Abstract
This article comments on:
Jessica Valdovinos-Ayala, Catherine Robles, Jaycie C. Fickle, Gonzalo Pérez-de-Lis, R. Brandon Pratt and Anna L. Jacobsen, Seasonal patterns of increases in stem girth, vessel development, and hydraulic function in deciduous tree species, Annals of Botany, Volume 130, Issue 3, 1 September 2022, Pages 355–365 https://doi.org/10.1093/aob/mcac032
Keywords: Tree vascular systems, hydraulic function, drought
Trees are amazing organisms that have evolved to accommodate changes in their environment at various time scales, from evolutionary to seasonal. In recent years, climate change and associated water stress have been pushing some forests to the limit (Adams et al., 2009). For example, in California over 129 million trees have died during recent droughts (California Department of Forestry and Fire Protection, 2018). Given the central role of trees in mitigating and responding to climate change, it is surprising that there are still fundamental features of how trees grow, adjust their development and physiology for environmental conditions, and transport water through their woody bodies that remain poorly understood. The new paper by Valdovinos-Ayala et al. (2022) in this issue provides new insights into the seasonal cycles of perennial growth in angiosperm trees, and how this is related to the development and function of water-conducting tissues. Along the way, this work also presents new questions and exposes basic knowledge gaps regarding the annual growth cycles of trees and their ability to transport water and mitigate variations in the environment.
Temperate forest trees undergo annual cycles of perennial growth. Some aspects of these annual cycles are well known and obvious, such as the flushing of buds in spring to produce new branches and leaves, or leaf senescence during autumn. What is not as obvious is what is happening inside the tree, under the bark. Some clues can be gleaned from looking at the familiar annual rings on stumps of felled trees. Variation can be seen in the width of annual rings, reflecting how favourable a given year’s environment was for growth. A primary component of environmental influence on growth is water availability, with drier years resulting in more narrow rings. However, if one looks with a magnifying glass there is also observable variation within an annual growth ring. The ‘pores’ seen in a cross-section of wood are actually the water-conducting conduits. A dramatic illustration of within-year variation is seen for so-called ‘ring porous’ woods such as is found in oaks, where at the start of the growing season large-diameter pores develop, while later in the season under heat and water stress small-diameter pores form. However, even in ‘diffuse porous’ woods more subtle variation can be seen between ‘early wood’ and ‘late wood’, with smaller pores in late wood. Indeed, it is known that these differences in the arrangements and diameters of ‘pores’ have major effects on water transport capacity and resistance to cavitation during drought (Hacke et al., 2017; Rodriguez-Zaccaro and Groover, 2019). While it would be tempting to assume that these ‘pores’ become active in conducting water as soon as the cells are produced in order to cope with the environmental conditions under which they were produced, the story is apparently much more complex, pointing to another way that trees may be adjusting development to alter water transport physiology in response to the environment.
The vascular cambium is the lateral meristem responsible for radial growth of stems, including production of secondary xylem (wood) and secondary phloem (inner bark). In these angiosperm species, the cambium contains both fusiform initials and ray initials (Larson, 1994). Ray initials divide to produce radially organized strands of cells that transverse the phloem, cambium and secondary xylem. The fusiform initials divide to produce daughters that go on to divide and differentiate into axially aligned cells including fibres, parenchyma and vessel elements. Vessel elements are the primary water-conducting cells of most angiosperms. Vessel elements undergo a predictable set of developmental steps, including initial expansion, secondary cell wall formation and programmed cell death, to produce a hollow cell corpse that conducts water (Groover and Jones, 1999). Vessel elements are produced in interconnected files to produce longer structures, vessels, responsible for high-capacity water transport.
Valdovinos-Ayala et al. undertook laborious experiments to follow the dynamics of annual growth in three angiosperm trees: maple, oak and poplar. Traits measured included diameter of growth, production of water vessel elements, and the time that vessels actually completed differentiation and then became part of functional vessels. The authors detailed the timing of cambial growth and production of vessel elements relative to bud break and leaf-out in spring. For all species, radial growth preceded leaf-out, with growth of radial branches preceding the main trunk by ~2–4 weeks. Radial growth included production of vessel elements well in advance of leaf-out. One might assume this is strategic – to have new water conduits ready to supply new, vigorous growth. However, this was not what was observed. Instead, the full maturation of vessel elements occurred 2–6 weeks after initial radial growth. Surprisingly, there was an additional period of up to 4 weeks before vessels actually began transporting water. These observations raise several questions about the strategies that have evolved for trees to transport water, with respect to both annual cycles of growth and environmental conditions within a growth cycle.
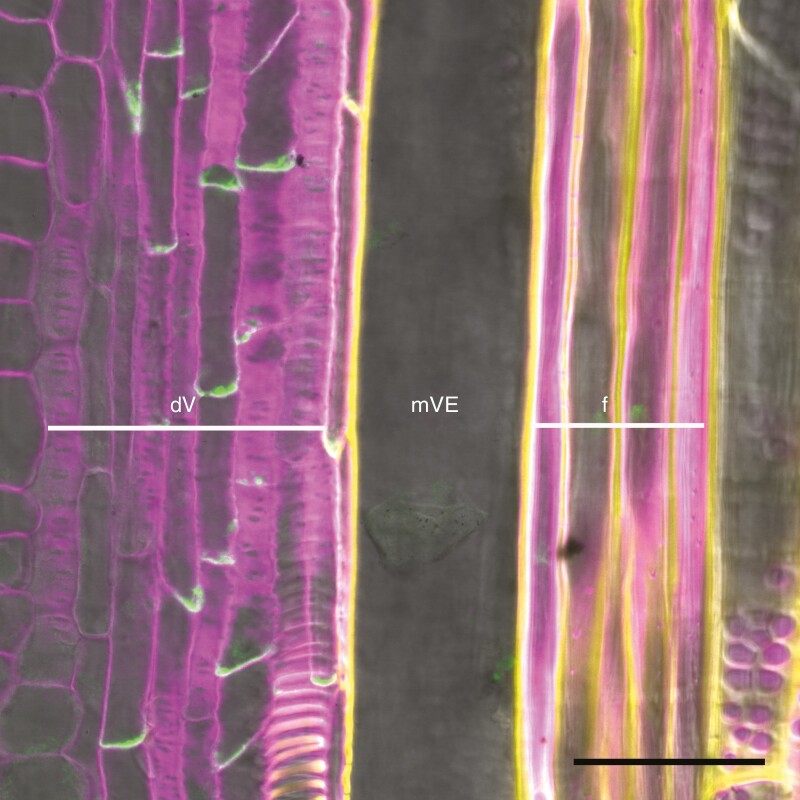
There are many interesting observations shown by Valdovinos-Ayala et al. that, if explained at a molecular or cell biology level, could provide fundamental new insights into how trees create functional water conduits that are optimized for both current and potential environments. For example, molecular information might provide clues as to why there is a delay in vessel element maturation. There are ample data describing gene expression at different developmental stages from cultures of both zinnia and Arabidopsis vessel elements (Demura et al., 2002; Kondo et al., 2015). There are multiple techniques that could be used to assay gene expression already associated with specific stages of vessel element differentiation within stem samples. Questions that could be addressed include at what stages vessel elements pause prior to undergoing programmed cell death and clearing the lumen of the water-conducting cell through hydrolysis. Could part of the pause in development reflect time needed to interconnect files of vessel elements into functional vessels? To answer this question, examination of auxin efflux PIN proteins responsible for canalization might be informative (Figure 1). Variation in the size and frequency of vessel elements produced across a growing season is another key part to understanding the results shown by Valdovinos-Ayala et al., and recent studies are identifying the genes and mechanisms underlying this variation (e.g. Li et al., 2019).
Fig. 1.
Immunolocalization of a poplar orthologue of PIN1 auxin efflux transporter protein in developing xylem. An antibody recognizing a poplar orthologue of PIN1 was used to label the protein. This longitudinal section of a poplar stem shows PIN localization (green signal) to the basal end of still living vessels, consistent with a role in canalization as in Arabidopsis. dV, developing vessels; mVE, mature vessel element; f, fibres. Photo: Lijuan Wang, Chinese Academy of Forestry (Wang, Gerttula and Groover, unpubl.).
What are the next steps?: interconnecting molecular and in planta studies. For example, using gene expression and molecular markers to understand where in the developmental programme vessel elements pause before undergoing programmed cell death. Molecular insights might provide understanding of the functional, physiological and ecological consequences of pausing. Additional tools that are needed include new approaches for live cell imaging deep under the bark, which presents many technical challenges. Perhaps the biggest message from studies such as that of Valdovinos-Ayala et al. is that we still have much to learn about the evolution, physiology and environmental responses of trees.
LITERATURE CITED
- Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, et al. 2009. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proceedings of the National Academy of Sciences USA 106: 7063–7066. doi: 10.1073/pnas.0901438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Forestry and Fire Protection. 2018. Over 129 million dead trees in California between 2010–2017. http://calfire-forestry.maps.arcgis.com/apps/MapJournal/index.html?appid=3457736fb0dd45f98d41ab4030ebf048.
- Demura T, Tashiro G, Horiguchi G, et al. 2002. Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells. Proceedings of the National Academy of Sciences USA 99: 15794–15799. doi: 10.1073/pnas.232590499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover A, Jones A. 1999. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiology 119: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke U, Spicer R, Schreiber SG, Plavcova L. 2017. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell & Environment 40: 831–845. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Fujita T, Sugiyama M, Fukuda H. 2015. A novel system for xylem cell differentiation in Arabidopsis thaliana. Molecular Plant 8: 612–621. doi: 10.1016/j.molp.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Larson, PR. 1994. The vascular cambium. Berlin: Springer-Verlag. [Google Scholar]
- Li S, Lin Y-CJ, Wang P, et al. 2019. The AREB1 transcription factor influences histone acetylation to regulate drought responses and tolerance in Populus trichocarpa. The Plant Cell 31: 663–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Zaccaro FD, Groover A. 2019. Wood and water: How trees modify wood development to cope with drought. Plants, People, Planet, 1: 346–355. [Google Scholar]
- Valdovinos-Ayala J, Robles C, Fickle JC, Perez-de-Lis G, Pratt RB, Jacobsen AL. 2022. Seasonal patterns of increases in stem girth, vessel development and hydraulic function in deciduous tree species. Annals of Botany 130: 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]