Abstract
Background
Stomatal pores in many species are separated from the atmosphere by different anatomical obstacles produced by leaf epidermal cells, especially by sunken stomatal crypts, stomatal antechambers and/or hairs (trichomes). The evolutionary driving forces leading to sunken or ‘hidden’ stomata whose antechambers are filled with hairs or waxy plugs are not fully understood. The available hypothetical explanations are based mainly on mathematical modelling of water and CO2 diffusion through superficial vs. sunken stomata, and studies of comparative autecology. A better understanding of this phenomenon may result from examining the interactions between the leaf cuticle and stomata and from functional comparisons of sunken vs. superficially positioned stomata, especially when transpiration is low, for example at night or during severe drought.
Scope
I review recent ideas as to why stomata are hidden and test experimentally whether hidden stomata may behave differently from those not covered by epidermal structures and so are coupled more closely to the atmosphere. I also quantify the contribution of stomatal vs. cuticular transpiration at night using four species with sunken stomata and three species with superficial stomata.
Conclusions
Partitioning of leaf conductance in darkness (gtw) into stomatal and cuticular contributions revealed that stomatal conductance dominated gtw across all seven investigated species with antechambers with different degrees of prominence. Hidden stomata contributed, on average, less to gtw (approx. 70 %) than superficial stomata (approx. 80 %) and reduced their contribution dramatically with increasing gtw. In contrast, species with superficial stomata kept their proportion in gtw invariant across a broad range of gtw. Mechanisms behind the specific behaviour of hidden stomata and the multipurpose origin of sunken stomata are discussed.
Keywords: Sunken stomata, leaf, epidermis, stomatal antechamber, stomatal encryptation, nocturnal transpiration, cuticular transpiration, stomatal transpiration, trichomes, Nerium oleander, Ficus elastica, Olea europaea, Clusia rosea, Arabidopsis thaliana, Capsicum annuum, Brassica oleracea
INTRODUCTION
Sunken stomata
Architectural characteristics of the stomatal apparatus are of profound importance for its function – prevention of fatal water loss while allowing sufficient carbon gain. During the light period of a day, stomata represent the most important plant–atmosphere interface, dynamically balancing the rates of transpiration and photosynthesis of a cuticle-sealed leaf. In an optimal mode, they are hypothesized to maximize CO2 influx for a given physiologically tolerable water loss (Cowan and Farquhar, 1977; Vico et al., 2013). At night, with stomata presumably closed, the cuticle and its cuticular wax are barriers that restrict water loss to a safe value that is not subjected to short-term control by the plant. Because of their key role in controlling CO2 influx and water efflux, the stomata and cuticle are assumed to be the primary targets of evolutionary adaptations to varying environmental conditions.
The position of stomata relative to the leaf surface has been a matter of interest to plant physiologists and anatomists for decades (Weyers and Meidner, 1990; Willmer, 1996; Larcher, 2001). In most species, a leaf cuticle that covers all epidermal cells forms external ledges arching over the stomatal pore, and the variable stomatal pore delimited by guard cells sits beneath the rigid ledges (Turner, 1994; Willmer, 1996). With this general arrangement, the central point of guard cells can protrude above, be in line with or be sunken below the surface of the epidermis. In the latter case, guard cells are located at the bottom of depressions, deep pits or crypts forming stomatal antechambers.
There is currently no consensus regarding the functional significance of sunken stomata. Traditionally, the stomatal antechamber has been considered as an adaptation to dry environments. However, the current distribution of species with sunken stomata outside of dry habitats as well as fossil records indicate that the water deficit is not the sole and perhaps not even the primary factor promoting sunken stomata. It appears that more than one evolutionary factor may favour the location of stomata in recesses within leaves. What underlies the evolutionary success of stomata seated at the bottom of an epidermal recess?
In the first part of this study, an overview of stomatal antechamber anatomy and ecology is presented, followed by a short review of current ideas on the benefits conferred by stomatal antechambers. In the second part, original experimental results are presented, addressing the environmental response of various types of sunken or encrypted stomata that are relatively isolated from the external environment.
Architecture of stomatal antechambers
Hidden stomata are usually classified according to the ratio of the width of the aperture of the stomatal antechamber relative to its depth, by the antechamber volume or shape and/or by the number of stomata in the chamber. Two basic categories can be distinguished (Jordan et al., 2008): (1) stomata encrypted in deep pits, crypts or longitudinal grooves and (2) stomata in shallow recesses protected by overarching external cuticular ledges
The term ‘sunken stomata’ in the following text, especially in the experimental part, includes both the above categories of stomatal antechamber, the deep crypts as well as the shallow pits covered with rigid ledges (papillae). In contrast, stomata with guard cells located at the same level as epidermal cells and without a discrete antechamber are called ‘superficial’ stomata.
Type (1) stomata in crypts can be formed by invaginations of the leaf epidermis protruding into abaxial mesophyll tissue or by entire leaf margins that curl tightly toward the middle vein. They can have different sizes and shapes, accommodating from one to many (8–35) stomata in one crypt as, for example, in six Banksia species investigated by Hassiotou et al. (2009a), or an average of 40 stomata per crypt in fossils of Banksia paleocarpa (Carpenter et al., 2014). Here, the large crypts (approx. 300 µm in diameter) are often filled with a mesh of trichomes, which may obscure the aperture of the pit. Both the hairs and crypts have an uncertain role in hindering water loss (see below). Crypts evolved several times in the Australian Proteaceae from wet as well as presumably dry climates (Jordan et al., 2008). However, several lines of evidence discussed below indicate that the location of stomata in deep grooves or crypts provides a selective advantage in xeric climates.
Type (2) stomata in shallow epidermal depressions covered partly by cuticular ledges (papilla) are common in most vascular plants. They can have different forms, for example pits where stomata appear sunken due to papillae surrounding the stomatal apparatus. In the Proteaceae, an abundance of species with papillose pits does not correspond to the level of aridity within their habitats. Jordan et al. (2008) argue that papillae and other epidermal features that increases the roughness of the leaf surface decrease its wettability, similar to trichomes in crypts and on the leaf surface. Lower wettability provides protection against waterlogging of stomatal pits, and hairs may prevent fungal growth into the stomatal antechamber and leaf interior via the stomatal pore. These evolutionary advantages may dominate in species with a paracytic stomatal complex, characterized by a lateral pair of subsidiary cells that arch over the stomatal pore, creating a stomatal antechamber (Gray et al., 2020).
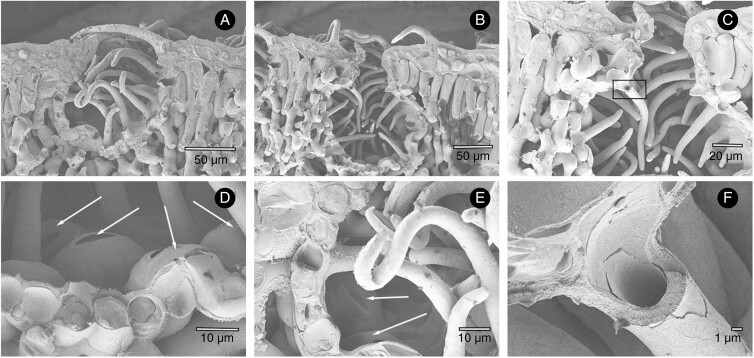
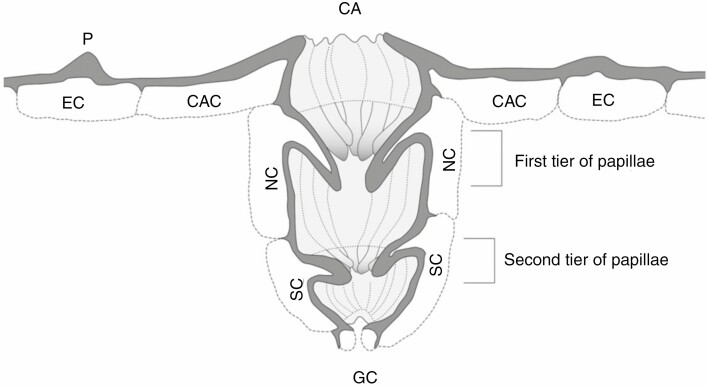
Antechambers forming deep crypts have been of greatest interest to physiologists and plant anatomists. Crypts are the air-filled, roughly spherical or cylindrical spaces invaginating into mesophyll tissue with an opening to the atmosphere that is smaller than their diameter. Each usually accommodates a number of stomata located at the bottom and along the crypt walls (Fig. 1A–F). It is assumed that the walls are covered with a cuticle and do not represent any significant source of transpired water. Crypts are typical for xerophyllous or sclerophyllous species of dry habitats, commonly exemplified by Nerium oleander or many Banksia species. For example, the visible crypt openings (crypt pores) in Banksia ilicifolia occupy about 20 % of the total abaxial leaf surface area, and the internal surface area of the crypts exceeds the crypt pore area by five times (Roth-Nebelsick et al., 2009). Thus, the crypt walls may enlarge the leaf area 100 % compared with the projected area. An interesting, extremely pronounced form of crypt was described recently in fossil needles of Cryptokerpia sarlaccophora (gymnosperm) from Permian deposits in Jordan (Blomenkemper et al., 2019). The crypts are three cells deep and possess two additional papillae rings formed by neighbouring and subsidiary cells arranged in a circle (Fig. 2). The hook-shaped papillae restrict the diffusive path in two tiers, like a double bottleneck, and presumably protect a single stoma at the bottom of the crypt.
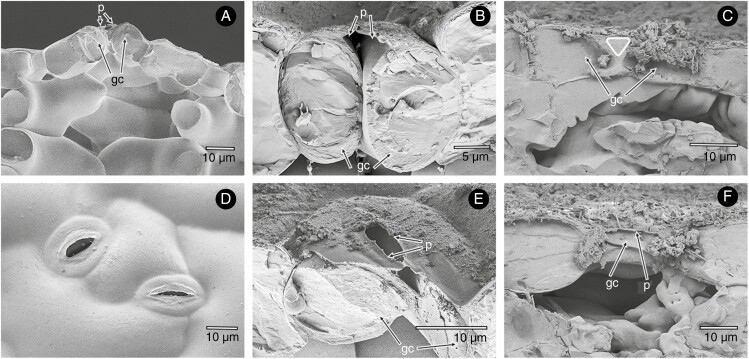
Fig. 1.
Crypts with stomata and hairs in Nerium oleander. Cryo-scanning electron microscopy (SEM) images of transects through the whole crypts on the abaxial side of an N. oleander leaf, with crypt walls, internal trichomes and trichomes overarching the crypt aperture (A, B). Details of the crypt interior showing numerous stomata at the crypt bottom (arrows in D, E) and fractured crypt walls. Hollow trichomes are protruding from the individual cells forming the wall (C, F).
Fig. 2.
Reconstruction of the stomatal crypt of Cryptokerpia sarlaccophora. CA, crypt aperture; CAC, crypt aperture cell; EC, epidermal cell; NC, neighbouring cell; SC, subsidiary cell; GC, presumed position of guard cells. According to Blomenkemper et al. (2019).
Crypts can be filled with trichomes, as is the case with Banksia or N. oleander, with stomata clustered along the walls of the crypt. The role of trichomes is enigmatic. Based on isoline estimates of water vapour concentration, they do not appear to hinder diffusion of water from the crypt (Roth-Nebelsick et al., 2009). Haworth and McElwain (2008) proposed that they could protect stomata from mechanical damage, provide UV protection, shield against excessive irradiation and heat, or block the formation of water layers across the crypt pore. These and other commonly accepted ecophysiological functions of leaf hairs are likely to exist (Bickford, 2016). Interestingly, the hairs are hollow (Fig. 1C, F) and probably filled with air; unfortunately, data about the permeability of hair surfaces for water are lacking. The dramatic increase of the abaxial leaf surface by crypt walls and trichome surfaces raises a question about a possible role for these surfaces as water sources that moisten the air close to stomata, and thus change the crypt’s microclimate. Could stomata profit from such a crypt-specific microclimate? In what other ways might the presence of crypts benefit stomata, leaf and plant fitness?
Benefits of sunken stomata
Adaptation to drought by reducing water loss more strongly than CO2 uptake.
Antechambers are located in the diffusion pathways for both water vapour and CO2. Consequently, the increment in length of the diffusion pathway for both gases through the antechamber should be the same. However, because CO2 diffusion does not end at the evaporation surfaces within the leaf but continues beyond, into mesophyll cells and chloroplasts, the diffusion path length for CO2 is longer than that for water molecules. Antechambers per se thus increase the diffusive path length for water vapour proportionally more than for CO2. Following this principle, Jeffree et al. (1971) estimated that a modelled stomatal antechamber in Sitka spruce increased diffusion resistance for water by about the same magnitude as open stomatal pores. Diffusion is hindered further by wax occlusions – amorphous or crystalline structures occurring inside and around the antechambers (Kim et al., 2010). They reduce the effective cross-section for free diffusion in air; moreover, if the distance between wax particles is close to or less than the mean free path of the diffusing molecules (approx. 100 nm), the free diffusion regime changes to much slower Knudsen diffusion. With Knudsen diffusion, the gas molecules collide more frequently with wax particles and hairs than with each other, and the diffusion coefficients decrease substantially (Cussler, 1987). With the lengthening of the common water and CO2 diffusion path lengths and the introduction of wax occlusions, the placement of stomata in antechambers selectively promotes CO2 uptake over water loss and, therefore, increases the instantaneous water use efficiency of photosynthesis. Using a simple mathematical model, Jeffree et al. (1971) showed that the antechambers of spruce, plugged with wax, formed two-thirds of the total needle resistance to water loss while they accounted for only one-third of the resistance to CO2 uptake. An even greater effect of wax plugs on stomatal conductance was estimated by Brodribb and Hill (1997) for a group of Southern Hemisphere conifers. Partitioning of the total leaf diffusion conductance to crypts and stomata in Banksia revealed a dominant role for stomata (77 %) over crypts (23 %) and a 7–11 % improvement in water use efficiency compared with leaves with superficial stomata (Hassiotou et al., 2009a).
If this principle works on an evolutionary time scale, the number of species with sunken stomata may be expected to increase with greater aridity in their environment. Jordan et al. (2008) observed this phenomenon in the fossil record of Proteaceae species exhibiting deep stomatal crypts. Deep crypts appeared relatively recently after the aridification of Australia had started. Other epidermal features considered as antitranspirants are papillae and hairs overhanging stomatal crypts. However, the paleorecord of their occurrence in an extinct Cretaceous conifer (Pseudofrenelopis) was not consistent with a change in paleoclimate aridity (Haworth and McElwain, 2008). Also, numerical modelling of water diffusion through the crypts of Proteaceae render the significance of their transpiration-reducing role doubtful (Roth-Nebelsick et al., 2009). In addition, stomata sunken in pits also occur in species native to habitats well supplied with water or even occasionally flooded, mainly in conifers (Waldhoff et al., 2002).
Facilitation of CO2 access in thick leaves.
Groups of stomata buried in deep grooves or crypts are also common in sclerophytes that cope with a deficiency of nutrients rather than with water shortage (Salleo et al., 1997). These observations indicate that selective pressures other than drought probably contributed to the evolution of hidden stomata. Mohammadian (2005) and Hassiotou et al. (2009a) showed for a set of ten Banksia species that crypt depth correlated significantly with leaf thickness and dry mass per area (LMA). The volume of crypts and density of stomata inside the crypts, especially at their bottom, also increased with LMA and leaf thickness. This indicates that deep, voluminous crypts with walls covered with numerous stomata may function as porous ‘pipelines’ facilitating diffusion of CO2 by shortening the distance to densely packed mesophyll and especially palisade tissue. Mathematical simulation of diffusion through the stomatal complex (antestomatal chamber, stomatal pore and sub-stomatal cavity) showed that the volume and shape of the antestomatal cavity are important parameters. An increase in both the area of the bottom of a crypt and density of stomata at the bottom should facilitate CO2 uptake relative to water loss (Roth-Nebelsick, 2007) because the bottom part represents the internal air–mesophyll inteface with the highest CO2 demand.
Protection of stomata from formation of water films in fog-rich environments.
Leaf surfaces have water-repellent properties that prevent formation of water films over stomatal pores, which would hinder CO2 influx into the leaf and slow photosynthetic CO2 assimilation. Diffusion of CO2 through water is almost 10 000 times slower than through air (Nobel, 1991), so waterlogging of stomatal pores or even stomatal antechambers would effectively uncouple the leaf from the ambient atmosphere. Hydrophobicity of waxes deposited inside and on the top of the cuticular membrane extending through antestomatal chambers and stomatal pores reduces the risk of water-plugged stomata. Stomatal papillae, elevating the aperture of the stomatal antechamber above the general surface of the epidermis, are also considered as one of the adaptive traits avoiding stomatal waterlogging (Hill, 1998). Without wax and papillae, this phenomenon could frequently occur, especially in cloud-rich, foggy or rainy environments.
In some species, typically conifers, antestomatal chambers are completely filled with porous plugs of wax (Jeffree et al., 1971; Brodribb and Hill, 1997; Kim et al., 2010). This leaf trait was traditionally assumed to be an adaptation reducing transpiration and increasing water use efficiency (Jeffree et al., 1971); however, fossil records show that the plugs appeared first in wet environments (Hill, 1998), and work with living species indicates that stomatal plugs inefficiently protect trees from water loss. Removal of stomatal plugs in Drimys winteri, an angiosperm tree from the wet tropics, did not increase the transpiration rate (Feild et al., 1998). Wax plugs in Sequoia seem inefficient in preventing transpiration when stomata remain partially open. Burgess and Dawson (2004) found that night-time transpiration could often amount to 20 % of maximum daytime summer transpiration in S. sempervirens, a tree of coastal vegetation subjected to frequent occurrence of fog. These findings indicate that the plugs played only a minor role in saving water, especially at high air humidity that is common in the tree’s habitat. Instead of saving water, the main advantage of the wax plugs is likely to be prevention of continuous water films forming on the leaf surface. Therefore, the antechambers with waxy plugs obscuring the stomata could be be most adaptive in wet habitats and cloud forest vegetation.
Protection against environmental stress factors beyond drought.
Investigations of the epidermis morphology of Erica arborea leaves fumigated with toxic volcanic gases revealed that stomata occurred deeper in the leaf mesophyll than in control leaves from non-polluted locations (Bartiromo et al., 2013). These results demonstrate another potential function of stomata encryptation – protection against harmful effects of polluted air. A specific action of small aerosols (<2 µm) such as soot, ash and fine dust is that the particles can settle on leaves and pass through stomatal openings, and, when moistened, can form a continuous film of water extending from the leaf surface through the stomata and into the leaf interior. This can create a liquid water connection between the leaf apoplast and the external atmosphere, forming in essence a wick that can transport liquid water directly from the internal epidermis or mesophyll cell walls. In doing so, stomatal control can be bypassed by what has been termed hydraulic activation of stomata (HAS) (Burkhardt, 2010). Extended boundary layer and micromorphological obstacles provided by papillae or trichomes arching over the antestomatal aperture can prevent deposition of fine airborne particles, reduce HAS and, consequently, save water. Consistent with this possibility, species with sunken stomata are common in fire-prone environments where smoke can regularly deposit fine aerosols.
Elevated leaf surface temperature is another factor which probably contributes to sunken stomata in leaves. For example, the depth of stomata in leaves from 23 Banksia species was correlated with the maximum annual temperatures in their respective habitats but not with minimum annual rainfall (Ratnawati, 2001). Higher leaf temperature increases leaf to air water vapour pressure difference (VPD), which as a driving force of transpiration could increase water loss in warm environments. A dual adaptation to increased leaf temperature and VPD can also explain the high abundance of deeply encrypted stomata in fossil leaves of vegetation from open habitats (Jordan et al., 2014).
Protection against pathogen attack
. Spores and bacteria accumulate around stomata and can invade the leaf interior via stomatal pores (Kroupitski et al., 2009). Siting stomata deep below the epidermal leaf surface, and especially obstructions in the the aperture of antechambers such as waxy plugs, can prevent hyphae of fungi and other pathogens from entering the leaf (Mohammadian et al., 2009). Fungal invasion is common in the humid environment of rain forest trees and, consistently, waxy plugs are frequently found in stomatal antechambers in leaves from these habiats (Mohammadian, 2005). Such features are postulated to increase leaf longevity (England and Attiwill, 2006). Stomatal wax repels water from the vicinity of the antestomatal cavity and prevents germination of fungal spores. However, the antestomatal cavity and wax plug can also provide a niche that enhances survival of phyllosphere fungi. Deckert et al. (2001) examined this plant–fungus relationship on needles of Pinus strobus but found no clear evidence of any mutual benefit.
Creation of a humid micro-environment and improved hydraulic ‘safety’.
The stomatal antechamber represents an additional, invariable and, especially in the case of CO2, a moderating element in the chain of diffusion resistances into or out of a leaf. Numerical modelling has shown that the zone of steep vertical humidity depression above a superficially positioned stomatal pore (cup-shaped concentration isolines) is significantly reduced when the stoma is buried in the antechamber (Roth-Nebelsick, 2007). As a result, relative humidity near the sunken guard cells increased to 75 %, compared with 65 % for a non-sunken stoma. Hassiotou et al. (2009a) speculated that the additional fixed resistance imposed by the antechamber creates a safety buffer, which attenuates negative impacts of rapid variation in the ambient environment, such as from wind effects that reduce boundary layer thickness or sunflecks that suddenly warm leaves. A more humid and stable microclimate in stomatal crypts filled with hairs and/or waxy plugs may thus be possible, thereby enabling greater stomatal conductance at higher leaf temperatures and leaf to air VPDs. In experiments with the rain forest conifer Agathis robusta, Mohammadian (2005) observed maximum photosynthesis at a higher temperature (30 °C) in leaves with intact waxy plugs than in leaves where the plugs had been artificially removed (25 °C). He suggested that stomata in the empty crypts were exposed to less humid air and had partially closed, relative to the situation where plugs were intact. An additional benefit of the buffering effect of sunken stomata is that mesophyll resistance to CO2 entry could be reduced with respect to LMA, given the disproportional shift of the overall resistance in the diffusion path to the water flux pathway that occurs with sunken stomata (Hassiotou et al., 2009b).
Numerical 3-D simulation of water vapour diffusion across crypts of Banksia ilicifolia showed that the crypts cannot be considered as an invariable (fixed) conductance element (Roth-Nebelsick et al., 2009). Their conductance depends not only on their shape and dimensions but also on the level of stomatal opening and relative humidity inside the crypt: the crypt conductance decreases with increasing stomatal conductance and with rising relative humidity. For example, the authors showed that crypt conductance decreased by 30, 21 and 10 % for narrow, standard and large stomatal pore size, respectively, when relative humidity inside the crypt increased from 50 % to 80 %. In spite of the generally small effect of crypts on transpiration rate (10–15 % reduction) for narrow and intermediate stomatal openings, which the authors predicted, a much higher reduction (48 %) was calculated for an extremely large stomatal conductance. However, the ‘large’ values of conductance used in the simulation model were about ten times higher than those measured by gas exchange in B. ilicifolia, which makes a reduction of almost 50 % unrealistic. The lack of information about water sources inside the crypts, i.e. stomata, hairs and cuticle, and about the actual relative humidity inside crypts leaves the role of crypts in controlling transpiration rate unresolved.
Do stomata in deep pits or crypts respond differently from those located superficially? The solution to this problem is currently restricted by the lack of methods allowing separation of stomatal and cuticular contributions to leaf surface transpiration and conductance in planta. Without accounting for cuticular conductance, it is hardly possible to make any judgements about stomata, especially when fluxes through both the cuticle and stomata are of the same order of magnitude, as is the case in darkness or in drought-stressed plants.
Here, I hypothesize that the increased level of humidity within the stomatal crypt, or any other specific trait of the antechamber climate, could modify the behaviour of encrypted stomata. For example, stomata could remain open for an extended period of time during the day, contributing to increased carbon gain. Also a larger opening at night could bring benefits, especially for plants that suffer from nutrient deficiency and depend for their supply on continuous nocturnal xylem flux (Scholz et al., 2007; Rohula et al., 2014). Shortage of nutrients on nutrient-poor soils leads to the evolution of sclerophyllous species with thick and hard leaves and well-developed papillae around the stomata. In order to test the hypothesis of a specific behaviour of sunken stomata at night, a technique allowing partitioning of the leaf surface conductance in the dark into its components was developed and employed here. The technique is described in the Appendix. Stomatal and cuticular conductance at the dark–light and light–dark transitions was measured in seven species with antestomatal chambers with different degrees of prominence.
MATERIALS AND METHODS
Plant species and growth conditions
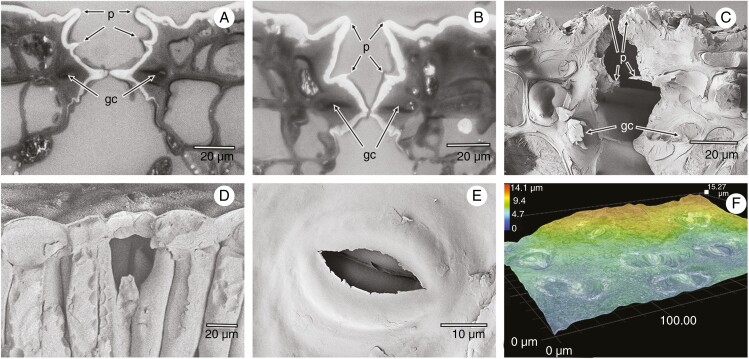
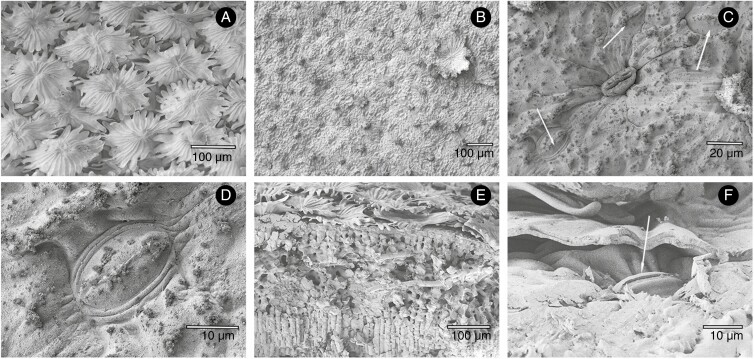
To test the above hypothesis, the following set of species with well-pronounced antechambers of different types was investigated: Nerium oleander L. (oleander), a Mediterranean sclerophyte shrub with typically voluminous crypts, each accommodating numerous stomata and filled with a dense mesh of trichomes (Fig. 1A–F); Ficus elastica Roxb. ex Hornem. (fig tree), a hemi-epiphyte native to tropical Asia, having large antechambers with one stoma at the bottom and no trichomes inside the crypt (Fig. 3A–C); Clusia rosea Jacq. (autograph tree), a tropical tree native to the Caribbean, with smooth leaves covered by a deep layer of epicuticular wax and medium-sized stomatal antechambers (Fig. 3D, F); and Olea europaea L. (olive tree), a sub-tropical dry-forest tree native to Africa, Asia Minor and the Mediterranean region, whose abaxial leaf side is fully covered with disc-shaped trichomes hiding the stomata beneath (Fig. 4A–F). All four species are hypostomatous, with thick leaves and relatively slow growth. For comparison, I included a set of three fast-growing annual species with thin amphistomatous leaves bearing relatively small-volume stomatal antechambers formed only by the papillae and the wall of thin epidermal cells: Arabidopsis thaliana L. (arabidopsis, Fig. 5A, D), Capsicum annuum L. (pepper, Fig. 5B, E) and Brassica oleracea (broccoli, Fig. 5C, F).
Fig. 3.
Antestomatal chambers of Ficus elastica (A–C) and Clusia rosea (D–F) leaves. Optical microscope images of semi-thin leaf transversal sections (A, B) and cryo-SEM image (C) of transects through stomatal antechamber pits of F. elastica, guard cells and through the underlying substomatal cavity. The pits are arched with external papillae (p). Guard cells (gc), located about 20–40 µm deep at the bottom of pits, produce internal papillae (p). The whitish layer in (A) and (B) covering the leaf surface and antechamber walls and protruding into the sub-stomatal cavity consists of epicuticular wax, indicating the existence and extent of an internal cuticle. Cryo-SEM images (D, E) and the digital microscope (Keyence WHX-7000) relief map (F) of C. rosea show the sub-stomatal cavity (D), upper view into the antestomatal chamber (E) and shallow elliptical furrows around the external stomatal papillae (F).
Fig. 4.
Abaxial surface and section through an Olea europaea leaf. The leaf is covered with flat, disc-shaped trichomes (A), which fully cover the stomata underneath (arrows in C, F and D) and form a special type of crypt that can be uncovered when the trichome is broken (F). The disc trichomes can be removed (torn off) with adhesives (here: collodion) and the stomata exposed (B–D). The abaxial surface and a transect through mesophyll tissue is shown in (E).
Fig. 5.
Surface and transect cryo-SEM views of leaves of Arabidopsis thaliana (A, D), Capsicum annuum (B, E) and Brassica oleracea (C, F). Abaxial surface of A. thaliana with transect of guard cells (gc) and papillae (p) (A) and a surface view of stomata (D) with the aperture formed by cuticular ledges (papillae) arching over a shallow antestomatal cavity. Freeze fracture through guard cells of C. annuum with papillae arching over a shallow antestomatal chamber (B, E). Freeze fracture of B. oleracea leaf showing a trans-sectional (C) and longitudinal (F) imprint of guard cells and papilla, delimiting a small antestomatal cavity (triangle in C) filled with cuticular wax. Epicuticular wax crystals are visible on the leaf surface.
Plants from the ‘large antechamber’ group were obtained from a local garden shop as 3- to 4-year-old saplings and grown in a glasshouse for 3–7 months (N. oleander and O. europaea) or for 3–5 years (C. rosea and F. elastica) before the gas exchange measurements. The F. elastica plants were acclimated to deep shade conditions [photosynthetic photon flux density (PPFD) 0–40 μmol m–2 s–1]. Plants from the ‘small antechamber’ group were grown from seeds in 1 L pots filled with garden substrate (C. annuum and B. oleracea) or in approx. 100 mL pots (A. thaliana) in a growth chamber (Percival Scientific, Inc, USA). The PPFD was 450 ± 50 μmol m–2 s–1 (C. annuum and B. oleracea) or 250 ± 50 m–2 s–1 (A. thaliana), day/night temperatures 25/17 °C at a 16 h photoperiod, and the free atmospheric CO2 concentration was 360–420 µmol mol–1. Four to six different plants from C. rosea, A. thaliana, C. annuum and B. oleracea were used, and one plant each from the other species. Four to twelve attached leaves per species were tested for nocturnal stomatal opening. All plants were regularly watered, keeping the soil surface moist.
Theory
The principle for estimating nocturnal stomatal conductance with gas exchange measurements is explained in the Appendix. Briefly, the light response of net CO2 exchange rate, A, [photosynthesis minus (photo-)respiration rate] was measured. The data were used to plot A vs. the leaf internal CO2 concentration, ci, showing the effects of both ci and light on the rate of net CO2 exchange. Typically, when the A(ci) relationship is analysed, ci is manipulated by varying the external CO2, ca. Here, ci varied in response to the light-dependent A, and both were recorded when the leaf total conductance for water vapour, gtw, reached a steady state. Thus, each point of the A vs. ci plot as shown in the Appendix (Fig. A1) was recorded at a different incident light intensity (PPFD). Special attention was given to the A and ci values at zero or very low light levels (0–5 μmol photons m–2 s–1), i.e. to the dark–light transitions in the morning (at artificial dawn) or the light–dark transitions after the daily light period (at artificial dusk). Typically, the A and ci values fall on a straight line, which indicates that the flux (A) is linearly proportional to its driving force (ca–ci) and the conductance invariant during the dark–light–dark transitions. The slope of the line corresponds to the stomatal (gaseous) conductance for CO2, gsc, in the dark, and can be converted to water vapour conductance of stomata, gsw, using the ratio of water and CO2 diffusivities (DH2O/DCO2 = 1.6). The calculations of ci used as a standard in gas exchange analysis are erroneous due to the incorrect assumption of zero water flux through the cuticle. However, the gsc slope is insensitive to the erroneous calculations of ci provided that the stomatal and cuticular conductances to water (gsw and gcw) do not change during the dawn or dusk measurements. The knowledge of stomatal conductance to water, together with that of leaf total conductance gtw as estimated with a standard gas exchange device, allows the calculation of cuticular conductance for water and partitioning of gtw.
Gas exchange measurements
The portable photosynthetic system LI-6800 (LI-COR, Lincoln, NE, USA) has been used to record the light-driven A(ci) relationship. Measurements started in the morning with the following leaf chamber settings: air flow through the chamber 250 μmol (air) s–1, chamber - ambient pressure difference (ΔP) 0.1 Pa, leaf–air water VPD 1.2 kPa, CO2 concentration in the leaf cuvette 410 μmol mol–1, leaf temperature 20 or 25 °C (specific for each species and selected for being closest to the ambient temperature) and fan speed 10 000 rpm. The 6 × 6 cm2 leaf chamber (6800-13) equipped with the large source (6800-03) of white light was used for all species. Typically, the following light levels were applied with 30–50 min intervals between the measurements: 0, 1, 3, 5, 10, 20, 50, 100, 300, 600, 1000, 1200, 7, 4, 2, 1 and 0 μmol m–2 s–1. Usually, recording the full light response took between 8 and 14 h so the light–dark transition was applied close to actual dusk. The leaf area exposed in the leaf chamber was estimated after the measurements by weighing a paper replica of the leaf, and data were recalculated accordingly. The high irradiance level (PPFD ≥ 1000 μmol m–2 s–1) was omitted in the low-light-acclimated species (F. elastica). To investigate the effect of the disc-shaped trichomes covering stomata on O. europaea leaves, the trichomes were removed (torn off, see Fig. 4B–D) using collodion (a solution of nitrocellulose in diethyl ether and ethanol, Merck, Germany). The collodion treatment and light response measurements were carried out with the same leaf, which was measured intact on the previous day.
Cryo-scanning electron microscopy
A fresh leaf sample was transferred onto a holder with a slit and clamping mechanism, glued on with Tissue-Tek and frozen by plunging it into liquid nitrogen slush. After freezing, the sample was transferred to a high vacuum cryo-preparation chamber (ALTO 2500, Gatan), where the sample was fractured at –160 °C, sublimated at –95 °C for 2 min and coated with 5 nm of gold at –135 °C. The coated sample was inserted into a Field Emission Electron Microscope (JSM-7401-F, JEOL) pre-cooled at –135 °C. Images were obtained using an Everhart–Thornley detector at an accelerating voltage of 1.5 kV in Gentle Beam (GB) low mode.
Data evaluation and statistics
Determination of the regression coefficients relating A to ci at the dark–light and light–dark transitions was the critical step in separation of cuticular and stomatal conductance. Usually, either three or four [A;ci] points were used for each artificial dawn and dusk transition. The slope of the best fit, with the coefficient of determination being nearest to one, was used for further evaluation. The slopes and coefficients of determination were determined using the MS Excel software package, which was also used for corrections of the slopes for CO2 permeability of the cuticle and the partitioning of leaf total conductance into its stomatal and cuticular fractions. Means and standard deviations were calculated using the MS Excel or SigmaStat packages.
RESULTS
Leaf water conductance in the dark (at dawn and dusk)
Leaf surface conductance, gtw, was higher at dawn than at dusk in all species (Fig. 6A, B, horizontal axis). The former ranged from 0.4 to 99.3 mmol m–2 s–1 and the latter from 0.2 to 86.6 mmol m–2 s–1 across all species except for B. oleracea. Dark conductance in B. oleracea ranged up to 362 mmol m–2 s–1 at dawn (Fig. 6A). The dawn conductance exceeded that at dusk by a factor of almost two in the sunken stomata group (gtw dawn = 100 ± 83 mmol m–2 s–1 and gtw dusk = 57 ± 71 mmol m–2 s–1, mean ± s.d.) while their ratio in arabidopsis was only 1.2 (gtw dawn = 286 ± 124 and gtw dusk = 222 ± 131 mmol m–2 s–1).
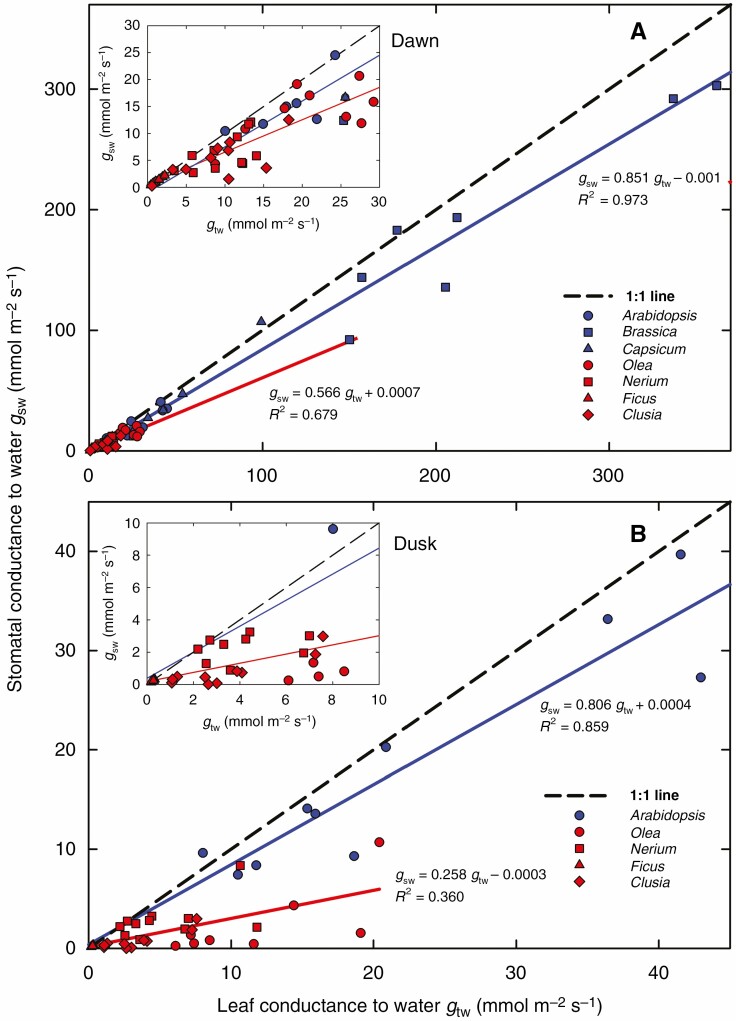
Fig. 6.
Contributions of stomatal and cuticular conductance to total leaf water conductance at the dark–light (dawn) and light–dark (dusk) transitions. Stomatal conductance gsw was estimated from the regression of A vs. ci as described in the Appendix [eqn (A7)] while the total conductance gtw was measured with a gas exchange device (LI-6800). Values found at artificial dawn (A) and dusk (B) are shown. Each point indicates a measurement on an individual leaf in one of four species characterized by well-pronounced stomatal antechambers (N. oleander, F. elastica, O. europaea and C. rosea, red symbols and red regression line) and in a group of species with superficial location of stomata (A. thaliana, C. annuum and B. oleracea, solid blue symbols and lines). The dusk data for superficial stomata include only A. thaliana. Insets depict low gtw ranges at higher resolution. The 1:1 lines (black dashed) indicate the theoretical situation where stomata fully account for total conductance gtw. Slopes of the regression lines indicate the estimated relative contributions of stomata.
Stomatal conductance at dawn and dusk
In all plant species and in the majority of measurements, the coefficients of determination of the regressions relating A to ci were close to 1 at dawn (R2 = 0.984 ± 0.024, n = 76) and slightly lower at dusk (0.946 ± 0.076, n = 53) (means ± s.d.). Slopes of the regression lines, which provide the stomatal conductance for CO2, gsc, were converted to stomatal conductance for water, gsw, using eqn (A7). In the vast majority of measurements, gsw was lower than the leaf conductance for water, gtw, obtained directly from standard gas exchange measurements, so almost all data points lie below the 1:1 line in Fig. 6A and B.
Stomatal conductance in the dark increased with increasing total leaf conductance in an approximately linear pattern for both the species with sunken stomata (red line in Fig. 6) and species with superficial stomata (blue line). The vertical distance between the coloured lines and the x-axis indicates the stomatal fraction of total leaf conductance, while the vertical distance between the coloured and 1:1 lines shows the cuticular fraction. The slopes of the regression lines indicate that stomata accounted for 85 and 57 % of leaf conductance at dawn in the superficial and sunken stomata groups, respectively (Fig. 6A), while at dusk the respective stomatal contributions decreased to 81 and 26 % (means across all species in the respective group).
Response of sunken stomata
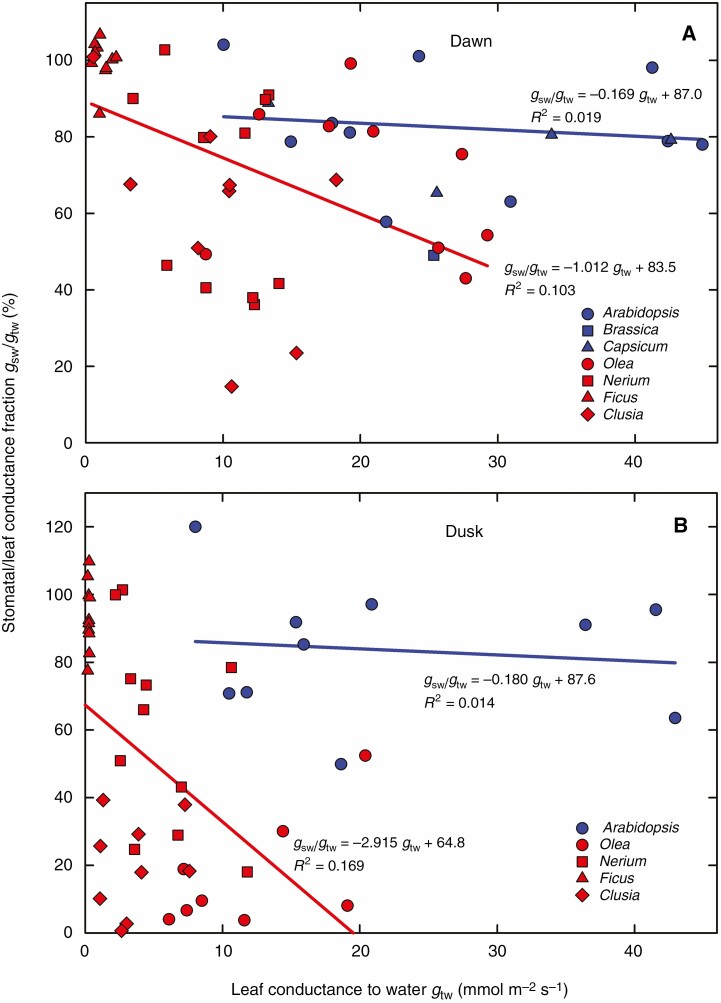
The linear approximations in Fig. 6 fail to uncover the group-specific stomatal behaviour, especially at dawn (Fig. 6A). A closer look at the stomatal contribution to leaf conductance in Fig. 7 reveals that the prevalence of stomatal over cuticular conductance in darkness persists in the species with superficial stomata over the whole range of leaf conductances (see blue lines in Fig. 7). In contrast, the fraction of stomatal over total leaf conductance decreased with increasing leaf conductance in species with sunken stomata (red lines). At both dawn and dusk, superficial stomata were responsible for about 80–87 % of leaf conductance. In contrast, the relative contribution of sunken stomata decreased with increasing total leaf conductance, and the sensitivity of stomata to gtw (slope of the red line) was even higher at dusk than at dawn.
Fig. 7.
Fraction of stomatal to total leaf conductance at dawn (A) and dusk (B). Points indicate individual measurements from all investigated species with well-pronounced stomatal antechambers (N. oleander, F. elastica, O. europaea and C. rosea, red symbols and red regression lines) and ten measurements on different A. thaliana leaves with a superficial location of stomata (solid blue symbols and lines). The ratio gsw/gtw is shown for the morning period – at dawn (A) – and for the evening when the measurements of light response of photosynthesis with the same leaf were concluding − at dusk (B).
Cuticular and stomatal conductance in the dark: interspecies comparison
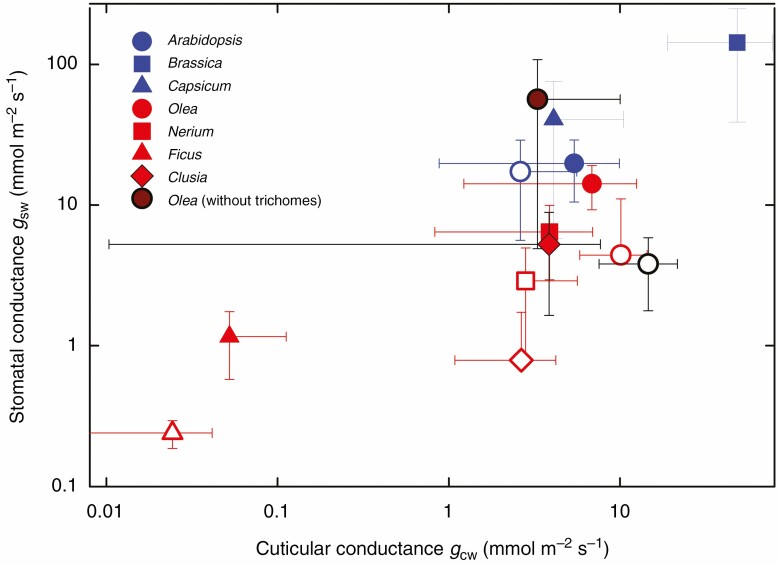
Stomatal conductances in darkness span almost three orders of magnitude across all seven species (0.24–143 mmol m–2 s–1, see Fig. 8). Cuticular conductance was proportional to stomatal conductance, but its range of absolute values was somewhat less (0.05–48.5 mmol m–2 s–1). In both cases, the extremes belong to F. elastica and B. oleracea. The means of cuticular conductance in the other five species ranged within a relatively narrow interval from 1 to 10 mmol m–2 s–1, while stomatal conductance was between 5 and 40 mmol m–2 s–1. After the leaf trichomes on O. europaea have been removed, gsw at dawn increased by a factor of 4 and returned to the value of the non-treated leaves at dusk. In contrast, cuticular conductance decreased to one half in the leaves without trichomes at dawn and increased 3-fold at dusk. Interestingly, the gsw values at dawn were higher than those at dusk in each species and in the vast majority of leaves that were measured, but the pattern was ambiguous in the case of cuticular conductance without any trend of gcw between or within species (Supplementary data Fig. S1).
Fig. 8.
The relationship between stomatal and cuticular conductance in seven species with stomatal antechambers of different prominence. Means and standard deviations of gsw and gcw at dawn (solid symbols) and dusk (open symbols) of four species with well-pronounced stomatal antechambers (N. oleander, F. elastica, O. europaea and C. rosea) and three species with superficially located stomata with less pronounced low-volume antechambers (A. thaliana, C. annuum and B. oleracea) are presented. Only the dawn values for B. oleracea and C. annuum are shown. All means and standard deviations were calculated from 6–10 measurements on different leaves. Note that the scales on both axes are logarithmic. The black-rimmed circles indicate O. europea leaves with trichomes removed by collodion (see also Fig. 4B–D).
DISCUSSION
Sunken stomata have traditionally been examined by describing that what is readily visible – the antechamber cavity, its shape, volume or content (Jeffree et al., 1971; Weyers and Meidner, 1990; Willmer and Fricker, 1996; Brodribb and Hill, 1997; Larcher, 2001; Roth-Nebelsick, 2007; Haworth and McElwain, 2008; Hassiotou et al., 2009a; Roth-Nebelsick et al., 2009). The functional implications of the structure of the guard and subsidiary cells, and the mechanical and physiological consequences of their being sunken have been less investigated (Feild et al., 1998; Eamus et al., 2008; Mott and Peak, 2013). Here I address this deficiency by examining the behaviour of sunken and superficial stomatal complexes in response to identical environmental drivers. As a prerequisite, it was necessary to design a technique allowing measurements of stomatal conductance without the interference of cuticular water loss. It was more feasible to do this for darkened leaves. Therefore, the discussion starts with a phenomenon that is generally acknowledged and experimentally more accessible – nocturnal transpiration – and specific effects of the formation of crypts on stomatal and cuticular function on that process.
Stomatal conductance dominates nocturnal leaf conductance
Nocturnal transpiration is a phenomenon that has been explored frequently during the last decades but its significance and ecological diversity are still unclear (de Dios et al., 2019; Fricke, 2019). The leaf surface conductance (gtw) at dawn and dusk ranged, in the measurements presented here, from some tens to several hundred mmol (H2O) m–2 s–1 across seven selected species and was higher at dawn than at dusk in all but two of 49 measured intact leaves (Supplementary data Fig. S1). The dawn vs. dusk difference in gtw seems to be a ubiquitous feature, probably controlled by the endogenous circadian clock (Caird et al., 2007; Costa et al., 2015). Interestingly, the amplitude of the drawn–dusk oscillations was higher in species with sunken stomata. I can only speculate about the reasons, which might arise from the greater diurnal fluctuation of turgor in sclerophyllous vs. mesophytic leaves, a different type of osmoregulation in guard cells (Lasceve et al., 1997) and/or specific cuticular traits in species with sunken stomata. Dawn and dusk conductances correlated in the data here with maximum stomatal conductance (Supplementary data Fig. S2) and photosynthetic rate (not shown) during the light response measurements. The correlation supports the hypothesis that nocturnal transpiration promotes leaf metabolism and plant growth, possibly by flushing respiratory CO2 out of the leaf and avoiding toxic CO2 concentrations in mesophyll cells (Fricke, 2019).
The partitioning of conductance in (near)darkness was a prerequisite of the experimental part of this study. The method, which was designed and applied here (see Appendix), is in principle similar to that published recently by Marquez et al. (2022). Both methods use a standard commercial gas exchange device for in planta measurements, but the technique of Marquez et al. was developed for red-light-illuminated leaves while the technique presented here was for measurements in the dark or in light–dark transitions. Nevertheless, both methods yielded comparable results. For example, cuticular conductance for water in C. annuum leaves was about 8 mmol m–2 s–1 and 6.5 mmol m–2 s–1 in the measurements of Marquez et al. and in this study, respectively. Cuticular conductance at dawn and dusk averaged over all seven species measured here was 7.8 and 3.7 mmol m–2 s–1, respectively, which is in the range of values reported for 160 species in the recent review of Schuster et al. (2017).
I hypothesized that increased stomatal opening and transpiration in darkness could provide a selective advantage to plant species with sunken stomata. They often are epiphytes, or sclerophytes inhabiting nutrient-poor soils, and nocturnal transpiration could increase the efficiency of water used for nutrient uptake and/or increase nutrient acquisition per se (Scholz et al., 2007; Rohula et al., 2014). Leaf surface conductance in the dark, gtw, was high in three thin-leafed amphistomatous species with superficial stomata (arabidopsis, pepper and broccoli, 23–232 mmol m–2 s–1) and low in the group of thick-leafed hypostomatous species with encrypted stomata (fig tree, oleander, autograph tree and olive tree, 1–20 mmol m–2 s–1). Stomata were usually responsible for more than half of the leaf conductance in darkness but, contrary to my hypothesis, they dominated more when located on the leaf surface (about 80 % of gtw at both dawn and dusk) and less when embedded in antechambers (73 % of gtw on average across four species). The dominance of stomata in nocturnal transpiration has been assumed in the literature but, to date, experimental evidence had been lacking. The assumption was based on the comparison of relatively high nocturnal leaf conductance (median of 40 mmol m–2 s–1 in a global dataset, de Dios et al., 2019) with the substantially lower cuticular conductance assessed by various techniques [median of 0.74 mmol m–2 s–1 obtained from measurements of isolated cuticles or astomatous leaf sides of 57 species, Kerstiens (1996); median of 1.47 mmol m–2 s–1 across a set of 160 species, Schuster et al. (2017); mean of 4.89 mmol m–2 s–1 across 221 species, measured as mass loss from detached leaves, Duursma et al. (2019)]. Cuticular conductance in most of the species examined here ranged from 1 to 10 mmol m–2 s–1 except for F. elastica with a gcw of 0.05 and 0.02 mmol m–2 s–1 at dawn and dusk, respectively (Fig. 8).
Are stomata in crypts controlled by water loss across the cuticle?
As mentioned above, the sunken stomata were less open in darkness and, across four species, they accounted for a smaller fraction of total leaf conductance than the superficial stomata (Fig. 6). It seems that they are more conservative compared with the non-sunken stomata. However, when looking at the dynamics of their contribution to the total leaf conductance, the opposite picture emerges: stomata in deep voluminous pits control their fraction of dark transpiration more sensitively while superficial stomata keep their proportion invariable (Fig. 7). This seems to be a unique feature of sunken stomata across the four investigated species, though this feature is not well preserved intraspecifically, i.e. when each species is observed individually (Supplementary data Fig. S3). The explanation for the greater stomatal response among the four species with sunken stomata is not clear. It could possibly be based on stomata–cuticle interactions. Eamus et al. (2008) have shown that the stomatal aperture response to leaf-to-air VPD is mediated by cuticular permeability in Eucalyptus, Commelina and Vicia, and a similar response may explain the observations of Feild et al. (1998) in Drimys, a tropical wet-forest tree with encrypted stomata. The markedly enlarged area of the leaf cuticle in species with stomata sunken in crypts may enhance this effect (Roth-Nebelsick et al., 2009).
Total leaf conductance in the dark, gtw, and dark transpiration rate were proportional to each other under the experimental conditions here because the driving force for evaporation (VPD) was kept constant. Low gtw implies a low rate of water loss, which should increase in parallel with rising gtw. Results show that in species with sunken stomata, the contribution of stomatal conductance to gtw decreased relative to the contribution of cuticular conductance, gcw, at higher transpiration rates at night. In short, each increment in dark transpiration occurs more through the cuticle than through the stomata. This stomata–cuticle complementarity is much more pronounced among species with antechamber-embedded stomata and might result from the phenomenon traditionally called peristomatal transpiration (Maier-Maercker, 1983). As the term indicates, water evaporation directly from the exposed surface of guard cells is assumed to be higher than that from other epidermal cells. The sunken guard and/or subsidiary cells may be covered with a more permeable cuticle that induces closing of the stomatal pore when water flux through the cuticle is rising. Higher water permeability of the stomata-bearing cuticle compared with the astomatous one was reported for several species (Santrucek et al., 2004; Karbulkova et al., 2008; Marquez et al., 2022) and could close stomata by reducing guard cell turgor. Nevertheless, an unambiguous and truly reliable dataset that quantifies permeability of the guard cell and pavement cell cuticles is lacking. Cuticular permeability per se can also be affected by the humidity of the surrounding air. It has repeatedly been shown that the permeability increases at elevated water vapour pressure or relative humidity (Hoad et al., 1997; Schönherr and Merida, 1981; Schreiber et al., 2001). Cuticle shrinkage or swelling induced by changes in leaf turgor are accompanied by changes in cuticular permeability (Boyer, 2015b) and might also contribute to the lower stomatal and variable cuticular conductance at dusk compared with dawn (Fig. 8; Supplementary data Fig. S1).
Other mechanisms affecting sunken stomata
It has been shown several times that the response of stomata to humidity is affected predominantly by the water potential of neighbouring cells rather than directly by the humidity of the surrounding air (Nonami et al., 1991; Buckley, 2005). Guard cells located at the bottom of an antestomatal cavity may experience smaller fluctuations of water potential in response to variations in leaf energy budgets (resulting from changes in ambient temperature or irradiance). Their location deeper in the leaf interior results in a higher resistance to transfer of heat from the leaf surface to evaporation sites and, conversely, to a lower resistance for water vapour diffusing from evaporating sites to guard cells (Mott and Peak, 2013). These authors verified that the ratio of these two resistances is much higher in N. oleander with stomata in crypts than in Xanthium strumarium and Pastinaca sativa, with more superficially positioned stomata, and speculated that it could help the plants with sunken stomata to maintain an isohydric water regime in harsh conditions. Isohydry is characterized by a relatively stable mid-day leaf water potential independent of seasonal or daily fluctuations in pre-dawn water potential of the plant shoot, so stomata of isohydric species are more responsive to fluctuating transpiration or VPD (Franks et al., 2007). Isohydry typically occurs in trees of xeric environments, for example in savannah trees, and occurs in environments with high potential risk of embolism and high costs of its repair (Bucci et al., 2005). In contrast, stomata of anisohydric plant species, usually fast-growing crops or annuals such as sunflower, respond less sensitively to changes in transpiration rate or VPD. The stomatal responses of the two groups of species investigated here, one with sunken and the other with superficial stomata, resemble the stomatal response of iso- and anisohydric species, respectively. I can only speculate as to whether the apparent isohydry of the species with markedly sunken stomata is just a coincidence or whether there is a causal link between guard cells ‘hidden’ in deep antestomatal chambers and their response to evaporative demand.
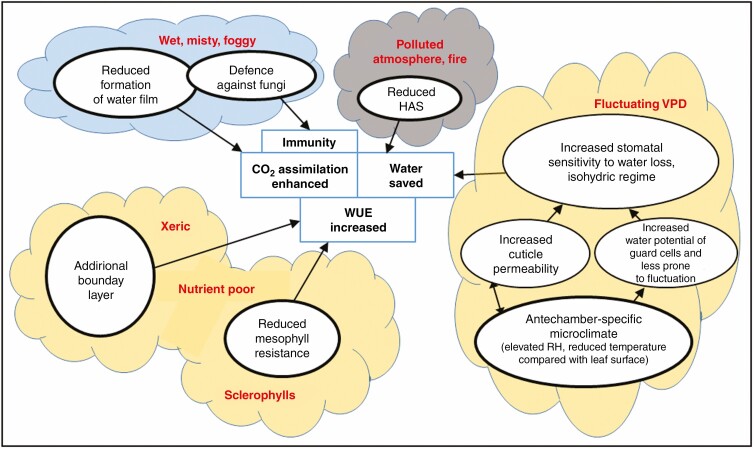
CONCLUSION
Sunken stomata have multiple functions. Their advantages in xeric and/or infertile conditions with fluctuating soil water availability and VPD are readily apparent, linked to water saving and avoidance of fatal, unrepairable depressions in leaf water potential and embolism (Fig. 9). Guard cells devoted to this purpose follow the isohydric mode of operation, i.e. they sensitively control transpiration, keeping the water potential stable and above the critical threshold for embolism (Tardieu and Simonneau, 1998). The results presented here indicate that, indeed, the species with sunken stomata control even the mild night-time transpiration more sensitively than the species with superficially located stomata.
Fig. 9.
The multipurpose character of antestomatal chambers. At least four different and/or subsequently acting selective presures contributed to evolution of antestomatal chambers: (i) xeric and/or nutrient-defficient soil environment; (ii) VPD fluctuating atmosphere; (iii) polluted atmosphere and fire-prone environment; and (iv) frequent occurrence of a water film on the leaf surface (see the four ‘clouds’ indicating relevant environmental conditions or leaf morphotypes, e.g. hard thick sclerophyllous foliage). Plant species adapted to the particular environment may profit from their antestomatal chambers via physical mechanisms affecting water, CO2 transport, stomatal behaviour or defending against leaf surface pathogens (ovals with bold lines) and leading to at least one of four benefits shown in the rectangular boxes. The underlying physical mechanisms including hydraulic activation of stomata (HAS) are described in detail in the text. The three main benefits to gas exchange provided by stomatal antechambers and sunken stomata can by coupled proportionally, e.g. water savings usually increase water use efficiency (WUE), but may act independently, optimize carbon gain or water loss depending on the environment and affect the plant physiology status. The arrows indicate the line and direction of causality in the short term; the long-term evolutionary feedbacks are not considered here. However, a mutual effect of cuticle permeability and antechamber climate can also be expected in the short-term range. Wax plugs, hairs or internal leaf cuticle often accompany stomatal antechambers and contribute to all physical mechanisms shown here. The antechamber-specific microclimate could increase stomatal sensitivity to the transpiration rate as indicated by experiments in this study. Two possible links between the stomatal sensitivity and antechamber climate, i.e. cuticle permeability increasing with relative humidity (RH) and guard cell water potential stabilized by relatively steady antechamber humidity and temperature, are suggested.
Stomatal antechambers evolved many times over and may have been shaped perhaps by selection for functions other than to resist xeric conditions, although this ‘pre-adaptation’ might have been valuable in later evolution (Mast and Givnish, 2002). It is possible that the dominant primary function was protection against the formation of continuous water films that could prevent assimilatory CO2 flux into the leaf (Fig. 9). Although this function is based on very different physical mechanisms, it results in a benefit almost identical to the previous one – keeping carbon flux for photosynthesis running. Both the functioning of encrypted guard cells and reduced leaf wettability share a common link in (epi)cuticular waxes, their structure and chemical composition, which brings another challenge for future research. Finally, the ‘sequestration’ of stomata in various leaf depressions, crypts or leaf grooves confers a broad range of protection from abiotic (mechanical, airborne particle, radiation or temperature) stresses and biotic attacks to stomata.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: the dawn–dusk effect on stomatal and cuticular conductance. Figure S2: the relationship between maximum leaf conductance at light saturation and leaf conductance in darkness. Figure S3: the percentage contribution of stomatal conductance to leaf surface conductance in darkness in seven plant species with stomatal antechambers differing in prominence.
Supplementary Material
ACKNOWLEDGEMENTS
I thank Petra Fialova and Jiří Vaněček for technical and SEM assistance. Special thanks are due to Gerhard Kerstiens (Lancaster, UK) for language revisions, editing and valuable comments to the manuscript, as well as to Rowan Sage, the handling editor of the manuscript, and two anonymous reviewers for their helpful comments and ideas.
Appendix
Partitioning of leaf water loss into its stomatal and cuticular components has been a matter of some interest for several decades, and various attempts to do so experimentally have been made, most recently by Marquez et al. (2022). Here, a gas exchange-based technique is suggested which enables partitioning total leaf transpiration into its cuticular and stomatal fractions and detecting incomplete stomatal closure at dark–light or light–dark transitions, i.e. at dawn and dusk.
Estimation of stomatal conductance for CO2
Net CO2 exchange between a leaf and its environment at steady state, A, can be expressed in its simplest form as
| (A1) |
where g and c are conductance and CO2 concentration, respectively, and the indexes t, c, a and i stand for ‘total’, ‘CO2’, ‘atmospheric’ and ‘leaf internal’, respectively. Total leaf conductance for CO2, gtc, is made up of the conductances of stomata, gsc, cuticle, gcc, and boundary layer, gbc; ci denotes CO2 concentration at surfaces evaporating water inside the leaf. The conductances gsc and gcc are arranged in parallel (CO2 can diffuse via either the stomata or the cuticle) and both the stomata and cuticle pathways are linked in series with the boundary layer (before diffusing through the stomata or cuticle, each CO2 molecule must pass the boundary layer). This arrangement requires for the total conductance for CO2:
| (A2) |
As a first approximation, we may ignore gbc, because it is 2–3 orders of magnitude higher in a ventilated leaf chamber than stomatal conductance in darkness. Further, the cuticle is almost impermeable for CO2 diffusion into or out of the leaf, so gcc is small relative to gsc (Boyer, 2015b) and can be neglected, too, in the first approximation (see below for a revision of this). Then, the total leaf conductance to CO2 can be approximated and substituted in eqn (A1) with stomatal conductance: gtc ≈ gsc.
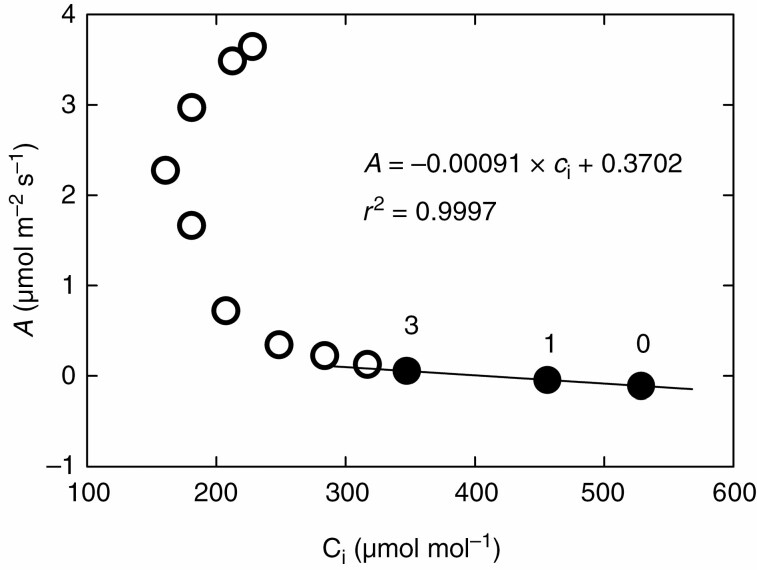
Provided stomata do not change their aperture during measurement, i.e. gsc is invariant, it is possible to determine gsc as the slope of the regression line relating A to the difference ca– ci in eqn (A1), or for constant ca, as the slope of the line relating A to ci. Presumably, stomata are not completely closed in the dark and they start to open further at low but non-zero irradiance. A typical example of such a dark–light transition in terms of the A(ci) response is shown in Fig. A1.
Fig. A1.
CO2 response of the photosynthesis rate at variable irradiance, following stomatal conductance reaching a steady state at each level. A Ficus elastica leaf was exposed to darkness for 40 min in the leaf chamber of a gas exchange system (LI-6800, USA) and, at that point, the photosynthesis rate (A) and leaf internal CO2 concentration (ci) were recorded (the data point marked as 0). Then, the light was increased in steps to 1, 3, 5, 7, 10, 20, 50, 100, 300, 600 and 1000 μmol m–2 s–1 with time intervals of 40 min between steps, and the A and ci values were recorded when the new steady state of leaf water conductance was reached. Other leaf environmental parameters (leaf temperature, leaf–air water vapour pressure difference and CO2 concentration in ambient atmosphere) were kept constant. The values recorded at the dark–light transition (solid circles with PPFD level indicated above them) yielded the parameters of the regression line shown in the graph. The slope of the regression line (–0.00091 mol m–2 s–1) gives the leaf conductance for CO2, gtc, as defined in eqn (A1), which is identical to stomatal conductance for CO2, gsc, if cuticular conductance for CO2 is neglected. The coefficient of determination (r2) being close to 1 indicates that stomata were not responding to low levels of irradiance (0–3 μmol m–2 s–1).
The calculation of ci via photosynthesis measurements is biased, especially at low degrees of stomatal opening, because the unknown contribution of cuticular conductance is ignored and cuticular transpiration is treated in calculations as stomatal water loss (Boyer, 2015a). The error in ci is usually small when stomata are open and their conductance for water vapour is much higher than the cuticular conductance (gsw >> gcw, where the index w indicates water). However in the dark, when stomata are almost closed, the error can be significant (Stinziano et al., 2020).
Is the gsc value, estimated as the slope of the A(ci) regression line shown in Fig. A1, affected by the erroneous calculation of ci yielded by a conventional gas exchange analysis? When the dilution effect of water vapour is ignored for simplicity’s sake, ci is calculated as
| (A3) |
The constant 1.6 represents the ratio of diffusion coefficients, D, of water and CO2 in air (Dw/Dc = gsw/gsc = 1.6). Total leaf conductance for water, gtw, is assessed from the measured leaf transpiration rate and leaf–air water vapour pressure difference (VPD). Cuticular conductance to water (gcw) is assumed to be zero, so gsw is (incorrectly) considered identical to gtw. This concept was first introduced by Moss and Rawlins (1963) and became the common algorithm used by contemporary gas exchange systems for calculating ci values. However, these values overestimate the real ones, which are lower at positive A (and higher at negative A) because gsw also includes gcw. Nevertheless, the gsc value, estimated as the slope of the A(ci) regression line shown in Fig. A1, is not affected by the erroneous calculation of ci in the case where all conductances to water (gsw, gcw and gtw) as well as ca do not change during the measurements for constructing the A(ci) plot. Differentiation of A in eqn (A3), with respect to ci, dA/dci, yields for the slope (gcw – gtw)/1.6, which can be expressed as –gsw/1.6, or –gsc. Provided that gtw, gsw and gcw do not change during the dark–light transition, the [A; ci] values should fall on a line having, in an ideal case, a coefficient of determination equal to 1 and a slope equal to –gsc.
Correction for CO2 flux across cuticle
CO2 diffuses across the lipidic cuticle at a much lower rate than water. Boyer (2015b) estimated that the cuticular conductance for CO2 across the astomatous upper side of grape leaves was about 40 times lower than for water. Total leaf conductance for CO2, gtc, estimated from the slope of the regression line in Fig. A1, should therefore be reduced to account for the cuticular CO2 leakage. Due to the parallel arrangement of stomatal and cuticular pathways, we can write for CO2:
| (A4a,b) |
In air-filled stomatal pores, the ratio of conductances to water and CO2 is equal to the ratio of their diffusion coefficients in air (Moss and Rawlins, 1963):
| (A5a) |
The ratio of the two conductances in the lipidic cuticle is (Boyer, 2015b):
| (A5b) |
Rearrangement of eqns (A4a, b) and (A5a, b) yields for gsc:
where gtc is the slope of the regression line in Fig. A1 and gtw is obtained from gas exchange measurements at the dark–light transition.
Partitioning of total leaf conductance for water vapour into stomatal and cuticular parts
The knowledge of stomatal conductance for CO2 (gsc) allows us to calculate stomatal conductance for water (gsw):
| (A6a) |
The gsw value represents stomatal conductance and, if additional water flows through the cuticle, gsw should always be lower than the leaf surface conductance gtw measured directly with a gas exchange device. Then, the residual conductance can be attributed to the cuticle (gcw):
| (A6b) |
The boundary layer of laminar air at the leaf surface affects water vapour diffusion and, thus, water conductance of the boundary layer, gbw, should to be taken into account. Then, eqn (A6a) modifies to:
| (A7) |
where the term 1.37 relates the rates of water and CO2 diffusing across the laminar boundary layer. The value of gbw is regularly estimated by gas exchange instruments and depends mainly on the speed of the air mixing fan in the leaf chamber.
FUNDING
The work was supported by the Czech Science Foundation (project no. 18-14704S) and also by the BC CAS core facility LEM supported by MEYS CR (LM2018129 Czech-BioImaging and OP VVV CZ.02.1.01/0.0/0.0/18_046/0016045).
LITERATURE CITED
- Bartiromo A, Guignard G, Lumaga MRB, et al. 2013. The cuticle micromorphology of in situ Erica arborea L. exposed to long-term volcanic gases. Environmental and Experimental Botany 87: 197–206. doi: 10.1016/j.envexpbot.2012.10.006. [DOI] [Google Scholar]
- Bickford CP. 2016. Ecophysiology of leaf trichomes. Functional Plant Biology 43: 807–814. doi: 10.1071/fp16095. [DOI] [PubMed] [Google Scholar]
- Blomenkemper P, Abu Hamad A, Bomfleur B. 2019. Cryptokerpia sarlaccophora gen. et sp. nov., an enigmatic plant fossil from the Late Permian Umm Irna Formation of Jordan. Palz 93: 479–485. doi: 10.1007/s12542-019-00466-x. [DOI] [Google Scholar]
- Boyer JS. 2015a. Impact of cuticle on calculations of the CO2 concentration inside leaves. Planta 242: 1405–1412. doi: 10.1007/s00425-015-2378-1. [DOI] [PubMed] [Google Scholar]
- Boyer JS. 2015b. Turgor and the transport of CO2 and water across the cuticle (epidermis) of leaves. Journal of Experimental Botany 66: 2625–2633. doi: 10.1093/jxb/erv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T, Hill RS. 1997. Imbricacy and stomatal wax plugs reduce maximum leaf conductance in Southern Hemisphere conifers. Australian Journal of Botany 45: 657–668. doi: 10.1071/bt96060. [DOI] [Google Scholar]
- Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Campanello P, Scholz FG. 2005. Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in neotropical savanna trees. Trees – Structure and Function 19: 296–304. [Google Scholar]
- Buckley TN. 2005. The control of stomata by water balance. New Phytologist 168: 275–292. doi: 10.1111/j.1469-8137.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- Burgess SSO, Dawson TE. 2004. The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration. Plant, Cell & Environment 27: 1023–1034. doi: 10.1111/j.1365-3040.2004.01207.x. [DOI] [Google Scholar]
- Burkhardt J. 2010. Hygroscopic particles on leaves: nutrients or desiccants? Ecological Monographs 80: 369–399. doi: 10.1890/09-1988.1. [DOI] [Google Scholar]
- Caird MA, Richards JH, Donovan LA. 2007. Nighttime stomatal conductance and transpiration in C-3 and C-4 plants. Plant Physiology 143: 4–10. doi: 10.1104/pp.106.092940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RJ, McLoughlin S, Hill RS, McNamara KJ, Jordan GJ. 2014. Early evidence of xeromorphy in angiosperms: stomatal encryptation in a new eocene species of Banksia (Proteaceae) from Western Australia. American Journal of Botany 101: 1486–1497. doi: 10.3732/ajb.1400191. [DOI] [PubMed] [Google Scholar]
- Costa JM, Monnet F, Jannaud D, et al. 2015. Open all night long: the dark side of stomatal control. Plant Physiology 167: 289–294. doi: 10.1104/pp.114.253369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan IR, Farquhar GD. 1977. Stomatal function in relation to leaf metabolism and environment. Symposia of the Society for Experimental Biology 31: 471–505. [PubMed] [Google Scholar]
- Cussler EL. 1987. Diffusion mass transfer in fluid systems. Cambridge: Cambridge University Press. [Google Scholar]
- Deckert RJ, Melville LH, Peterson RL. 2001. Epistomatal chambers in the needles of Pinus strobus L. (eastern white pine) function as microhabitat for specialized fungi. International Journal of Plant Sciences 162: 181–189. doi: 10.1086/317905. [DOI] [Google Scholar]
- de Dios VR, Chowdhury FI, Granda E, Yao YA, Tissue DT. 2019. Assessing the potential functions of nocturnal stomatal conductance in C-3 and C-4 plants. New Phytologist 223: 1696–1706. [DOI] [PubMed] [Google Scholar]
- Duursma RA, Blackman CJ, Lopez R, Martin-StPaul NK, Cochard H, Medlyn BE. 2019. On the minimum leaf conductance: its role in models of plant water use, and ecological and environmental controls. New Phytologist 221: 693–705. [DOI] [PubMed] [Google Scholar]
- Eamus D, Taylor DT, Macinnis-Ng CMO, Shanahan S, De Silva L. 2008. Comparing model predictions and experimental data for the response of stomatal conductance and guard cell turgor to manipulations of cuticular conductance, leaf-to-air vapour pressure difference and temperature: feedback mechanisms are able to account for all observations. Plant, Cell & Environment 31: 269–277. doi: 10.1111/j.1365-3040.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- England JR, Attiwill PM. 2006. Changes in leaf morphology and anatomy with tree age and height in the broadleaved evergreen species, Eucalyptus regnans F. Muell. Trees – Structure and Function 20: 79–90. [Google Scholar]
- Feild TS, Zwieniecki MA, Donoghue MJ, Holbrook NM. 1998. Stomatal plugs of Drimys winteri (Winteraceae) protect leaves from mist but not drought. Proceedings of the National Academy of Sciences, USA 95: 14256–14259. doi: 10.1073/pnas.95.24.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Drake PL, Froend RH. 2007. Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant, Cell & Environment 30: 19–30. doi: 10.1111/j.1365-3040.2006.01600.x. [DOI] [PubMed] [Google Scholar]
- Fricke W. 2019. Night-time transpiration – favouring growth? Trends in Plant Science 24: 311–317. doi: 10.1016/j.tplants.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Gray A, Liu L, Facette M. 2020. Flanking support: how subsidiary cells contribute to stomatal form and function. Frontiers in Plant Science 11: 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassiotou F, Evans JR, Ludwig M, Veneklaas EJ. 2009a. Stomatal crypts may facilitate diffusion of CO2 to adaxial mesophyll cells in thick sclerophylls. Plant, Cell & Environment 32: 1596–1611. doi: 10.1111/j.1365-3040.2009.02024.x. [DOI] [PubMed] [Google Scholar]
- Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR. 2009b. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. Journal of Experimental Botany 60: 2303–2314. doi: 10.1093/jxb/erp021. [DOI] [PubMed] [Google Scholar]
- Haworth M, McElwain J. 2008. Hot, dry, wet, cold or toxic? Revisiting the ecological significance of leaf and cuticular micromorphology. Palaeogeography Palaeoclimatology Palaeoecology 262: 79–90. doi: 10.1016/j.palaeo.2008.02.009. [DOI] [Google Scholar]
- Hill RS. 1998. Fossil evidence for the onset of xeromorphy and scleromorphy in Australian proteaceae. Australian Systematic Botany 11: 391–400. doi: 10.1071/sb97016. [DOI] [Google Scholar]
- Hoad SP, Grace J, Jeffree CE. 1997. Humidity response of cuticular conductance of beech (Fagus sylvatica L.) leaf discs maintained at high relative water content. Journal of Experimental Botany 48: 1969–1975. [Google Scholar]
- Jeffree CE, Johnson RPC, Jarvis PG. 1971. Epicuticular wax in stomatal antechamber of sitka spruce and its effects on diffusion of water vapour and carbon dioxide. Planta 98: 1–10. doi: 10.1007/BF00387018. [DOI] [PubMed] [Google Scholar]
- Jordan GJ, Weston PH, Carpenter RJ, Dillon RA, Brodribb TJ. 2008. The evolutionary relations of sunken covered, and encrypted stomata to dry habitats in proteaceae. American Journal of Botany 95: 521–530. doi: 10.3732/ajb.2007333. [DOI] [PubMed] [Google Scholar]
- Jordan GJ, Carpenter RJ, Brodribb TJ. 2014. Using fossil leaves as evidence for open vegetation. Palaeogeography Palaeoclimatology Palaeoecology 395: 168–175. doi: 10.1016/j.palaeo.2013.12.035. [DOI] [Google Scholar]
- Karbulkova J, Schreiber L, Macek P, Santrucek J. 2008. Differences between water permeability of astomatous and stomatous cuticular membranes: effects of air humidity in two species of contrasting drought-resistance strategy. Journal of Experimental Botany 59: 3987–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstiens G. 1996. Cuticular water permeability and its physiological significance. Journal of Experimental Botany 47: 1813–1832. doi: 10.1093/jxb/47.12.1813. [DOI] [Google Scholar]
- Kim KW, Kim DH, Han SH, Lee JC, Kim PG. 2010. Three-dimensional surface topography of the needle stomatal complexes of Pinus rigida and its hybrid species by complementary microscopy. Micron 41: 571–576. doi: 10.1016/j.micron.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Kroupitski Y, Golberg D, Belausov E, et al. 2009. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Applied and Environmental Microbiology 75: 6076–6086. doi: 10.1128/AEM.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher W. 2001. Physiological plant ecology. Berlin Heidelberg: Springer-Verlag. [Google Scholar]
- Lasceve G, Leymarie J, Vavasseur A. 1997. Alterations in light-induced stomatal opening in a starch-deficient mutant of Arabidopsis thaliana L deficient in chloroplast phosphoglucomutase activity. Plant, Cell & Environment 20: 350–358. doi: 10.1046/j.1365-3040.1997.d01-71.x. [DOI] [Google Scholar]
- Maier-Maercker U. 1983. The role of peristomatal transpiration in the mechanism of stomatal movement. Plant, Cell & Environment 6: 369–380. doi: 10.1111/j.1365-3040.1983.tb01269.x. [DOI] [Google Scholar]
- Marquez DA, Stuart-Williams H, Farquhar GD, Busch FA. 2022. Cuticular conductance of adaxial and abaxial leaf surfaces and its relation to minimum leaf surface conductance. New Phytologist 233: 156–168. [DOI] [PubMed] [Google Scholar]
- Mast AR, Givnish TJ. 2002. Historical biogeography and the origin of stomatal distributions in Banksia and Dryandra (Proteaceae) based on their cpDNA phylogeny. American Journal of Botany 89: 1311–1323. doi: 10.3732/ajb.89.8.1311. [DOI] [PubMed] [Google Scholar]
- Mohammadian MA. 2005. An investigation of the functions of leaf surface modifications in the Proteaceae and Araucariaceae. PhD Thesis, University of Adelaide, Australia.
- Mohammadian MA, Hill RS, Watling JR. 2009. Stomatal plugs and their impact on fungal invasion in Agathis robusta. Australian Journal of Botany 57: 389–395. doi: 10.1071/bt08175. [DOI] [Google Scholar]
- Moss DN, Rawlins SL. 1963. Concentration of carbon dioxide inside leaves. Nature 197: 1320–1321. doi: 10.1038/1971320a0. [DOI] [Google Scholar]
- Mott KA, Peak D. 2013. Testing a vapour-phase model of stomatal responses to humidity. Plant, Cell & Environment 36: 936–944. doi: 10.1111/pce.12026. [DOI] [PubMed] [Google Scholar]
- Nobel PS. 1991. Physicochemical and environmental plant physiology. San Diego: Academic Press. [Google Scholar]
- Nonami H, Schulze ED, Ziegler H. 1991. Mechanisms of stomatal movement in response to air humidity, irradiance and xylem water potential. Planta 183: 57–64. doi: 10.1007/BF00197567. [DOI] [PubMed] [Google Scholar]
- Ratnawati. 2001. Evidence of the morphological range, transition and evolution of stomatal protection mechanisms in some selected proteaceae. MSc Thesis, University of Tasmania, Tasmania.
- Rohula G, Kupper P, Raim O, Sellin A, Sober A. 2014. Patterns of night-time water use are interrelated with leaf nitrogen concentration in shoots of 16 deciduous woody species. Environmental and Experimental Botany 99: 180–188. [Google Scholar]
- Roth-Nebelsick A. 2007. Computer-based studies of diffusion through stomata of different architecture. Annals of Botany 100: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Nebelsick A, Hassiotou F, Veneklaas EJ. 2009. Stomatal crypts have small effects on transpiration: a numerical model analysis. Plant Physiology 151: 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S, Nardini A, LoGullo MA. 1997. Is sclerophylly of Mediterranean evergreens an adaptation to drought? New Phytologist 135: 603–612. [Google Scholar]
- Santrucek J, Simanova E, Karbulkova J, Simkova M, Schreiber L. 2004. A new technique for measurement of water permeability of stomatous cuticular membranes isolated from Hedera helix leaves. Journal of Experimental Botany 55: 1411–1422. [DOI] [PubMed] [Google Scholar]
- Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F. 2007. Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiology 27: 551–559. [DOI] [PubMed] [Google Scholar]
- Schönherr J, Merida T. 1981. Water permeability of plant cuticular membranes – the effects of humidity and temparature on the permeability of non-isolated cuticles of onion bulb scales. Plant, Cell & Environment 4: 349–354. [Google Scholar]
- Schreiber L, Skrabs M, Hartmann KD, Diamantopoulos P, Simanova E, Santrucek J. 2001. Effect of humidity on cuticular water permeability of isolated cuticular membranes and leaf disks. Planta 214: 274–282. [DOI] [PubMed] [Google Scholar]
- Schuster AC, Burghardt M, Riederer M. 2017. The ecophysiology of leaf cuticular transpiration: are cuticular water permeabilities adapted to ecological conditions? Journal of Experimental Botany 68: 5271–5279. [DOI] [PubMed] [Google Scholar]
- Stinziano JR, Tominaga J, Hanson DT. 2020. Where in the leaf is intercellular CO2 (Ci)? Considerations and recommendations for assessing gaseous diffusion in leaves. bioRxiv doi: 10.1101/2020.05.05.079053. [Preprint]. [DOI] [Google Scholar]
- Tardieu F, Simonneau T. 1998. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49: 419–432. [Google Scholar]
- Turner IM. 1994. Sclerophylly: primarily protective? Functional Ecology 8: 669–675. [Google Scholar]
- Vico G, Manzoni S, Palmroth S, Weih M, Katul G. 2013. A perspective on optimal leaf stomatal conductance under CO2 and light co-limitations. Agricultural and Forest Meteorology 182: 191–199. [Google Scholar]
- Waldhoff D, Furch B, Junk WJ. 2002. Fluorescence parameters, chlorophyll concentration, and anatomical features as indicators for flood adaptation of an abundant tree species in Central Amazonia: Symmeria paniculata. Environmental and Experimental Botany 48: 225–235. [Google Scholar]
- Weyers JDB, Meidner H. 1990. Methods in stomatal research. Harlow, UK: Longman Group. [Google Scholar]
- Willmer CM, Fricker M. 1996. Stomata. London: Chapman & Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.