Abstract
Background
A simplified Canadian definition was recently developed to enable identification of individuals with familial hypercholesterolemia (FH) and severe hypercholesterolemia in the general population. Our objective was to use a modified version of this new definition to assess contemporary disease prevalence, treatment patterns, and low-density lipoprotein cholesterol (LDL-C) control in Ontario, Canada.
Methods
We identified individuals aged 66 to 105 years who were alive as of January 1, 2011, using the Cardiovascular Health in Ambulatory Care Research Team (CANHEART) database, which was created by linking 19 population-based health databases in Ontario. Hypercholesterolemia was identified using LDL-C values. Cholesterol reduction and lipid-lowering treatment were assessed at time of diagnosis and after at least 2 and 5 years’ follow-up.
Results
Among 922,464 individuals, 2440 (0.26%) met criteria for definite or probable FH, and 72,893 (7.90%) for severe hypercholesterolemia. At diagnosis, mean LDL-C concentration was 9.52 mmol/L for those with definite FH, 5.83 mmol/L for those with probable FH, 5.73 mmol/L for those with severe hypercholesterolemia, and 3.33 mmol/L for all other individuals. After > 5 years, LDL-C concentration remained elevated at 3.58 mmol/L for those with definite FH, 2.72 mmol/L for those with probable FH, and 2.93 mmol/L for those with severe hypercholesteremia. Use of statin therapy was initially high (83% of those with definite FH, 78% of those with probable FH, 62% of those with severe hypercholesterolemia); however, fewer patients remained on statins at follow-up at > 5 years (62% of those with definite FH, 67% of those with probable FH, 58% of those with severe hypercholesterolemia).
Conclusions
Among older Ontarians, we estimated that 1 in 378 individuals had FH, and 1 in 13 had severe hypercholesterolemia. Despite being at substantially increased cardiovascular risk, these patients acheived suboptimal LDL-C level control and fewer were on medical therapy at follow-up.
Résumé
Introduction
Une définition canadienne simplifiée a récemment été élaborée pour permettre la détection des personnes atteintes d’hypercholestérolémie familiale (HF) et d'hypercholestérolémie grave au sein de la population générale. Notre objectif était d’utiliser la version modifiée de cette nouvelle définition pour évaluer la prévalence contemporaine de la maladie, les schémas de traitement et la maîtrise du cholestérol à lipoprotéines de faible densité (cholestérol LDL) en Ontario, au Canada.
Méthodes
Nous avons recensé les individus âgés de 66 à 105 ans qui étaient en vie au 1er janvier 2011 à partir de la base de données Cardiovascular Health in Ambulatory Care Research Team (CANHEART), qui a été créée par la liaison de 19 bases de données populationnelles de l’Ontario. L’hypercholestérolémie a été définie par les valeurs du cholestérol LDL. Nous avons évalué la diminution du cholestérol et le traitement hypolipémiant au moment du diagnostic et après au moins les suivis après 2 ans et après 5 ans.
Résultats
Parmi les 922 464 personnes, 2 440 (0,26 %) répondaient aux critères de diagnostic définitif ou de diagnostic probable d’HF, et 72 893 (7,90 %), aux critères d’hypocholestérolémie grave. Au diagnostic, les concentrations moyennes de cholestérol LDL étaient de 9,52 mmol/l chez ceux qui avaient un diagnostic définitif d’HF, de 5,83 mmol/l chez ceux qui avaient un diagnostic probable d’HF, de 5,73 mmol/l chez ceux qui avaient un diagnostic d’hypercholestérolémie grave et de 3,33 mmol/l chez toutes les autres personnes. Après > 5 ans, les concentrations de cholestérol LDL étaient demeurées élevées : 3,58 mmol/l chez ceux qui avaient un diagnostic définitif d’HF, 2,72 mmol/l chez ceux qui avaient un diagnostic probable d’HF et 2,93 mmol/l chez ceux qui avaient un diagnostic d’hypercholestérolémie grave. L’utilisation du traitement par statines était initialement élevée (83 % de ceux qui avaient un diagnostic définitif d’HF, 78 % de ceux qui avaient un diagnostic probable d’HF, 62 % de ceux qui avaient un diagnostic d’hypercholestérolémie grave). Toutefois, moins de patients avaient conservé les statines au suivi > 5 ans (62 % de ceux qui avaient un diagnostic définitif d’HF, 67 % de ceux qui avaient un diagnostic probable d’HF, 58 % de ceux qui avaient un diagnostic d’hypercholestérolémie grave).
Conclusions
Parmi les Ontariens âgés, nous avons estimé que 1 sur 378 personnes avaient une HF, et que 1 sur 13 avaient une hypercholestérolémie grave. Malgré le fait qu’ils sont exposés à un risque cardiovasculaire substantiellement élevé, ces patients ont atteint une maîtrise sous-optimale du taux de cholestérol LDL et moins d'entre eux étaient sous traitement médical au suivi.
Familial hypercholesterolemia (FH) is a common genetic disorder, predisposing affected individuals to lifelong elevations in low-density lipoprotein cholesterol (LDL-C) level and premature atherosclerotic cardiovascular disease (ASCVD).1 Yet, contemporary data suggest that the prevalence of FH is unknown in > 90% of the world’s population.2 Although national expert panels in several jurisdictions have recognized identification of these patients as an important priority,3, 4, 5 the complexity of frequently used diagnostic algorithms has made obtaining population estimates either challenging or impossible in many jurisdictions. Recently, a simplified Canadian definition was proposed that could be used to determine the population prevalence of FH and other forms of extreme cholesterol level elevation.6 The new criteria de-emphasize genetic testing and infrequent clinical manifestations (eg, corneal arcus, xanthelasma), and they were found to have excellent agreement when evaluated against the commonly used Simon Broome and Dutch Lipid Clinic Network (DLCN) criteria (Cohen’s κ = 0.969 and 0.966, respectively).6
In addition to early diagnosis, the clinical trajectories of patients with FH depend on adequate treatment and cholesterol control. Early initiation of statin (HMG CoA reductase inhibitor) therapies substantially reduces the risk of ASCVD and related mortality.7, 8, 9, 10 Left untreated, however, FH patients bear a greater than 90-fold increased risk of ASCVD mortality in young adulthood.11 Despite this risk, individuals remain suboptimally managed even in specialized centers, with nearly two-thirds of patients being untreated at the point of their initial assessments.12 Even fewer data have been collected regarding how patients with severe hypercholesterolemia are managed in routine clinical practice in the general population.
To address these gaps in knowledge, the first objective of our study was to estimate the prevalence of FH and severe hypercholesterolemia in the general population of Ontario, Canada. Secondary objectives of our study were to examine cholesterol levels after initial diagnosis and the use of statins and other lipid-lowering therapies in these patients.
Methods
Data sources
The Cardiovascular Health in Ambulatory Care Research Team (CANHEART) cohort was created by linking 19 population-based data sources in Ontario.13,14 Specific data sources essential to this study were the following: (i) the Ontario Laboratories Information System database and Dynacare Medical Laboratories database, which contain laboratory results from outpatient and hospital settings obtained since 2002; (ii) the Canadian Institute for Health Information Discharge Abstract Database, the Canadian Community Health Survey (CCHS), the Ontario Cancer Registry, the Ontario Hypertension Database, and the Ontario Diabetes Database, which contain information on cardiac risk factors and comorbidities; and (iii) the Ontario Drug Benefit Database, which contains data on prescription claims from individuals aged 65 and older. The use of the data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA) and does not require review by a research ethics board.
Study design
We identified Ontario residents with at least one LDL-C measurement taken between 2002 and 2010, who were aged 66 to 105 years at the time of their highest LDL-C measurement, and were alive and eligible for Ontario’s universal health insurance plan on January 1, 2011. We restricted our study population to this age range because we lacked information on statin and other lipid-lowering therapies in individuals aged < 66 years, as we therefore would not be able to estimate an untreated LDL-C level. For consistency with previous population-based studies, we excluded individuals with chronic kidney disease (ie, 2 consecutive measurements of estimated glomerular filtration rate < 60 mL/min per 1.73 m2 or albumin-creatinine ratio ≥ 3 mg/mmol over at least 90 days) given the potential for a falsely elevated LDL-C measurement.6
Defining FH and severe hypercholesterolemia
Some items used in the simplified Canadian definition (genetic testing, tendon xanthomas, and family histories of hypercholesterolemia or ASCVD) were not available in our data sources. Thus, our analyses used a modified version of the definition. A diagnosis of definite FH was based on a highest measured LDL-C level ≥ 8.5 mmol/L in individuals of all ages. Probable FH was diagnosed based on a history of premature ASCVD and an LDL-C level of 5.0-8.4 mmol/L. Premature ASCVD was defined as having documented hospitalization for myocardial infarction, stroke, peripheral arterial disease, percutaneous coronary intervention, coronary artery bypass grafting, or carotid endarterectomy at < 55 years of age for men and < 65 years for women.6 Severe hypercholesterolemia was diagnosed in those with an LDL-C level in the range 5.0-8.4 mmol/L and no premature ASCVD history. The general population in our study was defined as those not fulfilling any of the diagnostic criteria above. For individuals with a known statin prescription in the 100 days prior to their LDL-C test, we multiplied the LDL-C level by 1.43 to estimate the untreated LDL-C level. This method of adjusting the LDL-C levels corresponds to an approximate 30% reduction in LDL-C from baseline15 and is consistent with practice in several large-scale population-based reports of FH prevalence.16, 17, 18
Outcomes
We determined the prevalence and clinical characteristics of older Ontarians who met the criteria for FH and severe hypercholesterolemia.6 We assessed mean lipid concentrations in our study population at the index (diagnostic) cholesterol profile measurement. To evaluate cholesterol levels at follow-up, we also assessed lipid levels for those still alive after at least 2 years and 5 years. Finally, we examined the proportion of individuals filling prescriptions for statins (including high- and low-intensity) and other lipid-lowering therapies (fibrates, ezetimibe, bile-acid sequestrants, niacin) at index, 6 months after the index LDL-C measurement, and at the next LDL measurement at least 2 and 5 years later. The follow-up points of 2 and 5 years were chosen to examine shorter- and longer-term uptake, adherence, and impact of lipid-lowering therapies post-diagnosis, and to minimize the influence of cardiovascular events on individuals’ indications for lipid-lowering therapies.
Statistical analysis
Descriptive statistics were calculated for sociodemographic characteristics, plasma lipid concentrations, and cardiovascular risk factors of the cohort. Results for categorical variables were expressed as percentages and were compared using χ2 tests; continuous variables were reported as means and standard deviations (SDs), or medians (quartiles), and were compared using a 1-way analysis of variance. Two-tailed P values < 0.05 were considered significant. All datasets used were linked using unique encoded identifiers and analyzed at ICES (formerly the Institute for Clinical Evaluative Sciences) using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Study cohort
Of 9.4 million Ontario residents aged 20 to 105 years with a valid health card number who were alive on January 1, 2011, 4,769,444 without cholesterol measurements, 97,845 with chronic kidney disease, and 3,613,918 with a highest LDL-C level measured at age < 66 years were excluded, leaving 922,646 individuals who were included in the study.
Identification and prevalence of FH and severe hypercholesterolemia
We identified 2440 individuals with FH (968 definite; 1472 probable), corresponding to a prevalence of 0.26% (1 in 378; Table 1). An additional 72,893 individuals, or 7.90% (1 in 13), met the criteria for severe hypercholesterolemia. Mean ages were 72.4 years for the definite FH group, 70.1 years for probable FH, 72.8 years for severe hypercholesteremia, and 74.0 years for the general population; rates of women were 77.3% (definite FH), 76.2% (probable FH), 66.6% (severe hypercholesterolemia), and 53.4% (general population). Relative to the general population, individuals with FH and severe hypercholesterolemia had significantly higher burdens of hypertension, diabetes mellitus, coronary artery disease, current smoking, and obesity. Individuals with FH had the lowest rates of cancer.
Table 1.
Characteristics of patients aged ≥ 66 years with familial hypercholesterolemia (FH) and severe hypercholesterolemia in Ontario, Canada
| Characteristic | Definite FH |
Probable FH |
Severe hypercholesterolemia |
General population |
|---|---|---|---|---|
| n = 968 | n = 1472 | n = 72,893 | n = 847,313 | |
| Age, y | ||||
| Mean ± SD | 72.4 ± 5.4 | 70.1 ± 3.9 | 72.8 ± 5.6 | 74.0 ± 6.2 |
| Median (Q1–Q3) | 71 (68–75) | 69 (67–72) | 72 (68–76) | 73 (69–78) |
| Women | 748 (77.3) | 1121 (76.2) | 48,558 (66.6) | 452,481 (53.4) |
| Socioeconomic status | ||||
| Lowest income quintile | 192 (19.8) | 314 (21.3) | 13,366 (18.3) | 150,289 (17.7) |
| Highest income quintile | 175 (18.1) | 271 (18.4) | 14,884 (20.4) | 180,964 (21.4) |
| Cardiac risk factors | ||||
| Hypertension | 663 (68.5) | 1228 (83.4) | 49,351 (67.7) | 606,499 (71.6) |
| Diabetes mellitus | 233 (24.1) | 520 (35.3) | 15,448 (21.2) | 238,731 (28.2) |
| Premature ASCVD∗ | 30 (3.1) | 1472 (100.0) | 0 (0.00) | 18,491 (2.2) |
| Cancer | 109 (11.3) | 158 (10.7) | 8943 (12.3) | 123,261 (14.6) |
| Obesity, %† | 23.1 | 29.9 | 14.9 | 18.3 |
| Current smoker, %† | 16.6 | 6.2 | 8.0 | 8.5 |
| Lipid-lowering therapy use within 100 days of index cholesterol measurement | ||||
| Statins | 806 (83.3) | 1,141 (77.5) | 45,086 (61.9) | 366,984 (43.3) |
| High-intensity | 237 (24.5) | 319 (21.7) | 5088 (7.0) | 72,934 (8.6) |
| Low-intensity | 569 (58.8) | 822 (55.8) | 39,998 (54.9) | 294,050 (34.7) |
| Nonstatins | 102 (10.5) | 181 (12.3) | 2962 (4.1) | 42,489 (5.0) |
| Ezetimibe | 86 (8.9) | 135 (9.2) | 1998 (2.7) | 26,245 (3.1) |
| Other | 28 (2.9) | 65 (4.4) | 1157 (1.6) | 18,579 (2.2) |
Values are n (%), unless otherwise indicated.
ASCVD, atherosclerotic cardiovascular disease; Q1, quartile 1; Q3, quartile 3; SD, standard deviation.
Premature ASCVD defined as hospitalization for myocardial infarction, stroke, peripheral arterial disease, or prior percutaneous coronary intervention, coronary artery bypass grafting, or carotid endarterectomy at < 55 years of age in men and < 65 years in women.
Obesity and smoking status obtained from the Canadian Community Health Survey completed by 29,405 individuals in our study cohort. Estimates are weighted to be representative of the Ontario population.
By sex (Table 2), a lower prevalence was seen in men, compared with that in women, for definite FH (0.05% vs 0.15%), probable FH (0.08% vs 0.22%), and severe hypercholesterolemia (5.80% vs 9.66%).
Table 2.
Prevalence of familial hypercholesterolemia (FH) and severe hypercholesterolemia in Ontario, stratified by sex∗
| Groups | Number of subjects in the population | Definite FH | Probable FH | Severe hypercholesterolemia |
|---|---|---|---|---|
| Overall | 922,646 | 0.10 (0.10, 0.11) | 0.16 (0.15, 0.17) | 7.90 (7.84, 7.96) |
| Sex | ||||
| Men | 419,738 | 0.05 (0.05, 0.06) | 0.08 (0.08, 0.09) | 5.80 (5.73, 5.87) |
| Women | 502,908 | 0.15 (0.14, 0.16) | 0.22 (0.21, 0.24) | 9.66 (9.57, 9.74) |
Values indicate prevalence as a percentage, with (95% confidence interval), unless otherwise indicated.
Estimation of FH and severe hypercholesterolemia incorporated adjustment for statin therapy use. This was done by multiplying low-density lipoprotein-cholesterol (LDL-C levels) for individuals with a known statin prescription in the 100 days prior to their LDL-C test by 1.43 to estimate untreated LDL-C levels.
Cholesterol profile of individuals at diagnosis and follow-up
Full cholesterol profiles for the cohort at index and follow-up are described in Table 3. At initial diagnosis, mean LDL-C concentrations were 9.52 mmol/L for those with definite FH, 5.83 mmol/L for those with probable FH, 5.73 mmol/L for those with severe hypercholesterolemia, and 3.33 mmol/L for all other individuals. At follow-up of > 2 years (median follow up > 900 days), the mean LDL-C concentrations were lower, at 3.88 mmol/L for those with definite FH, 2.94 mmol/L for those with probable FH, 3.16 mmol/L for those with severe hypercholesterolemia, and 2.39 mmol/L for individuals in the general population. Five years following index cholesterol measurements (median follow-up > 2000 days) these values were even lower, at 3.58 mmol/L for those with definite FH, 2.72 mmol/L for those with probable FH, 2.93 mmol/L for those with severe hypercholesterolemia, and 2.27 mmol/L for the general population.
Table 3.
Cholesterol profile of patients aged ≥ 66 years with familial hypercholesterolemia (FH) and severe hypercholesterolemia at diagnosis and follow-up
| Characteristic | Definite FH |
Probable FH |
Severe hypercholesterolemia |
General population |
|---|---|---|---|---|
| n = 968 | n = 1,472 | n = 72,893 | n = 847,313 | |
| Cholesterol at index diagnosis, mmol/L∗ | ||||
| Total cholesterol | 9.35 ± 1.34 | 6.68 ± 0.94 | 6.84 ± 0.99 | 4.94 ± 1.08 |
| LDL-C | 7.03 ± 1.29 | 4.53 ± 0.81 | 4.71 ± 0.82 | 2.92 ± 0.89 |
| LDL-C (adjusted)† | 9.52 ± 1.19 | 5.83 ± 0.76 | 5.73 ± 0.70 | 3.33 ± 0.80 |
| HDL-C | 1.48 ± 0.39 | 1.39 ± 0.35 | 1.46 ± 0.36 | 1.40 ± 0.41 |
| Triglyceride | 2.09 ± 0.84 | 1.93 ± 0.82 | 1.75 ± 0.72 | 1.44 ± 0.68 |
| Cholesterol at 2+ years follow-up, mmol/L | ||||
| Number of individuals with follow-up | n = 953 | n = 1435 | n = 72,166 | n = 830,278 |
| Time of follow-up, d | 883 (784–1088) | 893 (790–1106) | 911 (791–1142) | 919 (784–1180) |
| Total cholesterol | 6.16 ± 1.79 | 5.11 ± 1.34 | 5.34 ± 1.28 | 4.43 ± 1.07 |
| LDL-C | 3.88 ± 1.64 | 2.94 ± 1.12 | 3.16 ± 1.11 | 2.39 ± 0.87 |
| HDL-C | 1.47 ± 0.36 | 1.38 ± 0.37 | 1.45 ± 0.37 | 1.40 ± 0.42 |
| Triglyceride | 1.75 ± 0.98 | 1.72 ± 0.94 | 1.58 ± 0.76 | 1.41 ± 0.75 |
| Control of LDL cholesterol at 2+ years follow-up | ||||
| LDL-C < 2.0 mmol/L | 71 (7.5) | 285 (19.9) | 9,764 (13.5) | 286,346 (34.5) |
| ≥ 50% reduction in LDL-C | 657 (68.9) | 730 (50.9) | 29,707 (41.2) | |
| Cholesterol values at 5+ years follow-up, mmol/L | ||||
| Number of individuals with follow-up | n = 896 | n = 1324 | n = 68,760 | n = 762,977 |
| Time of follow-up, in d | 2017 (1902–2227) | 2020 (1899–2221) | 2030 (1910–2246) | 2046 (1915–2284) |
| Total cholesterol | 5.85 ± 1.78 | 4.88 ± 1.32 | 5.11 ± 1.30 | 4.33 ± 1.09 |
| LDL-C | 3.58 (1.60) | 2.72 (1.11) | 2.93 (1.12) | 2.27 (0.89) |
| HDL-C | 1.48 ± 0.39 | 1.39 ± 0.38 | 1.46 ± 0.39 | 1.41 ± 0.43 |
| Triglyceride | 1.72 ± 1.02 | 1.71 ± 0.90 | 1.58 ± 0.78 | 1.44 ± 0.79 |
| Control of LDL cholesterol at 5+ years follow-up | ||||
| LDL-C < 2.0 mmol/L | 94 (10.5) | 355 (26.8) | 13,418 (19.5) | 291,369 (38.2) |
| ≥ 50% reduction in LDL-C | 638 (71.2) | 767 (57.9) | 32,641 (47.5) | |
Values are mean ± standard deviation, median (interquartile range), or n (%).
FH, familial hypercholesterolemia; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
SI conversion factors: to convert LDL-, HDL-, and total cholesterol and triglyceride levels to milligrams per decilitre, divide by 0.0259.
Adjusted LDL-C was estimated by multiplying LDL-C levels for individuals with a known statin prescription in the 100 days prior to their LDL-C test by 1.43 to estimate untreated LDL-C levels.
Two years following index cholesterol measurements, 68.9% of individuals with definite FH, 50.9% of those with probable FH, and 41.2% of those with severe hypercholesterolemia attained LDL-C values < 50% of baseline values. Very few individuals attained an LDL-C concentration of < 2.0 mmol/L—7.5% of those with definite FH, 19.9% of those with probable FH, and 13.5% of individuals with severe hypercholesterolemia. At 5-year follow-up, the percentage of those achieving reductions in LDL-C concentration of > 50% from baseline increased—71.2% of those with definite FH, 57.9% of those with probable FH, and 47.5% of individuals with severe hypercholesterolemia. An LDL-C concentration of < 2.0 mmol/L was attained by 10.5% of individuals with definite FH, 26.8% of those with probable FH, and 19.5% of those with severe hypercholesterolemia.
Use of cholesterol-lowering therapies during the study period
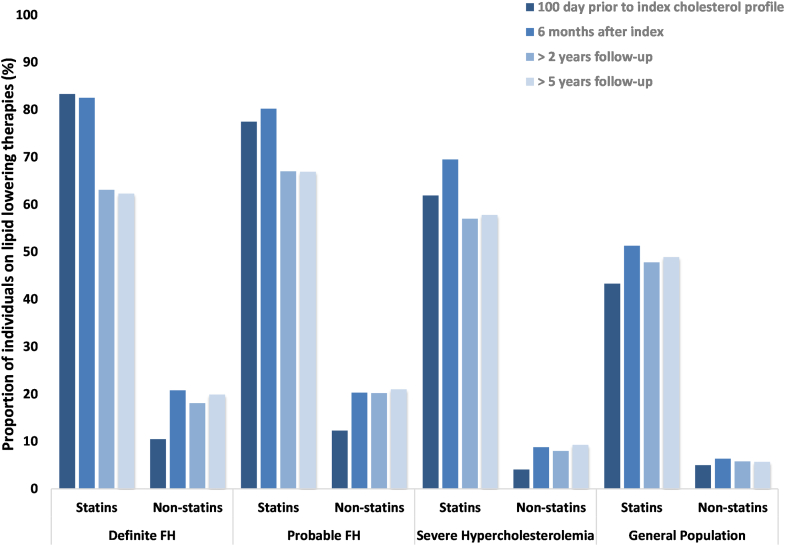
In our cohort, statins were used by 83.3% (24.5% high-intensity) of those with definite FH, 77.5% (21.7% high-intensity) of those with probable FH, 61.9% (7.0% high-intensity) of those with severe hypercholesterolemia and 43.3% (8.6% high-intensity) of the general population at initial diagnosis (Figs. 1 and 2). Nonstatin lipid-lowering therapies were used by 10.5% of individuals with definite FH, 12.3% of those with probable FH, 4.1% of those with severe hypercholesterolemia, and 5.0% of the general population. Use of statins, including high-intensity doses, and other lipid-lowering therapies increased slightly in the 6 months following index measurements.
Figure 1.
Use of statin therapy and other lipid-lowering therapy for patients (aged > 65 years) with familial hypercholesterolemia (FH) and severe hypercholesterolemia. Index values represent lipid-lowering prescription claims filled within 100 days prior to the index low-density lipoprotein-cholesterol measurement. Follow-up values indicate prescription claims filled within 100 days of the follow-up low-density lipoprotein-cholesterol measurement.
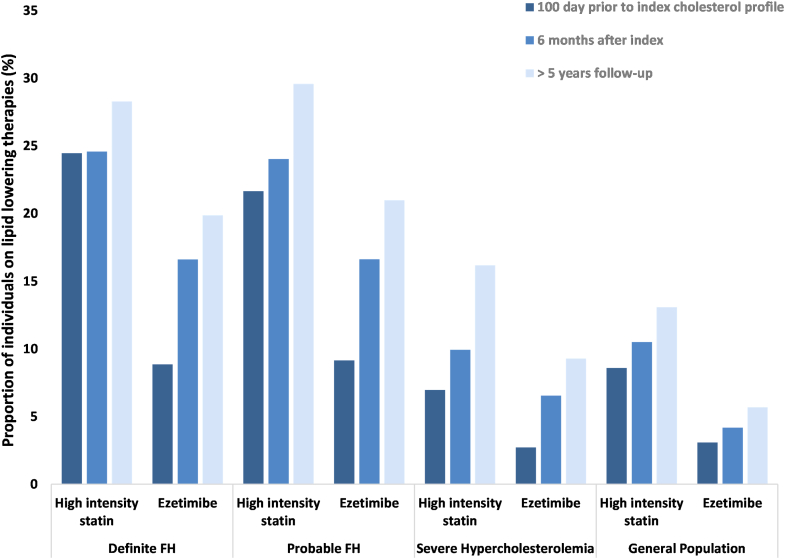
Figure 2.
Use of high-intensity statin therapy and ezetimibe in patients (aged > 65 years) with familial hypercholesterolemia (FH) and severe hypercholesterolemia. Index values represent lipid-lowering prescription claims filled within 100 days prior to the index low-density lipoprotein cholesterol measurement. Follow-up values indicate prescription claims filled within 100 days of the follow-up low-density lipoproteincholesterol measurement.
During the follow-up period of > 2 years, use of statins declined, with 63.1% of those with definite FH, 67.0% of those with probable FH, and 57.0% of those with severe hypercholesterolemia having a statin claim. Use of nonstatins also declined, with 18.1% of those with definite FH, 20.2% of those with probable FH, 8.0% of those with severe hypercholesterolemia, and 5.8% of the general population on these therapies at follow-up. Similar proportions of patients were on statins at follow-up at > 5 years (those with definite FH, 62.3%; those with probable FH, 66.9%; those with severe hypercholesterolemia, 57.8%; general population, 48.9%), although the use of high-intensity doses increased (those with definite FH, 28.3%; those with probable FH, 29.6%; those with severe hypercholesterolemia, 16.2%; general population, 13.1%). The proportions of patients on nonstatin therapies at 2 and 5 years were also similar (those with definite FH, 19.9%; those with probable FH, 21.0%; those with severe hypercholesterolemia, 9.3%; general population, 5.7%). However, notable increases were observed in the use of ezetimibe from index to > 5 year follow-up, as follows: those with definite FH, 8.9% vs 17.6%; those with probable FH, 9.2% vs 18.5%; those with severe hypercholesterolemia, 2.7% vs 8.3%; and the general population, 3.1% vs 4.4%.
Discussion
Applying a modified version of the simplified Canadian definition of FH to routinely collected health data, we were able to provide important insights on this group of patients at high risk of ASCVD. We estimated the prevalence of FH in older adults in Ontario to be at least 1 in 378, and the prevalence of severe hypercholesterolemia to be 1 in 13. Second, control of lipid levels was found to be suboptimal at follow-up. Only 68.9% and 50.9% of treated individuals with definite and probable FH, respectively, and 41.2% of individuals with severe hypercholesterolemia attained LDL-C values < 50% of baseline, as targeted by Canadian Cardiovascular Society guidelines. Third, despite being at high risk for ASCVD, among individuals older than 65 years, approximately two-fifths of those with FH and severe hypercholesterolemia went untreated with statins long-term.
Two recent large-scale meta-analyses2,19 have reported that FH affects approximately 1 in 300 individuals globally, with considerable variation across geographic regions and according to the diagnostic criteria employed. The prevalence estimates we obtained for FH among older adults (at 1 in 378) were similar to those found in these meta-analyses, as well as those from contemporary cohorts in nearby jurisdictions.17,18 However, a point to note is that we were unable to consider data on tendon xanthomas or first-degree relatives with elevated LDL-C level or premature ASCVD in identifying FH in our cohort. Additionally, screening for candidate mutations in FH is not routinely conducted in Ontario. Results from the Copenhagen General Population Study demonstrate that the combination of these genetic data with aforementioned clinical criteria led to greater capture of disease prevalence than either alone.20 In the absence of such data, FH diagnosis in our cohort relied solely on identified hypercholesterolemia and individual histories of premature cardiovascular disease. Contemporary cohorts of genetically defined FH heterozygotes suggest that only a small fraction of individuals with FH have LDL concentrations > 8.5 mmol/L; accordingly, our sample may be representative of only individuals with the most-deranged cholesterol. Taken together, these limitations mean that we likely have underestimated the true population prevalence in Ontario. However, the preponderance of contemporary estimates of FH are derived from mostly White European samples1,2,19; and the true prevalence in Ontario may be lower, given that people of non-European descent comprise over 28% of the population.13,21
Current Canadian guidelines recommend LDL-C level targets of < 2.0 mmol/L for FH patients with established ASCVD. For those without ASCVD, LDL-C reduction of > 50% has been recommended with a target of < 2.5 mmol/L.3 At 2 and 5 years following diagnostic lipid panels, LDL-C levels were persistently elevated in hypercholesteremic patients in Ontario. Even with treatment, 30%-50% of these patients failed to meet more-liberal primary prevention targets. These findings are not isolated. In the British Columbia Familial Hypercholesterolemia Registry, only one-third of patients achieved ≥ 50% reductions in LDL-C level from baseline at ∼10-year follow-up, despite using maximal-dose combination agents, and < 10% reached an LDL-C concentration < 2.0 mmol/L.12 Similar findings have been demonstrated in larger registries22 and population-based studies,23 in multiple jurisdictions,22, 23, 24, 25, 26, 27, 28, 29 with greater duration of follow-up.28 Patients with FH seem to have a response to lipid-lowering therapies similar to that of patients without FH.30 Thus, our findings may simply reflect and underscore the challenges of cholesterol reduction in these individuals, given such prominent baseline elevations.31 Future research exploring factors associated with treatment response in this population may improve success in achieving treatment targets.18,26
Our study found that although statin prescriptions increased after index lipid panels, including increasing use of high-dose statins, persistence on these medications declined in the hypercholesterolemic population long-term. A potential explanation for this finding might be the increased use of nonstatin alternatives (eg, ezetimibe), as observed in our study. Yet, these increases were marginal overall. Unfortunately, data suggest that most individuals affected by FH receive suboptimal treatment,4,20,32,33 with as many as half not receiving high-intensity statin-therapy.26 Even among patients receiving lipid-lowering treatment, some reports suggest that only two-thirds adhere to therapy long-term, and less than half (47%) remain on statins.34 Relative to other high-risk populations, patients with FH may actually demonstrate greater adherence.35 Still, due to elevated lipid levels from birth, FH contributes to higher ASCVD risk than other causes of dyslipidemia, making adequate treatment more important. Notable patient-level barriers to treatment adherence identified in this population include the following: mismatch between perceived and actual risk, concerns about use of medications, and inadequate/incorrect knowledge of treatment.36 Longitudinal and patient-specific education from care providers regarding therapeutic options may help overcome these challenges.37 At a provider level, interventions providing decision-support tools may also facilitate appropriate prescribing.38 Finally, tolerability and perceived tolerability remain significant barriers to statin treatment in these patients.25,26,39 Multi-drug therapy,40 including novel proprotein subtilisin/kexin type 9 (PSCK9)-inhibitors, may provide reasonable alternatives for those who are statin-intolerant.41,42
Although we were able to demonstrate the feasibility of using a modified version of the simplified Canadian definition to identify patients aged > 65 years with FH and assess lipid control, several limitations of our study merit consideration. First, as previously mentioned, we were unable to fully apply the Canadian definition due to the absence of some major (DNA mutations, tendon xanthomas) and minor (family history of hypercholesterolemia, ASCVD) diagnostic criteria from administrative databases, and thus may have underestimated the population prevalence of FH. Second, although some studies suggest the presence of a strong tendency toward lower incidences of traditional risk factors in the population with FH, we observed higher incidences of diabetes and hypertension among individuals classified into the probable FH group.43,44 Thus, although Canadian Cardiovascular Society Dyslipidemia Guidelines recommend lipid screening of all individuals aged ≥ 40 years, our study may be biased in the selection of individuals with a greater baseline risk for hypercholesterolemia and ASCVD, who therefore received lipid testing, which would bias our FH prevalence estimate upward. Third, we lacked medication data in individuals aged < 65 years, precluding estimation of prevalence and treatment patterns in this younger population. However, given that this is a genetic disorder with autosomal dominant transmission, the prevalence in younger individuals is likely comparable. Finally, our study period predates the advent of novel lipid-lowering therapies such as lomitapide and new proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. Further investigations on the impact of these medications on lipid control in the Ontario population are warranted.
Conclusions
We found that FH and severe hypercholesterolemia are common among older adults in the Ontario population, with notable proportions of those affected going untreated long-term. Greater use of current and emerging nonstatin therapies may provide additional LDL-C and ASCVD-risk reduction in this high-risk population.
Acknowledgements
This study was supported by the ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and Long-Term Care (MLTC). Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI) and Ontario Health. The analyses, opinions, results, statements, and conclusions expressed herein are solely those of the authors and do not reflect those of ICES, the funding or data sources; no endorsement is intended or should be inferred. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File, and Dynacare Medical Laboratories for providing access to the laboratory data being used in the CANHEART initiative.
Funding Sources
The authors are indebted to Dr Jack V. Tu (deceased May 30, 2018), who contributed to the conception and early work of this study. Funding of this study was from foundation grants (FDN 143313 and 154333) by the Canadian Institutes of Health Research (CIHR). L.A. was supported by the Comprehensive Research Education for Medical Students Scholar Program at the University of Toronto. H.A.-Q. is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada. J.G. holds the Novartis Chair in Medicine at McGill University. M.S. is funded by the Eliot Phillipson Clinician-Scientist Program at the University of Toronto and by a CIHR post-doctoral fellowship. J.U. is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada and an Ontario Clinician Scientist Award. H.W. was supported by a Phase 2 Clinician Scientist Award from the Heart and Stroke Foundation of Canada and a Tier II Canada Research Chair from the CIHR. D.L. is the Ted Rogers Chair in Heart Function Outcomes, a joint hospital-university chair of the University Health Network and the University of Toronto. D.K. is supported by the Jack Tu Research Chair in Cardiovascular Outcomes Research. The other authors have no funding sources to declare.
Disclosures
J.G. has received support from Aegerion, Amgen, Pfizer, Sanofi, and Valeant for FHCanada; honoraria from Amgen, Merck, Novartis, and Sanofi; and has collaborated with Amgen, Eli Lilly, Novartis, and Sanofi on clinical trials. J.A.U. has received consulting fees or honoraria from Amgen, Boehringer Ingelheim, Janssen, Merck, Novartis, and Sanofi, and grant support from AstraZeneca, Novartis, and Sanofi. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The use of the data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA) and does not require review by a research ethics board.
See page 746 for disclosure information.
References
- 1.Akioyamen L.E., Genest J., Shan S.D., et al. Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beheshti S.O., Madsen C.M., Varbo A., Nordestgaard B.G. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol. 2020;75:2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 3.Brunham L.R., Ruel I., Aljenedil S., et al. Canadian Cardiovascular Society position statement on familial hypercholesterolemia: update 2018. Can J Cardiol. 2018;34:1553–1563. doi: 10.1016/j.cjca.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard B.G., Chapman M.J., Humphries S.E., et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Eur Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson J.G. Management of familial hypercholesterolemia: a review of the recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Manag Care Pharm. 2013;19:139–149. doi: 10.18553/jmcp.2013.19.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruel I., Brisson D., Aljenedil S., et al. Simplified Canadian definition for familial hypercholesterolemia. Can J Cardiol. 2018;34:1210–1214. doi: 10.1016/j.cjca.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Besseling J., Hovingh G.K., Huijgen R., Kastelein J.J.P., Hutten B.A. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J Am Coll Cardiol. 2016;68:252–260. doi: 10.1016/j.jacc.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 8.Versmissen J., Oosterveer D.M., Yazdanpanah M., et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raal F.J., Pilcher G.J., Panz V.R., et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124:2202–2207. doi: 10.1161/CIRCULATIONAHA.111.042523. [DOI] [PubMed] [Google Scholar]
- 10.Luirink I.K., Wiegman A., Kusters D.M., et al. 20-year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019;381:1547–1556. doi: 10.1056/NEJMoa1816454. [DOI] [PubMed] [Google Scholar]
- 11.Neil A., Cooper J., Betteridge J., et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. 2008;29:2625–2633. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunham L.R., Cermakova L., Lee T., et al. Contemporary trends in the management and outcomes of patients with familial hypercholesterolemia in Canada: a prospective observational study. Can J Cardiol. 2017;33:385–392. doi: 10.1016/j.cjca.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Tu J.V., Chu A., Donovan L.R., et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART) Circ Cardiovasc Qual Outcomes. 2015;8:204–212. doi: 10.1161/CIRCOUTCOMES.114.001416. [DOI] [PubMed] [Google Scholar]
- 14.Ko D.T., Alter D.A., Guo H., et al. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J Am Coll Cardiol. 2016;68:2073–2083. doi: 10.1016/j.jacc.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Jones P.H., Davidson M.H., Stein E.A., et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR∗ Trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 16.Benn M., Watts G.F., Tybjærg-Hansen A., Nordestgaard B.G. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016;37:1384–1394. doi: 10.1093/eurheartj/ehw028. [DOI] [PubMed] [Google Scholar]
- 17.de Ferranti S.D., Rodday A.M., Mendelson M.M., et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES) Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 18.Bucholz E.M., Rodday A.M., Kolor K., Khoury M.J., de Ferranti S.D. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999–2014) Circulation. 2018;137:2218–2230. doi: 10.1161/CIRCULATIONAHA.117.032321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu P., Dharmayat K.I., Stevens C.A.T., et al. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease. Circulation. 2020;141:1742–1759. doi: 10.1161/CIRCULATIONAHA.119.044795. [DOI] [PubMed] [Google Scholar]
- 20.Benn M., Watts G.F., Tybjaerg-Hansen A., Nordestgaard B.G. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97:3956–3964. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 21.Tu J.V., Chu A., Rezai M.R., et al. The incidence of major cardiovascular events in immigrants to Ontario, Canada: the CANHEART immigrant study. Circulation. 2015;132:1549–1559. doi: 10.1161/CIRCULATIONAHA.115.015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez de Isla L., Alonso R., Watts G.F., et al. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART Registry follow-up. J Am Coll Cardiol. 2016;67:1278–1285. doi: 10.1016/j.jacc.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Zafrir B., Jubran A., Lavie G., et al. Clinical determinants and treatment gaps in familial hypercholesterolemia: data from a multi-ethnic regional health service. Eur J Prev Cardiol. 2017;24:867–875. doi: 10.1177/2047487317693132. [DOI] [PubMed] [Google Scholar]
- 24.Pijlman A.H., Huijgen R., Verhagen S.N., et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis. 2010;209:189–194. doi: 10.1016/j.atherosclerosis.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Hadfield S.G., Horara S., Starr B.J., et al. Are patients with familial hypercholesterolaemia well managed in lipid clinics? An audit of eleven clinics from the Department of Health Familial Hypercholesterolaemia Cascade Testing project. Ann Clin Biochem. 2008;45(Pt 2):199–205. doi: 10.1258/acb.2007.007078. [DOI] [PubMed] [Google Scholar]
- 26.deGoma E.M., Ahmad Z.S., O’Brien E.C., et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE-FH Registry. Circ Cardiovasc Genet. 2016;9:240–249. doi: 10.1161/CIRCGENETICS.116.001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leren T.P., Berge K.E. Subjects with molecularly defined familial hypercholesterolemia or familial defective apoB-100 are not being adequately treated. PloS One. 2011;6 doi: 10.1371/journal.pone.0016721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Béliard S., Carreau V., Carrié A., et al. Improvement in LDL-cholesterol levels of patients with familial hypercholesterolemia: Can we do better? Analysis of results obtained during the past two decades in 1669 French subjects. Atherosclerosis. 2014;234:136–141. doi: 10.1016/j.atherosclerosis.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Knickelbine T., Lui M., Garberich R., et al. Familial hypercholesterolemia in a large ambulatory population: statin use, optimal treatment, and identification for advanced medical therapies. J Clin Lipidol. 2016;10:1182–1187. doi: 10.1016/j.jacl.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 30.McGowan M.P., Hosseini Dehkordi S.H., Moriarty P.M., Duell P.B. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang J., Chan D.C., Watts G.F. The knowns and unknowns of contemporary statin therapy for familial hypercholesterolemia. Curr Atheroscler Rep. 2020;22:64. doi: 10.1007/s11883-020-00884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Z., Yuan B., Zhao D., et al. Familial hypercholesterolemia in China: prevalence and evidence of underdetection and undertreatment in a community population. Int J Cardiol. 2014;174:834–836. doi: 10.1016/j.ijcard.2014.04.165. [DOI] [PubMed] [Google Scholar]
- 33.Barkas F., Liberopoulos E., Liamis G., Elisaf M. Familial hypercholesterolemia is undertreated in clinical practice. Hell J Atheroscler. 2016;7:120–130. [Google Scholar]
- 34.Casula M., Scotti L., Tragni E., et al. Drug treatment and adherence of subjects < 40 years with diagnosis of heterozygous familial hypercholesterolemia. Atherosclerosis. 2016;254:172–178. doi: 10.1016/j.atherosclerosis.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 35.Jackevicius C.A. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 36.Kinnear F.J., Wainwright E., Perry R., et al. Enablers and barriers to treatment adherence in heterozygous familial hypercholesterolaemia: a qualitative evidence synthesis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gidding S.S., Champagne M.A., de Ferranti S.D., et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 38.Sparrow R.T., Khan A.M., Ferreira-Legere L.E., et al. Effectiveness of interventions aimed at increasing statin-prescribing rates in primary cardiovascular disease prevention: a systematic review of randomized clinical trials. JAMA Cardiol. 2019:1160–1169. doi: 10.1001/jamacardio.2019.3066. [DOI] [PubMed] [Google Scholar]
- 39.Wood F.A., Howard J.P., Finegold J.A., et al. N-of-1 trial of a statin, placebo, or no treatment to assess side effects. N Engl J Med. 2020;383:2182–2184. doi: 10.1056/NEJMc2031173. [DOI] [PubMed] [Google Scholar]
- 40.Duell P.B., Gidding S.S., Andersen R.L., et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: the CASCADE FH registry. Atherosclerosis. 2019;289:85–93. doi: 10.1016/j.atherosclerosis.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Nissen S.E., Stroes E., Dent-Acosta R.E., et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315:1580–1590. doi: 10.1001/jama.2016.3608. [DOI] [PubMed] [Google Scholar]
- 42.Moriarty P.M., Thompson P.D., Cannon C.P., et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758–769. doi: 10.1016/j.jacl.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Akioyamen L.E., Genest J., Chu A., et al. Risk factors for cardiovascular disease in heterozygous familial hypercholesterolemia: a systematic review and meta-analysis. J Clin Lipidol. 2019;13:15–30. doi: 10.1016/j.jacl.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Akioyamen L.E., Genest J., Shan S.D., et al. Anxiety, depression, and health-related quality of life in heterozygous familial hypercholesterolemia: a systematic review and meta-analysis. J Psychosom Res. 2018;109:32–43. doi: 10.1016/j.jpsychores.2018.03.170. [DOI] [PubMed] [Google Scholar]