Abstract
We have isolated the structural gene for translation initiation factor IF2 (infB) from the myxobacterium Myxococcus xanthus. The gene (3.22 kb) encodes a 1,070-residue protein showing extensive homology within its G domain and C terminus to the equivalent regions of IF2 from Escherichia coli. The protein cross-reacts with antibodies raised against E. coli IF2 and was able to complement an E. coli infB mutant. The M. xanthus protein is the largest IF2 known to date. This is essentially due to a longer N-terminal region made up of two characteristic domains. The first comprises a 188-amino-acid sequence consisting essentially of alanine, proline, valine, and glutamic acid residues, similar to the APE domain observed in Stigmatella aurantiaca IF2. The second is unique to M. xanthus IF2, is located between the APE sequence and the GTP binding domain, and consists exclusively of glycine, proline, and arginine residues.
Myxococcus xanthus is the best-characterized member of the myxobacteria family. These gram-negative soil bacteria are able to undergo a multicellular developmental program in response to starvation. Hundreds of thousands of bacteria glide to aggregation centers to form complex structures known as fruiting bodies. These specialized structures contain differentiated cells, the myxospores (9). During the developmental cycle of M. xanthus, cellular communication involving at least five different extracellular signals, known as A, B, C, D, and E, is required. The D signal corresponds to translational initiation factor 3 (IF3) (6, 7, 8), which contains a particular C-terminal extension absent in IF3s from other species. A mutation impeding development, mapping within this extension, suggested that the extension is necessary for developmental functions of the cell rather than for viability (8).
In prokaryotes, IF3 (encoded by the infC gene) is required for the initiation of translation with at least two other factors, IF1 (encoded by infA) and IF2 (encoded by infB), ribosomes, mRNA, fMet-tRNAfMet, and GTP (13). Our knowledge of this process comes essentially from studies with Escherichia coli. The three factors play essential roles in each step of translation initiation to control the correct entry into the first round of the elongation cycle (22). In E. coli, the infB gene is part of the nusA-infB operon (19, 23, 24, 29). IF2 is an essential GTP binding protein (20) which exists in two major forms in E. coli, IF2α (97.3 kDa) and IF2β (79.7 kDa). IF2β exists in two subforms (β1 and β2), which are barely distinguishable and which are due to two internal in-frame initiation codons separated by only 18 nucleotides (26). The expression of α and β forms of IF2 has also been observed in Bacillus subtilis (31). On the other hand, only one form seems to exist in other bacteria such as Bacillus stearothermophilus, Streptococcus faecium, and the myxobacterium Stigmatella aurantiaca (3, 4, 11). There is thus no obvious pattern to the occurrence of multiple forms of IF2 in gram-negative and gram-positive bacteria or even in closely related bacilli.
The fact that a C-terminal extension in M. xanthus IF3 appears necessary for developmental functions is intriguing given the very general role of IF3. We have previously identified a similar extension in the N-terminal portion of IF2 in the closely related bacterium S. aurantiaca, which hints that translation IFs, and hence translation itself, might play an important role in differentiation. In order to gain further insight into the existence and characteristics of peculiar protein domains in translation factors, so far only observed in myxobacteria, we decided to study the infB gene of M. xanthus. We took advantage of the sequence conservation between all known IF2 proteins, which covers the central GTP-binding domain and the C-terminal domain, to identify and clone the M. xanthus infB gene by cross-hybridization. The sequence analysis of the open reading frame revealed an N-terminal extension similar to that already observed in S. aurantiaca IF2 followed by a peculiar domain just upstream of the GTP binding site. The expression and the potential occurrence of multiple forms of IF2 in M. xanthus were investigated.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. M. xanthus DK101 was grown to late exponential phase in 1% Bacto Casitone (Difco) with 8 mM MgSO4 at 30°C and harvested at ∼5 × 108 cells/ml. E. coli strains were propagated at 30, 37, or 42°C in Luria-Bertani (LB) broth or on LB agar plates (1.5% [wt/vol]) (27). If required, ampicillin (100 μg ml−1), chloramphenicol (10 μg ml−1), isopropyl-thiogalactopyranoside (IPTG; 50 μg ml−1), and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 50 μg ml−1) were added. Growth of liquid cultures were monitored by measuring the optical density at 600 nm.
TABLE 1.
Bacteria and plasmids used in this study
| Plasmid or strain | Vector | Relevant genotype | Source or reference |
|---|---|---|---|
| Strains | |||
| M. xanthus | |||
| DK1622 | Wild type | 17 | |
| DK101 | sglA1 | 14 | |
| E. coli | |||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96, thi-1 relA1 | Bethesda Research Laboratory | |
| IBPC5321 | F−thi-1 argG6 argE3 his-4 xyl-5 tsx-29 rpsL ΔlacX74 | 25 | |
| CSR603 | F−recA1 uvrA6 phr1 leuB6 proA2 argE3 thi1 ara14 lacY1 galK2 xyl5 mtl1 rpsL31 tsx33 supE44 | 28 | |
| KW251 | F−supE44 galK2 galT22 metB1 hsdR2 mcrB1 mcrA [argA81:Tn10] recD1014 | Promega | |
| SL598R | F−thi-1 argG6 argE3 his-4 xyl-5 tsx-29 rpsL ΔlacX74 ΔinfB::cat2 recA1 (λGJ9-2) | 20 | |
| TLC7 | CSR603(pTrc99A) | ||
| TLC8 | CSR603(pB18-1) | This study | |
| TLC9 | CSR603(pTLC22) | This study | |
| TLC10 | DH5α(pTLC10) | This study | |
| TLC11 | DH5α(pTLC11) | This study | |
| TLC12 | DH5α(pTLC12) | This study | |
| TLC22 | DH5α(pTLC22) | This study | |
| TLC30 | DH5α(pTLC30) | This study | |
| TLC32 | DH5α(pTLC32) | This study | |
| SL18-1 | F−thi-1 argG6 argE3 his-4 xyl-5 tsx-29 rpsL ΔlacX74 ΔinfB::cat2 recA1 pB18-1 | This study | |
| SLT22 | F−thi-1 argG6 argE3 his-4 xyl-5 tsx-29 rpsL ΔlacX74 ΔinfB::cat2 recA1 pTLC22 | This study | |
| Plasmids | |||
| pBluescript II SK+ | bla ′lacZ | Stratagene | |
| pTrc99A | bla ′lacZ lacIq | Pharmacia | |
| pBR322 | bla tet | 2 | |
| pCL1921 | spec str ′lacZ | 21 | |
| pB18-1 | pBR322 | bla E. coli nusA E. coli infB | 24 |
| pTLC10 | pBluescript II SK+ | bla M. xanthus ′nusA M. xanthus ORF1 M. xanthus infB M. xanthus ORF3′ | This study |
| pTLC11 | pBluescript II SK+ | bla; 1,151-bp PstI-XhoI fragment of pTLC10 | This study |
| pTLC12 | pBluescript II SK+ | bla; 3,448-bp XhoI-XhoI fragment of pTLC10 | This study |
| pTLC22 | pTrc99A | bla; M. xanthus infB M. xanthus ORF3′ | This study |
| pLBYC8 | pTrc99A | bla; E. coli infB | 3 |
| pTLC30 | pCL1921 | spec str; 3.6-kb PleI fragment of M. xanthus infB | This study |
| pTLC32 | pTLC30 | spec str; M. xanthus infB M. xanthus ORF3′ | This study |
DNA manipulations, cloning, and transformation.
Total genomic DNA was isolated from M. xanthus DK101 by the method of Starich and Zissler (34). Plasmids from E. coli were extracted and purified as previously described by Sambrook et al. (27). Plasmid and genomic DNA was digested with restriction enzymes (Gibco-BRL, New England Biolabs Inc.) according to the supplier's recommendations. DNA restriction fragments were purified from agarose gels using the Qiaquick gel extraction kit (Qiagen). Ligation was obtained using the T4 DNA ligase (Gibco-BRL) in accordance with the manufacturer's recommendations. E. coli competent cells were prepared and transformed as described by Huff et al. (16) or by electroporation as described previously (1).
Plasmid constructs.
The 4.67-kb BamHI fragment containing the potential M. xanthus infB homolog (see Results and Fig. 1) was cloned in the pBluescript II SK+ vector (pTLC10).
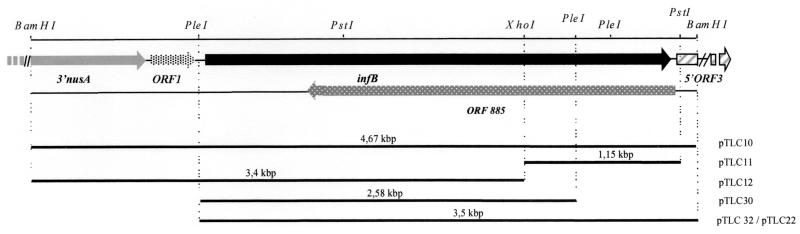
FIG. 1.
Overview of the genetic organization around the M. xanthus infB gene. Shown are the locations and the orientations of ORF1, the infB gene, the 3′ and the 5′ regions of two putative ORF (which are similar to the nusA and ORF3 genes) and an ORF present on the complementary strand encoding 885 aa. A partial restriction map of the 4.67-kb BamHI fragment from pTLC10 is presented at the top. Bars, restriction fragments cloned within the pBluescript II SK+ plasmid (for pTLC10, pTLC11, and pTLC12), plasmid pTrc99A (for pTLC22), and plasmid pCL1921 (for pTLC30 and pTLC32).
Plasmid pTLC10 containing the 4.67-kb BamHI insert was digested with the XhoI and PstI enzymes, giving two fragments of 726 bp and 1.15 kb, respectively. The 1.15-kb PstI-XhoI fragment was isolated and cloned in pBluescript II SK+ (pTLC 11; see Fig. 2). Plasmid pTLC12, a pBluescript II SK+ derivative, contained the 3.4-kb XhoI/BamHI insert.
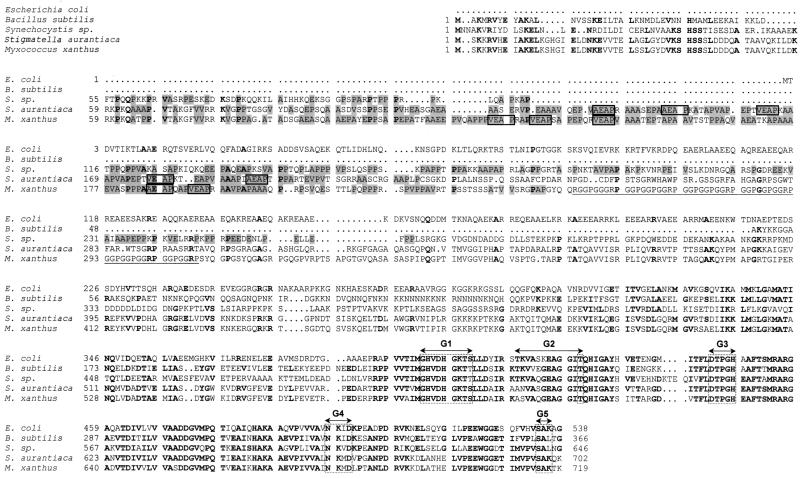
FIG. 2.
Comparison of amino acid sequences in the N-terminal and G domain regions of five bacterial species. XEAP motifs present in the APE region are boxed. The A, P, V, and E residues in the APE regions are shaded. The region rich in G, R, and P is underlined. Boldface characters identify identical amino acids at the same position in at least three IF2 species. Consensus sequences G1 to G5, found in the G domains of GTP-binding proteins, are also indicated. Swiss-Prot accession numbers for the E. coli, B. subtilis, Synechocystis sp., and S. aurantiaca IF2 proteins are P02995, P17889, P72689, and P55875, respectively.
In order to clone the infB gene without the upstream flanking sequence, we isolated a 2.6-kb PleI fragment from pTLC10. The PleI fragment was subcloned into the SmaI site of low-copy-number plasmid pCL1921 after filling in the PleI extremities with Klenow enzyme. This gave rise to pTLC30 (Table 1).
The 1.3-kb XhoI-BamHI infB fragment isolated from pTLC10 was inserted in pTLC30 also digested with XhoI-BamHI in order to reconstitute the entire infB gene in the same orientation as that of lacZ (pTLC32). pTLC32 was digested with EcoRI-BamHI to isolate an EcoRI-BamHI fragment containing the entire infB gene and the 5′ extremity of ORF3. This fragment was subcloned in expression vector pTrc99A (pTLC22).
Screening a M. xanthus λ Gem12 library.
The λ Gem12 library was kindly provided by J. Guespin-Michel (Rouen, France). This library was constructed by partially digesting M. xanthus genomic DNA with Sau3A and selecting fragments ranging from 9 to 23 kb. These fragments were filled in using the Klenow fragment and cloned into the filled XhoI site of λ Gem12. A 1.2-kb XhoI-PstI fragment containing the 3′ end of the S. aurantiaca infB gene (3) was labeled with [α-32P]dCTP (Amersham) by random priming (10) and used to screen around 80,000 clones by colony hybridization (27).
Hybridization.
Southern analysis of plasmid and chromosomal DNA fragments was performed as described previously (33).
DNA sequencing and computer analysis.
The inserts of plasmids pTLC10, -11, and -12 were in part sequenced using specific oligonucleotides. In addition, random nested deletions were created using the Exo III mung bean nuclease (Stratagene) to generate plasmids for sequencing. Both strands of the 4.67-kb BamHI region were sequenced with the dideoxy chain termination method (30) using an automated sequencer (ABI 310 Prism; Perkin-Elmer, Applied Biosystems Division) with Taq FS polymerase. Sequence analysis was performed with the program of the Genetics Computer Group (Madison, Wis.) sequence analysis software package. Preliminary sequence data concerning microbial genomes were obtained from The Institute for Genomic Research website at http: //WWW.tigr.org.
Maxicell analysis.
Expression of plasmid-encoded proteins in the strain CSR603 was analyzed as described previously (28).
Complementation of an E. coli infB null mutation.
The curing of the λ infB transducing phage (λGJ9-2) from strain SL598R carrying the various test plasmids and analysis of the survivors were performed as previously described (20).
Blotting and immunodetection of protein IF2.
The presence of IF2 was examined in cell extracts from M. xanthus and E. coli strains by immunoblotting, using an antibody to E. coli IF2 as described by Howe and Hershey (see Fig. 3B, lane 1, and Fig. 4) (15) or with antirabbit antibody horseradish peroxidase conjugate (Caltag Laboratories) revealed with peroxidase substrate (3-3′-diaminobenzidine tetrahydrochloride tablets; Sigma) (see Fig. 3B, lane 2).
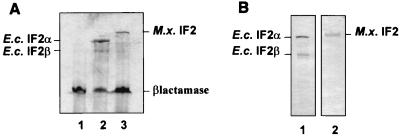
FIG. 3.
(A) Maxicell analysis. Cells were lysed by boiling them in sodium dodecyl sulfate (SDS) sample buffer. Proteins were separated by SDS–10% polyacrylamide gel electrophoresis. After being dried, the resulting gel was autoradiographed. Lanes: 1, TLC7; 2, TLC8; 3, TLC9. M.x., M. xanthus; E.c., E. coli. (B) Western blot analysis. Lane 1, E. coli crude cell extract; lane 2, M. xanthus crude cell extract.
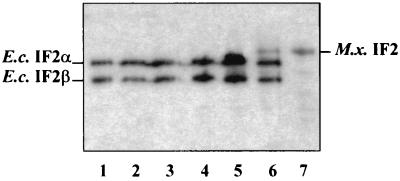
FIG. 4.
Western blot analysis of cured infB null mutant strains. Lanes: 1, E. coli SL598R at 30°C; 2, E. coli SL598R carrying pTRC99A at 30°C (before curing); 3, SL598R carrying pB18-1 at 30°C (before curing); 4, SLT18-1 at 37°C; 5, SL598R carrying pTLC22 at 30°C (before curing); 6, SL598R carrying pTLC22 at 30°C (before curing) with IPTG induction; 7, SLT22 at 37°C. M.x., M. xanthus; E.c., E. coli.
Nucleotide sequence accession number.
The overall nucleotide sequence for the 4.67-kb BamHI fragment from pTLC10 appears in the GenBank database under accession no. AF261103.
RESULTS
Identification and cloning of the infB locus.
We used the previously cloned S. aurantiaca infB gene to screen a λ Gem12 genomic library of M. xanthus DK101. A 1.12-kb XhoI-PstI fragment from the 3′ end of S. aurantiaca infB served as a hybridization probe and allowed us to detect several positive clones, one of them carrying a 13-kb insert. Southern blot analysis of a BamHI digest of this recombinant lambda phage identified a 4.67-kb BamHI fragment that hybridizes to the 1.12-kb C-terminal probe as well as to a 1.62-kb BamHI-SphI fragment corresponding to the N terminus of S. aurantiaca IF2 protein (3).
The 4.67-kb BamHI fragment was cloned in the pBluescript II SK+ vector to give plasmid pTLC10 and sequenced. A genetic map of this insert and the structure of several subclones are shown in Fig. 1. The nucleotide sequence has a high G+C content (69.5%), typical of genes of myxobacterial origin. It carries two complete open reading frames (ORF). The first ORF, ORF1 (336 nucleotides), extending from nucleotide 785 to 1120, codes for a hypothetical protein of 111 amino acids (aa). It is homologous to ORF1 in the B. subtilis and Thermus thermophilus nusA-infB operons and to the equivalent ORF in Mycobacterium tuberculosis and Deinococcus radiodurans (55 and 46% homology, respectively). A potential factor-independent terminator can be formed at the 3′ extremity of ORF1 placing the stop codon in the loop of the hairpin structure.
The second ORF, comprising 3,219 bp (nucleotides 1203 to 4415) and coding for a protein of 1,070 aa, displays extensive homologies to translation initation factor IF2 from a variety of organisms (Fig. 2). The putative AUG initiation codon (positions 1203 to 1205) is preceded by an AAGGG Shine-Dalgarno sequence (positions 1193 to 1197), and the ORF terminates with a UAG stop codon (positions 4413 to 4415). The G+C content of the wobble position is 84.69% for the infB gene and 73.21% for ORF1, a characteristic of myxobacterial genes. Downstream from the UAG stop codon we identified a strong putative transcription terminator (ΔG = −20.2 kcal). Upstream of ORF1, we localized the putative 3′ end of the nusA gene (Fig. 1) terminating with a UAA stop codon (positions 763 to 765). This sequence has 50% homology with the equivalent region of E. coli nusA. The sequence downstream from infB, starting at position 4478, shows homology to the 5′ half of ORF3 (Fig. 1) present in the same position with respect to infB in B. subtilis and S. aurantiaca (3, 32).
More surprisingly, we found a long ORF coding for a putative protein of 885 aa with an Mr of 94,500; this ORF would be transcribed in the opposite direction with respect to infB. The potential gene (ORF885) starts with an AUG start codon located, on the complementary strand, between the ORF3 Shine-Dalgarno sequence and initiation codon and extends all the way through codon 223 for IF2.
The protein sequence of IF2.
The M. xanthus infB gene encodes a protein with a deduced Mr of 111,800, making it the largest of IF2 proteins characterized to date. Compared to its counterparts from S. aurantiaca, E. coli, B. subtilis, B. stearothermophilus, and S. faecium, it contains 16, 180, 354, 328, and 285 additional amino acids, respectively (3, 4, 11, 23, 31). M. xanthus IF2 displays extensive homology to the C-terminal two-thirds of other IF2s, and this holds especially true for the central region (residues 571 to 717 of M. xanthus IF2; Fig. 2) corresponding to the GTP-binding domain (88% identical residues and 95% similarity with the equivalent region of S. aurantiaca IF2 and about 70% with that of the other bacteria cited above). On the other hand, the N-terminal sequences are surprisingly divergent. The increased size of M. xanthus IF2 with respect to other IF2s is exclusively due to an extension of its N-terminal region. However, a hydrophobic-cluster algorithm (12), designed to identify common conformational features among distantly related proteins, revealed that the first 90 residues of the N-terminal domain of M. xanthus IF2 display a highly hydrophobic structure (data not shown) similar to those described for other IF2s (3, 31). The adjacent region (residues 61 to 249) has an abnormal amino acid composition, consisting essentially (67%) of four different amino acids: 48 alanine, 44 proline, 19 valine, and 16 glutamic acid residues (Table 2). Due to the conserved high content in alanine, proline, and glutamic acid we have named this region the APE sequence (3). This domain is found in at least four myxobacterial proteins: the C-terminal portion of IF3 from M. xanthus, the ORF3 protein of S. aurantiaca, and the N-terminal domain of IF2 from M. xanthus and S. aurantiaca. Interestingly, in contrast to those of IF3 and the ORF3 protein, the APE sequences of M. xanthus and S. aurantiaca IF2 contain a high percentage of valine residues and the pattern XEAP is repeated five times, with X being alanine or valine (Fig. 2). Another protein domain immediately adjacent to the APE domain (residues 254 to 309) is found exclusively in IF2 of M. xanthus. This region of 56 aa comprises only glycine, proline, and arginine residues (33, 16, and 7, respectively). Named the GPR domain, it features a particular motif, GGRPGGPGGP, repeated five times (GGPGG six times and GRP six times). Both the APE and the GPR domains contain high levels of proline, 23 and 28% respectively, suggesting a high degree of flexibility for this portion of the protein. The remainder of the N-terminal portion of M. xanthus IF2 (residues 310 to 570) does not display any particular structural features.
TABLE 2.
Compositions of the APE regionsa
| Protein | Position of APE region | Length of APE region (aa) | % of aa that are:
|
||||
|---|---|---|---|---|---|---|---|
| A | P | E | V | A, P, E, or V | |||
| M. xanthus IF2 | 61–249 | 188 | 25.5 | 23 | 8.5 | 10 | 67 |
| M. xanthus IF3 | 181–247 | 66 | 34 | 11 | 14 | 1.5 | 60.5 |
| S. aurantiaca IF2 | 61–221 | 160 | 27 | 19 | 11 | 11 | 68 |
| S. aurantiaca ORF3 product | 76–195 | 119 | 12 | 10 | 18 | 2.5 | 42.5 |
| S. sp. IF2 | 57–266 | 210 | 12.5 | 26.5 | 7.5 | 4.5 | 51 |
Characterization of the putative M. xanthus infB gene product.
In order to assess whether the cloned M. xanthus chromosomal fragment actually codes for a gene product of the expected size, we performed a Maxicell analysis of an E. coli strain (CSR603) harboring plasmid pTLC22, which contains the putative infB gene without extensive upstream flanking sequences under the control of the IPTG-inducible Trc promoter (see Materials and Methods). A protein of the expected size (Mr, 111,000) was expressed from the plasmid-encoded gene (Fig. 3A, lane 3). As a control, we used strain CSR603 containing plasmid pLBYC8, which expresses the two forms of E. coli IF2 (IF2α and IF2β) from the same promoter (Fig. 3A, lane 2) (3).
In accordance, Western analysis using antibodies raised against E. coli IF2 also detected only a single protein of the same size in crude extracts of M. xanthus DK1622 (Fig. 3B, lane 2).
M. xanthus infB complements an E. coli infB null mutation.
We used the previously described “IF2 complementation strain” SL598R (20) to test whether expression of a plasmid-borne M. xanthus infB gene could assure survival of E. coli lacking its chromosomal infB gene. In this strain, a functional wild-type copy of infB is supplied in trans by a thermosensitive lysogenic λ phage integrated at att λ After heat induction at 42°C, the λ infB phage can be cured without killing the bacteria, provided a viable infB allele is supplied in trans.
Plasmid pTLC22 expressing M. xanthus infB and control plasmids pB18-1 and pTrc99A were used to transform SL598R. pB18-1 (Table 1) expresses IF2 to a level almost identical to that produced in a wild-type strain (25). After heat curing of the λ phage, survivors were detected only in strains carrying plasmid pB18-1 and pTLC22, not in strains with the vector alone (pTrc99A). The frequency of occurrence of successfully cured strains with pB18-1 was double that with pTLC22 (Table 3). Cured strains SL18-1 and SLT22, carrying pB18-1 and pTLC22, respectively, were stable through several rounds of purification at 42 and 37°C.
TABLE 3.
Analysis of the complemented strains
| Plasmid | Strain | Frequency of cured strainsa | Doubling timeb (min) at:
|
|
|---|---|---|---|---|
| 30°C | 37°C | |||
| pB18-1 | SL18-1 | 1 × 10−3 | 44 ± 4 | 29 ± 4 |
| pTLC22 | SLT22 | 6 × 10−4 | 59 ± 4 | 43 ± 5 |
| pTRC99A | No survivor | |||
Number of survivors at 42°C divided by number of bacteria exposed to 42°C.
Values are means ± standard deviations from six independent experiments.
Western analysis of crude cell extracts from several independent colonies of the complemented strain confirmed that only M. xanthus IF2 was expressed in SLT22 (Fig. 4, lane 7), thus indicating that the single form of the myxococcal protein can effectively replace E. coli IF2. In order to assess the efficiency of this functional replacement, we tested a representative number of SLT22 and SL18-1 clones for growth at 30 and 37°C in LB medium. At both temperatures strains relying on M. xanthus IF2 (SLT22) has a 1.5-fold-longer doubling time than strain SL18-1 expressing E. coli IF2 (Table 3).
DISCUSSION
Even though M. xanthus is a gram-negative organism, the genetic organization around the infB locus more closely resembles that of the gram-positive bacterium B. subtilis than that of E. coli. The intergenic region between nusA and infB in B. subtilis contains two ORF (31), ORF1 and ORF2, encoding two putative proteins named p10A and p11, which are absent in E. coli. Genes encoding proteins similar to the M. xanthus ORF1 product are present in the same chromosomal position in B. subtilis (48% homology), T. thermophilus, Helicobacter pylori, Mycobacterium leprae, and M. tuberculosis. Homologous regions in the products of various ORF1 reading frames are essentially located in the N-terminal halves of the proteins.
In B. subtilis, but not in E. coli, infB is followed by another gene encoding a protein of unknown function, ORF3, whose start codon overlaps the infB stop codon. A homologous gene is also located downstream of M. xanthus infB but is separated from it by a putative transcription terminator. Among the two potential translation start sites for ORF3, the downstream one has a significantly stronger Shine-Dalgarno sequence (AAGGGGG) and the derived N terminus more closely resembles that of other ORF3-encoded proteins from various species. In addition to B. subtilis, a gene homologous to ORF3 can also be found in the same chromosomal position in T. thermophilus and in the closely related myxobacterium S. aurantiaca, while it is present in different locations in several other gram-negative and gram-positive organisms such as Clostridium, Streptomyces, D. radiodurans, Thermotoga maritima, and M. leprae. All these quite-different organisms share nevertheless one common property: they all can differentiate to form spores and/or specific multicellular structures.
Rather unexpectedly, we found an extensive potential ORF (ORF885) on the complementary strand with respect to the infB coding sequence. This ORF codes for a hypothetical protein of 885 aa (Mr of 94,500) and extends from the start codon of ORF3 to codon 223 of infB. A similarity search identified several short potential ORF in the same location (complementary to a large part of infB) in a variety of eubacteria. The best score was obtained with Bordetella pertussis, where a simple frameshift would create an ORF encoding a protein of almost 600 aa with >40% identical residues. Similar frameshifts in other bacteria would also lead to production of this protein, suggesting that this might be the remnant of a gene that has lost its function during evolution.
Western blot analysis showed that M. xanthus expressed a single full-length form of IF2 whereas two or even three forms of the factor, differing in the lengths of their N-terminal regions, have been described for B. subtilis and E. coli, respectively (15, 26, 31).
The role of the N-terminal domain of IF2 is still unclear since, in E. coli, it does not appear to be essential for the initiation of protein synthesis in vivo and in vitro (5, 20, 26). The M. xanthus IF2 protein is significantly larger than its counterpart from E. coli. Nevertheless, it was capable of replacing the endogenous factor in this bacterium. The somewhat slower growth observed with a strain expressing M. xanthus IF2 instead of E. coli IF2 may not be very significant and might simply reflect the higher expression (approximately twofold; Fig. 4, compare lanes 6 and 7) suggested by the Western analysis. On the other hand, removal of the N-terminal extension of S. aurantiaca IF2 improved growth of E. coli somewhat, especially at 30°C (3).
With an Mr of 111,800, IF2 of M. xanthus is larger than all presently known IF2 proteins. This is essentially due to the insertion of a characteristic sequence between residues 61 and 249, which is also present in the S. aurantiaca protein but completely absent from the IF2 proteins from other bacteria, except the cyanobacterium Synechocystis (Fig. 2) (18). Like the myxobacteria, Synechocystis belongs to the group of gliding bacteria capable of forming multicellular structures, with the difference that individual cells fulfill different functions.
The main characteristic of the APE sequence is its abnormal amino acid composition. We now have knowledge of four myxobacterial proteins carrying such a domain. While alanine, proline, and glutamic acid are extremely enriched in all APE domains, valine is also very abundant in IF2 (11%).
The N-terminal APE sequence of M. xanthus IF2 is longer than those of M. xanthus IF3 and the product of S. aurantiaca ORF3 (188 aa versus 66 and 119 aa, respectively) and the repeated XEAP motif is specific to IF2. Despite the differences in size and the periodicity of motifs between the M. xanthus IF2 and IF3 APE sequences, continuous alignment of both regions reveals a 34% similarity. M. xanthus IF2 differs from all other proteins described here by the presence of an additional motif, GGRPGGPGGP, repeated five times. This motif is not only absent from all IF2 proteins known to date but also has no similarity with any protein in the data banks. For now, we can only suggest that this portion corresponds to a separation domain between the atypical N-terminal region and the central protein domain carrying the GTPase activity.
The finding that a developmental mutation in M. xanthus maps to the APE domain in IF3 (7) makes it thus very tempting to speculate that the atypical N-terminal IF2 extension in M. xanthus as well as Synechocystis could play a common but as yet unknown role in development. Mutational analysis of the peculiar M. xanthus IF2 N-terminal domains is in progress and will hopefully provide clues to a possible involvement of IF2 in the developmental process.
ACKNOWLEDGMENTS
We thank Harald Putzer for fruitful discussions and Ciaran Condon for critical reading of the manuscript.
This work was supported in part by the European program Human Capital Mobility (contract no. ERBCHRXCT 940529, Molecular mechanisms of initiation of prokaryotic translation) and by a Poitou-Charentes Region fellowship to E.T.-D.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1987. [Google Scholar]
- 2.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 3.Bremaud L, Laalami S, Derijard B, Cenatiempo Y. Translation initiation IF2 of the myxobacterium Stigmatella aurantiaca: presence of a single species with an unusual N-terminal sequence. J Bacteriol. 1997;179:2348–2355. doi: 10.1128/jb.179.7.2348-2355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brombach M, Gualerzi C O, Nakamura Y, Pon C L. Molecular cloning and sequence of Bacillus stearothermophilus translational initiation factor IF2 gene. Mol Gen Genet. 1986;205:97–102. doi: 10.1007/BF02428037. [DOI] [PubMed] [Google Scholar]
- 5.Cenatiempo Y, Deville F, Dondon J, Grunberg-Manago M, Sacerdot C, Hershey J W B, Hansen H F, Petersen H U, Clark B F C, Kjeldgaard M, La Cour T F M, Mortensen K K, Nyborg J. The protein synthesis initiation factor 2 G-domain. Study of a functionally active C-terminal 65-kilodalton fragment of IF2 from Escherichia coli. Biochemistry. 1987;26:5070–5076. doi: 10.1021/bi00390a028. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Y L, Kaiser D. dsg, a gene required for cell-cell interaction early in Myxococcus development. J Bacteriol. 1989;171:3719–3726. doi: 10.1128/jb.171.7.3719-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y L, Kaiser D. dsg, a gene required for Myxococcus development, is necessary for cell viability. J Bacteriol. 1989;171:3727–3731. doi: 10.1128/jb.171.7.3727-3731.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y L, Kalman L V, Kaiser D. The dsg gene of Myxococcus xanthus encodes a protein similar to translocation initiation factor IF3. J Bacteriol. 1994;176:1427–1433. doi: 10.1128/jb.176.5.1427-1433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin M. Recent advances in the social and developmental biology of the Myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–10. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich K, Brombach M, Pon C L. Identification, cloning and sequence of Streptococcus faecium infB (translational initiation factor) gene. Mol Gen Genet. 1988;214:595–600. doi: 10.1007/BF00330501. [DOI] [PubMed] [Google Scholar]
- 12.Gaboriaud C, Bissery V, Benchetrit T, Mornon J P. Hydrophobic cluster analysis: an efficient new way to compare and analyze amino acid sequences. FEBS Lett. 1987;224:149–155. doi: 10.1016/0014-5793(87)80439-8. [DOI] [PubMed] [Google Scholar]
- 13.Hershey J W B. Protein synthesis. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1987. pp. 613–647. [Google Scholar]
- 14.Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): genes controlling movement of single cells. Mol Gen Genet. 1979;171:167–176. [Google Scholar]
- 15.Howe J G, Hershey J W B. A sensitive immunoblotting method for measuring protein synthesis initiation factor levels in lysates of Escherichia coli. J Biol Chem. 1981;256:12836–12839. [PubMed] [Google Scholar]
- 16.Huff J P, Grant B J, Penning C A, Sullivan K F. Optimization of routine transformation of Escherichia coli with plasmid DNA. BioTechniques. 1990;9:570–577. [PubMed] [Google Scholar]
- 17.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 19.Kurihara T, Nakamura Y. Cloning of the nusA gene of Escherichia coli. Mol Gen Genet. 1983;190:189–195. doi: 10.1007/BF00330639. [DOI] [PubMed] [Google Scholar]
- 20.Laalami S, Putzer H, Plumbridge J, Grunberg-Manago M. A severely truncated form of translational initiation factor 2 supports growth of Escherichia coli. J Mol Biol. 1991;187:617–621. doi: 10.1016/0022-2836(91)90017-z. [DOI] [PubMed] [Google Scholar]
- 21.Lerner C G, Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990;15:4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy J E G, Gualerzi C O. Translational control of prokaryotic gene expression. Trends Genet. 1990;6:78–85. doi: 10.1016/0168-9525(90)90098-q. [DOI] [PubMed] [Google Scholar]
- 23.Plumbridge J A, Howe J G, Springer M, Touati-Schwartz D, Hershey J W B, Grunberg-Manago M. Cloning and mapping of a gene for translational initiation factor IF2 in Escherichia coli. Proc Natl Acad Sci USA. 1982;79:5033–5037. doi: 10.1073/pnas.79.16.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plumbridge J A, Springer M. Organization of the Escherichia coli chromosome around the genes for translation initiation factor IF2 (infB) and a transcription termination factor (nusA) J Mol Biol. 1983;167:227–243. doi: 10.1016/s0022-2836(83)80333-7. [DOI] [PubMed] [Google Scholar]
- 25.Plumbridge J A, Deville F, Sacerdot C, Petersen P, Cenatiempo Y, Cozzone A, Grunberg-Manago M, Hershey J W B. Effect of NusA protein on expression of the nusA, infB operon in E. coli. Nucleic Acids Res. 1985;13:3371–3388. doi: 10.1093/nar/13.9.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacerdot C, Vachon G, Laalami S, Morel-Deville F, Cenatiempo Y, Grunberg-Manago M. Both forms of translational factor 2 (α and β) are required for maximal growth of Escherichia coli. Evidence for two translational initiation codons for IF2β. J Mol Biol. 1992;225:67–80. doi: 10.1016/0022-2836(92)91026-l. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Sancar A, Hack A M, Rupp W D. Simple method for the identification of plasmid-coded proteins. J Bacteriol. 1979;137:692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sands J F, Regnier P, Cummings H S, Grunberg-Manago M, Hershey J W B. The existence of two genes between infB and rpsO in the Escherichia coli genome: DNA sequencing and S1 nuclease mapping. Nucleic Acids Res. 1988;16:10803–10816. doi: 10.1093/nar/16.22.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shazand K, Tucker J, Chiang R, Stansmore K, Sperling-Petersen H U, Grunberg-Manango M, Rabinowitz J C, Leighton T. Isolation and molecular genetic characterization of the Bacillus subtilis gene (infB) encoding protein synthesis initiation factor IF2. J Bacteriol. 1990;172:2675–2687. doi: 10.1128/jb.172.5.2675-2687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shazand K, Tucker J, Grunberg-Manago M, Rabinowitz J C, Leighton T. Similar organization of the nusA-infB operon in Bacillus subtilis and Escherichia coli. J Bacteriol. 1993;175:2880–2887. doi: 10.1128/jb.175.10.2880-2887.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 34.Starrich T, Zissler J. Movement of multiple DNA units between Myxococcus xanthus cells. J Bacteriol. 1989;171:2323–2336. doi: 10.1128/jb.171.5.2323-2336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]