Abstract
Purpose:
To assess the real-world efficacy and safety of aflibercept for the treatment of diabetic macular edema (DME).
Methods:
A systematic search was conducted across multiple databases. Articles were included if participants had DME and received aflibercept treatment for a minimum of 52 ± 4 weeks. Primary outcomes included changes in best-corrected visual acuity (BCVA) and central macular thickness (CMT). A risk of bias assessment of studies was completed, pooled estimates were obtained, and a meta-regression was performed. Information on adverse events was collected.
Results:
The search yielded 2112 articles, of which 30 were included. Aflibercept was more effective than laser photocoagulation functionally (12-month BCVA-weighted mean difference [WMD] = 10.77 letters, P < 0.001; 24 months = 8.12 letters, P < 0.001) and anatomically (12-month CMT WMD = –114.12 μm, P < 0.001; 24 months = –90.4 μm, P = 0.004). Compared to bevacizumab, aflibercept was noninferior at improving BCVA at 12 months (WMD = 1.71 letters, P = 0.34) and 24 months (WMD = 1.58 letters, P = 0.083). One study found that aflibercept was more effective than bevacizumab anatomically at 1 and 2 years (P < 0.001 at 12 and 24 months). Compared to ranibizumab, aflibercept rendered a greater improvement in BCVA at 1 year (WMD = 1.76 letters, P = 0.001), but not 2 years (WMD = 1.66 letters, P = 0.072). CMT was not significantly different between both therapies at 12 months (WMD = −14.30 μm, P = 0.282) and 24 months (P = 0.08). One study reported greater functional improvement with aflibercept compared with dexamethasone (P = 0.004), but inferiority in reducing CMT (P < 0.001). Meta-regression analysis demonstrated that dosing schedule was found to impact outcomes at 12 and 24 months, while study design and sample size did not impact outcomes at 12 months. There were minimal safety concerns using aflibercept therapy.
Conclusions:
Aflibercept is a safe and effective therapy option for DME in the clinical setting, performing superiorly to laser photocoagulation. Evidence regarding comparisons with bevacizumab, ranibizumab, and dexamethasone is mixed and limited.
Keywords: Aflibercept, Antivascular endothelial growth factor, Diabetic macular edema, Eylea, Retina
INTRODUCTION
Diabetic macular edema (DME) is a significant cause of vision loss, affecting 7% of diabetic individuals.1 The standard of care for DME was previously focal laser photocoagulation, and intravitreal corticosteroids have also been used.2 However, studies have demonstrated that antivascular endothelial growth factor (anti-VEGF) therapies have superior outcomes and an improved safety profile compared to laser photocoagulation.2 Bevacizumab (Avastin®) is an anti-VEGF therapy that was introduced for the treatment of colorectal cancer, currently used off-label for the treatment of macular edema.3 Ranibizumab (Lucentis®) is among the approved anti-VEGF treatments for DME, targeting neovascularization.4 Aflibercept (Eylea®) has also been approved for DME, bearing similar efficacy to ranibizumab.5,6 Aflibercept is a recombinant fusion protein that acts as a decoy receptor, with a higher affinity for VEGF-A, VEGF-B, and placental growth factor than their natural receptors.
Most reviews evaluating the efficacy of anti-VEGF treatments for DME have focused on randomized control trials (RCTs), as this is considered one of the highest levels of evidence.7 However, the external validity of RCTs is often limited due to strict inclusion/exclusion criteria and highly controlled testing parameters. A meta-analysis including non-RCT sources of information may, thus, highlight the real-world complexities of the disease and its management. In addition, the comparison of anti-VEGF, intravitreal corticosteroid, and laser photocoagulation therapy within a single meta-analysis has yet to be completed. This meta-analysis aims to evaluate the real-world efficacy of aflibercept for the treatment of DME in comparison to other DME treatments, including results from both RCT and non-RCT sources. The following questions were addressed:
What is the effect of aflibercept therapy on best-corrected visual acuity (BCVA), as a functional outcome, and central macular thickness (CMT), as an anatomical outcome, in patients with DME?
How does aflibercept compare to alternative therapies for DME?
Are there safety concerns associated with aflibercept use in DME?
METHODS
This research was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [Supplemental File 1]. A literature search was conducted on PubMed, Ovid MEDLINE, EMBASE, and ClinicalTrials.gov. The search strategy included key terms related to anti-VEGF, aflibercept, Eylea, diabetic retinopathy, and DME [Supplemental File 2]. The search included studies that were written in English or French and published before February 2020.
Supplemental File 1.
Prisma checklist
| Section/topic | Number | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: Background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to PICOS design | 3-4 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | N/A |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 4 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 4 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Supplemental File 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 4 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 4 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 4-5 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 5 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 5 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 5 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 5 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were prespecified | 6 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 6, Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | Supplemental File 3 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 6, Figures 2-4 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | Figures 6-9 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 6-13 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | Figure 5 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | Page 7, Supplemental File 4 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 13-15 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 15-16 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 16 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 17 |
PICOS: Participants, interventions, comparisons, outcomes, and study, NA: Not available
Supplemental File 2.
Sample search strategy for systematic review
| Number | Search terms |
|---|---|
| 1 | Anti-Vascular endothelial growth factor* |
| 2 | Anti-VEGF* |
| 3 | Aflibercept* |
| 4 | “Aflibercept” [Supplementary Concept] |
| 5 | Eylea* |
| 6 | 1 OR 2 OR 3 OR 4 OR 5 |
| 7 | "Diabetic retinopathy" [Mesh] |
| 8 | Diabetic retinopath* |
| 9 | “Macular Edema” [Mesh] |
| 10 | Macular Edema* |
| 11 | DME |
| 12 | Macular Oedema* |
| 13 | DMO |
| 14 | 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 |
| 15 | 6 AND 14 |
We searched PubMed, Ovid MEDLINE, EMBASE, and ClinicalTrials.gov. from inception to February 2020, for studies published in English or French, using the following strategy
Articles were included in the meta-analysis if participants had DME and received aflibercept in at least one of the study arms. Studies were required to report change in BCVA and/or anatomical retinal changes before and after treatment with a minimum follow-up duration of 52 weeks ± 4 weeks. The mean baseline and endpoint data with their respective standard deviations were required for aflibercept monotherapy statistical analysis purposes. The mean change in primary outcome with an associated standard deviation was required for comparative analysis purposes. Studies with incomplete data were included as part of the systematic review but excluded from the meta-analysis. Anatomical retinal changes were specific to the macular region, including the fovea (i.e., CMT, central foveal thickness, central subfield thickness, and central retinal thickness if only the macular region was considered [hereto collectively referred to as CMT]). Post hoc analyses, conference abstracts, viewpoints/opinion papers, reviews, meta-analyses, extension studies, and studies evaluating the efficacy of ziv-aflibercept were excluded. Data were extracted independently by two reviewers and organized into the following categories: study details, length of follow-up, treatment regimen, assessment of study endpoints, and adverse events.
Primary outcomes included changes in BCVA and CMT. Secondary outcomes consisted of safety, particularly ocular adverse events, Antiplatelet Trialists' Collaboration (APTC)-defined adverse events (myocardial infarction, stroke, or vascular death), serious/systematic adverse events, and death.
Version 2 of the Cochrane risk-of-bias tool assessed the risk of bias in RCTs. Each item was rated as low risk, having some concerns, or high risk. Total score was calculated by two reviewers based on the Cochrane Collaboration algorithm.8 Risk of bias in the non-RCTs was assessed using the Newcastle–Ottawa Scale (NOS) on a nine-point scale.9 A modified six-point version of the NOS was used to evaluate studies with no control group, omitting questions regarding the comparability of both the groups.10 An overall NOS score of 7 and above was considered low risk, 5–7 was considered to have some concerns, and under 5 was considered high risk. For the modified version, a score of 6 was considered low risk, 4–5 was considered to have some concerns, and 3 and under was considered high risk.
Estimates were pooled using the DerSimonian and Laird random-effects models with inverse variance weighting.11 Heterogeneity was quantitatively assessed using the I2 statistic. Subgroup analyses by comparator and further subdivision by randomization versus nonrandomization were performed. A meta-regression was performed to assess any factors that contributed to BCVA and CMT outcomes other than comparator group, evaluating the effect of dosing schedule, study design, and sample size. Publication bias was assessed via visual inspection of funnel plots.12 All analyses were performed using StataCorp. (2017). Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.13
RESULTS
The literature search of the selected databases resulted in 2112 titles (PubMed 548, Ovid MEDLINE 762, EMBASE 788, and ClinicalTrials.gov 14). After removal of 1255 duplicates, 857 studies remained. There was exclusion of 368 records based on title, 489 abstracts were screened, and 105 full-text articles were assessed for eligibility. Thirty studies were included in this review. The selection process is illustrated in Figure 1. Detailed study characteristics are given in Supplemental File 3.
Figure 1.

Flow diagram of studies included in this meta-analysis. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart outlining number of studies screened, assessed for eligibility, and included in review
Supplemental File 3.
Study details
| Author | Comparative therapy | Design | Duration of follow-up (months) | Sample size (eyes) | Dosing interval |
|---|---|---|---|---|---|
| Bahrami, et al., 201925 | None | Interventional (clinical trial) | 11 | 41 | 2q8 |
| Campos Polo, et al., 201846 | None | Interventional (clinical trial) | 12 | 29 | 2q8 |
| Terasaki et al., 201917 | None | Interventional (clinical trial) | 12 | 72 | 2q8 |
| Garweg et al., 201923 | None | Interventional (clinical trial) | 12 | 553 | 2q8 |
| Pak et al., 202037 | None | Interventional (clinical trial) | 12 | 46 | Treat and extend |
| Curry et al., 202032 | None | Interventional (clinical trial) | 24 | 26 | Treat and extend |
| Kaiho et al., 201733 | None | Retrospective case series | 12 | 51 | Every 4 weeks for 1-3 loading injections then PRN |
| Lukic et al., 202047 | None | Retrospective case series | 12 | 99 | Every 4 weeks for 5 loading injections then PRN |
| McCloskey, et al., 201815 | None | Retrospective case series | 24 | 18 | N/A |
| Tsapardoni et al., 201916 | None | Retrospective case series | 24 | 30 | 2q8 in 1st year, treat-and-extend in 2nd year |
| Kern et al., 202048 | None | Retrospective case series | 24 | 139 | Every 4 weeks for 3 loading injections then PRN |
| Khattab et al., 201918 | Nonea | RCT | 18 | 27 (in aflibercept monotherapy) | Every 4 weeks for 3 loading injections then PRN |
| Abouhussein and Gomaa, 202031 | Nonea | RCT | 12 | 20 (in aflibercept monotherapy) | Every 4 weeks for 3 loading injections then PRN |
| Do et al., 201230 | Laser photocoagulation | RCT | 12 | 0.5q4: 38, 2q4: 33, 2q8: 34, 2PRN: 38, laser: 33 | 0.5q4, 2q4, 2q8, PRN |
| Chen et al., 202027 | Laser photocoagulation | RCT | 12 | Aflibercept 2q4: 122, 2q8: 116, laser: 117 | 2q4 or 2q8 |
| Korobelnik et al., 201429 | Laser photocoagulation | RCT | 12 | Combinedb: Aflibercept, 2q4: 290, 2q8: 286, laser: 286 | 2q4 or 2q8 |
| Brown et al., 201528 | Laser photocoagulation | RCT | 23 | Combinedb: Aflibercept, 2q4: 291, 2q8: 287, control: 287 | 2q4 or 2q8 |
| Baker et al., 201926 | Laser photocoagulation | RCT | 24 | Aflibercept: 226, laser: 240, observation: 236 | PRN |
| Heier et al., 201619 | Laser photocoagulation | RCT | 34 | Combinedb: Aflibercept, 2q4: 291, 2q8: 287, laser: 287 | 2q4 or 2q8 |
| Ozsaygili and Duru, 202022 | Dexamethasone | RCT | 12 | Aflibercept: 50, dexamethasone: 48 | Every 4 weeks for 3 loading injections then PRN |
| Hernández-Bel et al., 201934 | Dexamethasone/aflibercept dual therapya | Retrospective cohort study | 12 | Aflibercept: 15, dexamethasone: 15 | 2q8 |
| Kaldırım, et al., 201920 | Ranibizumab | Prospective cohort study | 12 | Aflibercept: 30, ranibizumab: 30 | Every 4 weeks for 3 loading injections then PRN |
| Bhandari et al., 202038 | Ranibizumab | Prospective cohort study | 12 | Aflibercept: 217, ranibizumab: 166 | PRN |
| Ozkaya, et al., 202021 | Ranibizumab | Retrospective cohort study | 12 | Aflibercept: 20, ranibizumab: 26 | Every 4 weeks for 3 loading injections then PRN |
| Plaza-Ramos et al., 201936 | Ranibizumab | Retrospective cohort study | 12 | Aflibercept: 91, ranibizumab: 122 | Every 4 weeks for 3 loading injections then PRN |
| Schwarzer, et al., 201949 | Ranibizumab | Retrospective cohort study | 12 | Aflibercept: 34, ranibizumab: 41 | Treat and extend (average every 5.9 weeks) |
| Fouda and Bahgat, 201735 | Ranibizumab | RCT | 12 | Aflibercept: 35, ranibizumab: 35 | Every 4 weeks for 3 loading injections then PRN |
| Ciulla, et al., 201850 | Ranibizumab and bevacizumab | Retrospective cohort study | 24 | 12-months cohort Aflibercept: 1379, bevacizumab: 3109, ranibizumab: 1352 24-months cohort Aflibercept: 800, bevacizumab: 2403, ranibizumab: 1952 | N/A |
| Wells et al., 20155 | Ranibizumab and bevacizumab | RCT | 12 | Aflibercept: 208, bevacizumab: 206, ranibizumab: 206 | 2q4 |
| Wells et al., 201624 | Ranibizumab and bevacizumab | RCT | 24 | Aflibercept: 201, bevacizumab: 185, ranibizumab: 192 | 2q4 for 1 year, then PRN |
aOnly results relating to the aflibercept arm were considered, bThis study evaluated a combined database (VIVID and VISTA). 2q4: 2 mg every 4 weeks, 2q8: 2 mg every 8 weeks, 0.5q4: 0.5 mg every 4 weeks, PRN: Pro-re-nata (as needed), RCT: Randomized controlled trial, NA: Not available
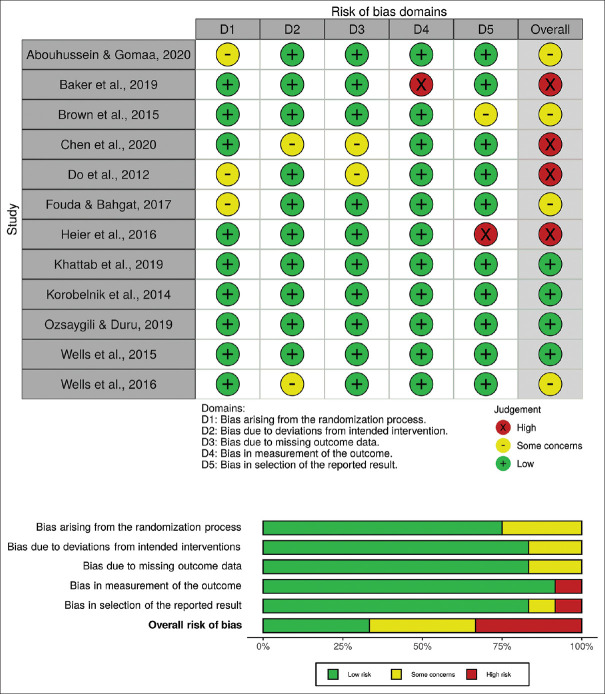
Bias among 12 RCTs was assessed using the Cochrane Collaboration's Risk of Bias 2 tool. Four studies were evaluated as having low risk of bias, four studies had some concerns, and four studies were high risk. A traffic plot and a summary plot of the included studies assessed using the Risk of Bias 2 tool are shown in Figure 2. The biases of seven cohort studies were assessed using the original NOS. Of a total score of 9, three studies were low-risk, and the remaining four studies had some concerns. A traffic plot and a summary plot of the included studies assessed using the NOS are shown in Figure 3. Of 11 noncohort studies, evaluated using the modified NOS, two studies were found to be low risk, and the remaining studies had some concerns. A traffic plot and a summary plot of the included studies assessed using the modified NOS are shown in Figure 4.14
Figure 2.
Traffic plot and summary plot of the included randomized controlled trials (RCTs). Risk of bias assessment of RCT studies using version 2 of the Cochrane risk-of-bias tool. Total score calculated based on the algorithm suggested by the Cochrane Collaboration.
Figure 3.

A traffic plot and summary plot of the included cohort studies. Risk of bias assessment of non-randomized controlled trial studies using the Newcastle-Ottawa Scale (NOS). An overall NOS score of 7 and above is considered low risk, a score of 5 to 7 is considered to have some concerns, and a score under 5 is considered high risk.
Figure 4.
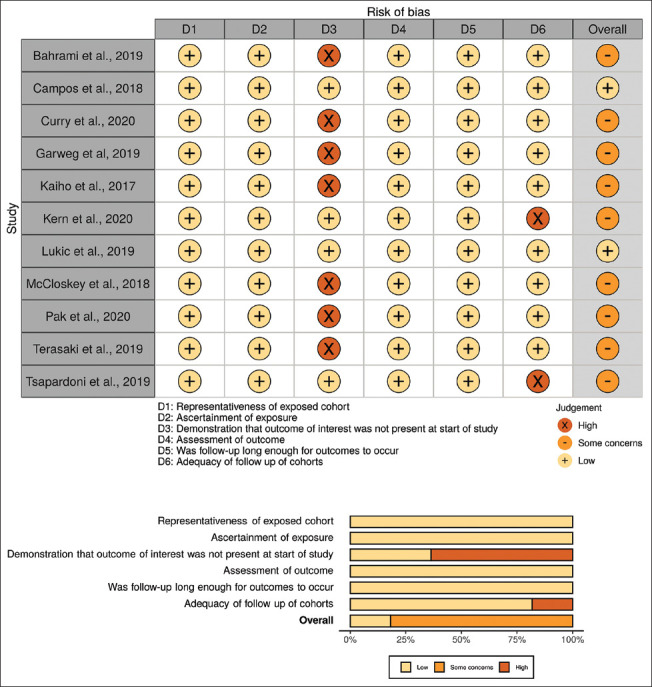
Traffic plot and summary plot of the included non-cohort studies. Risk of bias assessment of non-cohort, non-randomized controlled trial studies without a comparator group using a modified version of the Newcastle-Ottawa Scale. A score of 6 is considered low risk, a score of 4 or 5 is considered to have some concerns, and a score of 3 and under is considered high risk.
Meta-regression analysis demonstrated that dosing schedule was found to impact BCVA outcomes at 12 and 24 months and CMT outcomes at 12 and 24 months. Study design and sample size did not significantly impact results at 12 months for BCVA and CMT. There were insufficient data to perform study design and sample size analyses for 24-month data. Detailed results are given in Supplemental File 4.
Supplemental File 4.
Meta-regression results for various analysis strata
| BCVA | Multivariable regression | ||
|---|---|---|---|
|
| |||
| Beta | 95% CI | P | |
| BCVA 12 months | |||
| Dosing schedule | −1.97 | −3.82 - −0.12 | <0.0001 |
| Design (RCT vs. non-RCT) | 1.85 | −5.37 - 9.07 | 0.589 |
| Sample size | −3.44 | −9.03 - 2.16 | 0.207 |
| BCVA 24 months | |||
| Dosing schedule | −3.17 | −3.93 - −2.40 | <0.0001 |
| Design (RCT vs. non-RCT) | N/A | N/A | N/A |
| Sample size | N/A | N/A | N/A |
| BCVA all studies (all follow-up times) | |||
| Dosing schedule | −2.4 | −3.38 - −1.42 | <0.0001 |
| Design (RCT vs. non-RCT) | 1.4 | −3.75 - 6.55 | 0.58 |
| Sample size | −3.35 | −7.41 - 0.71 | 0.102 |
| CMT 12 months | |||
| Dosing schedule | 31.5 | 13.31 - 49.61 | 0.002 |
| Design (RCT vs. non-RCT) | 39.6 | −24.4 - 103.5 | 0.206 |
| Sample size | 23.9 | −28.6 - 76.29 | 0.346 |
| CMT 24 months | |||
| Dosing schedule | 35.6 | 26.3 - 50.9 | <0.0001 |
| Design (RCT vs. non-RCT) | N/A | N/A | N/A |
| Sample size | N/A | N/A | N/A |
| CMT all studies (all follow-up times) | |||
| Dosing schedule | 30.45 | 17.7 - 43.24 | <0.0001 |
| Design (RCT vs. non-RCT) | 19.3 | −36.1 - 74.7 | 0.477 |
| Sample size | 9.25 | −35.47 - 53.99 | 0.672 |
For the above analysis strata, the restricted maximum likelihood estimate of between-study variance (tau2) and joint test for all covariates with Knapp-Hartung modification are statistically significant (P=0.002-P <0.0001). All Betas are adjusted for other variables in each analysis strata. N/A: Not applicable, unable to estimate due to multi-collinearity or small number of studies, CI: Confidence interval, BCVA: Best-corrected visual acuity, RCT: Randomized clinical trial, CMT: Central macular thickness
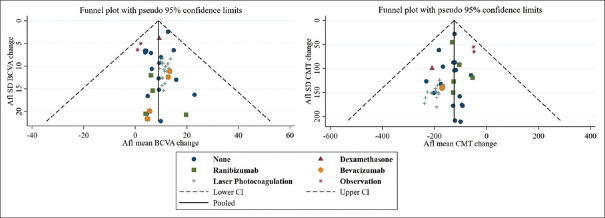
Visual examination of the funnel plots evaluating BCVA and CMT outcomes revealed symmetrical scattering of the included studies around the overall effect line, suggesting absence of publication bias [Figure 5].
Figure 5.
Funnel plots assessing publication bias of studies evaluating best-corrected visual acuity (pictured top) and central macular thickness (pictured bottom). Afl: Aflibercept, SD: Standard deviation, CI: Confidence interval
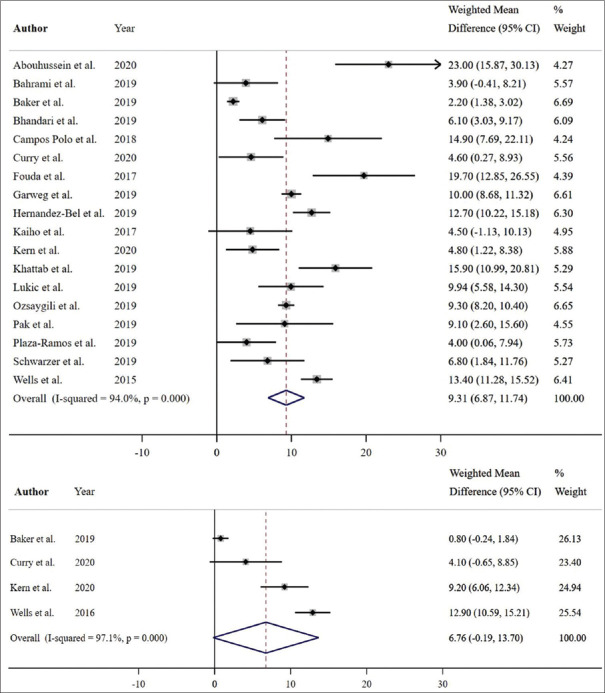
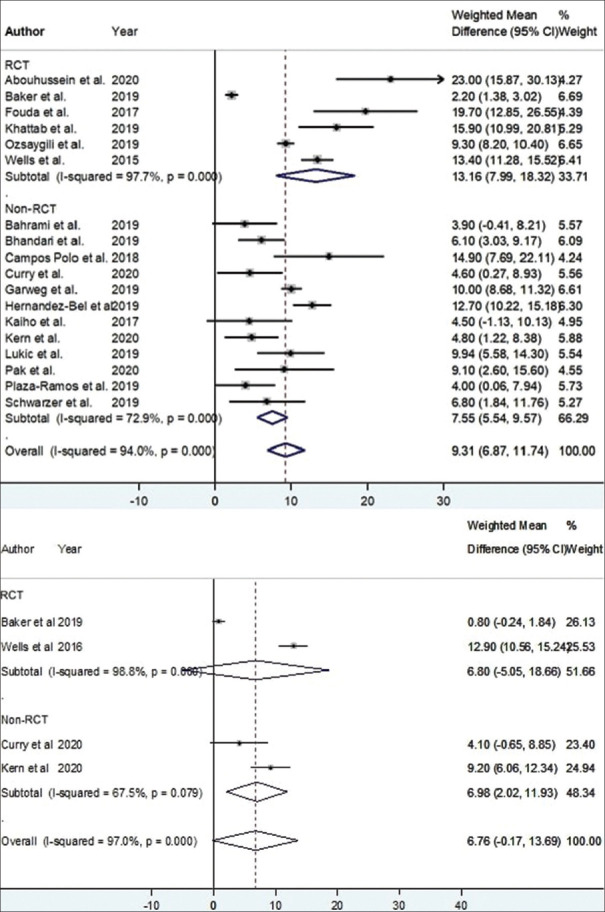
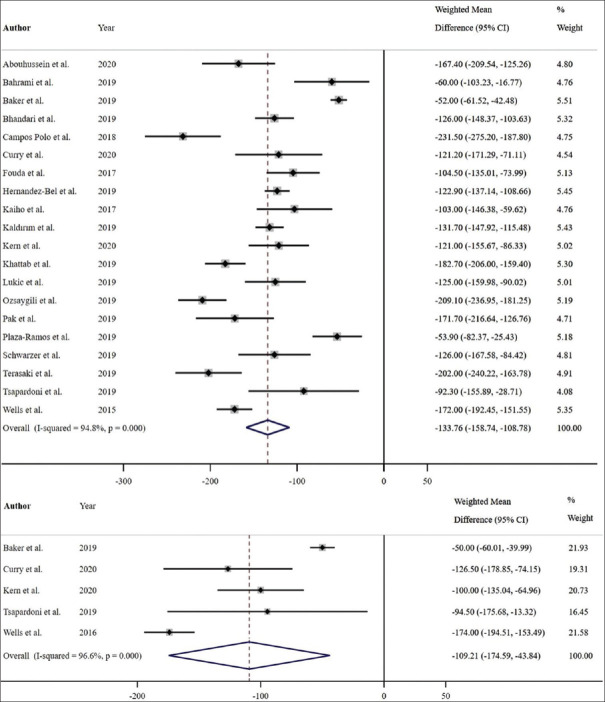
Of eighteen studies with complete 12-month data relating to BCVA, a significant pooled mean improvement of 9.31 Early Treatment Diabetic Retinopathy Study (ETDRS) letters was noted with aflibercept (weighted mean difference [WMD] = 9.31 ETDRS letters; 95% confidence interval [CI]: 6.87–11.74 ETDRS letters; P < 0.001, I2 = 94.0%) [Figure 6]. Three studies were not included in the 12-month analysis due to missing baseline, 12-month mean, or standard deviation data. Among these three studies, the mean BCVA was reported to be significantly improved with aflibercept monotherapy in two studies (mean difference of 6.1 ± 7.1 ETDRS letters, P = 0.006;15 mean difference of 3.7 ETDRS letters, P = 0.024),16 and an improvement in BCVA (with no reported P value) was found in one study (mean difference of 9.3 ± 9.3 ETDRS letters).17 Of the eighteen studies, six RCTs showed a significant improvement of 13.16 ETDRS letters with aflibercept (WMD = 13.16 ETDRS letters; 95% CI: 7.99–18.32 ETDRS letters; P < 0.001, I2 = 97.7%) [Figure 7], and twelve non-RCTs showed a significant improvement of 7.55 ETDRS letters with aflibercept (WMD = 7.55 ETDRS letters; 95% CI: 5.54–9.57 ETDRS letters; P < 0.001, I2 = 72.9%) [Figure 7].
Figure 6.
Estimated weighted mean difference of baseline to 12-month (pictured top), and baseline to 24-month best-corrected visual acuity (pictured bottom) with aflibercept therapy as measured using Early Treatment Diabetic Retinopathy Study (ETDRS) letters. Dashed line represents weighted mean difference. 12-month weighted mean difference: 9.31 ETDRS letters, P < 0.001. 24-month weighted mean difference: 6.76 ETDRS letters, P = 0.056. Values reported as crude mean difference, without adjustment for covariates. CI: Confidence interval
Figure 7.
Estimated weighted mean difference of baseline to 12-month (pictured top), and baseline to 24-month best-corrected visual acuity (pictured bottom) with aflibercept therapy stratified by study design as measured using Early Treatment Diabetic Retinopathy Study (ETDRS) letters. Dashed line represents weighted mean difference. 12-month weighted mean difference randomized controlled trial (RCT) 13.16 ETDRS letters, P < 0.001. 12-month weighted mean difference non-RCT: 7.55 ETDRS letters, P < 0.001. 24-month weighted mean difference RCT: 6.80 ETDRS letters, P = 0.261. 24-month weighted mean difference non-RCT: 6.98 ETDRS letters, P = 0.006. Values reported as crude mean difference, without adjustment for covariates. CI: Confidence interval.
Of four studies that reported complete 24-month mean BCVA outcomes, a marginally significant improvement in BCVA with a pooled mean gain of 6.76 ETDRS letters was noted with aflibercept (WMD = 6.76 ETDRS letters; 95% CI: –0.19–13.70 ETDRS letters; P = 0.056, I2 = 97.1%) [Figure 6]. Two studies were not included in the 24-month analysis due to missing data. Among these two studies, the mean BCVA was reported to be nonsignificantly increased with aflibercept monotherapy in one (mean difference of 6.4 ± 10.6 ETDRS letters, P = 0.1)15 and significantly increased in the other (mean difference of 4.9 ETDRS letters, P = 0.01).16 One study reported a statistically significant increase in BCVA at 18 months (mean difference 18.9 ± 7.0 ETDRS letters, P < 0.005).18 Of the four studies, two RCTs showed a mean improvement of 6.80 ETDRS letters with aflibercept (WMD = 6.80 ETDRS letters; 95% CI: –5.05–18.66 ETDRS letters; P = 0.261, I2 = 98.8%) [Figure 7] while two non-RCTs showed a mean improvement of 6.98 ETDRS letters (WMD = 6.98 ETDRS letters; 95% CI: 2.02–11.93 ETDRS letters; P = 0.006, I2 = 67.5%) [Figure 7].
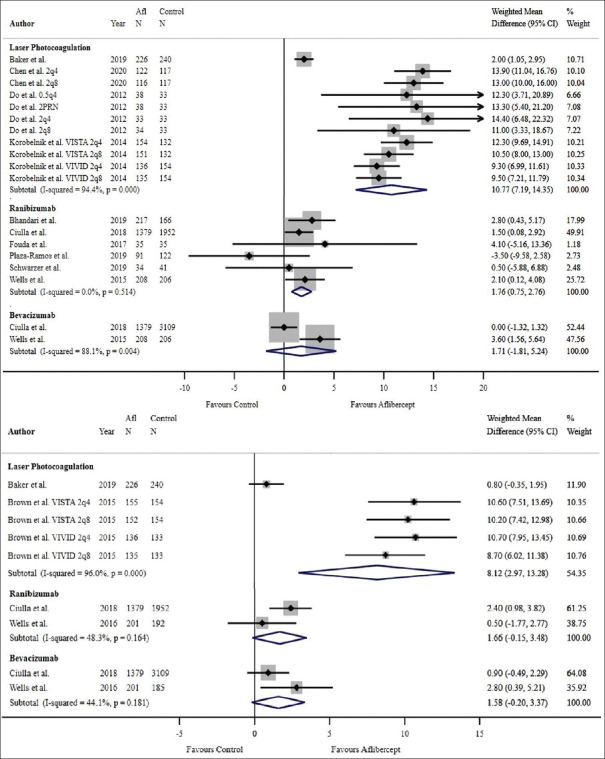
Among four studies comparing aflibercept and laser photocoagulation, aflibercept was superior in improving BCVA at 1 year (WMD = 10.77 ETDRS letters, 95% CI: 7.19–14.35 ETDRS letters, P < 0.001, I2 = 94.4%) [Figure 8]. All studies comparing aflibercept and laser photocoagulation were RCTs; therefore, weighted mean results remained the same with study type stratification. The 2-year results included two studies which continually evidenced the superiority of aflibercept (WMD = 8.12 ETDRS letters, 95% CI: 2.97–13.28 ETDRS letters, P < 0.001, I2 = 96.0%) [Figure 8]. Heier et al. reported a statistically significant improvement of BCVA with aflibercept over laser photocoagulation at 3 years (P < 0.001 for all aflibercept groups versus laser control, using the last observation carried forward [LOCF] method).19
Figure 8.
Estimated weighted mean difference of improvement in best-corrected visual acuity with aflibercept in comparison with other therapies at 12 months (pictured top) and 24 months (pictured bottom) as measured using Early Treatment Diabetic Retinopathy Study (ETDRS) letters. At 12 months: Weighted mean difference between aflibercept and laser photocoagulation: 10.77 ETDRS letters, P < 0.001, weighted mean difference between aflibercept and ranibizumab: 1.76 ETDRS letters, P = 0.001, weighted mean difference between aflibercept and bevacizumab: 1.71 ETDRS letters, P = 0.341. Single-study comparisons (not pictured): Weighted mean difference between aflibercept and dexamethasone: 2.90 ETDRS letters, P = 0.004. At 24 months: Weighted mean difference between aflibercept and laser photocoagulation: 8.12 ETDRS letters, P < 0.001, weighted mean difference between aflibercept and ranibizumab: 1.66 ETDRS letters, P = 0.072, weighted mean difference between aflibercept and bevacizumab: 1.58 ETDRS letters, P = 0.083. Note: Only comparator groups with more than one study were included in forest plots. Afl: Aflibercept, 2q4: 2 mg every 4 weeks, 2q8: 2 mg every 8 weeks, 0.5q: 0.5 mg every 4 weeks, 2PRN: 2 mg as needed; CI: Confidence interval.
Two trials compared visual outcomes related to aflibercept and bevacizumab at 1 and 2 years. No significant differences were found between the two therapies at 1 year (WMD = 1.71 ETDRS letters, 95% CI: –1.81–5.24 ETDRS letters, P = 0.341, I2 = 88.1%) [Figure 8] and 2 years (WMD = 1.58 ETDRS letters, 95% CI: –0.20–3.37 ETDRS letters, P = 0.083, I2 = 44.1%) [Figure 8]. There was insufficient power in the bevacizumab comparator group to undergo RCT versus non-RCT stratification analyses.
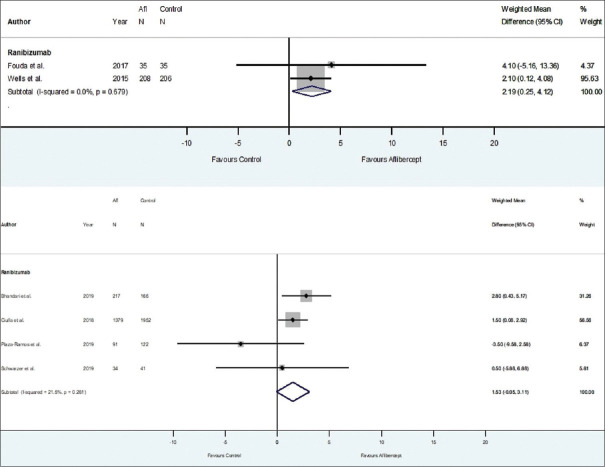
When comparing aflibercept with ranibizumab, the overall 1-year improvement in BCVA across all six studies was significantly better with aflibercept (WMD = 1.76 ETDRS letters, 95% CI: 0.75–2.76 ETDRS letters, P = 0.001, I2 = 0) [Figure 8]. Two studies were not included in the analyses due to missing standard deviation data; however, these studies reported no significant difference in BCVA improvement between aflibercept and ranibizumab at 12 months (P = 0.23720 and P = 0.8).21 Of the six studies, two RCTs demonstrated that the overall 1-year improvement in BCVA was significantly better with aflibercept (WMD = 2.19 ETDRS letters; 95% CI: 0.25–4.12 ETDRS letters; P = 0.027, I2 = 0.0%) [Figure 9] and four non-RCTs demonstrated a 1-year improvement in BCVA which was nonsignificantly better with aflibercept compared with ranibizumab (WMD = 1.53 ETDRS letters; 95% CI: –0.05–3.11 ETDRS letters; P = 0.058, I2 = 21.5%) [Figure 9]. At 2 years, visual outcomes related to aflibercept were no longer found to be superior to ranibizumab (WMD = 1.66 ETDRS letters, 95% CI: –0.15–3.48 ETDRS letters, P = 0.072, I2 = 48.3%) [Figure 8]. There was insufficient power in the 24-month ranibizumab comparator group to undergo RCT versus non-RCT stratification analyses.
Figure 9.
Estimated weighted mean difference of improvement in best-corrected visual acuity with aflibercept in comparison with ranibizumab at 12 months stratified by randomized controlled trial (RCT) (pictured top) vs. non-RCT (pictured bottom) as measured using Early Treatment Diabetic Retinopathy Study (ETDRS) letters. 12-month RCTs: Weighted mean difference between aflibercept and ranibizumab: 2.19 ETDRS letters, P = 0.027. 12-month non-RCTs: Weighted mean difference between aflibercept and ranibizumab: 1.53 ETDRS letters, P = 0.058. Note: Only comparator groups with more than one study in both RCT and non-RCT groups were represented in stratified analyses; thus, no comparator groups were represented in stratified analyses at 24 months. Afl: aflibercept, CI: Confidence interval.
One study compared the outcomes of aflibercept to dexamethasone, which evidenced superiority of aflibercept in improving BCVA at 1 year (P = 0.004).22 There was insufficient power in the dexamethasone comparator group to undergo RCT versus non-RCT stratification analyses.
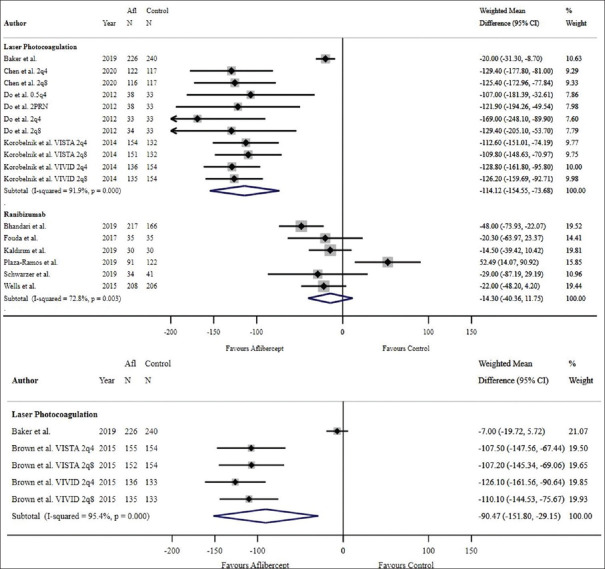
Of twenty studies that reported both baseline and 12-month mean CMT outcomes, a significant improvement in CMT with a pooled mean reduction of 133.76 μm was noted with aflibercept therapy (WMD = –133.76 μm; 95% CI: –158.74 to –108.78 μm; P < 0.001, I2 = 94.8%) [Figure 10]. In the two studies that were not included in the 12-month pre-post analysis due to missing baseline or 12-month mean or standard deviation, the mean CMT was significantly reduced with aflibercept monotherapy (mean difference –175.38 ± 132.62 μm, P = 0.006;23 mean difference –117.7 ± 103.3 μm, P = 0.0003).15 Of the twenty studies, six RCTs showed a significant pooled mean reduction of 147.52 μm with aflibercept (WMD = –147.52 μm; 95% CI: –211.45 to –83.60 μm; P < 0.001, I2 = 98.1%) [Figure 11] while fourteen non-RCTs showed a significant pooled mean reduction of 127.48 μm with aflibercept (WMD = –127.48 μm; 95% CI: –147.77 to –107.19 μm; P < 0.001, I2 = 83.3%) [Figure 11].
Figure 10.
Estimated weighted mean difference of baseline to 12-month (pictured top), and baseline to 24-month central macular thickness (μm) (pictured bottom). Dashed line represents weighted mean difference. 12-month weighted mean difference: –133.76 μm, P < 0.001. 24-month weighted mean difference: –109.21 μm, P = 0.001. Values reported as crude mean difference, without adjustment for covariates. CI: Confidence interval
Figure 11.
Estimated weighted mean difference of baseline to 12-month (pictured top), and baseline to 24-month central macular thickness (μm) (pictured bottom) stratified by study design. Dashed line represents weighted mean difference. 12-month randomized controlled trial (RCT) weighted mean difference: –147.52 μm, P < 0.001. 12-month non-RCT weighted mean difference: –127.48 μm, P < 0.001. 24-month RCT weighted mean difference: –111.66 μm, P = 0.072. 24-month non-RCT weighted mean difference: –106.64 μm, P < 0.001. Values reported as crude mean difference, without adjustment for covariates. CI: Confidence interval.
Of five studies that reported 24-month CMT outcomes, a significant improvement in CMT with a pooled mean reduction of 109.21 μm was noted with aflibercept therapy (WMD = –109.21 μm; 95% CI: –174.59 to –43.84 μm; P = 0.001, I2 = 96.6%) [Figure 10]. One study was not included in the meta-analysis due to missing endpoint standard deviation data; however, this study reported a significant reduction in CMT at 2 years (mean difference –123.3 ± 104.2 μm, P = 0.02).15 Khattab et al. reported an 18-month statistically significant reduction in CMT (mean difference –212.5 ± 55.7 μm, P < 0.005).18 Of the five studies, two RCTs showed a nonsignificant pooled mean reduction of 111.66 μm with aflibercept (WMD = −111.66 μm; 95% CI: –233.18–9.86 μm; P = 0.072, I2 = 99.1%) [Figure 11] while three non-RCTs showed a significant pooled mean reduction of 106.64 μm with aflibercept (WMD = −106.64 μm; 95% CI: –134.05 to –79.23 μm; P < 0.001, I2 = 0.0%) [Figure 11].
Aflibercept was superior to laser photocoagulation in improving CMT at both 1 year (WMD = −114.12 μm, 95% CI: –154.55 to –73.68 μm, P < 0.001, I2 = 91.9%) [Figure 12] and 2-year follow-ups (WMD = −90.47 μm, 95% CI = −151.80 to –29.15 μm, P = 0.004, I2 = 95.4%, respectively) [Figure 12]. Heier et al. reported a statistically significant improvement of CMT with aflibercept over laser photocoagulation at 3 years (P < 0.0001 for all aflibercept groups versus laser control, using the LOCF method).19 All studies comparing aflibercept and laser photocoagulation were RCTs; therefore, there was no subgroup analysis performed based on study design.
Figure 12.
Estimated weighted mean difference of reduction in central macular thickness (μm) with aflibercept in comparison with other therapies at 12 months (pictured top) and 24 months (pictured bottom). At 12 months: Weighted mean difference between aflibercept and laser photocoagulation: –114.12 μm, P < 0.001, weighted mean difference between aflibercept and ranibizumab: –14.30 μm, P = 0.282. Single-study comparisons (not pictured): Weighted mean difference between aflibercept and bevacizumab –68.00 μm, P < 0.001, weighted mean difference between aflibercept and dexamethasone: 108.40 μm, P < 0.001. At 24 months: Weighted mean difference between aflibercept and laser photocoagulation: –90.47 μm, P = 0.004. Single-study comparisons (not pictured): Weighted mean difference between aflibercept and ranibizumab: –22.00 μm, P = 0.008, weighted mean difference between aflibercept and bevacizumab: –45.00 μm, P < 0.001. Note: Only comparator groups with more than one study were included in forest plots. Afl: Aflibercept; 2q4: 2 mg every 4 weeks, 2q8: 2 mg every 8 weeks, 0.5q: 0.5 mg every 4 weeks, 2PRN: 2 mg as needed, CI: Confidence interval
Wells et al. reported a greater degree of CMT reduction with aflibercept compared with bevacizumab at both the one and 2-year time points (P < 0.001 for both years).5,24 There was insufficient power in the bevacizumab comparator group to undergo RCT versus non-RCT stratification analyses.
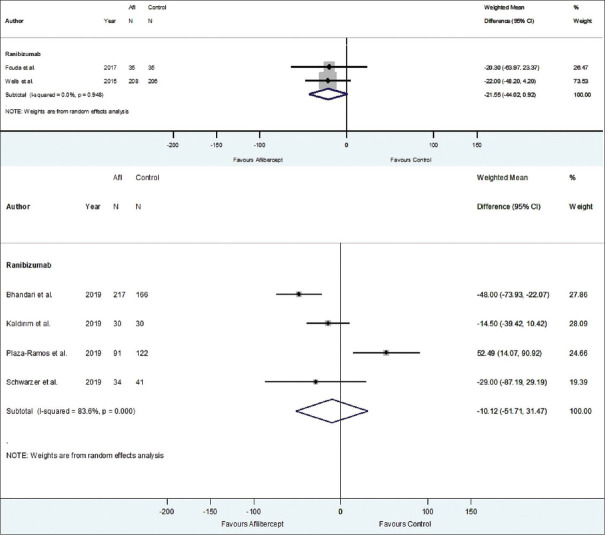
When comparing aflibercept with ranibizumab, the overall improvement in CMT across six studies at 1 year was not significantly different between the two groups (WMD = −14.30 μm, 95% CI = −40.36-11.76 μm, P = 0.282, I2 = 72.8%) [Figure 12]. A study by Ozkaya et al. displayed similar results at 1 year (P = 0.3).21 Of the six studies, two RCTs demonstrated a nonsignificantly increased reduction in CMT with aflibercept compared to ranibizumab (WMD = −21.55 μm, 95% CI = −44.02–0.92 μm, P = 0.060, I2 = 0.0%) [Figure 13]. Four non-RCTs demonstrated a nonsignificantly increased reduction in CMT with aflibercept compared to ranibizumab (WMD = −10.12 μm, 95% CI = −51.71–31.47 μm, P = 0.633, I2 = 83.6%) [Figure 13]. A nonsignificant difference in CMT outcomes was also reported at 2 years by Wells et al. (P = 0.08).24 There was insufficient power in the 24-month ranibizumab comparator group to undergo RCT versus non-RCT stratification analyses.
Figure 13.
Estimated weighted mean difference of improvement in central macular thickness (μm) with aflibercept in comparison with ranibizumab at 12 months stratified by randomized controlled trial (RCT) (pictured top) vs. non-RCT (pictured bottom). 12-month RCTs: Weighted mean difference between aflibercept and ranibizumab: –21.55 μm, P = 0.060. 12-month non-RCTs: Weighted mean difference between aflibercept and ranibizumab: –10.12 μm, P = 0.633. Note: Only comparator groups with more than one study in RCT and non-RCT groups were represented in stratified analyses; thus, no comparator groups were represented in stratified analyses at 24 months. Afl: Aflibercept, CI: confidence interval
Ozsaygili and Duru, in the same study that showed superiority of aflibercept in improving BCVA, demonstrated the superiority of dexamethasone over aflibercept in reducing the CMT at 1 year (P < 0.001).22 There was insufficient power in the dexamethasone comparator group to undergo RCT versus non-RCT stratification analyses.
Twenty-four studies commented on safety outcomes, including 14 studies reporting ocular adverse events5,17,18,19,23,24,25,26,27,28,29,30,31,32 and 10 reporting no ocular adverse events with aflibercept.15,16,20,21,22,33,34,35,36,37 The most commonly reported ocular adverse event was subconjunctival hemorrhage (164 cases of 2516 aflibercept-treated eyes with ocular safety data available, representing a prevalence of 6.5%).24,26,27,30,31,32 Twelve studies reported APTC-defined adverse events with aflibercept treatment.5,17,19,23,24,25,26,27,28,29,30,32 Four studies reported no APTC-defined events having occurred during the study with aflibercept treatment.15,16,35,37 The most commonly reported APTC-defined event was vascular or other unknown cause (107 cases reported of 2212 aflibercept-treated eyes, with APTC-defined data available, representing a prevalence of 4.8%).5,19,24,26,27,28,29,30 Twelve studies reported systemic or other serious adverse events associated with aflibercept treatment.5,17,19,23,24,25,26,27,28,29,30,32 Five studies reported no systemic or other serious events during the study.5,15,16,31,35,37 The most commonly reported adverse event was hypertension (52 cases reported of 2232 aflibercept-treated eyes with available data, representing a prevalence of 2.3%).19,26,27,30 All-cause mortality was reported in 7 studies,17,19,23,24,26,30,38 ranging from 0.9% to 5.5%, with one study reporting no deaths due to aflibercept treatment.35 In studies that reported the causes of death, most deaths were related to cardiovascular events.19,23,30 Among the studies comparing aflibercept with laser photocoagulation, most studies did not find a significant difference in adverse events between the two groups. Baker et al. reported a significantly increased intraocular pressure with aflibercept compared with observation.26 In the VISTA/VIVID studies, all-cause mortality was more frequent in the groups treated with aflibercept at the 100 and 148-week time points.19,28 Compared to aflibercept, there was a higher incidence of increased intraocular pressure and cataract formation in patients treated with dexamethasone.22,34 Wells et al., who compared safety outcomes between aflibercept, bevacizumab, and ranibizumab, found a higher rate of APTC-defined events for ranibizumab compared to aflibercept at 2 years,5,24 but no difference between groups in other categories of adverse events.
DISCUSSION
This meta-analysis summarizes the evidence regarding the efficacy and safety of aflibercept for treatment of DME. A wide variety of study types were incorporated, from controlled environments such as RCTs, to retrospective studies with heterogenous samples of DME patients in real-life clinical settings. Aflibercept therapy rendered a significant improvement in BCVA at 1 year and a marginally significant improvement at 2 years. Similar results were obtained at 12 months when studies were stratified by randomization status. However, at 24 months, RCTs demonstrated that the improvement in BCVA was nonsignificant while non-RCTs demonstrated a significant improvement. There was also a reduction in macular thickness at both 1 and 2 years. Similar to BCVA, the results obtained at 12 months with RCTs were similar to nonstratified results. Conversely, at 24 months, RCTs demonstrated that the improvement in CMT was nonsignificant while non-RCTs demonstrated that the improvement was significant. In comparison with laser photocoagulation, there was a significantly greater improvement in BCVA and anatomical outcomes with aflibercept therapy, similar to the results of previous meta-analyses comparing the two therapies.7,39,40
Aflibercept has been reported to have superior visual acuity outcomes compared to bevacizumab.40,41,42 Our analysis failed to reveal any differences between aflibercept therapy and bevacizumab with respect to the functional outcome. The lack of significant BCVA improvement in the present study suggests that aflibercept might be superior in a specific subpopulation of patients, such as patients with a poor baseline BCVA. Only one trial reported a significantly greater degree of CMT reduction with aflibercept compared with bevacizumab at both the 1 and 2-year time points.5,24
Aflibercept provided a significant advantage over ranibizumab therapy at 1 year in terms of BCVA, mirroring results from previous meta-analyses.41,42 When stratified based on randomization status, RCTs maintained this significance; however, non-RCTs reported nonsignificance. The overall 1-year advantage was no longer observed at the 2-year time point. It is important to consider that two studies were not included in the analyses due to missing standard deviation information, both of which reported no differences between aflibercept and ranibizumab BCVA outcomes.20,21 CMT outcomes were not significantly different at 1 or 2 years between ranibizumab and aflibercept, and these outcomes were consistent irrespective of study design.
Ozsaygili and Duru demonstrated functional superiority of aflibercept over dexamethasone; however, dexamethasone was shown to be anatomically superior to aflibercept.22 Although a correlation between visual acuity and macular thickness is usually observed, the relationship has been described as modest.43 This suggests that there are additional variables affecting BCVA in the setting of DME other than macular thickness, such as HbA1C and age.43 Damage to photoreceptors may also be irreversible; therefore, despite reduction in macular edema, visual acuity may remain compromised.
Aflibercept has demonstrated considerable safety across multiple study designs when compared to various treatment modalities. Two other meta-analyses found no significant adverse events occurring under aflibercept therapy.39,42 This contrasts to a safety study evaluating aflibercept, ranibizumab, and laser therapies found an increased risk of death, cerebrovascular accident, and vascular death among patients using aflibercept and ranibizumab compared with laser and sham.44 Zhang et al. found more frequent systemic adverse events in groups treated with anti-VEGF therapy, whereas steroid therapy resulted in more frequent ocular adverse events, including cataracts and increased intraocular pressure.41 Similarly, the present study found that in both studies evaluating dexamethasone, there were elevations in intraocular pressure after dexamethasone therapy. These findings suggest that steroid therapy should be employed cautiously.
In this study, meta-regression analysis demonstrated dosing schedule as a predictor of outcomes in addition to comparator group. This aligns with findings of previously published data demonstrating the variability of different dosing schedules on patient outcomes.45 It is important to consider that the risk of bias tools used to evaluate RCTs and non-RCTs vary in their standards of assessment. RCTs are inherently of higher quality; therefore, a low risk score for a non-RCT study may only equate to an RCT with some concerns. Although including multiple study designs resulted in a more comprehensive and clinically relevant review, there were several limitations: (1) studies with small sample sizes were not powered enough to undergo meaningful statistical analyses; (2) there were a limited number of comparison studies for certain treatments, particularly bevacizumab and dexamethasone; and (3) the methodological quality and the nonblinding of participants in non-RCTs may have introduced selection bias and confounding factors. However, the real-life data contributed by such studies provides a more realistic representation of the efficacy of aflibercept in practice; and (4) a significant amount of heterogeneity was present across comparator studies, likely due to differences in experimental protocols. To address this variability, a random-effects model was used to calculate pooled estimates.
Aflibercept is effective at improving visual and anatomical outcomes in patients with DME, both in highly controlled settings and in a real-life environment. Aflibercept shows greater improvements when compared to laser photocoagulation overall. Individual studies have shown an advantage of aflibercept when compared to bevacizumab anatomically, ranibizumab functionally at 1 year, and dexamethasone functionally. Aflibercept is shown to be noninferior compared to bevacizumab functionally and ranibizumab anatomically. Aflibercept demonstrates a considerable safety profile in patients with DME. Future research should assess the predictors of response to aflibercept. Furthermore, given the substantial differences in the cost associated with each treatment modality, cost-effectiveness studies taking into consideration the findings of the present study are warranted.
Availability of data and material
The present manuscript has data included as electronic Supplementary Material.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12:346–54. doi: 10.1007/s11892-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 2.Kim EJ, Lin WV, Rodriguez SM, Chen A, Loya A, Weng CY. Treatment of diabetic macular edema. Curr Diab Rep. 2019;19:68. doi: 10.1007/s11892-019-1188-4. [DOI] [PubMed] [Google Scholar]
- 3.Canada H, editor. Health Canada. Avastin. Drug Product Database. Ottawa, Ontario, Canada: Health Canada; 2005. [Google Scholar]
- 4.Lucentis. Government of Canada; 2007. [Last accessed on 2021 Jul 10]. Available from: https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=78217 .
- 5.Diabetic Retinopathy Clinical Research Network. Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eylea. Government of Canada; 2013. [Last accessed on 2021 Jul 10]. Available from: https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=90055 .
- 7.Régnier S, Malcolm W, Allen F, Wright J, Bezlyak V. Efficacy of anti-VEGF and laser photocoagulation in the treatment of visual impairment due to diabetic macular edema: A systematic review and network meta-analysis. PLoS One. 2014;9:e102309. doi: 10.1371/journal.pone.0102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 9.Wells GA, Tugwell P, O’Connell D, Welch V, Peterson J, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2015 [Google Scholar]
- 10.Jiang YQ, Tian Y, Zeng LJ, He SN, Zheng ZT, Shi L, et al. The safety and efficacy of hybrid ablation for the treatment of atrial fibrillation: A meta-analysis. PLoS One. 2018;13:e0190170. doi: 10.1371/journal.pone.0190170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Light R, Pillemer D. Cambridge, MA: Harvard University Press; 1984. Summing Up: The Science of Reviewing Research. [Google Scholar]
- 13.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 14.McGuinness LA, Higgins JP. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 15.McCloskey CF, Mongan AM, Chetty S, McAteer DM, Quinn SM. Aflibercept in diabetic macular oedema previously refractory to standard intravitreal therapy: An irish retrospective study. Ophthalmol Ther. 2018;7:173–83. doi: 10.1007/s40123-018-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsapardoni FN, Makri OE, Lagogiannis AP, Tsekouras IK, Chairas N, Pallikari A, et al. Functional and anatomic results of up to 24 months aflibercept treatment for diabetic macular edema in real-life setting. Hell J Nucl Med. 2019;22(Suppl 2):47–54. [PubMed] [Google Scholar]
- 17.Terasaki H, Shiraki K, Ohji M, Metzig C, Schmelter T, Zeitz O, et al. Efficacy and safety outcomes of intravitreal aflibercept focusing on patients with diabetic macular edema from Japan. Retina. 2019;39:938–47. doi: 10.1097/IAE.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khattab AM, Hagras SM, AbdElhamid A, Torky MA, Awad EA, Abdelhameed AG. Aflibercept with adjuvant micropulsed yellow laser versus aflibercept monotherapy in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2019;257:1373–80. doi: 10.1007/s00417-019-04355-6. [DOI] [PubMed] [Google Scholar]
- 19.Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123:2376–85. doi: 10.1016/j.ophtha.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 20.Kaldırım H, Yazgan S, Kırgız A, Atalay K, Savur F. A comparison study of ranibizumab and aflibercept in patients with naive diabetic macular edema in presence of serous retinal detachment. Curr Eye Res. 2019;44:987–93. doi: 10.1080/02713683.2019.1608260. [DOI] [PubMed] [Google Scholar]
- 21.Ozkaya A, Demir G, Kirmaci A. Comparison of aflibercept and ranibizumab in diabetic macular edema associated with subretinal detachment. Eur J Ophthalmol. 2020;30:363–9. doi: 10.1177/1120672119827855. [DOI] [PubMed] [Google Scholar]
- 22.Ozsaygili C, Duru N. Comparison of intravitreal dexamethasone implant and aflibercept in patients with treatment-naive diabetic macular edema with serous retinal detachment. Retina. 2020;40:1044–52. doi: 10.1097/IAE.0000000000002537. [DOI] [PubMed] [Google Scholar]
- 23.Garweg JG, Stefanickova J, Hoyng C, Schmelter T, Niesen T, Sowade O, et al. Vision-related quality of life in patients with diabetic macular edema treated with intravitreal aflibercept: The AQUA study. Ophthalmol Retina. 2019;3:567–75. doi: 10.1016/j.oret.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahrami B, Hong T, Schlub TE, Chang AA. Aflibercept for persistent diabetic macular edema: Forty-eight-week outcomes. Retina. 2019;39:61–8. doi: 10.1097/IAE.0000000000002253. [DOI] [PubMed] [Google Scholar]
- 26.Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, et al. Effect of initial management with aflibercept vs. laser photocoagulation vs. observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: A randomized clinical trial. JAMA. 2019;321:1880–94. doi: 10.1001/jama.2019.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YX, Li XX, Yoon YH, Sun X, Astakhov Y, Xu G, et al. Intravitreal aflibercept versus laser photocoagulation in asian patients with diabetic macular edema: The VIVID-east study. Clin Ophthalmol. 2020;14:741–50. doi: 10.2147/OPTH.S235267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658–65. doi: 10.1016/j.ophtha.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Abouhussein MA, Gomaa AR. Aflibercept plus micropulse laser versus aflibercept monotherapy for diabetic macular edema: 1-year results of a randomized clinical trial. Int Ophthalmol. 2020;40:1147–54. doi: 10.1007/s10792-019-01280-9. [DOI] [PubMed] [Google Scholar]
- 32.Curry BA, Sanfilippo PG, Chan S, Hewitt AW, Verma N. Clinical outcomes of a treat and extend regimen with intravitreal aflibercept injections in patients with diabetic macular edema: Experience in clinical practice. Ophthalmol Ther. 2020;9:87–101. doi: 10.1007/s40123-019-00224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiho T, Oshitari T, Tatsumi T, Takatsuna Y, Arai M, Shimizu N, et al. Efficacy of one-year treatment with aflibercept for diabetic macular edema with practical protocol. Biomed Res Int. 2017;2017:7879691. doi: 10.1155/2017/7879691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández-Bel L, Cervera-Taulet E, Navarro-Palop C, Castro-Navarro V, Chiarri-Toumit C, Montero-Hernández J. Sequential dexamethasone and aflibercept treatment in patients with diabetic macular edema: Structural and functional outcomes at 52 weeks. Ophthalmologica. 2019;241:98–104. doi: 10.1159/000489345. [DOI] [PubMed] [Google Scholar]
- 35.Fouda SM, Bahgat AM. Intravitreal aflibercept versus intravitreal ranibizumab for the treatment of diabetic macular edema. Clin Ophthalmol. 2017;11:567–71. doi: 10.2147/OPTH.S131381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plaza-Ramos P, Borque E, García-Layana A. Evaluation of ranibizumab and aflibercept for the treatment of diabetic macular edema in daily clinical practice. PLoS One. 2019;14:e0223793. doi: 10.1371/journal.pone.0223793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pak KY, Shin JP, Kim HW, Sagong M, Kim YC, Lee SJ, et al. One-year results of treatment of diabetic macular edema with aflibercept using the treat-and-extend dosing regimen: The VIBIM study. Ophthalmologica. 2020;243:255–62. doi: 10.1159/000504753. [DOI] [PubMed] [Google Scholar]
- 38.Bhandari S, Nguyen V, Fraser-Bell S, Mehta H, Viola F, Baudin F, et al. Ranibizumab or aflibercept for diabetic macular edema: Comparison of 1-year outcomes from the fight retinal blindness! Registry. Ophthalmology. 2020;127:608–15. doi: 10.1016/j.ophtha.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen CL, Lindsay A, Wong E, Chilov M. Aflibercept for diabetic macular oedema: A Meta-analysis of randomized controlled trials. Int J Ophthalmol. 2018;11:1002–8. doi: 10.18240/ijo.2018.06.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussain RM, Ciulla TA. Treatment strategies for refractory diabetic macular edema: Switching anti-VEGF treatments, adopting corticosteroid-based treatments, and combination therapy. Expert Opin Biol Ther. 2016;16:365–74. doi: 10.1517/14712598.2016.1131265. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Wang W, Gao Y, Lan J, Xie L. The efficacy and safety of current treatments in diabetic macular edema: A systematic review and network meta-analysis. PLoS One. 2016;11:e0159553. doi: 10.1371/journal.pone.0159553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: A network meta-analysis. Cochrane Database Syst Rev. 2017;6:CD007419. doi: 10.1002/14651858.CD007419.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diabetic Retinopathy Clinical Research Network. Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avery RL, Gordon GM. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: A systematic review and meta-analysis. JAMA Ophthalmol. 2016;134:21–9. doi: 10.1001/jamaophthalmol.2015.4070. [DOI] [PubMed] [Google Scholar]
- 45.Ziemssen F, Schlottman PG, Lim JI, Agostini H, Lang GE, Bandello F. Initiation of intravitreal aflibercept injection treatment in patients with diabetic macular edema: A review of VIVID-DME and VISTA-DME data. Int J Retina Vitreous. 2016;2:16. doi: 10.1186/s40942-016-0041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campos Polo R, Rubio Sánchez C, García Guisado DM, Díaz Luque MJ. Aflibercept for clinically significant diabetic macular edema: 12-month results in daily clinical practice. Clin Ophthalmol. 2018;12:99–104. doi: 10.2147/OPTH.S154421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukic M, Williams G, Shalchi Z, Sim D, Patel PJ, Keane PA, et al. Intravitreal aflibercept for diabetic macular oedema: Moorfields' real-world 12-month visual acuity and anatomical outcomes. Eur J Ophthalmol. 2020;30:557–62. doi: 10.1177/1120672119833270. [DOI] [PubMed] [Google Scholar]
- 48.Kern C, Schiefelbein J, Fu DJ, Schworm B, Sim D, Herold T, et al. Two year visual acuity and structural outcomes in patients with diabetic macular oedema treated with intravitreal aflibercept – A retrospective cohort study. Clin Ophthalmol. 2020;14:533–41. doi: 10.2147/OPTH.S237586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarzer P, Ebneter A, Munk M, Wolf S, Zinkernagel MS. One-year results of using a treat-and-extend regimen without a loading phase with anti-VEGF agents in patients with treatment-naive diabetic macular edema. Ophthalmologica. 2019;241:220–5. doi: 10.1159/000495623. [DOI] [PubMed] [Google Scholar]
- 50.Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retina. 2018;2:1179–87. doi: 10.1016/j.oret.2018.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The present manuscript has data included as electronic Supplementary Material.