Abstract
Few investigations examine patterns of opioid and nonopioid analgesic prescribing and concurrent pain intensity ratings before and after institution of safer prescribing programs such as the October 2013 Veterans Health Administration system-wide Opioid Safety Initiative (OSI) implementation. We conducted a quasi-experimental pre–post observational study of all older U.S. veterans (≥50 years old) with osteoarthritis of the knee or hip. All associated outpatient analgesic prescriptions and outpatient pain intensity ratings from January 1, 2012 to December 31, 2016, were analyzed with segmented regression of interrupted time series. Standardized monthly rates for each analgesic class (total, opioid, nonsteroidal anti-inflammatory drug, acetaminophen, and other study analgesics) were analyzed with segmented negative binomial regression models with overall slope, step, and slope change. Similarly, segmented linear regression was used to analyze pain intensity ratings and percentage of those reporting pain. All models were additionally adjusted for age, sex, and race. Before OSI implementation, total analgesic prescriptions showed a steady rise, abruptly decreasing to a flat trajectory after OSI implementation. This trend was primarily due to a decrease in opioid prescribing after OSI. Total prescribing after OSI implementation was partially compensated by continuing increased prescribing of other study analgesics as well as a significant rise in acetaminophen prescriptions (post-OSI). No changes in nonsteroidal anti-inflammatory drug prescribing were seen. A small rise in the percentage of those reporting pain but not mean pain intensity ratings continued over the study period with no changes associated with OSI. Changes in analgesic prescribing trends were not paralleled by changes in reported pain intensity for older veterans with osteoarthritis.
Keywords: Geriatric, Veterans, Osteoarthritis, Opioids, Analgesics, Nonopioid analgesics, Pain intensity ratings, Quasiexperimental design, Segmented regression, Negative binomial regression, Observational study, Analgesic prescribing trends
1. Introduction
Although nationally the amounts of prescribed opioids peaked about 8 years ago, opioid overdose deaths and related adverse outcomes continue to rise and have emerged as a public health emergency in the United States.2,3,5,12 Earlier studies demonstrated increased trends in opioid prescribing and a concomitant increase in other sedative/stimulant prescription rates paralleled by adverse events, particularly hospitalizations and overdose deaths, in various populations.11,14,15 In addition, evidence from studies have started to question the effectiveness of opioids in treating some types of pain, including osteoarthritis.7 For these reasons, national policies, guidelines, and initiatives have been developed and implemented over the past several years to help clinicians decrease opioid use and to use multiple recommended risk mitigation strategies to reduce rates of associated harms.
Recent studies have noted these initiatives to be effective with a downward trend in opioid prescribing in some settings.6,9,13,16,20 As an example, the Veterans Health Administration (VHA) launched a system-wide Opioid Safety Initiative (OSI) in October 2013 to educate prescribers about safer opioid prescribing practices, followed by a sustained organizational effort to attenuate opioid prescribing. A recent review of opioid prescription rates in the VHA system by Lin et al.9 delineated the decreasing trend in opioid prescribing since the OSI rollout.9,17 Investigators demonstrated changes in both rates of prescribing as well as reductions in opioid doses (quantifiable as morphine equivalents) since the initiation of the OSI.
Many studies, however, have not evaluated the impact of changing opioid prescribing practices on use of other nonopioid analgesics nor concurrent changes in reported pain intensity. Because of the continued public health threat opioids present, most studies have focused solely on opioid prescribing.6,8 As policies and trends in opioid prescribing change, this will likely impact use of alternative analgesic options, especially nonopioid analgesic prescribing. These changes in practice may impact patient-reported pain intensity. In addition, limitations of previous studies have assumed stable underlying populations, reporting total number of prescriptions by year or by month instead of calculating prescription rates.1,9,11 Researchers commonly have not corrected for the varying length of each month (as much as 10%, 28–31 days) when calculating either counts or rates.
The Evaluating Arthritis Analgesic Safety and Effectiveness (EAASE) project is an ongoing multicenter observational study evaluating the safety and effectiveness of analgesic medications prescribed to older veterans who have been diagnosed with osteoarthritis (1 IO1 HX000911–01A2). Using national data collected as part of this study, we had the opportunity to evaluate national trends in opioid and nonopioid analgesic prescribing before and after the VHA OSI initiative. Our hypothesis is that with guidance limiting or decreasing use of opioid therapy, clinicians may increase prescribing of nonopioid analgesics. To determine if such changes in published analgesic policies and initiatives indirectly affected patient outcomes, concurrent pain intensity ratings will be evaluated.
2. Methods
2.1. Study design and data sources
We conducted a retrospective, interrupted time-series, segmented regression model of aggregated monthly national data available from the VHA Corporate Data Warehouse (CDW), a national repository of patient-level medical records.18 Data for this investigation were part of an observational study evaluating the safety and effectiveness of analgesics in older veterans with arthritis of the knee or hip. Outpatient prescription and pain intensity ratings and demographic covariate data from VHA CDW files18 included name of analgesic and prescription release date, as well as any documented pain intensity ratings. Data were then aggregated monthly over a 5-year study period and prescribing rates and summary pain intensity ratings calculated for entry into segmented regression models controlled for sociodemographic characteristics. Study protocols were approved by the VA Central Institutional Review Board (VA Central IRB Study 13–31, 1 I01 HX000911–01A2, IIR 12–106).
2.2. Veterans Health Administration Opioid Safety Initiative
To study the overall trends in analgesic prescribing and the impact of a guideline-based analgesic safety initiative, total number of analgesic prescriptions and pain intensity ratings were evaluated in the 2 years before and 3 years after the VHA OSI. The OSI was a national VHA clinical initiative to promote safe opioid-related prescribing that completed rollout to all VHA facilities in October 2013.9,17
2.3. Subjects
A national sample of all VHA patients aged 50 years or older from January 1, 2012 to December 31, 2016, diagnosed with osteoarthritis of the knee and/or hip (ICD9: 715.15, 715.16, 715.25,715.26, 715.35, 715.36, 715.95, 715.96; ICD10: M16.0, M16.1, M16.10, M16.11, M16.12, M16.2, M16.3, M16.30, M16.31, M16.32, M16.4, M16.5, M16.50, M16.51, M16.52, M16.6, M16.7, M16.9, M17, M17.0, M17.1, M17.10, M17.11, M17.12, M17.2, M17.3, M17.30, M17.31, M17.32, M17.4, M17.5, M17.9, M13.15, M13.151, M13.152, M13.159, M13.16, M13.161, M13.162, M13.169, M13.85, M13.851, M13.852, M13.859, M13.86, M13.861, M13.862, M13.869) was identified. Two outpatient visits or one inpatient encounter noting these International Classification of Disease (ICD) codes were required for inclusion. Sociodemographic variables included age (in years), sex, and race (white or non-white). Rates used for outcomes and covariates are calculated as the monthly outcomes or covariates (numerator) divided by the number of unique veterans (denominator) in a given month.
2.4. Outcomes
Monthly rates of musculoskeletal analgesic prescriptions were calculated for all outpatient analgesic prescriptions for a 5-year period (January 1, 2012-December 31, 2016) and were recorded and aggregated as counts by study month. Musculoskeletal analgesics were categorized as: opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and other study analgesic prescriptions. The denominator for the calculation of prescription rates was the number of unique patients aged 50 years or older, diagnosed with osteoarthritis of the knee or hip in a given month during the study period. Outcomes were adjusted for sociodemographic covariates, age, sex, and race.
Opioids were defined as all opioid agonists including tramadol and their fixed nonopioid combinations with a few exceptions. Opioid and nonopioid cough preparations were excluded. Nonsteroidal anti-inflammatory drugs included celecoxib, diclofenac, etodolac, ibuprofen, indomethacin, ketoprofen, ketorolac, meloxicam, naproxen, piroxicam, salsalate, sulindac, and tolmetin. Nonsteroidal anti-inflammatory drugs and acetaminophen were counted as separate prescriptions when used as single agents but not when in combination with opioids. Other study analgesics included menthol with and without salicylate, capsaicin, and local anesthetics.
Pain intensity ratings are collected as part of routine outpatient clinical care in the VHA. This is usually documented as “the presence and intensity of pain” with responses provided by veterans in response to the question, “Please rate your pain right now on a 0 (no pain) to 10 (worst pain imaginable) scale”. Following recommendations for optimal distributions and best-fitting models when using pain score data, we followed recommendations by Goulet et al.4 Only nonmissing pain intensity ratings were included in analyses (ie, missing was not counted as no or “zero” pain). Outpatient pain intensity ratings (excluding those from inpatient encounters) during the same study period were collected and summarized following recommended calculations for optimal model fit for zero-inflated Poisson distributions4: percentage of those reporting pain (0 = no pain or 1 = any pain) and the mean pain intensity rating of those reporting pain (rating, range 1–10 highest).
2.5. Analyses
Analyses followed a similar approach to that of the study by Lin et al. that modeled VHA high-dose opioid therapy and concurrent benzodiazepine prescriptions9,19 using segmented regression analyses of interrupted time series.
All variables were examined for missing values and for appropriate distributions and summarized as mean and SDs for continuous and percentages for categorical measures.
For the evaluation of pain intensity, only outpatient ratings were used. A total of 10,350,959 separate outpatient pain intensity ratings over the 5-year period were summarized as monthly values: proportion of those reporting pain (0 vs 1–10) and mean of those reporting pain (1–10 scale). Pain intensity ratings were also summarized as percent reporting mild (1–3), moderate (4–6), and severe (7–10) pain. As common to all observational studies involving pain ratings, participants having more pain would more likely receive both stronger medication as well as more frequent appointments and assessments of pain intensity. However, these are recognized limitations of using observational data. Distributions were compared with the results of previous VHA system-wide analysis of pain score distributions4 to validate the consistency of pain intensity ratings over time.
The sample sociodemographic characteristics were examined. Raw counts for each category of study analgesic prescriptions (total study analgesics, opioid, NSAID, other study analgesics, and acetaminophen) were computed for each month. These counts were standardized by dividing by the number of days in each month and then multiplying by the length of an average month (365.25/12 = 30.44 days) and rounded to a whole number. Raw and standardized counts were examined and graphed. Finally, rates were calculated as follows: the number of prescriptions of each analgesic type within a standard month was divided by the number of unique patients (50+ years of age and meeting the diagnostic criteria) for the entire VHA within that month and multiplied by 100 to achieve a standardized monthly rate in mean prescriptions per 100 person-months.
For analgesic prescribing, Poisson regression was performed to model the standardized monthly prescription count (offset by the log [number of unique patients in that month]). All models exhibited highly significant overdispersion and required negative binomial regression methods.
Subsequently, negative binomial regression models were performed with the following variables: sociodemographic covariates (mean age [years], sex [% male], race [% white]); time (in months) for overall model linear slope; an indicator variable for OSI (pre or post) or step; and an interaction term between time and the indicator variable for OSI implementation to assess the possible change in slope (Δβ). A significant step term would signify a sharp increase or decrease–a step change—in prescribing associated with the OSI. Of note, the changes in slope (interaction term), slope change (Δβ), from the pre- to post-OSI periods are of more substantive interest in demonstrating the effect of the OSI intervention.
Autocorrelation was investigated using several methods (harmonic terms and ACF/PACF graphs). Because of the lack of consistent autocorrelation, we ultimately decided to use Poisson-family models that resulted in best fit with negative-binomial models (see below response) with no adjustments for autocorrelation/seasonality.
Finally, pain intensity ratings were analyzed similarly with covariate-adjusted linear regression. Because pain intensity ratings typically conform to a zero-inflated Poisson, negative binomial distribution, or a hurdle Poisson/negative binomial distribution,4 the monthly average ratings were summarized by 2 complementary methods: proportion reporting pain (1–10 vs 0) and mean pain intensity rating for those reporting pain (1–10). All models were additionally adjusted for the same 3 covariates: mean age (years), sex (% male), and race (% white). As with the negative binomial models, a step change would indicate an increase or decrease in pain intensity ratings associated with the OSI, whereas significant interaction term or change in slope would signal an increasing or decreasing trend in the level of pain after OSI compared to before OSI. In addition, the percent of mild (1–3), moderate (4–6), and severe (7–10) pain were analyzed and graphed as multivariable linear regression.
Effect sizes for each of the prescription and pain intensity models were determined as follows. The predicted values and 95% confidence intervals for the end of the study (month 60, December 2016) was calculated from both the pre-OSI model and the full (pre-/post-OSI) model. These separate estimates were used to calculate the percent difference between the 2 prediction points with the pre-OSI prediction as the reference. A contrast was then performed for the pre-/post-OSI model to decide significance (Hochberg adjusted) of these differences. The resulting predicted lines for both models were graphed along with unadjusted rates for comparison.
2.6. Sample size and probability adjustments
Because the analyses involve counts in the thousands and denominators in the hundreds of thousands, like many previous studies of national databases, we did not perform an a priori sample size assessment.9 However, these aggregate summary measures (rates and means) comprise the data points for the analyses. We used a quasi-experimental design (segmented regression of interrupted time series), in which it is generally accepted that more than a dozen points on either side of the “event”/inflection point of interest are enough to provide stable slope estimates.
Nevertheless, for the negative binomial sample size calculation, we performed a post hoc calculation for the total prescription rate results using R package, power.nb.test, showing that, indeed, 12 values on each side of the inflection point are sufficient for 80% power (mu0 = 26.6, mu1 = 33.0, RR = 1.011 (pre-/post-OSI), theta = 632, duration = 1, α = 0.05/2-sided). We had 22 pre-OSI and 38 post-OSI values.
For the linear regression models, we used G*Power version 3.1.9.2 for overall R2: multiple linear regression using α = 0.05, power = 0.80, n predictors = 6, and effect size = 0.35 (large), a sample size of 46 (data points) is required. N = 60 for our analysis. The range of effect sizes we saw for the linear regression (R2) was 0.29 to 0.91 (Table 2).
Table 2.
Summary of multivariable regression models for VHA analgesic prescribing and pain intensity scores in older veterans with osteoarthritis.
| Analgesic prescription category | Negative binomial models* (estimate, P) | |||
|---|---|---|---|---|
| Time, β | Step | Slope change, Δβ | Model AIC | |
| Total study analgesics | 0.012, 0.003 | 0.319, <0.0001 | −0.010, <0.0001 | 1220.5, <0.0001 |
| Opioid | 0.018, <0.0001 | 0.578, <0.0001 | −0.019, <0.0001 | 1161.8, <0.0001 |
| NSAID | 0.002, 0.743 | 0.013, 0.743 | −0.001, 0.743 | 1073.1, <0.0001 |
| Other study analgesics | 0.012, 0.0002 | 0.029, 0.743 | −0.001, 0.743 | 939.5, <0.0001 |
| Acetaminophen | 0.006, 0.255 | −0.145, 0.004 | 0.005, 0.047 | 866.7, <0.0001 |
| Pain intensity scores | Linear regression models† (estimate, P) | |||
| Time, β | Step | Slope change, Δβ | Model adj. R2 | |
| Percent reporting pain (1–10 v. 0) | 0.089, 0.038 | 0.926, 0.271 | −0.029, 0.662 | 0.91, <0.0001 |
| Percent with mild pain (1–3) | −0.016, 0.729 | 0.363, 0.170 | −0.011, 0.662 | 0.29, <0.001 |
| Percent with moderate pain (4–6) | 0.046, 0.047 | −0.010, 0.963 | −0.002, 0.963 | 0.88, <0.0001 |
| Percent with severe pain (7–10) | 0.059, 0.109 | 0.572, 0.613 | −0.016, 0.963 | 0.86, <0.0001 |
| Mean reporting pain (1–10) | 0.003, 0.613 | 0.015, 0.963 | 0.000, 0.963 | 0.70, <0.0001 |
Hochberg adjustment of study probabilities made within each model type (negative binomial and linear regression).
Negative binomial models with terms for overall slope (time), step, and change of slope (pre-/post-OSI), additionally adjusted for mean age (years), % male sex, and % white race, along with model Akaike Information Criterion (AIC) and likelihood ratio probability (compared with null model).
Linear regression models with terms for overall slope (time), step, and change of slope (pre-/post-OSI), additionally adjusted for mean age (years), % male sex, and % white race, along with model adjusted R2 and F-statistic probability.
NSAID, nonsteroidal anti-inflammatory drug; VHA, Veterans Health Administration.
Hochberg adjustments were applied to the resulting probabilities within the estimates for the negative binomial models and within the linear regression models.
All analyses were performed with RStudio (Version 1.0.153, Boston, MA) using R Version 3.4.2 and SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
During the study period, there were a total of 8,384,564 prescriptions written for 348,787 unique patients who met inclusion criteria (Table 1). Mean age for this cohort was 63.4 years (SD, 8.6) ranging from 50 to 104 years. Mean age for the cohort increased slightly over the study period from 64.3 to 66.3 years (β = 0.03, P < 0.0001—not shown). Percent male sex (mean, 93.3%; range 92.4–93.6; β = −0.02, P < 0.0001—not shown) and percent white race (mean, 69.2%; range 67.9–70.7; β = −0.04, P < 0.0001—not shown) demonstrated small but significant decreases over the same period. The denominator for the monthly calculation of prescription rates was all veterans older than 50 years with the diagnosis of osteoarthritis of the knee or hip, including those who did not receive any analgesic prescriptions, in the study period (overall, 499,243 unique patients).
Table 1.
EAASE national sample characteristics.
| Total | |
|---|---|
| Acetaminophen | 426,940 |
| Unique patients (receiving a prescription), n | 348,787 |
| Age (y), mean (SD) (range: 50–104) | 63.4 (8.6) |
| Proportion of white race (%) | 69.2 |
| Proportion of male sex (%) | 93.3 |
| Average prescriptions/person, mean (SD) | 7.2 (7.1) |
| Pain intensity ratings among those reporting pain, median (IQR) | 6 (4–8) |
IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug.
3.1. Study analgesics
3.1.1. Total study analgesics
For total study analgesic prescriptions, there was a positive (increasing) trend before OSI (βtime = 0.012, P = 0.003) (Tables 2 and 3 and Fig. 1). At the initiation of the OSI, there was a significant step (0.319, P < 0.0001) as well as a pronounced negative change in slope (Δβ = −0.010, P < 0.0001). The post-OSI slope was not significantly different from zero or a flat trajectory. Total study analgesic prescriptions increased until the OSI inflection point, after which it displayed a flat trajectory (Fig. 1). The effect size demonstrated a 30.6% (P < 0.001) drop in total analgesic prescribing compared to what would have been predicted (estimate, 95% CI) under the pre-OSI trend, 48.1 (41.9–55.2) vs 33.4 (31.3–35.7) prescriptions/100 person-months.
Table 3.
Effect sizes for VHA analgesic prescribing and pain intensity scores in older veterans with osteoarthritis.
| Analgesic prescription category | Negative binomial models* | |||
|---|---|---|---|---|
| Predicted (95%) 12/2016 under pre-OSI model | Predicted (95% CI) 12/2016 under full model | Effect size change (%) | P | |
| Total study analgesics | 48.1 (41.9–55.2) | 33.4 (31.3–35.7) | −30.6 | <0.001 |
| Opioid | 32.4 (28.3–37.1) | 16.9 (15.7–18.2) | −47.8 | <0.001 |
| NSAID | 10.7 (9.3–12.4) | 10.3 (9.7–11.0) | −3.7 | 0.748 |
| Other study analgesics | 4.4 (3.8–5.2) | 4.3 (4.0–4.6) | −2.3 | 0.748 |
| Acetaminophen | 1.9 (1.6–2.2) | 2.1 (2.0–2.3) | 10.5 | 0.003 |
| Pain intensity scores | Linear regression models† | |||
| Predicted (95%) 12/2016 under pre-OSI model | Predicted (95% CI) 12/2016 under full model | Effect size change (%) | P | |
| Percent reporting pain (0 vs 1–10) | 62.0 (60.7–63.2) | 60.8 (60.4–61.1) | −1.9 | 0.096 |
| Percent with mild pain (1–3) | 13.3 (12.7–13.9) | 13.2 (13.1–13.3) | −0.8 | 0.070 |
| Percent with moderate pain (4–6) | 23.1 (22.3–23.9) | 22.8 (22.6–22.9) | −1.3 | 0.952 |
| Percent with severe pain (7–10) | 25.5 (24.5–26.6) | 24.8 (24.6–25.1) | −2.7 | 0.196 |
| Mean reporting pain (1–10) | 5.7 (5.6–5.8) | 5.7 (5.7–5.7) | 0.1 | 0.870 |
Units are prescriptions/100-person years for analgesic prescription categories, percent reporting pain where indicated, and units of pain intensity rating (1–10 scale) for mean reporting pain. Hochberg adjustment of study probabilities made within each model type (negative binomial and linear regression).
Negative binomial models with terms for overall slope (time), step, and change of slope (pre-/post-OSI), additionally adjusted for mean age (years), % male sex, and % white race, along with model Akaike Information Criterion (AIC) and likelihood ratio probability (compared with null model).
Linear regression models with terms for overall slope (time), step, and change of slope (pre-/post-OSI), additionally adjusted for mean age (years), % male sex, and % white race, along with model adjusted R2 and F-statistic probability. CI, confidence interval; NSAID, nonsteroidal anti-inflammatory drug; OSI, Opioid Safety Initiative; VHA, Veterans Health Administration.
Figure 1.
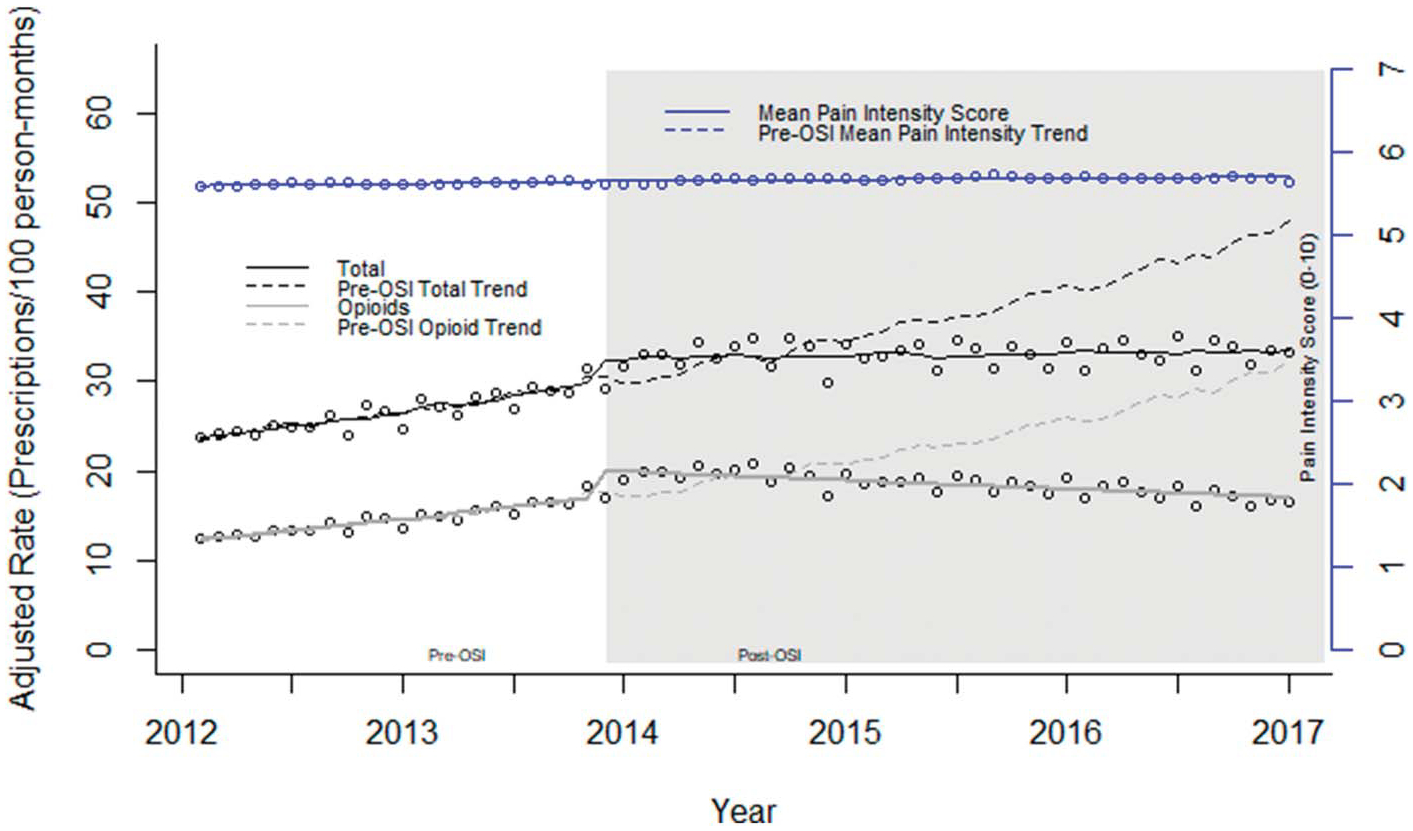
EAASE National Cohort, rates for total analgesic prescriptions, opioid prescriptions, and mean pain intensity score for those reporting pain (2012–2017). Prescription classes as rates (mean count/100 person-months). Counts were adjusted according to the length of a standard month (365.25 days/12 = 30.44 days) to account for the varying length of each month. Rates and superimposed lines from multivariable negative binomial models for prescription rates and a multivariable linear regression model for mean pain intensity including terms for slope, step change (pre-/post-OSI), and change in slope. Pre-OSI models and predicted values (dashed lines) include only term for slope. All models additionally adjusted for age (years), male sex (%), and white race (%). OSI, Opioid Safety Initiative.
3.1.2. Opioids
Before OSI, there was an increase in opioid prescribing (βtime = 0.018, P < 0.0001) along with an increase in prescribing at about the time of the OSI (step change: 0.578, P < 0.0001) (Tables 2 and 3 and Fig. 1). These trends were followed by a negative Δβ (−0.019, P < 0.0001). Figure 1 shows the pre-OSI increase in opioid prescribing followed by a pronounced sustained decrease after OSI. Extrapolating the pre-OSI trend would predict 32.4 (28.3–37.1) prescriptions/100 person-months compared with 16.9 (15.7–18.2) predicted under the full model (post-OSI), a significant −47.8% change (P < 0.001).
3.1.3. Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drug prescriptions were stable over the study period (βtime = 0.002, P = 0.743), showing no change in either step or slope change in the post-OSI period (Δβ = −0.001, P = 0.743) (Tables 2 and 3 and Fig. 2). Figure 2 illustrates an unchanging nonsignificant increase in NSAID prescribing over the entire study period without any associated perturbation related to the OSI. Similarly, the predicted lines from both the pre-OSI model and the full model show considerable overlap and no significant effect size changes, pre-OSI trend 10.7 (9.3–12.4) vs post-OSI trend 10.3 (9.7–11.0) with overlapping 95% confidence intervals and a small relative effect size (−3.7%, P = 0.748).
Figure 2.
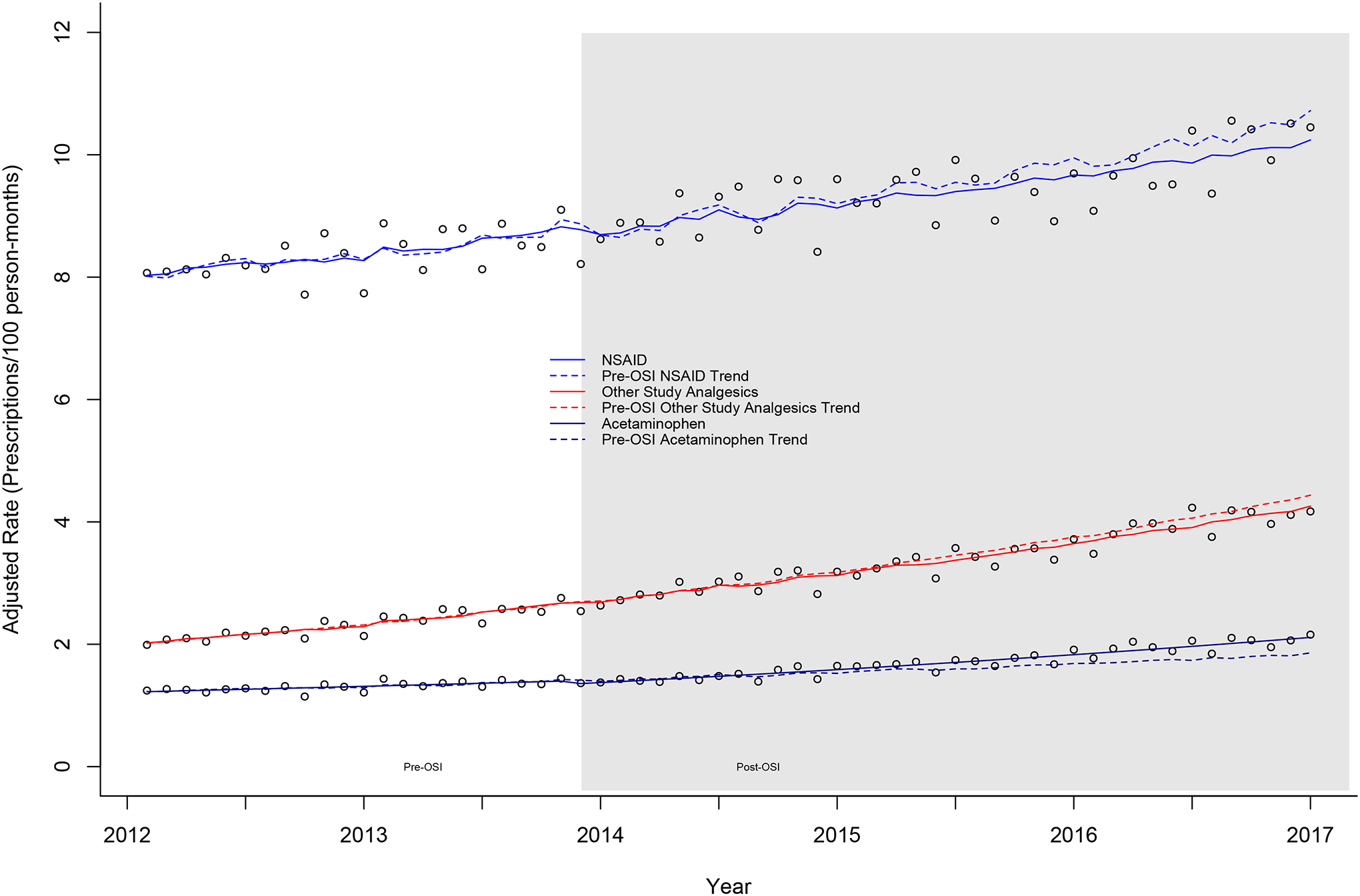
EAASE National Cohort, rates for nonsteroidal anti-inflammatory drugs (NSAIDs), other study analgesics, and acetaminophen (2012–2017). Prescription classes as rates (mean count/100 person-months). Counts were adjusted according to the length of a standard month (365.25 days/12 = 30.44 days) to account for the varying length of each month. Rates and superimposed lines from multivariable negative binomial models including terms for slope, step change (pre-/post-OSI), and change in slope. Pre-OSI models and predicted values (dashed lines) include only term for slope. All models additionally adjusted for age (years), male sex (%), and white race (%). OSI, Opioid Safety Initiative
3.1.4. Other study analgesics
Other study analgesic prescriptions increased modestly over the study period (βtime = 0.012, P < 0.0002) (Tables 2 and 3 and Fig. 2). However, there was no step change (0.029, P = 0.743) or change in slope in the post-OSI period (Δβ = −0.001, P = 0.743). Other study analgesics showed increasing prescribing with no post-OSI changes. End of study predictions resulted in only a −2.3% and nonsignificant difference (P = 0.748).
3.1.5. Acetaminophen
Acetaminophen prescriptions demonstrated a flat trajectory over the study period (βtime = 0.006, P = 0.255) (Tables 2 and 3 and Fig. 2). However, a small and modestly significant step decrease was noted (step = −0.145, P = 0.004) and a very modest significant increase in slope in the post-OSI period was noted (Δβ = 0.005, P = 0.047). The effect size calculation mirrored these results with a modestly significant (10.5%, P = 0.003) increase in acetaminophen prescribing at the end of the study compared with the pre-OSI trend, post-OSI: 2.1 (2.0–2.3) vs 1.9 (1.6–2.2) prescriptions/100 person-months, respectively.
3.2. Pain intensity measures
Figures 1 and 3 illustrate the pain intensity measures and their trajectories related to OSI implementation over the study period.
Figure 3.
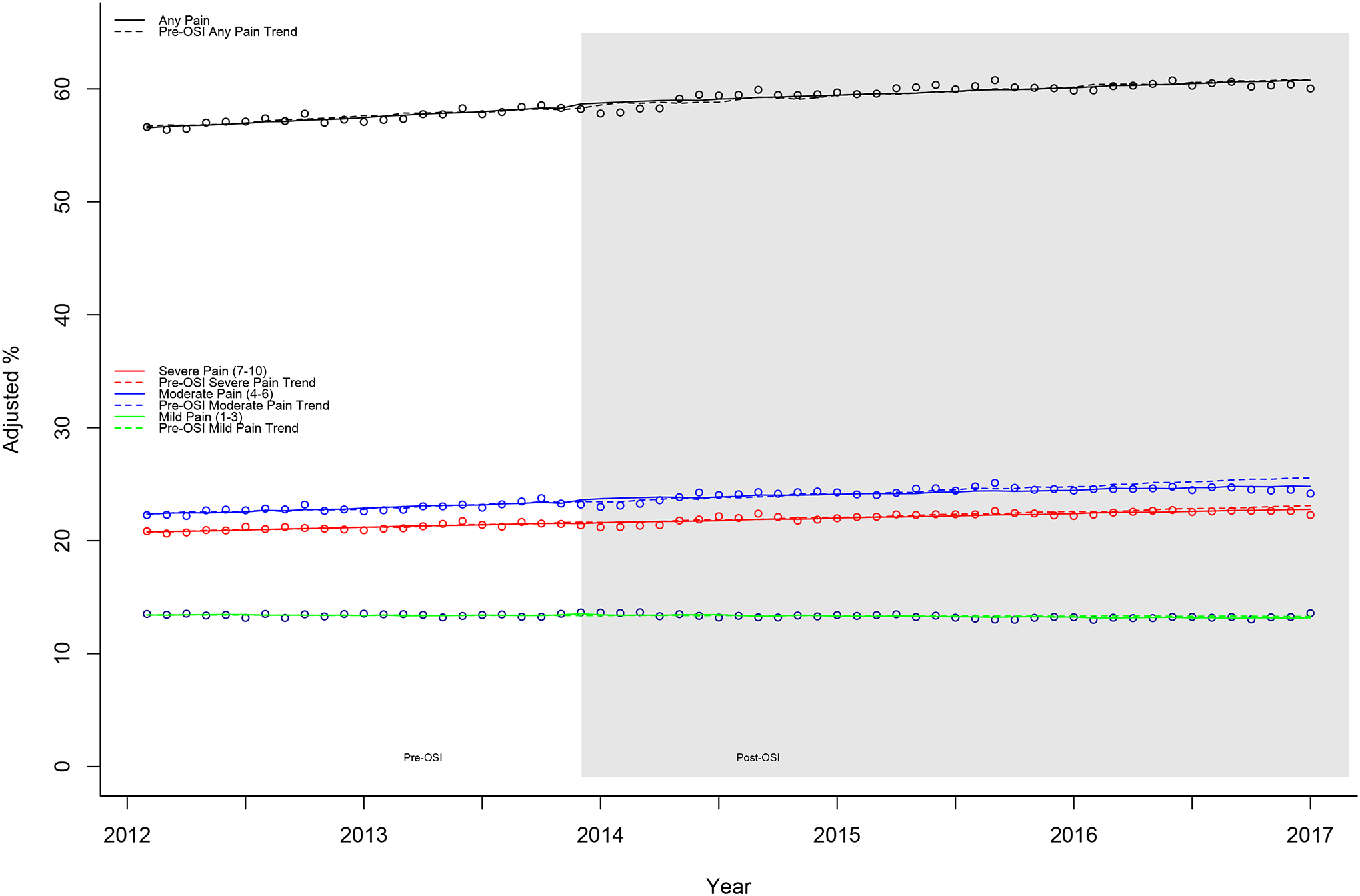
EAASE National Cohort, percent values for pain intensity measures: percentages reporting pain, mild pain (1–3), moderate pain (4–6), and severe pain (7–10) (2012–2017). Monthly mean percentages and superimposed lines from multivariable linear regression models including terms for slope, step change (pre-/post-OSI), and change in slope. Pre-OSI models and predicted values (dashed lines) include only term for slope. All models additionally adjusted for age (years), male sex (%), and white race (%). OSI, Opioid Safety Initiative.
3.2.1. Pain intensity in those reporting pain
The slope of pain intensity in those reporting pain was unchanged over the study period (βtime = 0.003, P = 0.613) and showed no changes related to OSI (step = 0.015, P = 0.963; Δβ = 0.000, P = 0.963) (Tables 2 and 3 and Fig. 1). Effect size calculations showed a nonsignificant 0.1% (P = 0.870) increase in pain intensity in those reporting pain between the pre and pre/post (full) models.
3.2.2. Percentage reporting pain
The percentage reporting pain increased gradually throughout the study period (βtime = 0.089, P = 0.038) (Tables 2 and 3 and Fig. 3). As with the other pain intensity measures (Figs. 1 and 3), there were no step (0.926, P = 0.271) or slope changes (Δβ = −0.029, P = 0.662) associated with OSI. The effect size calculation for the end of the study was nonsignificant (−1.9%, P = 0.096).
3.2.3. Percent reporting mild, moderate, and severe pain
Categorizing those reporting pain into mild (1–3), moderate (4–6), and severe (7–10) pain revealed that there was a significant steady upward trend in those reporting moderate pain (βtime = 0.046, P = 0.047) (Tables 2 and 3 and Fig. 3). Mild pain exhibited a small, nonsignificant decrease over the study period (−0.016, P = 0.729). Severe pain showed no significant slope over the study (0.059, P = 0.109). The changes associated with OSI were small (all <3% change), with no measure demonstrating any significant change associated with OSI (ie, no significant step, Δβ, or effect size change).
4. Discussion
4.1. Opioid and nonopioid analgesics
This study described covariate-adjusted outpatient analgesic trends (opioid prescribing, nonopioid analgesic prescribing, and pain intensity ratings) to understand more comprehensively prescribing trends for an older group of osteoarthritis patients before and after implementation of the VHA OSI.
We found a rise in total analgesic prescriptions before OSI, driven primarily by increasing opioid prescriptions with lesser contributions from other study analgesics and acetaminophen. In the post-OSI era, there was a dramatic reduction in overall analgesic prescribing. This trend occurred because of the pronounced decrease in opioid prescribing in the post-OSI period, which was partially compensated by increased prescriptions from the other categories, a general increase in other study analgesics prescribing over the entire study period, and a modest increase in acetaminophen prescribing after OSI. Nonsteroidal anti-inflammatory drug prescribing seemed to continue unchanged through the study period.
The changes in these analgesic prescribing patterns did not parallel changes in overall reported pain intensity in this sample. Covariate-adjusted pain intensity measures (percentage reporting pain, but not pain intensity ratings in those reporting pain) show a clinically small, but significant steady increase over the entire study period. This was apparently due to a growing number of those experiencing moderate pain over the entire study period, compared with those experiencing mild or severe pain. No step or slope changes associated with OSI were seen with any pain intensity measures.
Previous studies have demonstrated inflection points6,9,16 with opioid prescribing, albeit at different times. Kazanis et al.,6 for example, using time-series forecasting models with both military and civilian data showed increasing and then decreasing prescriptions for opioids (inflection point about 2011).6 We did not find a decrease in the 2011 to 2013 period as they did. Other studies have demonstrated that educational programs, state monitoring programs, and a “best practices initiative” continue to be effective in reducing opioid prescribing.16 Our findings mirrored those of Lin et al.9 using VHA data, demonstrating the increase and subsequent decrease in opioid prescribing before and after the 2013 OSI.
As the opioid epidemic continues to be a public health threat with risk of adverse outcomes from prescription opioids, clinicians will increasingly be challenged to balance adequately treating patient pain while limiting the use of opioid analgesics. As previously mentioned, other studies indict and challenge the benefits of opioids in various disease states, including osteoarthritis.7 Findings from this study demonstrate that with programs to ensure safer opioid prescribing practices, there has been an effective reduction in the use of opioids but also a concurrent increase in the use of nonopioid analgesic alternative medications. Acetaminophen showed a very modest increase in prescribing after OSI. There continues to be a steady increase in other study analgesic use, but not one that is at a greater rate of increase than before the OSI. If these practice trends continue, there should be awareness and monitoring of the impact that the greater use these analgesic medications may have on safety, effectiveness, and clinical outcomes for the patients being treated.10,21 Clinicians will need to be informed of the risks and benefits of these analgesic alternatives in older patients. Future studies should evaluate the safety and effectiveness of not only opioid analgesic use, but also these alternative nonopioid analgesic medications that seem to be increasingly used, as indicated by this study, to treat pain in older persons.
4.2. Limitations
This study has several limitations. Data and results do not reflect causality because this was an observational study. In addition, findings from VHA data, particularly in this older subset, may not be generalizable to other health care settings and patient populations. Although the emphasis was on evaluation of prescribing trends before and after OSI implementation, undoubtedly, concurrent national policies related to analgesic prescribing, as well as public awareness of risks, may have also impacted changes in prescribing trends.
In this investigation, data on non-VHA prescribed medications and self-administration of over-the-counter medications or alternative therapies were not available. We did not evaluate coprescribing of sedative medications nor calculate the doses of prescribed opioids (as in morphine equivalent doses) but confined our analyses to prescription rates. Finally, the presence of comorbid medical (such as cancer or other terminal) and mental health conditions was assumed to be randomly distributed and stable over the study period.
In the future, an interrupted time-series analysis will be used to investigate seasonality, produce forecasts to compare with subsequent data, as well as execute a more comprehensive model to associate all the other nonopioid analgesic medications with opioid trends.
5. Summary
In conclusion, recent trends and opioid prescribing safety initiatives have been effective and as demonstrated by this and other studies, there are decreasing rates of opioid prescribing for older veterans with osteoarthritis of the knee and hip. The decrease in opioid prescribing is only partially compensated by the prescribing of other non-opioid analgesics in older patients. However, changes in analgesic prescribing do not seem to be associated with concurrent changes in reported pain intensity by older veterans with osteoarthritis. Future studies should investigate potential risks and benefits of these changing rates of opioid and nonopioid analgesic medications and the impact these have on patient safety and pain intensity outcomes.
Acknowledgements
The EAASE (Evaluating Arthritis Analgesic Safety and Effectiveness) team includes Ruth Balk, Rachel Dismore, Vera Gaetano, Dorian Gittleman, David Leverty, Erin Linden, Brittany Majeski, Ebony Manuel, Kai Monde, Diana Natividad, Grace Polusny, Anthony Rinaldi, Daniel Signor, and Lee Stefanis.
This work was supported by Award Number 1 I01 HX000911-01A2 (Hwang) from the Health Services Research & Development Service of the VA Office of Research and Development. The contents of this article are solely the responsibility of the authors and do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- [1].Ackerman IN, Zomer E, Gilmartin-Thomas JF, Liew D. Forecasting the future burden of opioids for osteoarthritis. Osteoarthr Cartil 2018;26:350–5. [DOI] [PubMed] [Google Scholar]
- [2].Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305:1315–21. [DOI] [PubMed] [Google Scholar]
- [3].Compton WM, Boyle M, Wargo E. Prescription opioid abuse: problems and responses. Prev Med 2015;80:5–9. [DOI] [PubMed] [Google Scholar]
- [4].Goulet JL, Buta E, Bathulapalli H, Gueorguieva R, Brandt CA. Statistical models for the analysis of zero-inflated pain intensity numeric rating scale data. J Pain 2017;18:340–8. [DOI] [PubMed] [Google Scholar]
- [5].Hargan ED. Determination that a public health emergency exists. Washington: Department of Health and Human Services, 2017. [Google Scholar]
- [6].Kazanis W, Pugh MJ, Tami C, Maddry JK, Bebarta VS, Finley EP, McGeary DD, Carnahan DH, Potter JS. Opioid use patterns among active duty service members and civilians: 2006–2014. Mil Med 2018;183:e157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, Kroenke K, Bair MJ, Noorbaloochi S. Effect of opioid vs. nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE Randomized Clinical Trial. JAMA 2018;319:872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].La Frenais FL, Bedder R, Vickerstaff V, Stone P, Sampson EL. Temporal trends in analgesic use in long-term care facilities: a systematic review of international prescribing. J Am Geriatr Soc 2018;66:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin LA, Bohnert AS, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the Opioids Safety Initiative on opioid-related prescribing in veterans. PAIN 2017;158:833–9. [DOI] [PubMed] [Google Scholar]
- [10].Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CC, Day RO, McLachlan AJ, Ferreira ML. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 2015;350:h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Vander Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004–2012. J Gen Intern Med 2014;30: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Office of the Assistant Secretary for Planning and Evaluation. Opioid abuse in the U.S. and HHS actions to address opioid-drug related overdoses and deaths. Washington: U.S. Department of Health & Human Services. ASPE Issue Brief, 2015. Available at: ///C:/Users/trent/Documents/VA%20Research/EAASE_TS/Pain%20Journal/Bibliography/ib_OpioidInitiative.pdf. Accessed October 10, 2018. [Google Scholar]
- [13].Patrick SW, Fry CD, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reduction in opioid-related death rates. Health Aff (Millwood) 2016;35:1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paulozzi LJ, Rudd RA, Jones CM, Mack KA. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. Morb Mortal Wkly Rep 2011;60:1487–92. [PubMed] [Google Scholar]
- [15].Rudd RA, Aleshire N, Zibbel JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000–2014. Morb Mortal Wkly Rep 2016;60:1487–92. [DOI] [PubMed] [Google Scholar]
- [16].Sun BC, Lupulescu-Mann N, Charlesworth CJ, Kim H, Hartung DM, Deyo RA, John McConnell K. Impact of hospital “Best Practice” mandates on prescription opioid dispensing after an emergency department visit. Acad Emerg Med 2017;24:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].U.S. Department of Veterans Affairs. VHA pain management. Available at: https://www.va.gov/PAINMANAGEMENT/Opioid_Safety_Initiative_OSI.asp. Accessed August 6, 2018.
- [18].VA Informatics and Computing Infrastructure (VINCI). Corporate Data Warehouse (CDW).
- [19].Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Therap 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- [20].Weiner SG, Baker O, Poon SJ, Rodger AF, Garner C, Nelson LS, Schuur JD. The effect of opioid prescribing guidelines on prescriptions by emergency physicians in Ohio. Ann Emerg Med 2017;70:799–808. [DOI] [PubMed] [Google Scholar]
- [21].Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis 2018;9:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


