Abstract
Background
Thyrotoxicosis is a clinical syndrome produced by a multitude of disorders. Thyrotoxicosis is a serious medical condition that, if left untreated, can lead to a fatal illness. This review of recent evidences give additional input for perioperative management of thyrotoxic patients.
Methods
The literatures were found with Boolean operators in the form of thyrotoxicosis AND anesthesia, antithyroid medications AND perioperative optimization AND beta blockers OR calcium channel blockers in electronic data base sources such as the Cochrane library, PubMed, and Google scholar. This review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement.
Conclusions
and recommendations: Before surgery and anesthesia, manifestation of thyrotoxicosis including palpitation, irritability etc should be ruled out.
Keywords: Management, Medications, Perioperative, Thyroid function tests, Thyrotoxicosis
Highlights
-
•
Thyrotoxicosis is a clinical syndrome produced by a multitude of disorders.
-
•
Hyperthyroidism is a prevalent clinical condition that raises the risk of complications.
-
•
Surgery and anesthesia should be postponed in elective thyrotoxicosis patients.
-
•
Patients with thyrotoxicosis symptoms and elevated thyroid function tests should be optimized for 12–18 months.
1. Background
Thyrotoxicosis is frequently confused with hyperthyroidism, however the two are not synonymous because hyperthyroidism is defined as an increase in thyroid hormone levels in the thyroid gland's secretion or synthesis whereas thyrotoxicosis is a group of signs and symptoms induced by thyroid hormone's improper activity in the tissue [1].
Thyrotoxicosis is a clinical syndrome produced by a multitude of disorders that causes an excess in thyroid hormone in the tissue or in the thyroid gland [2]. It is a serious clinical problem that can be caused by a variety of factors, including toxic multinodular goiter, toxic adenoma, gestational trophoblastic disease, drug-induced hyperthyroidism, grave disease, genetic, environmental, and endogenous factors may all play a role in the development of thyrotoxicosis [3].
Hyperthyroidism is a prevalent clinical condition that raises the risk of complications and necessitates of surgery with a cumulative incidence of 0.2–1.3% in iodine-rich areas, the prevalence may rise in iodine-poor areas, in addition to this a total frequency of 1.3% was found in a nationwide survey in the United States, while an average prevalence of 0.75% was found in a European study [4]. In Africa, the prevalence of endemic goiter ranges from 1% to 90%, while the incidence of hyperthyroidism ranges from 13% to 43.7%, when come to Ethiopia the prevalence of endemic goiter was 39.9%, with the most prevalent is thyrotoxicosis at 43.7% [5]. A study conducted at the University of Gondar compressive specialized hospital screened patients showed that 14.6% develop hyperthyroidism [6].
If left untreated, thyrotoxicosis can produce a variety of symptoms such as tachycardia, tremor, palmar sweating, eye problems, irritability, altered behavior, hot intolerance, fatigability, palpitation, increased appetite, and weight loss [7]. It also increase the complication rate including atrial fibrillation, ventricular dysfunction, heart failure and fasten morbidity and mortality of the patient [8]. Thyrotoxicosis may also end up with severe form called thyroid storm in uncontrolled patient and resulting impatient mortality, increased hospital stay, ventilation requirement, cardiovascular as well central nervous system complication [9]. Patients with thyrotoxicosis may require surgery and anesthesia, and they face additional risks and complications in the perioperative periods as a result of the increased thyroid hormone, which affects every body system which indicated that patients with suspected thyrotoxicosis require adequate perioperative preparation to mitigate the adverse effects and improve the patient's outcome [10].
2. Rationale
Thyrotoxicosis is a common finding during a preoperative assessment. Preoperative optimization of thyrotoxicosis patients is crucial for overcoming the difficulties/problems faced during perioperative period, and there is also unnecessary patient postponement, which has a direct influence on the patients' and their parents' economic, social, and psychological load. In addition, inadequate thyrotoxicosis perioperative optimization will result in unwanted complications, greater hospitalization, and a negative impact on patients, parents, and healthcare delivery. There is still debate about perioperative optimization of thyrotoxicosis patients undergoing surgery and anesthesia, so this review of recent evidences give additional input for perioperative management of thyrotoxicosis patients to achieve a uniform level of care for uncontrolled thyrotoxicosis patients.
3. Methods
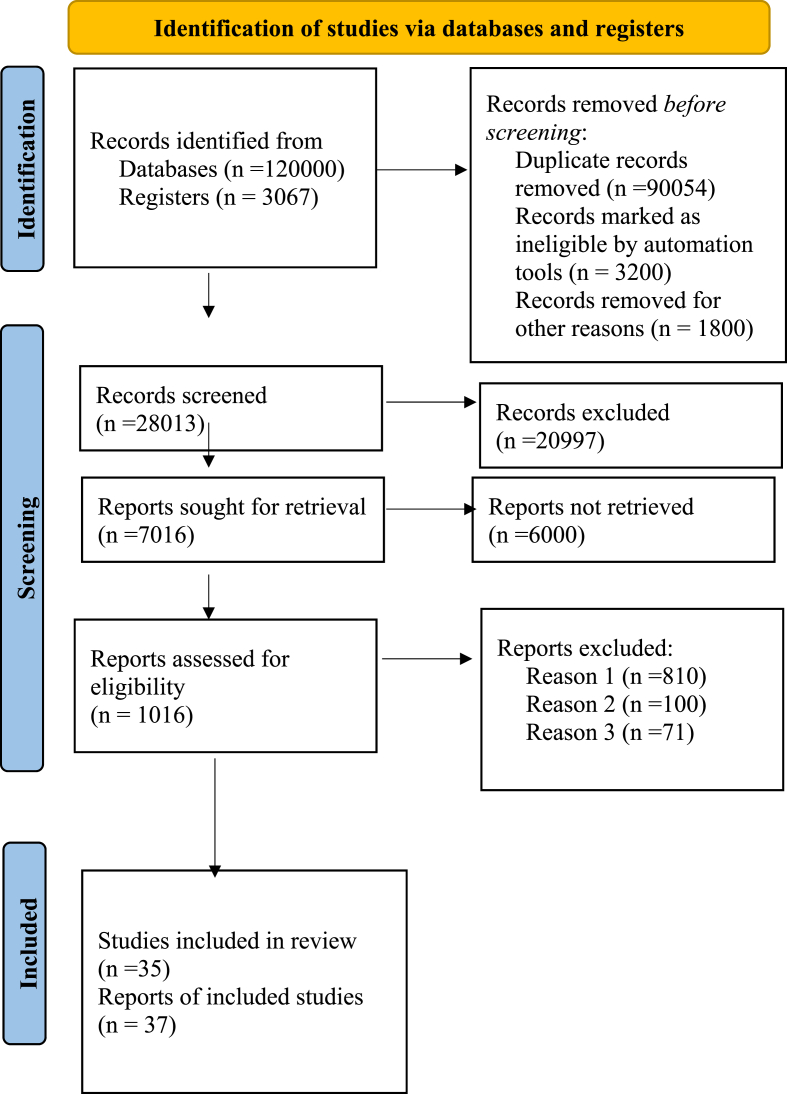
The literatures were found with Boolean operators in the form of thyrotoxicosis AND anesthesia, antithyroid medications AND perioperative optimization AND beta blockers OR calcium channel blockers in electronic data base sources such as the Cochrane library, PubMed, and Google scholar. The literatures were extracted using preferred reporting item for systematic review and meta-analysis (PRISMA) format (Fig. 1). The literatures comprised thyrotoxicosis patients in English version from the last ten years were included in this document. Duplication was deleted from all of the materials and entered into the endnote version ×8 software and finally 35 pieces of literatures were incorporated into the final output. Following a thorough literature review, the following recommendations were derived from the degree and quality of evidence level using good clinical practice (GCP), world health organization (WHO), 2011 with 1a-meta-analysis, evidence-based guideline, and systematic review of randomized controlled trials (RCTs), 1 b-randomized controlled trial (RCT), 2a-systematic review of cohort and case control studies and 3a-case reports and case series (Table 1). The evidence-based summary was created by examining the risk and benefit, cost, and available resources for thyrotoxicosis management and optimization. This review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [11]. This paper also registered in research registry with identifying number reviewregistry1408 with a link: https://www.researchregistry.com/browse-the-registry#registryofsystematicreviewsmeta-analyses/
Fig. 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA-2020).
Table 1.
Level of evidences and recommendations.
| Level | Types of evidence | Degree of recommendation |
|---|---|---|
| 1a | Meta-analysis, evidence-based guideline, systematic review of RCT | Strongly recommended, directly applicable |
| 1b | Systematic review of one RCT | Strongly recommended and applicable |
| 1c | Randomized clinical trail | Recommended, applicable |
| 2a | Systematic review of case control, cohort and retrospective and prospective cohort study | Extrapolated evidence from other study |
| 3a | Case series, case report | Extrapolated evidence from other study |
Source: Good Clinical Practice, GCP, WHO, 2011
We evaluated our systematic review compliance by using AMSTAR 2 criteria and fell in moderate quality [12].
4. Areas of controversies
There are numerous debates over how to manage and optimize thyrotoxicosis patients. Thyrotoxicosis is caused by a combination of factors, from which an RCT found that continuing antithyroid drug (ATD) for 60–120 months is more effective than 12–18 months [12]. Another systematic review and meta-analysis of the effects of long-term ATD treatment (more than 24 months) found that it is effective and safe to use ATD for such a long time [13]. According to a nationwide survey conducted in Italy in 2014 on the length of ATD, the majority of patients in clinical practice are taking the medicine for 12–24 months to achieve euthyroid status prior to surgery [13].
According to a 2018 European thyroid association guideline on ATD for adult patients is a therapeutic option, if relapse occurs even after ATD is completed, revising the treatment regimen or changing the type of drug is a good option, and if the above treatment modality is not working, definitive therapy is the mainstay of treatment of choice [14].
The length of ATD is determined by the patient's condition and the normalization of thyroid function tests, according to a recommendation published by the American thyroid association in 2016 the drug should be continued for 12–18 months, but if the patient is pregnant, the drug should be stopped and replaced with propylthiouracil (PTU), if the patient has a recurrence, ATD must be continued for another 18 months [15]. A randomized control trial on the effect of amiodarone on amiodarone-induced thyrotoxicosis found that amiodarone should be stopped to make the patient euthyroid due to the increased severity and time it takes to become euthyroid [16].
The preferred treatment option for amiodarone caused thyrotoxicosis is glucocorticoid, according to a European thyroid association recommendation published in 2018. Based on a risk-benefit analysis and the severity of cardiac dysfunction in this patient, the decision to discontinue amiodarone was made [17].
5. Discussion
A study done on preoperative evaluation in patients with subclinical hyperthyroidism, and the patient has signs and symptoms of thyrotoxicosis, a full preoperative evaluation of each organ system is required, including laboratory and imaging tests such as thyroid function tests, complete blood counts, and ECG (2a),[15].
Frans Brandt and colleagues conducted a review and meta-analysis of case control and cohort studies in Denmark on the association between overt hyperthyroidism and mortality which found that there is a devastating complication of thyrotoxicosis, which increases the patient's mortality rate by 20% when compared to euthyroid patients (1a) [16].
Yang LB and colleagues conducted a meta-analysis of cohort studies to determine whether subclinical hyperthyroidism increases the risk of cardiovascular complications, mortality, and morbidity found that patients with subclinical hyperthyroidism have a 19% risk of cardiovascular disease, 52% risk of cardiovascular mortality, and 25% risk of cardiovascular morbidity (1a) [18].
A systematic review and meta-analysis on the length of ATD found that extended administration of ATD for more than 24 months is related with a lower rate of relapse and complication as compared with shorter duration of taking ATD [19] (1a).
A retrospective cohort study conducted on thyrotoxicosis patients at University of Gondar Comprehensive and Specialized Hospital found that patients receiving ATD for a short period of time do not fully return to normal, thyroid function test (TFT), but as the duration of ATD use increases TFT fully normalizes, due to this, patients who take ATD for more than one year had better TFT normalization than those who take it for less than one year [20] (2a).
Jackie Gilbert conducted a review on the optimization of high-risk patients, including increasing age, male sex, and underlying cardiovascular illness which found that atrial fibrillation is a significant risk factor for increased mortality and in order to avoid a crisis medications such as propranolol, atenolol, and calcium channel blockers such as Diltiazem and verapamil are used as an alternative if beta blockers are not tolerated (2a) [21].
A review done by Carina P. Himes' on the optimization of thyrotoxicosis patients, If the surgery is elective, it should be optimized until euthyroid, but if it is an emergency, intravenous beta blockers, corticosteroids, oral ATD, and antihypertensive medication should be on hand (2a) [22].
A review done by Maguy Chiha and colleagues in the United States found that around 10% of thyroid storm deaths were documented and this is strongly linked to uncontrolled hyperthyroidism that requires surgery and to reduce the mortality and morbidity early diagnosis and early treatment before surgery is necessary (2a) [23].
A study done in Denmark on the comparison of treated and untreated hyperthyroidism patient states that well controlled hyperthyroidism has a significant reduction of mortality when compared with untreated patients (2a) [24].
Rodolfo J Galindo and colleagues found that patients who develop thyroid storm have a 12-fold increased mortality when compared to thyrotoxicosis without thyroid storm, this shows that patients with thyrotoxicosis need to be treated to avoid life-threatening complications (2a) [25].
Claire L Wood et al. conducted on drug dose titration and block and replace on thionamide for patients identified and treated for thyrotoxicosis found that there is no difference in biochemical stability between block and replace and dose titration (1c) [26].
Eskes SA et al. conducted a multicenter RCT on the continuation of amiodarone in amiodarone-induced thyrotoxicosis and the treatment modality utilized, which was divided into three groups: prednisone, sodium perchlorate, and perchlorate. Finally, the results suggest that euthyrodism can be achieved even if amiodarone is continued in patients with thyrotoxicosis caused by amiodarone, and that prednisone, a regularly used medicine, is preferred over the other two medications (1c) [27].
A study on the continuation of amiodarone in amiodarone induced thyrotoxicosis type two patients treated with prednisone for cardiovascular system abnormalities, there is recurrence and severity of thyrotoxicosis due to amiodarone continuation, so it conclude that treatment with prednisone for amiodarone induced thyrotoxicosis patient and continuing amiodarone in this patient, it delay normalization period of thyrotoxicosis (1c) [16].
A randomized controlled trial conducted by Tetsuya Tagami et al. on the effect of beta blockers and antithyroid drugs in new onset thyrotoxicosis for grave disease randomized on 28 patients adding beta blockers to ATD has no effect on thyroid function reduction but it does stabilize sympathetic hyperactivity (1c) [28].
Another study using beta blockers to manage thyrotoxicosis patients undergoing non-thyroid and thyroid surgery has a significant effect in reducing sympathetic activity in the cardiac system, particularly in the preoperative period when ATD is not being used (2a) [29].
A review on emergency thyroid storm management, a combination of treatment approaches is required to reduce patient morbidity and mortality including supportive care, antithyroid medication, adrenergic blocker, corticosteroid, paracetamol, and treatment of the cause is the mainstay of thyroid crisis management (2a) [30].
Marcia Rashelle Palace study on perioperative optimization of thyrotoxicosis patients, who are scheduled for surgery should be preoperatively prepared for euthyroid to reduce thyrotoxic crisis, including thyroid storm and cardiac problems and thyrotoxic patients were given ATD, such as PTU 100–150 mg every 6–8 h, propranolol 10–40 mg, and calcium channel blockers as an alternative. Both ATD and beta blockers were continued postoperatively, but ATD was discontinued following thyroidectomy, in addition to this Lugols solution or potassium iodide, beta blocker, and glucocorticoid are the drugs of choice if the surgery is emergency to make the patient hemodynamically stable (2a) [31].
A study done on thyroid storm management states that giving ATD like PTU 600 mg loading and 200–300 mg maintenance every 6hr and propranolol 40–80 mg every 4hr and hydrocortisone 100 mg every 8hr and as well as hemodynamic support [32].
A study states that thyrotoxicosis causes catastrophic cardiac complications that are difficult to manage, such as atrial fibrillation and heart failure, so preoperative optimization of thyrotoxic patients is required to reduce this problem (3a) [33].
A study done on anesthesia management of thyrotoxicosis patient come for surgery incorporated under consideration including reduction of stress response condition, reducing sympatitic hyper activity drugs, making anesthesia deep, using standard monitoring if available invasive monitoring and reserve intensive care bed (3a) [34].
A substantial cardiac consequence involving shortness of breath, abrupt heart failure, dilated cardiomyopathy, atrial fibrillation, and cardiomegaly was reported with uncontrolled toxic goiter without a previous history of cardiac problems (3a) [35].
A review on the optimization of thyrotoxic patients undergoing surgery on the day before surgery, patient must be euthyroid in order to lower the risk of thyrotoxicosis complications by using antithyroid medicine, beta blockers, radioiodine, and potassium iodide (2a) [36].
A case report and literature review on the anesthesia implications of severe hyperthyroidism secondary to molar pregnancy found that preoperative optimization of uncontrolled thyrotoxicosis patients secondary to molar pregnancy is necessary, and regional anesthesia is the most important anesthesia technique used to overcome this complication (3a) [37]. The results of the reviewed literatures were summarized below (Table 2).
Table 2.
The results of the reviewed literatures.
| Authors | Year | Title | Study design | Intervention | Outcome | recommendation | Level of evidence |
|---|---|---|---|---|---|---|---|
| Ross DS et al. | 2016 | Diagnosis and management of hyperthyroidism and other cause of thyrotoxicosis | Evidence based guideline | Use of ATD, beta blocker | Diagnosis and management of thyrotoxicosis | Strongly recommended and directly applicable | 1a |
| Maia AL et al. | 2013 | Diagnosis and treatment of hyperthyroidism | evidence guideline | Use of ATD, beta blocker | Diagnosis and treatment of hyperthyroidism | Strongly recommended and directly applicable | 1a |
| Brandt F | 2011 | Association between overt hyperthyroidism and mortality | Review and meta-analysis | – | Increase mortality rate | Strongly recommended and directly applicable | 1a |
| Yang LB et al. | 2012 | Subclinical hyperthyroidism and the risk of cardiovascular events and all-cause mortality | Meta-analysis of cohort study | – | Mortality rate | Extrapolated evidence from other study | 1a |
| Wood CL et al. | 2020 | Randomized trial of block and replace vs dose titration thionamide | RCT | Comparison | Effect of dose titration over block replace | Recommended and applicable | 1c |
| Tagami et al. | 2012 | Short-term effects of β-adrenergic antagonists and methimazole in new-onset thyrotoxicosis caused by Graves' disease | RCT | Comparison of beta blocker and metamizole | No effect on combination treatment for thyrotoxicosis but stable cardiovascular system | Strongly recommender and applicable | 1c |
| Sadiq AM et al. | 2021 | Challenges in the Management of Thyrotoxicosis Associated with Atrial Fibrillation and Heart Failure | Case report | Propranolol, digoxin, Carbimazole, and furosemide | Management of cardiac problem due to thyrotoxicosis | Weakly recommender | 3a |
| Al Jassim A | 2018 | do patients with graves' disease need to be euthyroid prior to surgery | Cohort | Beta blocker, PTU, potassium iodide, and steroid | Development of thyroid storm | Extrapolate evidence from other study | 2a |
| Marcia Rashelle Palace | 2017 | Perioperative optimization of thyrotoxicosis patient | Review | Beta blocker, ATD, | Continuation of beta blocker postoperatively and discontinuation of ATD | Extrapolated evidence from other study | 2a |
| Azizi F et al. | 2017 | Long term ATD treatment | Systematic Review and meta-analysis | ATD | Duration of ATD | Strongly recommender& directly applicable | 1a |
| Eyob Alemayehu Gebreyohans et al. | 2019 | Normalization of TFT in thyrotoxicosis patient | Retrospective cohort | PTU, atenolol | Normalization of TFT | Extrapolate evidence from other study | 2a |
ATD; Antithyroid Drug, PTU; propylthiouracil, TFT: Thyroid function test.
6. Conclusions and recommendations
Thyrotoxicosis is a serious medical condition that, if left untreated, can lead to a fatal illness. Before surgery and anesthesia, manifestation of including palpitation, irritability, lack of concentration, hot intolerance, weight loss, increased appetite, and signs such as tachycardia, atrial fibrillation, palmar sweating, ataxia tremor, pulmonary embolism, stroke, and hypertension must all be ruled out. In addition to the history and physical examination, a patient-centered laboratory and imaging modality, such as a whole blood count, thyroid function tests (TSH, T3 and T4), ECG and x-ray, and organ function tests, are required, depending on the patient's condition (age, comorbidity).
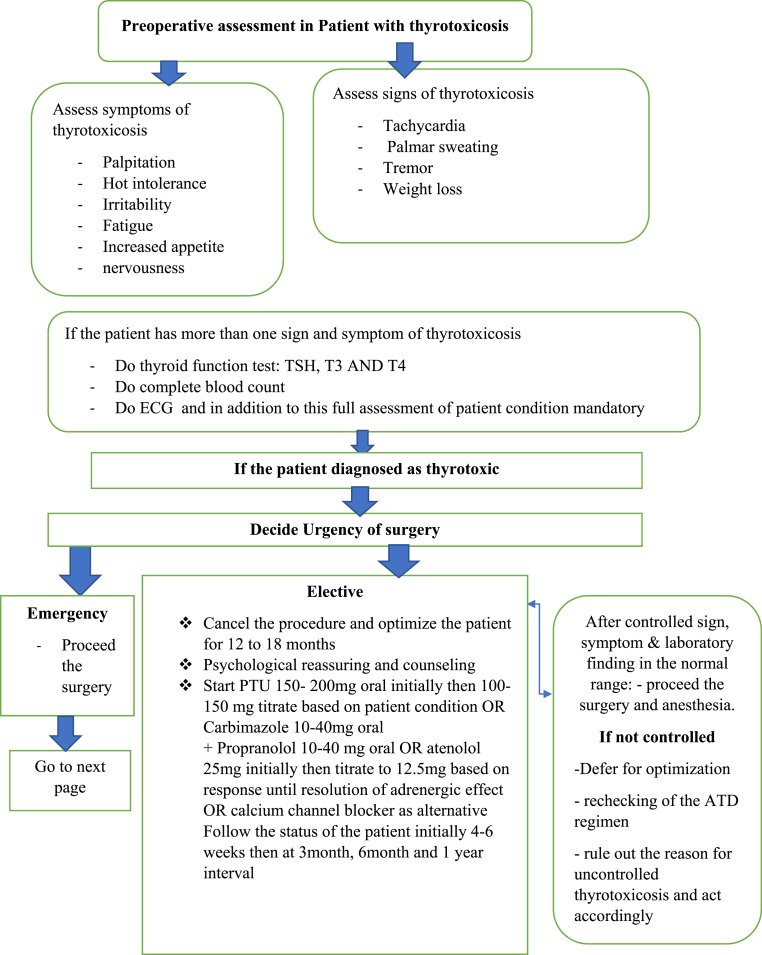
Surgery and anesthesia should be postponed in elective thyrotoxicosis patients, and the patient should be optimized with PTU 100–200 mg for 12–18 months, propranolol 10–40 mg until sympathetic activity stabilizes, Carbimazole 10–40 mg, and potassium iodide for 10 days, so thyroid function tests must be repeated every 4–6 weeks, followed by three-month and six-month intervals; if toxic manifestations are reduced, ATD must be titrated to reduce drug side effects, in addition to this patient counseling, psychological reassurance, and follow-up are required to achieve good thyrotoxicosis control. If the situation is an emergency, adequate preparation for a thyrotoxicosis crisis is required. Premedicate with propranolol 0.1–0.15 mg/kg iv, PTU 200–400 mg oral, diazepam 5–10 mg, and prepare hydralazine, Lidocaine, corticosteroid such as hydrocortisone 100 mg or dexamethasone 2 mg every 4–6hr for 72 h.
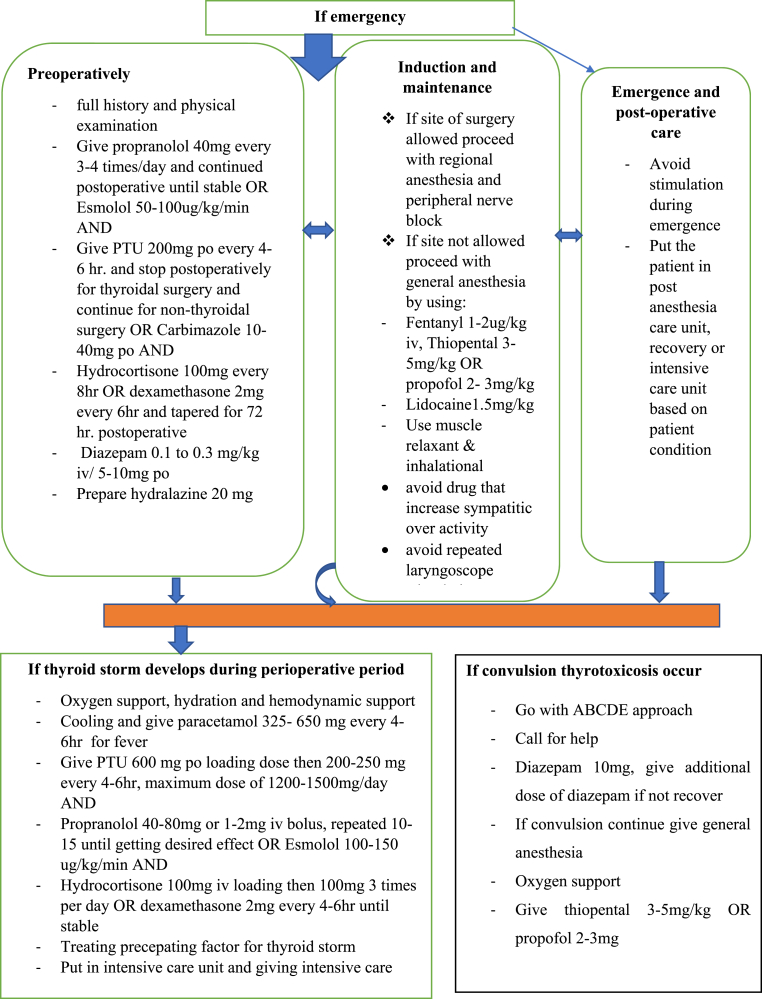
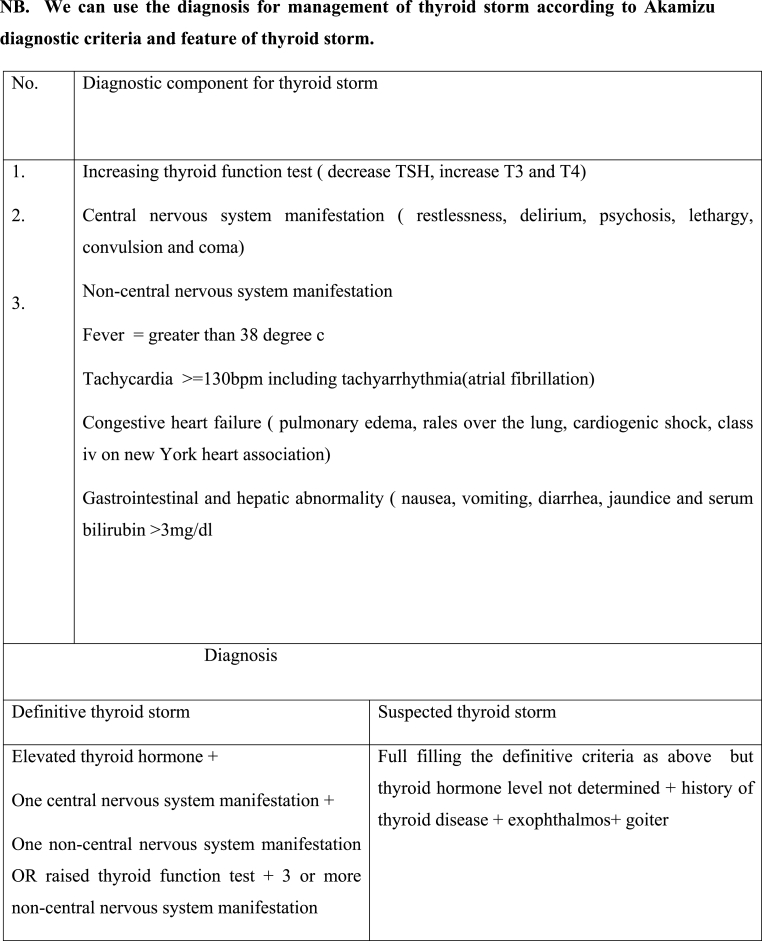
If the type of surgery allows it, regional anesthesia and peripheral nerve block are preferable to general anesthesia. If not, proceed with general anesthesia at a deep and smooth level using fentanyl 1-2μg/kg, thiopental 3–5 mg/kg, or propofol 2–3 mg/kg, Lidocaine 1.5 mg/kg, halothane, and muscle relaxant with suxamethonium and vecuronium. Avoid drugs or using with caution that has symptomimic effect. If a thyrotoxicosis crisis such as a thyroid storm occurs manage it accordingly. First, determine whether or not there is thyrotoxicosis by using the Akamizu diagnostic criteria for thyroid storm, then follow the ABCDE approach, call for help, give paracetamol 325–650 mg every 6hr, hydrocortisone 2–4 mg/kg, propranolol 0.15 mg/kg or 40–80 mg, PTU 400–600 mg, cooling and hydration is required, and if convulsion thyrotoxicosis occurs, a good approach is to use diazepam 10 mg, ventilation, oxygenation, and mechanical support in the intensive care unit. If you experience a cardiac arrhythmia, follow the cardiac life support guidelines and antiarrhythmic management protocol.
Patients with thyrotoxicosis symptoms and elevated thyroid function tests should be optimized for 12–18 months, or until the patient is in a euthyroid state. Consider the patient's overall condition, as well as risk-benefit analysis, when performing perioperative optimization (Fig. 2).
Fig. 2.
Flow diagram of perioperative management of thyrotoxic patients.
Ethical approval
Not required.
Sources of funding
Not funded
Author contribution
This work was carried out in collaboration among all authors. Misganew Terefe and Debas Yaregal Melesse contributed to the conception of the review and interpreted the literatures based on the level of evidence and revised the manuscript. Yosef Belay Bizuneh and Yonas Addisu Nigatu participate in reviewing preparation of the manuscript. Both authors participate in preparation and critical review of the manuscripts. In addition, all authors read and approved the manuscript.
Registration of research studies
1 Name of the registry: reviewregistry
2 Unique Identifying number or registration ID: reviewregistry1408.
3 Hyperlink to your specific registration (must be publicly accessible and will be checked):https://www.researchregistry.com/browse-the-registry#registryofsystematicreviewsmeta-analyses/
Guarantor
Misganew Terefe, Debas Yaregal Melesse (D.Y. Melesse), Yosef Belay Bizuneh (Y. B. Bizuneh), Yonas Addisu Nigatu.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
No conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104487.
Contributor Information
Misganew Terefe, Email: misganewte@gmail.com.
Yosef Belay Bizuneh, Email: phanuelyosef@gmail.com.
Yonas Addisu Nigatu, Email: yonasaddisu71@gmail.com.
Debas Yaregal Melesse, Email: dabyyaregal82@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sharma A., Stan M.N. Thyrotoxicosis: diagnosis and management. Mayo Clin. Proc. 2019;94(6):1048–1064. doi: 10.1016/j.mayocp.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Ross D.S., Burch H.B., Cooper D.S., Greenlee M.C., Laurberg P., Maia A.L., et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 3.Łacka K., Fraczek M.M. [Classification and etiology of hyperthyroidism]. Polski merkuriusz lekarski : organ Polskiego. Tow. Lekarskiego. 2014;36(213):206–211. [PubMed] [Google Scholar]
- 4.Taylor P.N., Albrecht D., Scholz A., Gutierrez-Buey G., Lazarus J.H., Dayan C.M., et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018;14(5):301–316. doi: 10.1038/nrendo.2018.18. [DOI] [PubMed] [Google Scholar]
- 5.Ogbera A.O., Kuku S.F. Epidemiology of thyroid diseases in Africa. Indian J. Endocrinol. Metabol. 2011;15(Suppl 2):S82–S88. doi: 10.4103/2230-8210.83331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asmelash D., Tesfa K., Biadgo B. Thyroid dysfunction and cytological patterns among patients requested for thyroid function test in an endemic goiter area of gondar, North west Ethiopia. Int. J. Endocrinol. 2019;2019 doi: 10.1155/2019/9106767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maia A.L., Scheffel R.S., Meyer E.L.S., Mazeto G.M., Carvalho GAd, Graf H., et al. The Brazilian consensus for the diagnosis and treatment of hyperthyroidism: recommendations by the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism. Arquivos Brasileiros Endocrinol. Metabol. 2013;57(3):205–232. doi: 10.1590/s0004-27302013000300006. [DOI] [PubMed] [Google Scholar]
- 8.Ertek S., Cicero A.F. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch. Med. Sci. 2013;9(5):944–952. doi: 10.5114/aoms.2013.38685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angell T.E., Lechner M.G., Nguyen C.T., Salvato V.L., Nicoloff J.T., LoPresti J.S. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J. Clin. Endocrinol. Metabol. 2015;100(2):451–459. doi: 10.1210/jc.2014-2850. [DOI] [PubMed] [Google Scholar]
- 10.Palace M.R. Perioperative management of thyroid dysfunction. Health Serv. Insights. 2017;10 doi: 10.1177/1178632916689677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 12.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed) 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartalena L., Burch H., Burman K., Kahaly G.A. 2013 European survey of clinical practice patterns in the management of Graves' disease. Clin. Endocrinol. 2016;84(1):115–120. doi: 10.1111/cen.12688. [DOI] [PubMed] [Google Scholar]
- 14.Kahaly G.J., Bartalena L., Hegedüs L., Leenhardt L., Poppe K., Pearce S.H. 2018 European thyroid association guideline for the management of graves' hyperthyroidism. Eur. Thyroid J. 2018;7(4):167–186. doi: 10.1159/000490384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross D.S., Burch H.B., Cooper D.S., Greenlee M.C., Laurberg P., Maia A.L., et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 16.Bogazzi F., Bartalena L., Tomisti L., Rossi G., Brogioni S., Martino E. Continuation of amiodarone delays restoration of euthyroidism in patients with type 2 amiodarone-induced thyrotoxicosis treated with prednisone: a pilot study. J. Clin. Endocrinol. Metabol. 2011;96(11):3374–3380. doi: 10.1210/jc.2011-1678. [DOI] [PubMed] [Google Scholar]
- 17.Bartalena L., Bogazzi F., Chiovato L., Hubalewska-Dydejczyk A., Links T.P., Vanderpump M. 2018 European thyroid association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. Eur. Thyroid J. 2018;7(2):55–66. doi: 10.1159/000486957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L.B., Jiang D.Q., Qi W.B., Zhang T., Feng Y.L., Gao L., et al. Subclinical hyperthyroidism and the risk of cardiovascular events and all-cause mortality: an updated meta-analysis of cohort studies. Eur. J. Endocrinol. 2012;167(1):75–84. doi: 10.1530/EJE-12-0015. [DOI] [PubMed] [Google Scholar]
- 19.Azizi F., Malboosbaf R. Long-term antithyroid drug treatment: a systematic review and meta-analysis. Thyroid. 2017;27(10):1223–1231. doi: 10.1089/thy.2016.0652. [DOI] [PubMed] [Google Scholar]
- 20.Gebreyohannes E.A., Ayele E.M., Tesfaye S.A., Seid M.A. Normalization of thyroid function tests among thyrotoxicosis patients attending a University Hospital in North-West Ethiopia. Thyroid Res. 2019;12(1):1–8. doi: 10.1186/s13044-019-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert J. Thyrotoxicosis - investigation and management Clinical medicine (London, England) 2017;17(3):274–277. doi: 10.7861/clinmedicine.17-3-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himes C.P., Ganesh R., Wight E.C., Simha V., Liebow M., editors. Perioperative Evaluation and Management of Endocrine Disorders. Mayo Clinic proceedings; 2020. Elsevier. [DOI] [PubMed] [Google Scholar]
- 23.Chiha M., Samarasinghe S., Kabaker A.S. Thyroid storm: an updated review. J. Intensive Care Med. 2015;30(3):131–140. doi: 10.1177/0885066613498053. [DOI] [PubMed] [Google Scholar]
- 24.Lillevang-Johansen M., Abrahamsen B., Jørgensen H.L., Brix T.H., Hegedüs L. Excess mortality in treated and untreated hyperthyroidism is related to cumulative periods of low serum TSH. J. Clin. Endocrinol. Metabol. 2017;102(7):2301–2309. doi: 10.1210/jc.2017-00166. [DOI] [PubMed] [Google Scholar]
- 25.Galindo R.J., Hurtado C.R., Pasquel F.J., García Tome R., Peng L., Umpierrez G.E. National trends in incidence, mortality, and clinical outcomes of patients hospitalized for thyrotoxicosis with and without thyroid storm in the United States, 2004-2013. Thyroid. 2019;29(1):36–43. doi: 10.1089/thy.2018.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood C.L., Cole M., Donaldson M., Dunger D.B., Wood R., Morrison N., et al. Randomised trial of block and replace vs dose titration thionamide in young people with thyrotoxicosis. Eur. J. Endocrinol. 2020;183(6):637–645. doi: 10.1530/EJE-20-0617. [DOI] [PubMed] [Google Scholar]
- 27.Eskes S.A., Endert E., Fliers E., Geskus R.B., Dullaart R.P., Links T.P., et al. Treatment of amiodarone-induced thyrotoxicosis type 2: a randomized clinical trial. J. Clin. Endocrinol. Metabol. 2012;97(2):499–506. doi: 10.1210/jc.2011-2390. [DOI] [PubMed] [Google Scholar]
- 28.Tagami T., Yambe Y., Tanaka T., Tanaka T., Ogo A., Yoshizumi H., et al. Short-term effects of β-adrenergic antagonists and methimazole in new-onset thyrotoxicosis caused by Graves' disease. Intern. Med. (Tokyo) 2012;51(17):2285–2290. doi: 10.2169/internalmedicine.51.7302. [DOI] [PubMed] [Google Scholar]
- 29.Tay S., Khoo E., Tancharoen C., Lee I. Beta-blockers and the thyrotoxic patient for thyroid and non-thyroid surgery: a clinical review. OA Anaesth. 2013;1(1):1–6. [Google Scholar]
- 30.Idrose A.M. Acute and emergency care for thyrotoxicosis and thyroid storm. Acute Med Surg. 2015;2(3):147–157. doi: 10.1002/ams2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palace M.R. Perioperative management of thyroid dysfunction. Health Serv. Insights. 2017;10 doi: 10.1177/1178632916689677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hampton J. Thyroid gland disorder emergencies: thyroid storm and myxedema coma. AACN Adv. Crit. Care. 2013;24(3):325–332. doi: 10.1097/NCI.0b013e31829bb8c3. [DOI] [PubMed] [Google Scholar]
- 33.Sadiq A.M., Chamba N.G. Challenges in the management of thyrotoxicosis associated with atrial fibrillation and heart failure: two case reports. Clin. Med. Insights Case Rep. 2021;14 doi: 10.1177/1179547621994573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reber A., Valenti L., Müller S. A patient with graves' disease scheduled for thyroidectomy with high risk for thyroid storm caused by severe medication nonadherence: anaesthetic and surgical considerations. Case Reports in Anesthesiology. 2019;2019 doi: 10.1155/2019/4781902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witczak J.K., Ubaysekara N., Ravindran R., Rice S., Yousef Z., Premawardhana L.D. Significant cardiac disease complicating Graves' disease in previously healthy young adults. Endocrinol. Diabetes Metabol. Case Rep. 2020:2020. doi: 10.1530/EDM-19-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piantanida E. Preoperative management in patients with Graves' disease. Gland Surg. 2017;6(5):476–481. doi: 10.21037/gs.2017.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swaminathan S., James R.A., Chandran R., Joshi R. Anaesthetic implications of severe hyperthyroidism secondary to molar pregnancy: a case report and review of literature. Anesth. Essays Res. 2017;11(4):1115–1117. doi: 10.4103/aer.AER_38_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.