Abstract
Purpose:
To evaluate the total corneal thickness distribution pattern using a high-resolution spectral-domain optical coherence tomography (HR SD-OCT) for distinguishing normal eyes from keratoconus (KCN).
Methods:
One hundred and forty-four patients were enrolled in three groups (55 normal, 45 mild KCN, and 44 moderate-to-severe KCN eyes) in this prospective diagnostic test study. Total corneal thickness was measured in 8 semi-meridians using HR SD-OCT (Heidelberg Engineering, Heidelberg, Germany) in 5 and 7 mm zones. The central corneal thickness (CCT), corneal focal thinning (minimum thickness [Min], min minus median and maximum [Min-Med, Min-Max]), and asymmetry indices (inferior minus superior [I-S] and supranasal minus infratemporal [SN-IT]) were calculated. One-way analysis of variance and the area under the receiver operating characteristic curve (AUC) were used for the analysis.
Results:
Thinner CCT, lower Min thickness, more negative Min-Max, Min-Med, and greater I-S and SN-IT were found in KCN eyes compared to the control group (P < 0.001). The inferior and IT semi-meridians were the thinnest locations in KCN cases in the 5 mm central zone (P < 0.001). CCT followed by Min-Med had the highest discriminative ability for differentiating mild KCN (AUC, sensitivity and specificity: 0.822, 87.0%, 60.37% and 0.805, 82.93%, 66.0%, respectively) and moderate-to-severe KCN (0.902, 87.82%, 73.08% and 0.892, 85.37%, and 78.85%, respectively) from normal corneas.
Conclusion:
The inferior and IT sectors of the cornea with the largest thickness changes in the 5 mm zone are the most common thinning sites in keratoconic corneas, and CCT and Min-Med are the most sensitive indices for the diagnosis of KCN.
Keywords: Corneal thickness, Keratoconus, Spectral-domain optical coherence tomography
INTRODUCTION
Keratoconus (KCN) is a bilateral noninflammatory ectatic corneal disorder characterized by asymmetry in corneal pachymetry and corneal thinning, increased curvature, and the development of irregular astigmatism.1,2 Because KCN is a common implication for corneal transplantation,3 a comprehensive corneal assessment is important in the timely diagnosis and management of the disease. Corneal thickness is considered an important parameter in KCN diagnosis,2 and accurate corneal thickness measurement at the central (CCT) and thinnest points is important for refractive surgery screening.4 This parameter associated with peripheral corneal thickness is important for corneal cross-linking and intrastromal corneal ring implantation in KCN management.5
There are different methods to measure corneal thickness, including ultrasound pachymetry,6 confocal microscopy,7 and slit scanning, Scheimpflug tomography.8,9 The first two are contact methods that measure CCT at certain points and are associated with a possible risk of infection transmission.10 In addition, their precision is operator dependent and measurements may not be accurate if the probe is not positioned accurately.11 Corneal thickness measurement using tomography imaging systems is preferred to other methods because they can provide a thickness map of the cornea to assess the normality of the thickness map pattern; however, these devices still have some disadvantages such as resolution limitation, which may lead to underestimation of the corneal thickness, especially in the postrefractive assessments.12 In addition, in advanced KCN cases, these devices may fail to provide acceptable maps due to the extensive irregularity of the corneal surface and difficulty in the fixation of KCN eyes.13 Furthermore, they can hardly differentiate corneal irregularity caused by tear film problems or corneal scars from true KCN.14
Today, anterior segment optical coherence tomography (AS-OCT) can assess the anterior and posterior corneal surfaces with a high resolution (HR) based on the reflectivity difference in tissues using time-domain and spectral-domain (SD) methods. The SD method, also known as Fourier-domain, provides higher resolution images at a higher scanning speed and has a deeper penetration into the tissue15 so that corneal thickness measurement using this method may provide information that is not available by tomography technique alone.
Previous studies measured the corneal thickness using different AS-OCT devices;11,16,17 however, the exact distribution of corneal thickness along different meridians and the diagnostic ability of OCT parameters in differentiating on KCN from normal eyes have been rarely reported.
The purpose of this study was to evaluate the distribution pattern of total corneal thickness and determine the diagnostic ability of different corneal pachymetric parameters using a HR SD-OCT for distinguishing KCN from normal eyes.
METHODS
This diagnostic test study was a part of a prospective study conducted between February 20 and December 21, 2018, at Noor Eye Hospital. The research project was approved by the Ethics Committee of Tehran University of Medical Sciences (code: IR.TUMS.VCR.REC.1396.4815). All stages of the current study were conducted according to the principles of the Declaration of Helsinki. The objectives of this study were explained to the participants, and informed consent was obtained before the study. Participants were recruited from patients referred to the KCN clinic and those referred to the refractive surgery unit. If both eyes were eligible for inclusion, one eye was randomly selected.
All patients underwent complete ocular examinations including the measurement of corrected distance visual acuity using a Snellen chart, refractive assessment using retinoscopy by a Heine Streak Retinoscope, slit-lamp biomicroscopy, and fundoscopy. The anterior and posterior corneal surfaces were assessed using the Scheimpflug tomography by Pentacam HR (Oculus, Wetzlar, Germany) and AS-OCT technique using HR SD-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany; software version 3.1.0.55).
Diagnosis of KCN was based on the presence of scissoring reflex on retinoscopy, and at least one of the following slit-lamp signs including the Fleischer ring, Vogt striae, apical thinning, Munson's sign, Rizutti's sign, and the presence of abnormal topographic criteria using the Pentacam including a skewed asymmetric bow tie or inferior steepening or a claw pattern, SRAX >21°, anterior Kmax >49.0 diopter (D) or inferior-superior (I-S) value >1.9 D.1,18 Patients were assigned by an experienced corneal specialist with more than 10 years of experience.
The severity of KCN was classified based on Pentacam Topographic KCN Classification (TKC). TKC provides four grades (I–IV) for KCN classification. In some intermediate grades (e.g., III–VI), lower values were documented. The KCN Group 1 (G1) corresponded to Grade I, and the KCN Group 2 (G2) corresponded to Grades II–VI.
The normal group was selected from refractive surgery candidates who had a normal clinical examination based on retinoscopy and slit-lamp biomicroscopy, and no abnormal topographic criteria.1,18
The exclusion criteria were any history of ocular surgery, corneal cross-linking, ring implantation, corneal hydrops, corneal scar, any systemic diseases affecting the eyes, and signs and symptoms of dry eye. The patients with a history of contact lens wear in the past 4 weeks were also excluded.
Spectralis anterior segment module provides HR images (axial and transverse resolutions 3.9 and 14 μm, respectively) at a high scanning speed per second (40,000 A-scans/s) and can display the corneal thickness in detail.19,20 Moreover, this module provides angle-to-angle images for precise assessment of the corneal angles and scleral structure. During the imaging, patients were asked to fixate on a blue light target and blink at intervals between measurements to avoid dry eye. After proper fixation, an average of three measurements was taken in vertical, horizontal, and diagonal meridians were recorded. After imaging, the total corneal thickness was measured in 8 semi-meridians at 45° intervals in two zones of 5 and 7 mm centered on the corneal geometric center. Horizontal scans were used to measure along the nasal (0°) and temporal (180°) semi-meridians, vertical scans were applied to measure along with the superior (90°) and inferior (270°) semi-meridians, and diagonal scans were used to measure along the supranasal (45°), infratemporal (225°), supratemporal (135°), and infranasal (315°) semi-meridians [Figures 1 and 2].
Figure 1.
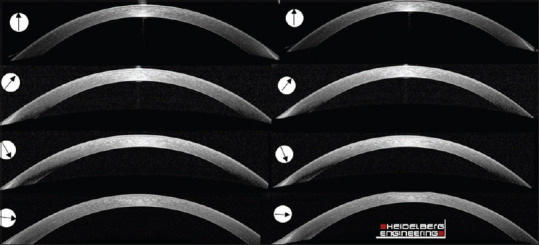
Cross-sectional optical coherence tomography images in all eight meridians in the keratoconus eye
Figure 2.
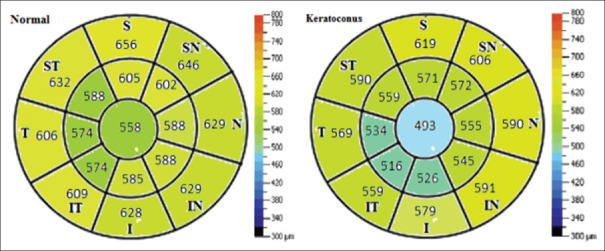
Sector map shows mean pachymetry in each zone in normal and keratoconus eyes (I: Inferior, S: Superior, T: Temporal, N: Nasal, IN: Infranasal, IT: Infratemporal, SN: Supranasal, ST: Supratemporal)
Moreover, the mean absolute CCT was calculated in semi-meridians in the geometric center of the cornea and recorded in the center of the annular rings. Then, the indices of focal thinning including minimum (Min) thickness, the difference between the minimum and median thickness (Min-Med), and the difference between the minimum and maximum thickness (Min-Max) were calculated for all measurement points. Corneal asymmetry indices including the difference between the mean thickness of the inferior and superior octants of the cornea (I-S), the difference between the mean thickness of supranasal and infratemporal octants of the cornea (supranasal-infratemporal [SN-IT]) were calculated for both 5 mm and 7 mm zones. For evaluation of the intraobserver repeatability test, all measurements were repeated 2 weeks apart by the same observer who was blind to the grouping of the cases. Because the corneal epithelium was not distinguishable from the tear film in some cases, for better interpretation and comparison of the results, the tear film (the first hyperreflective layer) was included in the thickness measurements (the tear film thickness is about 3–6 μ).21 All imaging techniques were performed in a dark room between 9 am and 5 pm by an experienced operator to avoid any bias.
SPSS 23 software (IBM Inc., New York, NY, USA) was used for statistical analyses. The normality of data was assessed using the Kolmogorov–Smirnov test. Descriptive statistics including mean, standard deviation, and range of corneal thickness were calculated for each semi-meridian in two 5 and 7 mm zones in both groups. The mean values of the variables were compared between the two groups using the independent t-test. A P < 0.05 was considered statistically significant. A receiver operating characteristic (ROC) curve analysis was performed for all variables to determine the area under the ROC curve (AUC) to evaluate the accuracy of different variables. The Youden index was applied to calculate the optimum cut-off value. To compare the diagnostic ability of each parameters, sensitivity (true positive/[true positive + false negative]) and specificity (true negative/[true negative + false positive]) were reported for all variables. The intraclass correlation coefficient (ICC) was performed to assess the reliability of the repeated measurements. An ICC > 0.80 was considered excellent repeatability.19
RESULTS
A total of 144 eyes (55 normal, 45 mild KCN, and 44 moderate-to-severe KCN eyes) were included in the study. KCN groups included 45 eyes (50.56%) with Grade I (G1), 27 eyes (30.33%) with Grade II, and 17 eyes (19.11%) with Grade III (G2) KCN. Females accounted for 58.2% of normals, whereas 62.9% of the patients with KCN (G1 and G2) were male. The demographic characteristics of the participants are summarized in Table 1, and the mean values of pachymetric parameters are presented in Table 2.
Table 1.
Demographic characteristics of study groups
| Variables | Mean±SD (range) | P (ANOVA) | |||
|---|---|---|---|---|---|
|
| |||||
| Normal (n=55) | KCN G1 (n=45) | KCN G2 (n=44) | Total (n=144) | ||
| Age | 31.07±7.06 (19-49) | 32.66±4.70 (25-47) | 31.68±5.97 (24-46) | 31.81±6.02 (19-49) | 0.493 |
| Sphere (D) | −2.44±2.27 (−6.00 - +8.00) | −1.92±1.95 (−6.75 - +1.00) | −2.81±3.53 (−13.50 - +3.75) | −2.41±2.62 (−13.5 - +8.00) | 0.271 |
| Cylinder (D) | −1.73±1.68 (0.00 - −7.25) | −2.42±1.55 (0.00 - − 8.50) | −3.69±1.99 (−0.75 - − 10.75) | −2.48±1.88 (0.00 - −10.75) | <0.001 |
| CDVA, logMAR | 0.01±0.05 (0.00 - +0.30) | 0.13±0.19 (0.10 - + 0.70) | 0.37±0.19 (0.10 - +0.80) | 0.16±0.21 (0.00 - +0.80) | <0.001 |
**The significant variables highlighted (ANOVA). ANOVA: Analysis of variance, SD: Standard deviation, KCN: Keratoconus, KCN G1: Mild KCN group, KCN G2: Moderate and severe KCN groups, D: Diopter, CDVA: Corrected distance visual acuity
Table 2.
Mean and standard deviation of corneal thickness parameters separately in normal and keratoconus eyes
| Variable (µm) | Mean±SD (range) | P (ANOVA) | P (normal versus G1) | P (normal versus G2) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Normal (n=55) | KCN G1 (n=45) | KCN G2 (n=44) | ||||
| Central corneal thickness | 558.49±37.59 (473-689) | 512.24±32.93 (439-583) | 486.02±43.38 (413-641) | <0.001 | <0.001 | <0.001 |
| Minimum thickness | 531.88±40.29 (443-662) | 485.73±30.98 (413-556) | 461.87±42.78 (383-617) | <0.001 | <0.001 | <0.001 |
| Minimum-median thickness | −39.03±9.97 (−75 - −19) | −50.23±13.73 (−97 - −24) | −66.18±19.83 (−102 - −22) | <0.001 | 0.001 | <0.001 |
| Minimum-maximum thickness | −98.50±19.22 (−138 - −62) | −113±26.78 (−201 - −69) | −124.26±26.38 (−189 - −77) | <0.001 | 0.007 | <0.001 |
| Total corneal thickness (5 mm) | ||||||
| S | 585.03±40.08 (500-659) | 559.19±39.57 (451-648) | 555.95±45.27 (450-681) | <0.001 | 0.010 | 0.003 |
| I | 564.81±39.08 (488-669) | 518.73±35.68 (430-585) | 501.92±48.81 (414-661) | <0.001 | <0.001 | <0.001 |
| N | 569.00±40.25 (499-645) | 537.04±32.93 (444-605) | 538.43±44.22 (451-675) | <0.001 | <0.001 | 0.001 |
| T | 552.56±36.49 (459-639) | 519.46±35.28 (423-592) | 513.07±44.89 (431-660) | <0.001 | <0.001 | <0.001 |
| SN | 580.33±37.27 (511-657) | 551.36±35.45 (456-625) | 553.07±46.55 (469-694) | <0.001 | 0.002 | 0.004 |
| IT | 552.50±38.81 (472-629) | 509.0±34.42 (433-577) | 494.0±44.81 (406-619) | <0.001 | <0.001 | <0.001 |
| ST | 571.62±36.75 (480-642) | 545.85±34.54 (438-618) | 541.21±44.84 (453-677) | <0.001 | 0.005 | 0.001 |
| IN | 566.81±38.71 (566-659) | 529.56±33.09 (450-606) | 525.19±44.63 (432-657) | <0.001 | <0.001 | <0.001 |
| I-S | −19.62±20.74 (−75-29) | −37.17±35.82 (−131-91) | −52.90±33.85 (−124-11) | <0.001 | 0.017 | <0.001 |
| SN-IT | 28.00±24.22 (−53-79) | 46.41±26.65 (−2-138) | 62.27±32.42 (−8-138) | <0.001 | 0.005 | <0.001 |
| Total corneal thickness (7 mm) | ||||||
| S | 638.09±42.20 (564-724) | 613.02±43.07 (489-687) | 603.26±47.08 (507-721) | 0.001 | 0.021 | 0.001 |
| I | 608.28±43.91 (528-717) | 557.53±36.66 (458-629) | 558.07±46.90 (477-706) | <0.001 | <0.001 | <0.001 |
| N | 610.05±47.71 (511-713) | 578.17±34.20 (490-665) | 577.48±42.59 (481-709) | <0.001 | 0.001 | 0.001 |
| T | 586.84±40.80 (490-684) | 567.21±37.78 (465-636) | 553.14±42.55 (475-672) | <0.001 | 0.002 | <0.001 |
| SN | 624.71±41.72 (520-713) | 597.09±40.04 (489-687) | 590.60±49.21 (487-731) | <0.001 | 0.009 | <0.001 |
| IT | 588.52±41.46 (520-679) | 548.56±35.17 (465-625) | 542.30±42.18 (462-636) | <0.001 | <0.001 | <0.001 |
| ST | 607.43±45.68 (519-694) | 580.46±39.82 (482-672) | 576.68±42.97 (492-706) | 0.001 | 0.010 | 0.002 |
| IN | 607.20±43.47 (517-710) | 573.41±33.34 (481-660) | 576.07±43.33 (474-697) | <0.001 | <0.001 | 0.001 |
| I-S | −29.81±27.89 (−99-35) | −44.29±31.34 (−126-24) | −43.92±32.10 (−111-24) | 0.030 | 0.069 | 0.080 |
| SN-IT | 35.25±30.68 (−41-100) | 49.36±25.15 (−13-112) | 50.87±38.77 (−48-137) | 0.034 | 0.107 | 0.064 |
**The significant variables highlighted (ANOVA, Bonferroni). ANOVA: Analysis of variance, SD: Standard deviation, KCN: Keratoconus, KCN G1: Mild KCN group, KCN G2: Moderate and sever KCN groups, S: Superior, I: Inferior, N: Nasal, T: Temporal, SN: Supranasal, IT: Infratemporal, ST: Supratemporal, IN: Infranasal, I-S: Inferior-superior, SN-IT: Supranasal-infratemporal
The cornea was thinner in the center and became thicker toward the periphery; corneal pachymetric indices were thinner in 5 mm zone compared to 7 mm zone (P < 0.001). The mean CCT was 558.49 ± 37.59, 512.24 ± 32.93, and 486.02 ± 43.38 μm in normal, G1 and G2 KCN eyes, respectively (P < 0.001). Evaluation of the total corneal thickness indices in both 5 and 7 mm zones showed that all parameters were significantly thinner in KCN eyes (P < 0.001). The mean thickness was thinner in inferior and temporal semi-meridians compared to superior and nasal semi-meridians at 5 mm and 7 mm zones in all groups. In KCN groups, the thinnest pachymetric values were found in the inferior and IT semi-meridians, and the thickest corneal area was seen in the superior semi-meridian in both diameter zones.
Evaluation of the corneal focal thinning and asymmetric parameters showed lower Min thickness, greater I-S and SN-IT at 5 and 7 mm, and more negative Min-Max and Min-Med in the KCN eyes compared to the control group (P < 0.001); however, corneal asymmetric parameters were higher in 5 mm zone than the 7 mm zone in KCN group (P < 0.001). The total and central corneal thickness (CCT) measurements showed excellent reliability in all groups (ICC >0.80).
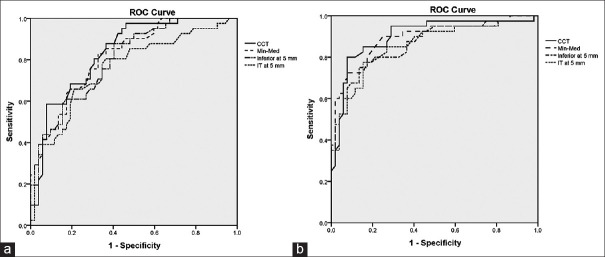
The ROC curve analysis is shown in Table 3. The highest discriminative ability was found for CCT in G1 (AUC 0.822, sensitivity 87.0%, and specificity 60.37%) and G2 (AUC 0.902, sensitivity 87.82%, and specificity 73.08%), followed by the Min-Med index in G1 (AUC 0.805, sensitivity 82.93%, and specificity 66.0%) and G2 (AUC 0.892, sensitivity 85.37%, and specificity 78.85%). The inferior and IT semi-meridians had the highest AUCs compared to other sectors in both 5 mm and 7 mm zones [Table 3]. ROC curves of thickness parameters with the highest diagnostic ability are demonstrated in Figure 3.
Table 3.
The result of receiver operating characteristics analysis to differentiate eyes with keratoconus from normal eyes
| Variable (µm) | Normal versus KCN G1 | Normal versus KCN G2 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| AUC (95% CI) | Cut-off | Sensitivity | Specificity | AUC (95% CI) | Cut-off | Sensitivity | Specificity | |
| Central corneal thickness | 0.822 (0.730-0.893) | ≤520 | 87.80 | 60.37 | 0.902 (0.826-0.956) | ≤508 | 87.82 | 73.08 |
| Minimum thickness | 0.759 (0.660-0.842) | ≤516 | 51.22 | 88.68 | 0.865 (0.782-0.929) | ≤497 | 56.34 | 96.50 |
| Minimum–median thickness | 0.805 (0.711-0.880) | ≤−40 | 82.93 | 66.0 | 0.892 (0.813-0.948) | ≤−45 | 85.67 | 78.85 |
| Minimum–maximum thickness | 0.679 (0.575-0.772) | ≤−123 | 41.46 | 88.68 | 0.779 (0.678-0.856) | ≤−106 | 80.5 | 61.5 |
| Total corneal thickness (5 mm) | ||||||||
| S | 0.685 (0.581-0.777) | ≤565 | 63.40 | 77.70 | 0.701 (0.605-0.798) | ≤560 | 56.1 | 76.92 |
| I | 0.793 (0.697-0.870) | ≤542 | 80.49 | 64.15 | 0.862 (0.779-0.928) | ≤529 | 75.6 | 86.5 |
| N | 0.721 (0.619-0.808) | ≤552 | 75.60 | 66.04 | 0.704 (0.607-0.800) | ≤547 | 68.3 | 73.1 |
| T | 0.742 (0.641-0.826) | ≤724 | 58.5 | 79.2 | 0.768 (0.675-0.854) | ≤530 | 65.9 | 75.0 |
| SN | 0.708 (0.606-0.798) | ≤577 | 82.9 | 56.6 | 0.697 (0.593-0.789) | ≤565 | 67.5 | 67.3 |
| IT | 0.789 (0.692-0.866) | ≤540 | 80.41 | 60.38 | 0.855 (0.766-0.920) | ≤518 | 77.5 | 84.62 |
| ST | 0.699 (0.597-0.791) | ≤548 | 56.10 | 77.36 | 0.773 (0.626-0.815) | ≤539 | 58.5 | 70.1 |
| IN | 0.764 (0.666-0.846) | ≤547 | 75.61 | 67.9 | 0.776 (0.678-0.856) | ≤527 | 63.41 | 88.46 |
| I-S | 0.694 (0.593-0.787) | ≤−40 | 48.78 | 86.80 | 0.807 (0.701-0.873) | ≤−41 | 68.50 | 86.54 |
| SN-IT | 0.694 (0.590-0.785) | >37 | 63.4 | 67.3 | 0.80 (0.706-0.879) | >42 | 77.50 | 74.50 |
| Total corneal thickness (7 mm) | ||||||||
| S | 0.647 (0.538-0.740) | ≤632 | 73.2 | 52.8 | 0.717 (0.614-0.805) | ≤642 | 80.0 | 53.66 |
| I | 0.758 (0.658-0.840) | ≤592 | 80.0 | 56.98 | 0.799 (0.709-0.879) | ≤595 | 85.2 | 59.62 |
| N | 0.693 (0.589-0.784) | ≤608 | 80.0 | 47.17 | 0.704 (0.600-0.794) | ≤609 | 82.93 | 46.2 |
| T | 0.707 (0.604-0.796) | ≤548 | 46.3 | 88.8 | 0.738 (0.637-0.824) | ≤541 | 46.3 | 92.3 |
| SN | 0.686 (0.582-0.778) | ≤625 | 79.9 | 52.8 | 0.724 (0.622-0.812) | ≤624 | 85.4 | 53.8 |
| IT | 0.746 (0.646-0.830) | ≤578 | 80.4 | 54.72 | 0.772 (0.680-0.859) | ≤585 | 85.3 | 53.8 |
| ST | 0.671 (0.566-0.764) | ≤608 | 78 | 56 | 0.708 (0.605-0.798) | ≤608 | 85.4 | 57.7 |
| IN | 0.731 (0.566-0.764) | ≤577 | 63.4 | 75.5 | 0.716 (0.607-0.800) | ≤575 | 58.5 | 78.8 |
| I-S | 0.640 (0.536-0.738) | ≤−53.4 | 41.46 | 80.0 | 0.636 (0.519-0.724) | ≤−22 | 78.0 | 45.0 |
| SN-IT | 0.633 (0.526-0.730) | >28 | 80.11 | 44.24 | 0.640 (0.533-0.738) | >43 | 65.0 | 60.78 |
**The highest AUCs highlighted. AUC: Area under the receiver operating characteristic curve, CI: Confidence interval, KCN: Keratoconus, KCN G1: Mild KCN group, KCN G2: Moderate and sever KCN groups, S: Superior, I: Inferior, N: Nasal, T: Temporal, SN: Supranasal, IT: Infratemporal, ST: Supratemporal, IN: Infranasal, I-S: Inferior-superior, SN-IT: Supranasal-infratemporal
Figure 3.
Comparison of parameters with the highest area under the receiver operating characteristic curve to differentiate mild (a), and moderate-to-severe (b) keratoconus from normal eyes (CCT: Central corneal thickness, Min-Med: Minimum-median thickness, IT: Infratemporal)
DISCUSSION
Regarding thickness parameters in normal eyes, the mean CCT in the current study (558.49 ± 37.59 μm) was close to the value reported by López de la Fuente et al.19(555.5 ± 29.64 μm) using the same technique but higher than the value reported by Catalan et al.22(537.6 ± 30.66 μm). Moreover, the values of focal thinning and asymmetric parameters in the normal group were larger than the results of a study by Kanellopoulos and Asimellis23 using the RTVue OCT in normal corneas. It seems that differences in the results are due to different measurement techniques.
In the present study, the measured thickness parameters in KCN eyes for CCT, Min thickness, Min-Med, and Min-Max were larger than the results of a study reported by Catalan et al.22 and Kanellopoulos et al.23 However, evaluation of corneal asymmetry indices showed lower values of I-S and SN-IT at 5 mm zone in the KCN group compared to Temstet et al.'s24 study reports, which could be due to the differences in the severity of KCN in different populations. However, it is noteworthy that in recent studies, these parameters had significantly larger mean differences in KCN versus normal eyes.
In general, higher mean thickness values in the present study compared to some previous studies that used other methods of corneal thickness measurement may probably be due to including the tear film in the thickness measurement. In the presence of corneal ectasia, especially in severe KCN cases, it is not possible to differentiate the precorneal tear film (PTF) of the epithelium layer. Therefore, in the current study, tear film was included in corneal thickness components to enhance the accuracy of the measurement. Including PTF in measurements has a low coefficient of variation and high intraobserver repeatability and interobserver reproducibility.19 The present study also showed high repeatability in measuring the total corneal thickness.
Few studies have reported corneal pachymetric parameters in different meridians separately. Comparison of thickness in the opposite semi-meridians showed a thinner thickness in the inferior versus superior, temporal versus nasal, and IT versus SN semi-meridians in KCN and normal eyes which was similar to the findings reported by Dutta et al.10 in KCN eyes and Li et al.25 in normal eyes in different meridians using RTVue OCT. However, the present study showed that the inferior and IT semi-meridians in the 5 mm zone had the thinnest thickness in KCN cases. Thinner corneas in the inferior and IT semi-meridians could be due to the inferior displacement of the corneal cone in KCN eyes, and more exposure of these corneal parts to trauma from eye rubbing.26,27 Abnormal eye rubbing may lead to the progression of KCN,28 increased oxidative stress, loss of keratocytes, and stromal scarring.29
Although KCN is a disease with a “pancorneal” pathology30 and the paracentral and peripheral corneal thickness can provide valuable information about the progression of corneal thinning, the largest mean difference in pachymetric indices between normal and keratoconic corneas was in the 5 mm zone.
ROC analysis showed that CCT and focal thinning parameters, especially Min-Med thickness, had the best diagnostic ability for differentiating KCN from normal corneas. Among total corneal thickness parameters in different semi-meridians, inferior, and IT semi-meridians in the 5 mm zone showed the highest accuracy for KCN detection. Reviewing the literature showed that few studies have investigated the accuracy of focal and total corneal thickness in different semi-meridians, and most of these studies have focused on the epithelial thickness map, which is not available in all clinical centers. Catalan et al.22 studied 22 KCN and 104 normal eyes using the RTVue OCT and found that the Min-Med parameter had the highest diagnostic accuracy (AUC = 0.974). In addition, they found that S-I, with an AUC similar to Min-Med, was the best distinguishing index in both groups; however, this parameter showed less accuracy in the present study. In another study, Li et al.31 compared 37 KCN and 36 normal eyes using the Visante OCT system and found that Min thickness had the highest accuracy (AUC = 0.954). These studies used different measurement techniques from our study and evaluated the cornea from the center to the 5 mm zone, while we also studied the 7 mm corneal zone. In contrast to the present study, they did not investigate KCN corneas in different grades.
One of the limitations of the current study was the manual measurement of parameters; however, the results of some parameters were similar to studies using automated measurements. In addition, this method represents a simple measurement for quick evaluation of patients in clinical practice.
In summary, the results highlighted an increase in both corneal thickness asymmetry and focal thinning indices and a decrease in corneal thickness in different semi-meridians, especially the inferior and IT sectors of the cornea, in ectatic versus normal eyes. Evaluation of the total corneal thickness showed that the largest thickness changes probably occur in the 5 mm zone and, therefore, this zone is accurate enough for KCN diagnosis.
Among all studied indices, the CCT and Min-Med thickness index for assessment of total corneal thickness, and inferior and the IT semi-meridians thickness, especially in the 5 mm zone may be helpful for screening and early detection of KCN eyes with high accuracy. These parameters can be used as complementary diagnostic indices in Scheimpflug tomography devices that provide the corneal thickness map.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Mehrnaz Akhavan Bsc., Noor Eye Hospital, Tehran, Iran for providing assistance with OCT measurement.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 3.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167–73. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 4.Price FW, Jr, Koller DL, Price MO. Central corneal pachymetry in patients undergoing laser in situ keratomileusis. Ophthalmology. 1999;106:2216–20. doi: 10.1016/S0161-6420(99)90508-0. [DOI] [PubMed] [Google Scholar]
- 5.Abdelmassih Y, El-Khoury S, Dirani A, Antonios R, Fadlallah A, Cherfan CG, et al. Safety and efficacy of sequential intracorneal ring segment implantation and cross-linking in pediatric keratoconus. Am J Ophthalmol. 2017;178:51–7. doi: 10.1016/j.ajo.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Kozak I, Hornak M, Juhas T, Shah A, Rawlings EF. Changes in central corneal thickness after laser in situ keratomileusis and photorefractive keratectomy. J Refract Surg. 2003;19:149–53. doi: 10.3928/1081-597X-20030301-10. [DOI] [PubMed] [Google Scholar]
- 7.MeenakshiSundaram S, Sufi AR, Prajna NV, Keenan JD. Comparison of in vivo confocal microscopy, ultrasonic pachymetry, and scheimpflug topography for measuring central corneal thickness. JAMA Ophthalmol. 2016;134:1057–9. doi: 10.1001/jamaophthalmol.2016.2183. [DOI] [PubMed] [Google Scholar]
- 8.Ambrósio R, Jr, Alonso RS, Luz A, Coca Velarde LG. Corneal-thickness spatial profile and corneal-volume distribution: Tomographic indices to detect keratoconus. J Cataract Refract Surg. 2006;32:1851–9. doi: 10.1016/j.jcrs.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Auffarth GU, Wang L, Völcker HE. Keratoconus evaluation using the Orbscan Topography System. J Cataract Refract Surg. 2000;26:222–8. doi: 10.1016/s0886-3350(99)00355-7. [DOI] [PubMed] [Google Scholar]
- 10.Dutta D, Rao HL, Addepalli UK, Vaddavalli PK. Corneal thickness in keratoconus: Comparing optical, ultrasound, and optical coherence tomography pachymetry. Ophthalmology. 2013;120:457–63. doi: 10.1016/j.ophtha.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Nam SM, Im CY, Lee HK, Kim EK, Kim TI, Seo KY. Accuracy of RTVue optical coherence tomography, Pentacam, and ultrasonic pachymetry for the measurement of central corneal thickness. Ophthalmology. 2010;117:2096–103. doi: 10.1016/j.ophtha.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Park SH, Choi SK, Lee D, Jun EJ, Kim JH. Corneal thickness measurement using Orbscan, Pentacam, Galilei, and ultrasound in normal and post-femtosecond laser in situ keratomileusis eyes. Cornea. 2012;31:978–82. doi: 10.1097/ICO.0b013e31823d03fc. [DOI] [PubMed] [Google Scholar]
- 13.Wu W, Wang Y, Xu L. Meta-analysis of Pentacam vs. ultrasound pachymetry in central corneal thickness measurement in normal, post-LASIK or PRK, and keratoconic or keratoconus-suspect eyes. Graefes Arch Clin Exp Ophthalmol. 2014;252:91–9. doi: 10.1007/s00417-013-2502-5. [DOI] [PubMed] [Google Scholar]
- 14.Yip H, Chan E. Optical coherence tomography imaging in keratoconus. Clin Exp Optom. 2019;102:218–23. doi: 10.1111/cxo.12874. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Xia X, Tian B, Zhou S. Comparison of Fourier-domain and time-domain optical coherence tomography in the measurement of thinnest corneal thickness in keratoconus. J Ophthalmol. 2015;2015:402925. doi: 10.1155/2015/402925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonul S, Koktekir BE, Bakbak B, Gedik S. Comparison of central corneal thickness measurements using optical low-coherence reflectometry, Fourier domain optical coherence tomography, and Scheimpflug camera. Arq Bras Oftalmol. 2014;77:345–50. doi: 10.5935/0004-2749.20140087. [DOI] [PubMed] [Google Scholar]
- 17.Jhanji V, Yang B, Yu M, Ye C, Leung CK. Corneal thickness and elevation measurements using swept-source optical coherence tomography and slit scanning topography in normal and keratoconic eyes. Clin Exp Ophthalmol. 2013;41:735–45. doi: 10.1111/ceo.12113. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadpour M, Heidari Z. Pentacam. In: Mohammadpour M, editor. Diagnostics in Ocular Imaging. Cham, Switzerland: Springer; 2021. pp. 65–162. [Google Scholar]
- 19.López de la Fuente C, Sánchez-Cano A, Segura F, Hospital EO, Pinilla I. Evaluation of total corneal thickness and corneal layers with spectral-domain optical coherence tomography. J Refract Surg. 2016;32:27–32. doi: 10.3928/1081597X-20151207-03. [DOI] [PubMed] [Google Scholar]
- 20.Pang CE, Vanathi M, Tan DT, Mehta JS. Evaluation of corneal epithelial healing under contact lens with spectral-domain anterior segment optical coherence tomography (SD-OCT) Open Ophthalmol J. 2011;5:51–4. doi: 10.2174/1874364101105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaya S, Schmidl D, Schmetterer L, Witkowska KJ, Unterhuber A, Aranha Dos Santos V, et al. Effect of hyaluronic acid on tear film thickness as assessed with ultra-high resolution optical coherence tomography. Acta Ophthalmol. 2015;93:439–43. doi: 10.1111/aos.12647. [DOI] [PubMed] [Google Scholar]
- 22.Catalan S, Cadarso L, Esteves F, Salgado-Borges J, Lopez M, Cadarso C. Assessment of corneal epithelial thickness in asymmetric keratoconic eyes and normal eyes using Fourier domain optical coherence tomography. J Ophthalmol. 2016;2016:5697343. doi: 10.1155/2016/5697343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanellopoulos AJ, Asimellis G. OCT-derived comparison of corneal thickness distribution and asymmetry differences between normal and keratoconic eyes. Cornea. 2014;33:1274–81. doi: 10.1097/ICO.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 24.Temstet C, Sandali O, Bouheraoua N, Hamiche T, Galan A, El Sanharawi M, et al. Corneal epithelial thickness mapping using Fourier-domain optical coherence tomography for detection of form fruste keratoconus. J Cataract Refract Surg. 2015;41:812–20. doi: 10.1016/j.jcrs.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Tang M, Zhang X, Salaroli CH, Ramos JL, Huang D. Pachymetric mapping with Fourier-domain optical coherence tomography. J Cataract Refract Surg. 2010;36:826–31. doi: 10.1016/j.jcrs.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dursun D, Piniella AM, Pflugfelder SC. Pseudokeratoconus caused by rosacea. Cornea. 2001;20:668–9. doi: 10.1097/00003226-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 27.McMonnies CW. Abnormal rubbing and keratectasia. Eye Contact Lens. 2007;33:265–71. doi: 10.1097/ICL.0b013e31814fb64b. [DOI] [PubMed] [Google Scholar]
- 28.Moran S, Gomez L, Zuber K, Gatinel D. A case-control study of keratoconus risk factors. Cornea. 2020;39:697–701. doi: 10.1097/ICO.0000000000002283. [DOI] [PubMed] [Google Scholar]
- 29.Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, et al. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–7. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 30.Brautaset RL, Nilsson M, Miller WL, Leach NE, Tukler JH, Bergmanson JP. Central and peripheral corneal thinning in keratoconus. Cornea. 2013;32:257–61. doi: 10.1097/ICO.0b013e31825240d7. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Meisler DM, Tang M, Lu AT, Thakrar V, Reiser BJ, et al. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology. 2008;115:2159–66. doi: 10.1016/j.ophtha.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]