culture and tracheal aspirate.
The sum does not add up to 100 due to missing data to categorize the source of onset.
1192 ESBL-K. pneumoniae, 325 ESBL-E. coli, and 114 ESBL-producing other Enterobacterales. The P-value is from the Kruskal-Wallis test or Wilcoxon rank-sum tests as appropriate.
A majority of the CRE cases were K. pneumoniae (84.8%) and E. coli (11.1%). Urine culture was the source of specimen in nearly half of the cases (Table 2). Patients presented with urinary tract infections in 47% of the cases, followed by surgical site or wound infections (14.2%), and healthcare-associated or community-acquired pneumonia (13.9%). A total of 680/4236 (16.1%) of the cases were carbapenemase positive (CPE), with 184 (4.3%) being non-carbapenemase-producing CRE, but 80% of the cases had unknown carbapenemase status. This may be because not all CRE cases were subjected to molecular testing. As a result, the proportion of CPE cases presented here does not represent the actual prevalence. A majority of the CPE cases were KPC-producing CRE (77.2%), followed by NDM (6.7%). Furthermore, there were organisms identified with dual carbapenemases (including KPC and OXA-48, KPC and NDM, and OXA-48 and NDM) and imipenamase (IMP)-producing K. pneumoniae (Table 2). The majority of NDM-producing CRE were K. pneumoniae (67.3%) and E. coli (19.2%), whereas 94% of KPC was K. pneumoniae.
Further, some of the less common Enterobacterales were identified with rare genes, including NDM-producing Serratia marcescens (two cases), KPC-producing Proteus mirabilis (two cases), NDM-producing E. cloacae (five cases), and VIM-producing E. cloacae (one case). Also, extended-spectrum-β-lactamase (ESBL) production by the same CRE organism or another co-existing organism was reported in 38.4% of the cases.
Over 85% of the cases were admitted to ACH at the time of specimen collection, and 32% had indwelling medical devices. About 75% of cases were either healthcare-acquired or healthcare-associated community-onset, whereas 24% were community-associated infections. Markedly, 35% of cases were classified as community-onset healthcare-associated infections, which were attributed to previous hospitalizations or the transferring facility, implying potential inter-facility transfer related transmission of the infections (Table 2).
In multivariable analysis, the incidence rate of CRE was associated with race/ethnicity, carbapenemase production, source of the case report, having an indwelling medical device, and underlying medical conditions (Table 3). The annual incidence rate of CRE among African Americans was 1.22 (95% CI: 1.05–1.42) times that of Asian Americans, and the incidence rate of CRE among White Americans was 1.23 times (95% CI: 1.05–1.43) that of Asians, after controlling for the source of patient transfer or admission, age, race, indwelling device use, carbapenemase production, underlying disease, and correlated outcome data. In addition, the incidence rate of CRE cases reported at ACH or LTAC was 16% (95% CI: 4%–30%) higher than the rate of cases from LTCF. Also, the incidence rate of carbapenemase positive CRE was 1.19 (95% CI: 1.07–1.32) times the incidence rate of carbapenemase negative CRE. However, the incidence rate of CRE was not associated with the source of patient transfer. Surprisingly, the incidence rate of CRE among patients who had indwelling medical devices was 15% (95% CI: 8%–21%) lower compared to the incidence rate among patients without indwelling medical devices (P < 0.001).
Table 3.
Multivariable model of predictors of the rate of CRE in the Greater Houston region, Texas, 2015–2020
| Factors | Adjusted rate ratio | 95% CI | Adjusted P value |
|---|---|---|---|
| Race/Ethnicity | |||
| Asian | 1.0 | 1 | |
| Black | 1.22 | 1.05–1.42 | 0.011 |
| White | 1.23 | 1.05–1.43 | 0.008 |
| Hispanic | 1.06 | 0.89–1.25 | 0.525 |
| Other or mixed | 0.99 | 0.80–1.23 | 0.949 |
| Unknown | 1.07 | 0.90–1.27 | 0.429 |
| Carbapenemase production | |||
| Carbapenemase positive | 1.19 | 1.07–1.32 | 0.002 |
| Carbapenemase unknown | 1.61 | 1.48–1.75 | < 0.001 |
| Carbapenemase negative | 1.0 | 1 | |
| Case reported from | |||
| Acute care hospital | 1.16 | 1.04–1.30 | 0.008 |
| Long-term acute | 1.16 | 1.04–1.30 | 0.008 |
| Long-term care facility | 1.0 | 1.00–1.00 | 0.000 |
| Home healthcare | 1.01 | 0.80–1.27 | 0.902 |
| Outpatient clinic | 1.0 | 1 | |
| Case admitted from | |||
| Acute care hospital | 0.91 | 0.80–1.04 | 0.166 |
| Long-term acute | 0.93 | 0.81–1.08 | 0.347 |
| Long-term care facilities | 1.0 | 1.00–1.00 | 0.000 |
| Home or home healthcare | 1.0 | 1 | |
| Indwelling medical devices (yes vs. no) | 0.85 | 0.79–0.92 | < 0.001 |
| Underlying illness or chronic disease (yes vs. no or unknown) | 1.15 | 0.98–1.17 | 0.140 |
NOTE: The model was based on the GEE method and used to account for within-subject measures using an exchangeable correlation structure with the negative binomial distribution assumption.
4. Discussion
This study showed that the rate of reported CRE cases has been increasing steadily since 2015, particularly with a marked rise since 2018, and identified clinically and statistically meaningful predictors of the incidence rate of CRE. The trajectory of cases over the last 3 years has generally been alarming, with a sharp rise observed from April 2019 to September 2019. This trend could be explained by the occurrence of regional outbreaks or an increase in laboratory testing. However, there was a sharp decline in the number of CRE cases reported in 2020. One possible reason for the decrease is the COVID-19 pandemic, where reports of cases other than COVID-19 were likely relegated. It is also possible that the COVID-19 infection control measures abated CRE transmissions.
While about 24% of the cases were estimated to be community-associated infections, nearly 35% were healthcare-associated community-onset infections, implying that cases were either attributed to prior hospitalization, transferring facilities, or indirect transfer. This suggests the burden of inter-facility case transfer and potential inter-facility transmission of infections. Other studies have shown that interhospital patient transfer has significant potential for infection transmission, where a patient may be directly or indirectly transferred between facilities carrying infectious agents [26]. Although details of community-associated CRE infections and transmission are largely unknown and depend on geographic locations, previous estimates from U.S.-based studies showed that community-associated CRE infections range from 5.6% to 10.8% [27]. However, most of them were population-based studies.
K. pneumoniae was the most common CRE, and KPC-producing CRE was the most frequently identified CPE. Several studies have also reported that K. pneumoniae and KPC-producing CRE are pre-dominant in the U.S. [4, 13, 28]. These findings are contrary to a recent study, which reported that 43% of CRE cases were K. pneumoniae followed by 27% E. coli and 30.3% being KPC-producing CRE [29]. This contradictory finding may be due to the small sample size of that study, which included only 42 cases (43%) of K. pneumoniae. Furthermore, most of the organisms identified were less commonly reported. Additionally, sporadic emergence of both class B and class D β-lactamase producing CRE infections were noted, including NDM-producing CRE, 45 (6.7%), and a few cases of IMP, VIM, OXA-48, and co-production of KPC and OXA-48, KPC and NDM, and OXA-48 and NDM.
Less commonly reported Enterobacterales-producing carbapenemases, including NDM-producing S. marcescens, KPC-producing P. mirabilis, NDM-producing E. cloacae, and VIM-producing E. cloacae, which are rarely reported in the U.S., were also documented. It is important to note that these organisms are not included in the Texas CRE case definition, and they are not among the reportable organisms; however, these CRE organisms were reported to the local health departments when unusual resistant mechanisms or cluster transmissions were suspected. Emergence of rare organisms with rare resistance mechanisms requires special attention.
In general, acute care hospitals were the most common source of CRE cases, followed by long-term acute care hospitals. The rate of CRE cases reported from acute care hospitals or long-term acute care facilities was 1.6 times higher than the rate of cases from long-term care facilities. A previous study also found that CRE infections were most commonly reported from long-term care facilities [15]. Surprisingly, the incidence rate of CRE among patients who had indwelling medical devices was 15% lower than patients without indwelling medical devices. The lower rate of CRE among patients with indwelling medical devices may be attributed to aggressive infection control measures, and this may result in reduction of device-associated infections. Thus, patients without invasive devices, such as those who are not residents of healthcare facilities may have received less attention with regard to the prevention of antimicrobial-resistance compared with patients with invasive devices.
This study is subject to some limitations. First, the study used surveillance data that was clinical laboratory–based CRE testing and may not detect carriers [30]. Therefore, the results may not accurately reflect the true rate of CRE in the region. However, the snapshot of cases reported through surveillance is informative and can be very useful in estimating and understanding the burden of disease as well as trends of CRE cases in the region. Second, the study was based on secondary data and may be prone to reporting and documentation bias. Nevertheless, every effort was made to abstract accurate data from the available records to minimize errors and missing data. Lastly, surveillance bias may have caused underreporting in one group of cases over the other, leading to biased estimates.
Acknowledgments
This study was possible with the support of the University of Texas Health Sciences Center School of Public Health at Houston, Texas. We would like to appreciate the Houston Health Department and Fort Bend County Health and Human Services for approving the use of the surveillance data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. CD was supported by NIH/NIAID grants R01AI116914 and R01AI150685.
Abbreviations:
- CRE
Carbapenem-Resistant Enterobacterales
- KPC
Klebsiella pneumoniae carbapenemase
Footnotes
Ethical approval
This study protocol was reviewed and approved as exempt by the institutional review board of the University of Texas Health Science Center in Houston, Texas.
Competing interests
None declared.
Reference
- [1].Woodworth KR, Walters MS, Weiner LM, Edwards J, Brown AC, Huang JY, et al. Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms—United States, 2006–2017. MMWR Morb Mortal Wkly Rep, 67; 2018. p. 396–401. doi: 10.15585/mmwr.mm6713e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].CDC Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: US: Department of Health and Human Services; 2019 [Google Scholar]
- [3].CDCGuidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep 2009;58:256–60. [PubMed] [Google Scholar]
- [4].van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017;8:460–9. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008;29:1099–106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- [6].Mathers AJ, Cox HL, Bonatti H, Kitchel B, Brassinga AKC, Wispelway B, et al. Fatal cross infection by carbapenem-resistant Klebsiella in two liver transplant recipients. Transpl Infect Dis 2009;11:257–65. doi: 10.1111/j.1399-3062.2009.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011;55:4943–60. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Iovleva A, Doi Y. Carbapenem-resistant Enterobacteriaceae. Clin Lab Med 2017;37:303–15. doi: 10.1016/j.cll.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu MC, Tang HL, Chiou CS, Wang YC, Chiang MK, Lai YC. Clonal dissemination of carbapenemase-producing Klebsiella pneumoniae: two distinct sub-lineages of sequence type 11 carrying bla KPC-2 and bla OXA-48. Int J Antimicrob Agents 2018;52:658–62. doi: 10.1016/j.ijantimicag.2018.04.023 [DOI] [PubMed] [Google Scholar]
- [10].Cerqueira GC, Earl AM, Ernst CM, Grad YH, Dekker JP, Feldgarden M, et al. Multi-institute analysis of carbapenem resistance reveals remarkable diversity, unexplained mechanisms, and limited clonal outbreaks. Proc Natl Acad Sci U S A 2017;114:1135–40. doi: 10.1073/pnas.1616248114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Landman D, Bratu S, Kochar S, Panwar M, Trehan M, Doymaz M, et al. Evolution of antimicrobial resistance among Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae in Brooklyn, NY. J Antimicrob Chemother 2007;60:78–82. doi: 10.1093/jac/dkm129. [DOI] [PubMed] [Google Scholar]
- [12].Agarwal M, Shiau S, Larson EL. Repeat gram-negative hospital-acquired infections and antibiotic susceptibility: a systematic review. J Infect Public Health 2018;11:455–62. doi: 10.1016/j.jiph.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 2018;66:1290–7. doi: 10.1093/cid/cix893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 2007;20:440–58. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Livorsi DJ, Chorazy ML, Schweizer ML, Balkenende EC, Blevins AE, Nair R, et al. A systematic review of the epidemiology of carbapenem-resistant Enterobacteriaceae in the United States. Antimicrob Resist Infect Control 2018;7:55. doi: 10.1186/s13756-018-0346-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prabaker K, Lin MY, McNally M, Cherabuddi K, Ahmed S, Norris A, et al. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae: a multihospital study. Infect Control Hosp Epidemiol 2012;33:1193–9. doi: 10.1086/668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McKinnell JA, Miller LG, Singh RD, Gussin G, Kleinman K, Mendez J, et al. High prevalence of multidrug-resistant organism colonization in 28 nursing homes: an ‘iceberg effect. J Am Med Dir Assoc 2020;21:1937–43. doi: 10.1016/j.jamda.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Won SY, Munoz-Price LS, Lolans K, Hota LB, Weinstein RA, Hayden MK, et al. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2011;53:532–40. doi: 10.1093/cid/cir482 [DOI] [PubMed] [Google Scholar]
- [19].Thaden JT, Lewis SS, Hazen KC, Huslage K, Fowler VG, Moehring RW, et al. Rising rates of carbapenem-resistant Enterobacteriaceae in community hospitals: a mixed-methods review of epidemiology and microbiology practices in a network of community hospitals in the southeastern United States. Infect Control Hosp Epidemiol 2014;35:978–83. doi: 10.1086/677157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Duin D, Perez F, Rudin SD, Cober E, Hanrahan J, Ziegler J, et al. Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 2014;58:4035–41. doi: 10.1128/AAC.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 2017;215(suppl 1):S28–36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kanamori H, Parobek CM, Juliano JJ, van Duin D, Cairns BA, Weber DJ, et al. A prolonged outbreak of KPC-3-producing Enterobacter cloacae and Klebsiella pneumoniae driven by multiple mechanisms of resistance transmission at a large academic burn center. Antimicrob Agents Chemother 2017:61. doi: 10.1128/AAC.01516-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hargreaves ML, Shaw KM, Dobbins G, Vagnone PMS, Harper JE, Boxrud D, et al. Clonal dissemination of Enterobacter cloacae harboring blaKPC-3 in the upper Midwestern United States. Antimicrob Agents Chemother 2015;59:7723–34. doi: 10.1128/AAC.01291-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gupta V, Ye G, Olesky M, Lawrence K, Murray J, Yu K. Trends in resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States: 2013–2017. BMC Infect Dis 2019;19:742. doi: 10.1186/s12879-019-4387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].United States Census Bureau. Population estimates. https://www.census.gov/topics/population.html. [accessed 10 October 2021].
- [26].Huang SS, Avery TR, Song Y, Elkins KR, Nguyen CC, Nutter SK, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol 2010;31:1160–9. doi: 10.1086/656747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kelly AM, Mathema B, Larson EL. Carbapenem-resistant Enterobacteriaceae in the community: a scoping review. Int J Antimicrob Agents 2017;50:127–34. doi: 10.1016/j.ijantimicag.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kutlu HH, Us E, Tekeli A. Investigation of carbapenemase genes and molecular epidemiology of Enterobacteriaceae strains isolated between 2010–2014 in a university hospital. Mikrobiyol Bul 2018;52:1–12. doi: 10.5578/mb.66156 [DOI] [PubMed] [Google Scholar]
- [29].Black CA, So W, Dallas SS, Gawrys G, Benavides R, Aguilar S, et al. Pre-dominance of non-carbapenemase producing carbapenem-resistant Enterobacterales in south Texas. Front Microbiol 2020;11:623574. doi: 10.3389/fmicb.2020.623574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee BY, Bartsch SM, Wong KF, Kim DS, Cao C, Mueller LE, et al. Tracking the spread of carbapenem-resistant Enterobacteriaceae (CRE) through clinical cultures alone underestimates the spread of CRE even more than anticipated. Infect Control Hosp Epidemiol 2019;40:731–4. doi: 10.1017/ice.2019.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


