Abstract
Further characterization of the genetic environment of the gene encoding the Escherichia coli extended-spectrum β-lactamase, blaVEB-1, revealed the presence of a plasmid-located class 1 integron, In53, which carried eight functional resistance gene cassettes in addition to blaVEB-1. While the aadB and the arr-2 gene cassettes were identical to those previously described, the remaining cassettes were novel: (i) a novel nonenzymatic chloramphenicol resistance gene of the cmlA family, (ii) a qac allele encoding a member of the small multidrug resistance family of proteins, (iii) a cassette, aacA1b/orfG, which encodes a novel 6′-N-acetyltransferase, and (iv) a fused gene cassette, oxa10/aadA1, which is made of two cassettes previously described as single cassettes. In addition, oxa10 and aadA1 genes were expressed from their own promoter sequence present upstream of the oxa10 cassette. arr-2 coded for a protein that shared 54% amino acid identity with the rifampin ADP-ribosylating transferase encoded by the arr-1 gene from Mycobacterium smegmatis DSM43756. While in M. smegmatis, the main inactivated compound was 23-ribosyl-rifampin, the inactivated antibiotic recovered from E. coli culture was 23-O-ADP-ribosyl-rifampin. The integrase gene of In53 was interrupted by an IS26 insertion sequence, which was also present in the 3′ conserved segment. Thus, In53 is a truncated integron located on a composite transposon, named Tn2000, bounded by two IS26 elements in opposite orientations. Target site duplication at both ends of the transposon indicated that the integron likely was inserted into the plasmid through a transpositional process. This is the first description of an integron located on a composite transposon.
Integrons are genetic elements that consist of an integrase gene with adjacent gene cassettes that commonly contain antibiotic resistance genes. Several classes of integrons have been established based on the structure of the integrase (56). The most commonly encountered integrons are those of class 1. They are characterized by a 5′ conserved segment (5′-CS), which contains the int gene, encoding the integrase which catalyzes site-specific recombination (12, 13), and in most cases a 3′ conserved segment (3′-CS), which carries qacEΔ1, a functional deletion derivative of the qacE gene, which specifies resistance to antiseptics and disinfectants, the sul1 gene, which confers sulfonamide resistance, and an open reading frame (ORF), orf5, of unknown function (22, 48, 62). Integrons can integrate gene cassettes, by site-specific recombination, at a recombination site called attI1 (23, 56). Gene cassettes are individual mobile units bounded by integrase recombination core sites and have conserved features at the 3′ ends of the cassettes with an inverse core site and a 59-base element (21, 61). The consensus core site sequence is GTTRRRY (R is a purine, and Y is a pyrimidine) (61). Integrons have been found in a variety of gram-negative species, including Pseudomonas aeruginosa (33, 38, 56). They are often part of transposons or plasmids (33, 56). Integron-located genes other than those conferring antibiotic resistance have been described, such as qacE, which encodes an exporter protein mediating resistance to antiseptics and disinfectants (31, 48, 50, 54). Cassettes are always integrated in the same orientation and are cotranscribed from one or two common promoters located in the 5′-CS (14, 32). However, the qacE and the cmlA gene cassettes carry their own promoter sequences (3, 50, 52, 60).
Rifampin is a valuable antibiotic for treating infections such as tuberculosis, staphylococcal infections, and some infections caused by gram-negative organisms (e.g., Neisseria meningitidis and Acinetobacter spp.). Its antimicrobial activity is mediated by inhibition of prokaryotic DNA-dependent RNA polymerases, and most rifampin-resistant Mycobacterium tuberculosis and Mycobacterium leprae strains have an alteration in the β-subunit of this enzyme (25, 47). However, in some resistant organisms, such as Nocardia, Bacillus, and Pseudomonas spp. and nontuberculosis species of mycobacteria, the resistance to rifampin is not usually due to mutations in the rpoB gene (68). Several other resistance mechanisms have been identified, including a rifampin efflux (11) and inactivation of rifampin by decomposition (16), glycosylation (63), phosphorylation (69), and ribosylation (15, 55). The first reported case of rifampin inactivation by ribosylation has been described for M. smegmatis DSM43756, a bacterial strain that is naturally resistant to rifampin (15, 55). In this strain, rifampin is modified first to ADP-ribosylated rifampin (RIP-TAs) and then to ribosylated rifampin (RIP-Mb) (28, 42). The gene responsible for the rifampin inactivation in M. smegmatis, arr-1, is chromosomally located. Recently, another chromosomal gene, arr-2, which is 54% identical to arr-1, was found in P. aeruginosa, where it conferred resistance to rifampin (67).
Escherichia coli MG-1, which was previously shown to be resistant to multiple antibiotics, was isolated from a clinical sample from a South Asian patient who had been hospitalized in France (51). Since the gene cassette, blaVEB-1, encoding a novel extended-spectrum β-lactamase of clinical relevance was identified, we characterized the genetic environment of this gene in order to predict its potential for spreading. We report here a novel integron, In53, which is part of a composite transposon that is inserted on a large self-transferable plasmid. Detailed characterization of the nine gene cassettes of In53 is provided, as well as the determination of the physiological effect mediated by one of the integrated genes, arr-2.
MATERIALS AND METHODS
Enzymes and chemicals.
T4 DNA ligase and restriction endonucleases were used according to the manufacturer's recommendations (Amersham Pharmacia Biotech, Orsay, France). Cetyltrimethylammonium bromide (CTAB), ethidium bromide (EtBr), chloramphenicol, rifampin, and kanamycin were from Sigma (Sigma, St. Quentin Fallavier, France). Taq DNA polymerase was from Perkin Elmer (Perkin Elmer, Les Ullis, France). Antibiotic disks were used for routine antibiograms (Sanofi-Diagnostics Pasteur, Marnes-la-Coquette, France). The antimicrobial agents and their sources have been described elsewhere (51).
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used in the study are listed in Table 1. Cells were grown at 37°C under aerobic conditions in Trypticase soy (TS) broth (Gibco-BRL-Life Technologies, Eragny, France) containing the appropriate antibiotic or on Mueller-Hinton (MH) agar plates (Sanofi-Diagnostics Pasteur). Antibiotic concentrations for selection were as follows: ampicillin, 100 μg/ml; chloramphenicol, 15 μg/ml, kanamycin, 50 μg/ml; and rifampin, 200 μg/ml. Antibiotic susceptibility was determined by disk diffusion on MH agar (51). The method of Steers et al. (59) was used to determine the MICs. For each strain, 104 CFU per spot were delivered onto MH plates containing antibiotics in twofold dilutions. The MIC was determined as the lowest concentration of an antibiotic at which no visible growth was observed after 20 h of incubation at 37°C. Induction of chloramphenicol resistance by growth in the presence of 1 μg of chloramphenicol per ml was performed as described previously (17).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotypea | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH10B | F′ mcrA Δ(mrr-hsdRMS mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 araΔ139 Δ(ara leu)7697 galU galK λ− rpsL endA1 nupG Strr | Life Technologies |
| E. coli MG-1 | Extended-spectrum cephalosporin resistance, Rifr Kanr Tobr Genr Amkr Sptr Strr Sulr Tetr Cmr | 51 |
| Plasmidsb | ||
| pPCR-script Amp (SK+) | Ampr | Stratagene |
| pPCR-script Cm (SK+) | Cmr | Stratagene |
| PBK-CMV | Neor Kanr | Stratagene |
| pNLT1 | Natural plasmid of E. coli MG-1 containing blaVEB1 ESBL, Rifr Kanr Tobr Genr Amkr Sptr Strr Sulr Cmr | 51 |
| pRLT2 | pBKCMV recombinant plasmid containing an 11-kb Sau3A fragment with blaVEB-1 ESBL, Kanr Tmr Amkr Netr Cmr Sulr | This work |
| pRLT3 | pBKCMv recombinant plasmid containing a 13-kb Sau3A fragment with blaVEB-1 ESBL, Kanr Tmr Amkr Netr Cmr | This work |
| pRLT4a and b | pPCRscript recombinant plasmid containing a 1.3-kb PCR fragment with qacI; a, EtBrr CTABr; b, Etbrr CTABr | This work |
| pRLT5a and b | pPCRscript recombinant plasmid containing a 1.8-kb PCR fragment with aacA1/orfG; a, Kanr Tmr Amkr Netr; b, no resistance observed | This work |
| pRLT6a and b | pPCRscript recombinant plasmid containing a 2.7-kb PCR fragment with arr-2; a, Rifr; b, no resistance observed | This work |
| pRLT7a and b | pPCRscript recombinant plasmid containing a 3.8-kb PCR fragment with cmlA5; a, Cmr; b, Cmr | This work |
| pRLT8a and b | pPCRscript recombinant plasmid containing a 3.2-kb PCR fragment with oxa10/aadA1; a, Ampr Strr Sptr; b, Ampr Strr Sptr | This work |
| pRLT9a and b | pPCRscript recombinant plasmid containing a 1.9-kb PCR fragment with the oxa10/aadA1 fusion cassette; a, Ampr Strr Sptr; b, Ampr Strr Sptr | This work |
| pRLT10a and b | pPCRscript recombinant plasmid containing a 0.9-kb PCR fragment with the oxa10 cassette; a, Ampr; b, no resistance observed | This work |
Abbreviations: Kan, kanamycin; Tm, tobramycin; Net, netilmycin; Str, streptomycin; Spt, spectinomycin; Amk, amikacin; Cm, chloramphenicol; Tet, tetracycline; Sul, sulfonamide; Rif, rifampin; ESBL, extended-spectrum β-lactamase.
For plasmids with “a” and “b” designations, “a” indicates recombinant plasmids where the inserted genes are in the same orientation as the Plac promoter of pPCRscript and “b” indicates recombinant plasmids where the inserted genes are in the orientation opposite that of the Plac promoter of pPCRscript.
DNA techniques.
Electrotransformation of E. coli DH10B was performed as described previously (43). Whole-cell DNA preparation, small- and large-scale plasmid DNA preparations, and agarose electrophoresis were also done as described previously (43, 58).
Standard PCR experiments were performed as described previously (29, 58). For each PCR amplification experiment, 500 ng of total DNA of E. coli MG-1 was used in a standard PCR mixture of 100 μl with the following amplification program: 10 min, 94°C; 35 cycles of 1 min at 94°C, 1 min at 55°C, and 3 min at 72°C; and a final extension of 10 min at 72°C. The sequences of the PCR primers are available upon request. PCRs were performed in a DNA thermal cycler 9600 (Perkin-Elmer).
Cloning, DNA sequencing, and sequence analysis.
Sau3AI fragments of E. coli MG-1 were size selected and cloned into the pBKCMV vector by selecting E. coli DH10B transformants on TS plates supplemented with 100 μg of ampicillin (51). Sequencing of the inserts on both strands was performed using laboratory-designed primers on an Applied Biosystem sequencer (ABI 377; PE-Biosystem, Les Ullis, France). Nucleotide and amino acid sequences were analyzed by using the software available online over the Internet at Pedro's Biomolecular Research Tools website (http://www.fmi.ch/biology/research_tools.html) and the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). Multiple sequence alignment of deduced peptide sequences was carried out over the Internet at the University of Cambridge website using ClustalW (http://www.ebi.ac.uk/clustalW/). Promoter prediction by neural network was carried out over the Internet at http://www.fruitfly.org/seq_tools/promoter.html.
Parts of the identified class 1 integron were PCR amplified, and the DNA fragments were purified using the Qiaquick PCR purification kit (Qiagen, Courtaboeuf, France) and subsequently cloned in both orientations into the pPCRscript vector (Ampr or Cmr) (Stratagene, Amsterdam, The Netherlands). The inserts of these resulting recombinant plasmids were resequenced.
Biochemical characterization of ARR-2 and structural analysis of the inactivated product.
E. coli DH10B harboring recombinant plasmid pRLT-6a was grown in 200 ml of TS broth with 100 μg of ampicillin per ml on a Brunswick rotary shaker for 18 h at 37°C. Cells were collected by centrifugation and resuspended in 5 ml of extraction buffer (20 mM Tris-HCl [pH 8.0], 1 mM EDTA, 5 mM dithiothreitol). The cell suspension was sonicated three times on ice for 30 s at 40 W (Bioruptor; Cosmo Bio, Tokyo, Japan). To 4 ml of this cell homogenate, 4 ml of reaction mixture containing 60 mg of rifampin and 100 mg of NADH was added. After incubation at 37°C for 3 h, the reaction mixture was centrifuged and the supernatant was freeze-dried. Dried samples were extracted with 8 ml of methanol, and the extract was chromatographed on an LH-20 Sephadex column (28 by 290 mm; Amersham Pharmacia Biotech) with methanol.
The molecular weight and molecular formula of RIP-TAs were determined by positive- and negative-ion fast atom bombardment mass spectrometry (FAB-MS) and high-resolution FAB-MS (HRFAB-MS) on a JEOL JMS-HX110 instrument (JEOL, Tokyo, Japan) (42). The inactivated antibiotic compounds were analyzed by reverse-phase thin-layer chromatography (KC18F; J. T. Baker, Inc., Tokyo, Japan) with a development solvent system of 0.2 M sodium chloride–dimethyl sulfoxide–acetonitrile (4:1.5:4). Analysis of the inactivated compounds was also done by reverse-phase high-pressure liquid chromatography on a LiChrosphere 100 RP-18(e) column (Cica-Merck, Tokyo, Japan; column dimensions, 4.6 by 150 mm; eluent, 38% acetonitrile [CH3CN] with 0.05% trifluoroacetic acid) at a flow rate of 1 ml per min, with a detection system set at UV 270 nm (28, 42).
Nucleotide sequence accession number.
The nucleotide sequence of the entire In53 integron along with flanking sequence has been deposited in the GenBank database under the accession no. AF205943.
RESULTS AND DISCUSSION
Antibiotic resistance of E. coli MG-1 is of plasmidic origin.
In addition to having a previously described extended-spectrum resistance to β-lactams, E. coli MG-1 was resistant to tetracycline, netilmicin, tobramycin, amikacin, gentamicin, CTAB, EtBr, sulfonamide, streptomycin, spectinomycin, rifampin, and chloramphenicol. Except for tetracycline, these resistance markers were transferred from E. coli MG-1 to E. coli JM109 at a frequency of 10−8, similar to that of the extended-spectrum β-lactamase gene blaVEB-1 (data not shown). The transconjugants were resistant to netilmicin, tobramycin, amikacin, gentamicin, CTAB, EtBr, streptomycin, spectinomycin, rifampin, and sulfonamides. They were also resistant, albeit at a lower level, to chloramphenicol (Table 2). DNA analysis of the transconjugants revealed the presence of a large plasmid with an estimated size of 160 kb that was able to confer the extended-spectrum resistance profile along with the other resistance markers. This plasmid was previously named pNLT-1 (51). The resistance determinant of tetracycline was harbored on a second plasmid along with a blaTEM-1 resistance gene, encoding a narrow-spectrum penicillinase.
TABLE 2.
In vitro susceptibility to various antimicrobial agents of E. coli MG-1, E. coli DH10B, E. coli DH10B harboring natural plasmid pNLT1 and of E. coli DH10B harboring recombinant plasmids that display some of the resistance markers of In53
| Antibiotic | MIC (μg/ml) fora:
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli MG-1b |
E. coli DH10B
|
E. coli DH10B | |||||||||||||||||
| pNLT1c | pRLT2d | pRLT3d | pRLT4e
|
pRLT5e
|
pRLT6e
|
pRLT7f
|
pRLT8e
|
pRLT9e
|
pRLT10e
|
||||||||||
| a | b | a | b | a | b | a | b | a | b | a | b | a | b | ||||||
| Imipenem | 0.25 | 0.25 | 0.25 | 0.25 | — | — | — | — | — | — | — | — | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Ceftazidime | 256 | 256 | >256 | >256 | — | — | — | — | — | — | — | — | 4 | 4 | 4 | 4 | 4 | 0.25 | 0.25 |
| Amoxicillin | >512 | >512 | >512 | >512 | — | — | — | — | — | — | —g | —g | >512 | >512 | >512 | >512 | >512 | 2 | 2 |
| Cephalothin | >256 | 128 | 128 | 128 | — | — | — | — | — | — | — | — | 256 | 256 | 256 | 256 | 256 | 2 | 2 |
| Streptomycin | >400 | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h | —h |
| Spectinomycin | >400 | >400 | >400 | >400 | — | — | — | — | — | — | — | — | >400 | >400 | >400 | >400 | — | — | 4 |
| Kanamycin | >256 | >256 | —g | —g | — | — | >128 | — | — | — | — | — | — | — | — | — | — | — | <0.5 |
| Gentamicin | 32 | 16 | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | <0.5 |
| Tobramycin | 128 | 64 | — | — | — | — | >128 | — | — | — | — | — | — | — | — | — | — | — | <0.5 |
| Amikacin | 64 | 32 | — | — | — | — | <128 | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Netilmicin | 32 | 32 | — | — | — | — | >128 | — | — | — | — | — | — | — | — | — | — | — | <0.5 |
| Chloramphenicol | 16 | 16 | 32 | 32 | —g | —g | —g | —g | —g | —g | 32 | 16 | —g | —g | —g | —g | —g | —g | 2 |
| Tetracycline | >32 | 2 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 2 |
| CTAB | 300 | 300 | 300 | 300 | 400 | 400 | — | — | — | — | — | — | — | — | — | — | — | — | 100 |
| EtBr | 200 | 200 | 200 | 200 | 250 | 250 | — | — | — | — | — | — | — | — | — | — | — | — | 75 |
| Rifampin | >256 | >256 | >256 | >256 | — | — | — | — | >256 | 8 | — | — | — | — | — | — | — | — | 8 |
| Sulfonamide | >1,000 | 1,000 | >1,000 | >1,000 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 15 |
For “a” and “b” plasmids, the gene is inserted in the same orientation as the Plac promoter and in the opposite orientation, respectively. —, not assayed.
E. coli MG-1 produces VEB-1 β-lactamase along with a TEM-1 penicillinase.
Natural plasmid containing blaVEB-1.
Recombinant plasmid backbone is pBKCMV (Kmr).
Recombinant plasmid backbone is pPCRscript-Cam (Cmr).
Recombinant plasmid backbone is pPCRscript-Amp (Ampr).
Resistance carried on the cloning vector.
Resistance carried by the host strain.
The blaVEB-1 gene cassette of pNLT-1 is inserted into a class 1 integron.
In order to determine the genetic environment of blaVEB-1, whole-cell DNA from E. coli MG-1 was partially digested with Sau3AI and cloned into pBKCMV. Only recombinant clones with large inserts were selected upon a first screening. Two of the largest recombinant plasmids conferring ampicillin resistance, pRLT-2 and pRLT-3 (Fig. 1A and B), containing, respectively, an 11-kb and a 13-kb insert, were retained for further study. The sequences of the inserts present in pRLT-2 and pRLT-3 were determined, and their genetic organization is shown in Fig. 1A and B. Besides the classical ORFs encountered in class 1 integrons, 10 additional ORFs were present. At the 5′ and 3′ ends of the insert, the sequences were identical to parts of the 5′- and 3′-CSs of class 1 integrons (4).
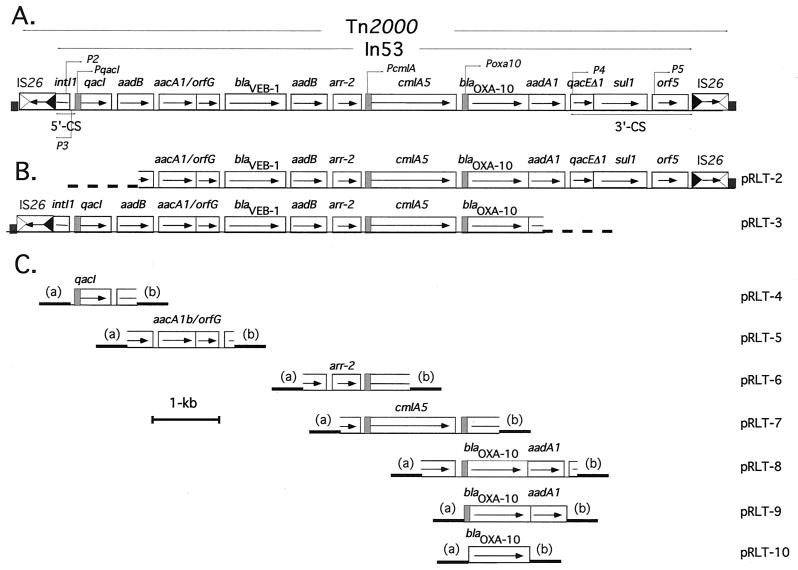
FIG. 1.
(A) Schematic representation of Tn2000 that contained In53 from E. coli MG-1. The 5′ and the 3′-CSs are indicated by a double arrow. ORFs or genes are shown as boxes with an arrow indicating the orientation of the coding sequence and with the gene name above the corresponding box. The different promoter sequences, P2, P3, P4, P5, PqacI, PcmlA, and Poxa10 are represented with a gray box and with an arrow. The hatched boxes at each end of the transposon represent the target site duplication. IS26-related inverted right and left repeats are shown by empty and filled triangles, respectively. The coding orientation of the IS26 transposase gene is represented by an arrow. The Tn2000 and the In53 structures are indicated between two divergent arrows. (B) Schematic representation of pRLT-2 and pRLT-3, two plasmids carrying large DNA inserts from E. coli MG-1 cloned into pBKCMV. The thick dotted line represents the pBKCMV vector sequence. (C) Schematic representation of pRLT-4 to -10, which correspond to plasmids constructed from various PCR products cloned into pPCRscript vectors (either Ampr or Cmr). Each construct exists in both orientations (a and b) with respect to Plac, the plasmid-located promoter.
The 5′-CS was truncated, and only the first 200 bp of the class 1 integrase gene was present, resulting in a nonfunctional integrase (4). Therefore, the cassettes are likely unable to move by means of this integrase. While the cassette promoter P2 located in the integrase gene was still present, the promoter P1 was deleted. The expression of the inserted genes was likely driven by this P2 promoter (−35, TTGTTA, and −10, CACAGT), which was in its strong promoter configuration; this configuration arose by means of a three-guanosine insertion between the −35 and −10 boxes, bringing its spacing to an optimal 17 bp (14, 32) (Fig. 2). However, this −10 box differs from the known −10 boxes of P2 promoters by a T-to-C replacement at the first position (14, 32). This position is the most conserved position in the −10 boxes of prokaryotic promoter sequences (14, 32). Sequence analysis of the DNA sequence further upstream of the truncated integrase gene revealed the presence of an insertion sequence, IS26 (41) (Fig. 1 and 2). Although a −35 sequence in the inverted repeat of IS26 can form a promoter when juxtaposed to a −10 sequence (37, 43), this is not the case in In53. Therefore, it is likely that the cassettes are primarily expressed from the P2 promoter.
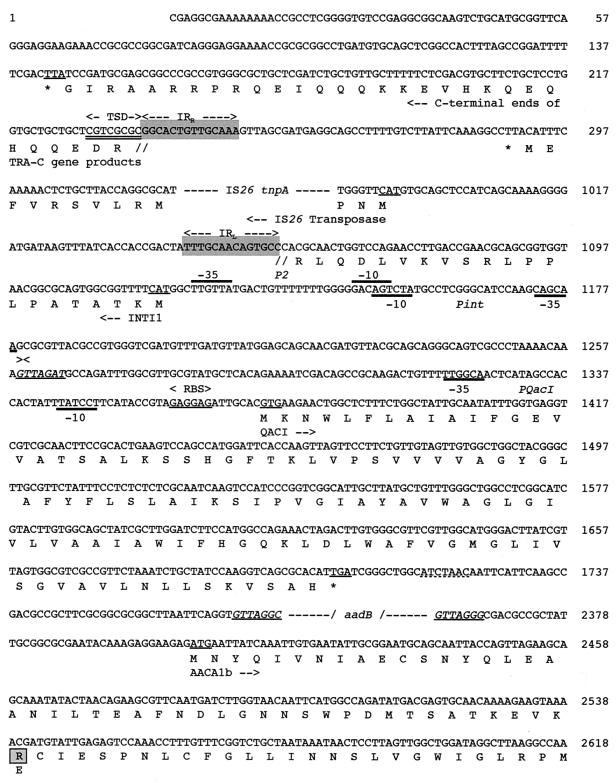
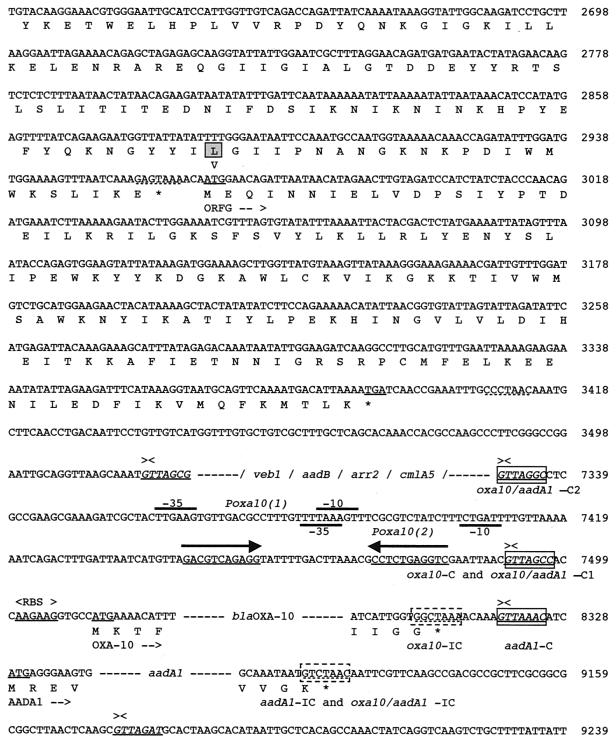
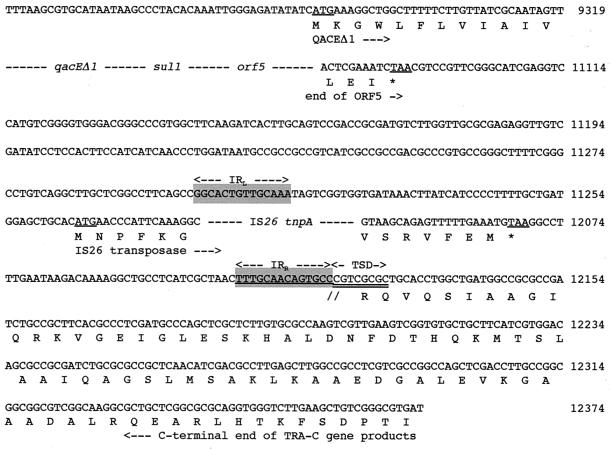
FIG. 2.
Nucleotide sequence of a 12,374-bp fragment of In53 surrounded by the two insertion sequences, IS26, part of intI1, attI1, and some flanking sequences. The deduced amino acid sequence is designated in single-letter code below the nucleotide sequence. Slashes flanked by dashes indicate break points within the cassette sequences, while dashes alone represent sequence breakpoints within coding sequences. Facing arrows indicate gene cassette boundaries. The start and stop codons of the various genes of interest are underlined. Gene names followed by arrows indicating their translational orientation are below their initiation codons. Right and left IS-related inverted repeats (IRR and IRL) are shown by gray boxes and by two divergent arrows with appropriate labels. IS-related target site duplications (TSD) are double underlined. The various oxa10, aadA1, and oxa10/aadA1 fusion cassette core and inverse core sites are indicated with boxes and dashed boxes, respectively. C, core site; IC, inverse core site; RBS, ribosome-binding site. The computer-predicted promoter Poxa10(1), Poxa10(2), P2, and Pint sequences are represented, with Poxa10(1) and P2 indicated by thick overlining and Poxa10(2) and Pint indicated by thick underlining. The two divergent arrows above underlined sequences represent a symmetry element generally encountered in 59-base elements. Within the amino acid sequence of AACA1b, the modified positions with respect to AACA1 are boxed, and the amino acid found in AACA1 (64) is indicated below these positions.
The 3′-CS consisted of qacEΔ1 fused to the sul1 gene and of an entire orf5 typical of sul1-associated integrons (22). The 3′-CS contained the same sequence as that found in the 3′-CS of In5 (22) except that the 3′-CS of In53 is 2,126 bp long (versus 2,386 bp in In5, which is the longest known 3′-CS [22]) and merged directly into an IS26 insertion sequence. The sul1 gene present in the 3′-CS of In53 is functional since E. coli DH10B(pRLT-2) exhibited decreased susceptibility to sulfonamides (Table 2).
These data indicate that the insert in plasmid pNLT-1 contains a truncated class 1 integron, designated In53, with an unusually large variable region and a sequence encoding an integrase that is not functional. PCR using integrase-specific primers failed to detect any intact type 1, type 2, or even type 3 integrase gene present in E. coli MG-1 (data not shown), suggesting that In53 is an inactive integron with respect to cassette movement, at least in E. coli MG-1.
The gene cassettes of In53.
The 5′- and 3′-CS flanked 10 ORFs. Gene cassettes are individual mobile units bounded by integrase recombination core sites and have conserved features at the 3′ ends of the cassettes with an inverse core site followed by a 59-base element (21, 61). The 59-base element is an imperfect inverted repeat sequence which acts as a recombination site. The recombination crossover occurs after the first guanine of the conserved GTTRRRY (R = purine, Y = pyrimidine) core site, located at the end of the 59-base element (21, 60). At the 3′ end of most ORFs, structures homologous to the 59-base element were present, suggesting that most ORFs were part of gene cassettes (13, 60). Two ORFs lacked a 59-base element sequence, suggesting that they may belong to fused gene cassettes.
(i) The aadB cassettes.
In53 contained two aadB cassette versions that differ only by one base pair, which remains silent in terms of amino acid sequence (9) (Fig. 1 and 2). These cassettes are widespread among gram-negative bacteria and confer resistance to kanamycin, tobramycin, and gentamicin (38, 44, 56). The presence of two almost identical cassettes in integrons has been reported for other gene cassettes, such as oxa2 (56, 66). It is interesting that in all the blaVEB-1-containing integrons described to date (44, 51, 67), an aadB gene cassette is found 3′ to blaVEB-1 (38).
(ii) The qacI cassette.
The first cassette contained an ORF of 345 nucleotides (Fig. 1A and 2). A putative initiation GTG codon was preceded at 7 bp by a ribosome-binding site-like sequence (Fig. 2). The coding sequence, designated qacI, which may direct the synthesis of a 110-amino-acid protein, had 90% identity with qacF from Enterobacter aerogenes (50) and 67.8% identity with the sequence of the qacE gene (48). The qacE gene specifies an exporter protein that mediates resistance to intercalating dyes and quaternary ammonium compounds and that has been found in the class 1 integron of transposon Tn402, later designated Tn5090 (54). The qacI and qacE cassettes diverge at their extremities and particularly at their 3′ ends; a 60-bp sequence was present downstream from qacI, whereas a 141-base element has been associated with qacE (54). The qacI 59-base element was identical to that of aadA6, which encodes an adenylyltransferase (45). A similar 59-base element was found at the 3′ end of qacF (50). The members of the family of 59-base elements are long imperfect inverted repeats that vary in length (from 60 bp up to 141 bp) but retain similarity to the consensus at their termini and are active in integrase-mediated site-specific recombination (13, 61). Hypotheses for the mechanisms of cassette movements have been proposed (56), but the question of whether the genes and the 59-base element have independent origins remains to be elucidated. Closely related genes associated with closely related 59-base elements have been described, e.g., catB3 and catB5 cassettes (8). In contrast, aadA6 and qacI gene cassettes represent an interesting example of 59-base elements associated with genes encoding different functions. A similar observation was made with the 90% sequence identity between the Vibrio cholerae repeated sequences and the 59-base element associated with blaP3, an integron-associated gene encoding a β-lactamase (39). These data may suggest that cassettes can exchange 59-base elements via integrase-mediated recombination at the internal boundaries of the two 59-base elements instead of the normal position at the outer boundaries of the 59-base element (56).
The long leader sequence in the qacE cassette has been shown to contain promoter sequences (32). These promoter sequences were also putatively identified in the long leader of the qacI cassette (Fig. 1A and C and 2). Computer-assisted promoter prediction programs by neural network identified these sequences as highly likely active promoter sequences (threshold, 0.98). The deduced protein, QacI, shares 90% amino acid identity with QacF, 75% identity with QacE (48), 37.6% with QacC (an antiseptic resistance protein from Staphylococcus aureus) (34), and 70.1% with EmrE (an E. coli protein mediating resistance to EtBr) (53) (Fig. 3). These proteins form a family of small multidrug export proteins that use proton motive force to energize transport and mediate resistance to antiseptics and disinfectants (48). In order to study the phenotype conferred by qacI, a 1.3-kb PCR fragment from pRLT-2 was cloned into pPCRscript, generating pRLT-4a and pRLT-4b, depending on the orientation of the insert with respect to the vector promoter sequence. The MICs of CTAB and EtBr for E. coli DH10B(pRLT-4a) and E. coli DH10B(pRLT-4b) were 100 and 400 μg/ml, respectively, indicating that QacI confers resistance to quaternary compounds. The fact that the MICs of the quaternary ammonium compounds and EtBr were the same for E. coli DH10B(pRLT-4a) and E. coli DH10B(pRLT-4b) is consistent with the hypothesis that there is a promoter sequence in the long leader sequence of qacI.
FIG. 3.
Comparison of the deduced amino acid sequence of the qacI product (QacI) with that of other proteins of the small multidrug resistance family (49). QacF is encoded by a gene cassette from In40 of Enterobacter aerogenes BM2688 (50); QacG is encoded by the gene cassette from In31 of P. aeruginosa 101/1477 (31); QacE is encoded by the gene cassette from In16 (48); QacEΔ1 is a QacE derivative encoded by the truncated qacEΔ1 allele found in the 3′-CS of several sulI-associated integrons (48, 62). EmrE is an ethidium efflux protein from E. coli (53); QacC is a protein from Staphylococcus aureus (34). Identical residues are indicated by dashes, and conserved residues are shown by asterisks. The underlined residues from QacEΔ1 indicate the deleted portion of the protein compared to QacE.
(iii) The cmlA5 cassette.
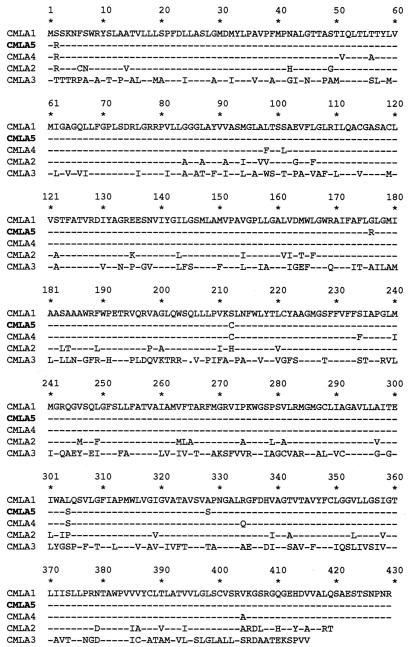
The seventh cassette, spanning 1,548 nucleotides (Fig. 1A and 2), contained an ORF of 1,230 nucleotides starting at a putative GTG initiation codon at position 5988 that was preceded at 8 bp by a ribosome-binding site-like sequence (AAGGAG) (data not shown). This coding sequence, designated cmlA5, shared 97% identity with the cmlA1 gene of the class 1 integron In4 in Tn1696, which confers a nonenzymatic chloramphenicol resistance (3, 60). The cmlA5 59-base element was 70 bp in length, and the sequence differs at two positions from that of the 59-base element of cmlA1 (data not shown). Eight DNA mismatches lead to five amino acid changes (Fig. 4). A 2.2-kb PCR fragment obtained with primers hybridizing within flanking sequence of cmlA5 (Table 1 and Fig. 1), thus containing the entire cmlA5 cassette, was cloned into the pPCRScript/Amp vector in both orientations. The resulting plasmids, pRLT7-a and pRLT7-b (Fig. 1C), conferred chloramphenicol resistance on E. coli DH10B at similar levels (MICs of 32 and 16 μg/ml, respectively) (Table 2). The deduced protein of 409 amino acids, CMLA5, shared 97% identity with CMLAl and 54% identity with the polypeptide predicted from the Salmonella enterica serotype Typhimurium gene, which confers resistance to florfenicol and chloramphenicol (2, 6, 7) (Fig. 4). CMLAl is an efflux protein of the major facilitator family and confers resistance by chloramphenicol efflux (3, 60).
FIG. 4.
Comparison of the deduced amino acid sequences of the cmlA5 product (CMLA5) with those of other proteins of the CMLA family (49). Dashes represent conserved amino acids, and one gap introduced for the alignment is indicated by a dot. While CMLA1 is encoded by a gene cassette from In4 (3), CMLA2 is encoded by a gene cassette from In40 (50), CMLA4 is encoded by a gene cassette from In52 (51), and the genes for CMLA3, CMLA3a and -3b (2, 6, 7) are chromosomal and not present on gene cassettes. CMLA3a and -b differ from CMLA3 by one single-amino-acid substitution each (L308F and V274A, respectively); only the sequence of CMLA3 is displayed.
While most gene cassettes are usually inserted in the same orientation and are under the control of the common promoters P1 and P2 located in the 5′-CS (14), a few cassettes described to date, including cmlA variants, qacE, and qacEΔ1, contain a promoter-like sequence (3, 19, 31, 50, 52, 60). Analysis of the region upstream from cmlA5 showed a putative promoter consisting of −35 (TCGCGG) and −10 (TACGAT) motifs separated by 17 nucleotides. The −10 and −35 motifs were identical to those proposed for cmlA1, which indicates that the cmlA5 gene may also be expressed from its own promoter. The region upstream from cmlA5 contains a small ORF that may encode a nine-amino-acid peptide that is closely related to the leader peptides of cat genes and that differs from that of cmlA1 by having a Lys instead of an Asn at position 6 (data not shown). This region also contains inverted repeats capable of forming alternate stem-loop structures (18, 60). These features are similar to those found upstream from the inducible cat and erm genes, which are regulated by transcriptional attenuation (18, 35). The cmlA5 gene in plasmid pRLT-7a and -7b conferred low-level chloramphenicol resistance on E. coli DH10B (Fig. 1C and Table 2). Inducibility of expression of CMLA1 from plasmid R26 in E. coli K-12 by subinhibitory concentrations of chloramphenicol has been demonstrated previously (17). The similarities in structure and in sequence of the two cassettes suggest that the regulation of cmlA5 might be similar to that of cmlA1. Indeed, when plasmid pRLT-7a or -7b was expressed in E. coli DH10B, a twofold increase in the MIC of chloramphenicol was observed upon induction.
(iv) The aacA1/orfG fusion cassette.
The aacA1 gene did not appear to possess an associated 59-base element but instead was followed 4 bp downstream by an additional ORF, named orfG (GenBank accession number AF047479) (A. Gravel, R. Parent, and P. H. Roy, unpublished data). Although most of the gene cassettes thus far discovered carry antibiotic resistance genes, there are a few other examples of cassettes that carry genes that are not involved in antimicrobial resistance or whose function remains unknown (56). The ORFG product falls in the latter category. ORFG is 142 codons long and is followed by a 59-base element of 107 bp. It is interesting that neither aacA1b nor ORFG has been found as a single gene cassette (64) (GenBank accession number AF047479) (Gravel et al., unpublished). The aacA1 and orfG genes have very similar G+C contents and codon usage, and thus they are likely to form a cassette as a single unit.
A 1.8-kb PCR fragment obtained with primers hybridizing within flanking sequences of the aacA1/orfG cassette (Table 1 and Fig. 1C) was cloned into the pPCRscript/Amp vector in both orientations. Only plasmid pRLT5-a, where the aacA1/orfG cassettes were colinear with the vector promoter (Fig. 1C), conferred kanamycin, tobramycin, amikacin, and netilmicin resistance on E. coli DH10B (MIC of >128 μg/ml). The deduced protein of 183 amino acids shared 98% identity (two amino acid changes) (Fig. 2) with AACA1 found in the Citrobacter diversus R plasmid and in In21 (GenBank accession number AF047479) (64; Gravel et al., unpublished) and thus was named AACA1b.
(v) Promoter/oxa10/aadA1 fusion cassette.
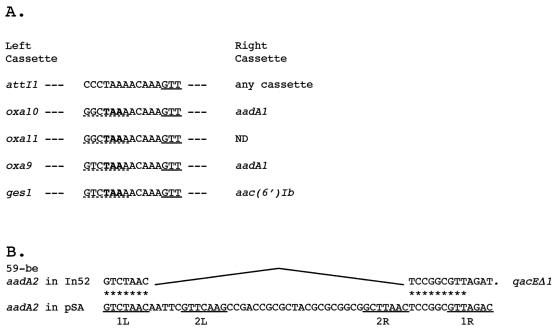
In In53, the oxa10 gene cassette did not appear to possess an associated 59-base element but instead was fused to an aadA1 cassette. The oxa10 coding sequence has so far been described only as a single gene cassette, while aadA1 has been found only once as a fused cassette in Tn1331 (26, 45, 56, 66). Loss of a 59-base element may have occurred in a variety of ways, e.g. slippage during DNA replication caused by stem-loop structure in a single-stranded 59-base element DNA template (56). For aadA2 in In52 the mechanism by which the fusion occurred may be proposed as an internal deletion at the two conserved regions 1L and 2R of the aadA2 59-base element (Fig. 5B) (61). Several other fused cassettes where one 59-base element has likely been deleted have been documented. This is the case for aadA1/oxa9 in Tn1331 (66) and for blaGES-1/aac(6′)Ib in In52 (52), where the sequence found between the two genes is identical to part of attI (Fig. 5A). In the oxa10/aadA1 fusion, the typical nine nucleotides that are closely related to attI were also found, indicating that the deletion may have arisen from an identical 59-base element and thus indicate a common process of 59-base-element deletion (Fig. 5A).
FIG. 5.
Various structures of fused gene cassettes. (A) Intergenic regions of fused cassettes in comparison with the attI1 sequence. Single underlining shows the recombination cleavage site (GTT). Dashed underlining shows the remainder of the inverse core site of the preceding cassette, and boldface shows the stop codon of the preceding gene. ND, not determined. (B) Structure of the sequence of a deleted 59-base element (59-be) found in In52 (52), which is likely to be the result of an integrase-mediated reaction between 1L and 2R regions. 1L, 2L, 2R, and 1R correspond to conserved regions within the 59-base elements (61) and are underlined. The origins of the displayed sequences are as follows: attI, references 56 and 61; oxa10/aadA1, present work; oxa11, references 20 and 46; oxa9/aadA1, reference 66; aadA2/qacEΔ1, reference 52; aadA2 in pSA, reference 5; and ges1/aac(6′)Ib, reference 52.
Two putative recombination core sites were found 5′ to the blaOXA-10 gene (Fig. 2). The most likely recombination core site for the fusion cassette corresponds to the closest site 5′ to the translational start site, oxa10/aadA1-C1 (GTTAGCC), which corresponds to the core site normally encountered in oxa10 gene cassettes. As expected for fusion cassettes, the core site oxa10/aadA1-C1 has one mismatch with the inverse core site of aadA1. The second possible recombination core site, oxa10/aadA1-C2 (GTTAGGC), which is located immediately after the cmlA5 59-base element, also has one mismatch with the inverse core site of aadA1. It seems that the cmlA and the oxa10/aadA1 cassettes are separated by 161 bp of unrelated sequence. A BLAST search against the GenBank database did not reveal homology with any known sequence, indicating that these 161 bp do not belong to either of the two cassettes. Computer-assisted promoter prediction programs by a neural network identified within these 161 bp revealed two putative promoter sequences, Poxa10(1) and Poxa10(2) (threshold, 0.99) (Fig. 2). How this sequence got inserted in front of blaOXA-10 is unknown. It could be hypothesized that it is the remnant of a deleted cassette that was inserted between the cmlA5 and oxa10 cassettes. This hypothesis is strengthened by the presence of a symmetry element, GACNTCAGAGG (whose complement is CCTCTGANGTC), that probably represents the center of a truncated 59-base element for the preceding sequence containing the promoters (Fig. 2). The promoter, whose sequence has not been seen in an integron before, may represent a new cassette with its structural gene and inverted core site deleted. Whether this truncated cassette may act as a mobile promoter cassette is unknown.
In In53, the oxa10 gene cassette was located downstream from the cmlA5 cassette and could be transcribed from the cmlA5 promoter. However, Ploy et al. have shown that the genes located downstream of cmlA2 are silent because of transcriptional silencing due to the cmlA2 terminator (50). In the case of cmlA5, the same phenomenon may be true, although E. coli MG-1 expressed OXA-10, according to isoelectric focusing results showing a pI value of 6.1, which is consistent with OXA-10 expression (data not shown). These results may indicate that the fused oxa10/aadA1 cassette may harbor an efficient E. coli promoter or that the cmlA5 silencer is not effective in E. coli MG-1. In order to test these hypotheses, three distinct PCR products were cloned in both orientations into pPCRscript. Plasmids pRLT-8a and pRLT-8b contained the 3′ half of the coding sequence of cmlA5 (including the transcriptional silencer) and the entire oxa10/aadA1 fusion cassette, pRLT-9a and -9b contained the entire oxa10/aadA1 fusion cassette including the preceding 161 bp, and pRLT-10a and -10b contained only the oxa10 cassette without the preceding 161 bp (Fig. 1C). The recombinant plasmids were tested for β-lactamase activity and for spectinomycin resistance. No significant difference in expression between the two orientations was observed for plasmid pRLT-8 and pRLT-9. pRLT-10a conferred ampicillin, ticarcillin, and cephalothin resistance on E. coli DH10B as expected for OXA-10 expression, while pRLT-10b failed to express OXA-10 since the gene is in antisense orientation with respect to the vector promoter. These results were consistent with the hypothesis that an active E. coli promoter is present in front of blaOXA-10 (Fig. 1C and Table 2). Therefore, it is very likely that the fused gene cassette oxa10/aadA1 harbored its own promoter, bringing the number of cassettes with long leader sequences harboring a promoter sequence to three.
(vi) The arr-2 cassette.
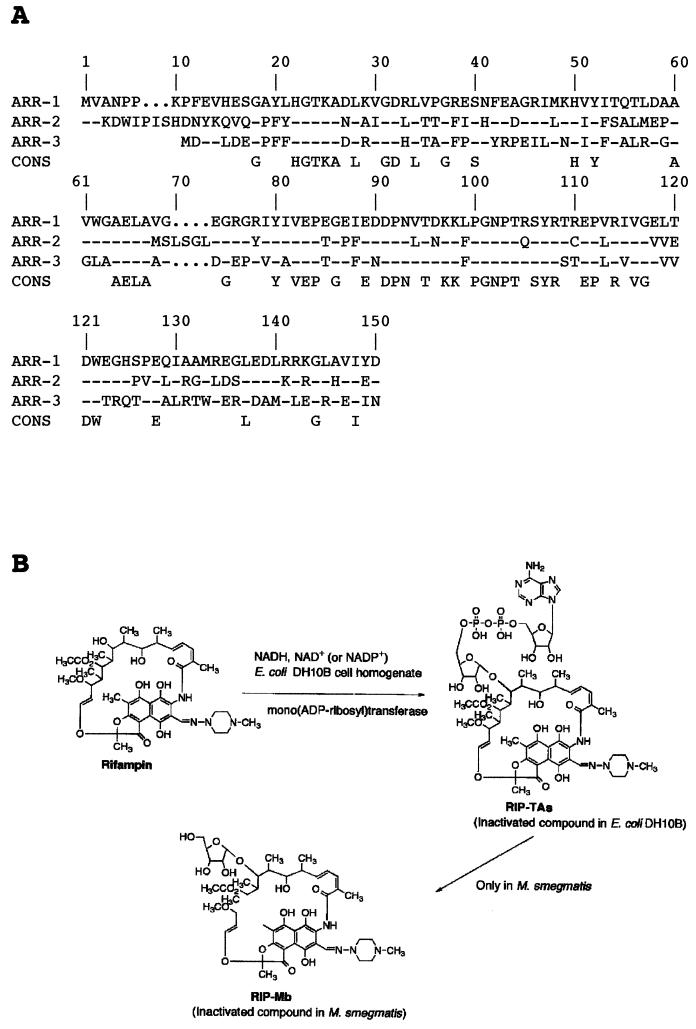
The DNA sequence around the rifampin resistance gene revealed characteristic gene cassette features. This gene was identical to the previously described arr-2 gene from P. aeruginosa (67), which implies interspecies transfer of that gene. This gene putatively coded for a 150-amino-acid protein that conferred rifampin resistance in P. aeruginosa and in E. coli (67). The closest homologues in the GenBank database were the ADP-ribosylating transferase encoded by the arr gene in M. smegmatis (55) and an unpublished sequence, arr-3 from Streptomyces coelicolor (57). ARR-2 showed 54% identity, with only two gaps in the alignment, with ARR-1 and 59% identity with ARR-3 from S. coelicolor (Fig. 6A). The mechanism of resistance of arr-1 is inactivation of rifampin by ribosylation (15, 28, 42). The MICs of rifampin increased to >256 μg/ml for E. coli DH10B with pRLT-2 or pRLT-6. The finding that arr-2 conferred rifampin resistance, in addition to its homology with arr-1, strongly suggests that the ARR-2 is an ADP-ribosylating transferase.
FIG. 6.
(A) Amino acid alignment of the three known ARR proteins: ARR-1 from M. smegmatis (15), ARR-2 from E. coli MG-1 and P. aeruginosa pTh2 (reference 67 and the present work), and ARR-3 from Streptomyces (57). Dashes represent identical amino acids. A consensus (CONS) sequence is derived from the alignment. (B) Schematic representation of the inactivation pathway of rifampin by ARR-2 and ARR-1 (28).
Production and extraction of rifampin inactivation product by E. coli DH10B(pRLT-4).
Although arr-2 had been previously identified, the physiological role of ARR-2 had not been investigated. When rifampin was added to the reaction mixture including a cell homogenate of E. coli DH10B harboring the recombinant plasmid pRLT-6 and NADH, the antibiotic was found to be inactivated within 2 h. To purify and to identify the inactivated compound, the reaction mixture was first extracted with ethyl acetate. No colored compounds were observed in the ethyl acetate phase, but the presence of colored rifampin-related compounds was suggested in the aqueous phase. After the pH of the fraction was changed to 7.0, the colored fraction was freeze-dried and extracted with methanol and the solvent extract was concentrated under vacuum. LH-20 Sephadex chromatography of the concentrated colored compound allowed us to obtain purified rifampin-related products. From 60 mg of rifampin, 43 mg of purified inactivated compound (designated RIP-TAs) was obtained. The reverse-phase thin-layer chromatography profile showed that the inactivated compound was identical to RIP-TAs, showing a retention front value of 0.8 (data not shown). The identity of the colored compound with RIP-TAs was also confirmed by reverse-phase high-pressure liquid chromatography, which showed the same retention time (7.3 min) as RIP-TAs. Positive- and negative-ion FAB-MS data for the purified inactivated product indicated the molecular mass to be 1,363 kDa, and based on the HRFAB-MS data, the molecular formula was determined to be C58H79N9O25P2. Taken together, these data suggested that the arr-2 gene encodes a mono(ADP-ribosyl)transferase that produces an inactivation product of RIP-TAs [23-(O-ADP-ribosyl)rifampin]. Although the main inactivated compound is 23-ribosyl-rifampin (RIP-Mb) in M. smegmatis DSM43756, interestingly, E. coli DH10B produces only RIP-TAs as the inactivated product. No other inactivation product, such as RIP-Mb, was observed. Therefore, these data suggested that E. coli DH10B has no enzyme which can remove the AMP and phosphate from RIP-TAs, as shown in Fig. 6B, to generate RIP-Mb (28, 42). In M. smegmatis the RIP-TAs is converted to ribosylated rifampin (RIP-Mb) by the action of an ADP-ribose phosphohydrolase (55). It seems that such an enzyme is absent from E. coli or simply is unable to perform the reaction on RIP-TAs, since the only inactivated product isolated from E. coli was RIP-TAs.
The mono(ADP-ribosyl) transferase (36) transfers the ADP-ribose moiety of NADH to acceptor molecules, usually proteins (24). Many bacterial mono(ADP-ribosyl)transferases are toxins, such as those of Corynebacterium diphtheriae, V. cholerae, Bordetella pertussis, and Clostridium botulinum (40). Endogenous mono-ADP-ribosylation has been demonstrated in several bacteria, including P. aeruginosa (24), but little is known about the physiological role of this modification process (24). In these cases, the acceptors were proteins. However, in the present study the acceptor was a low-molecular-weight antibiotic, rifampin, and to our knowledge, this is the second example of ADP-ribosylation as a mechanism of antibiotic inactivation and the first that is integron and plasmid located. Moreover, the ADP-ribosyl moiety is joined to an oxygen atom, in contrast to the examples cited above, where it is joined to a nitrogen atom (Fig. 6B).
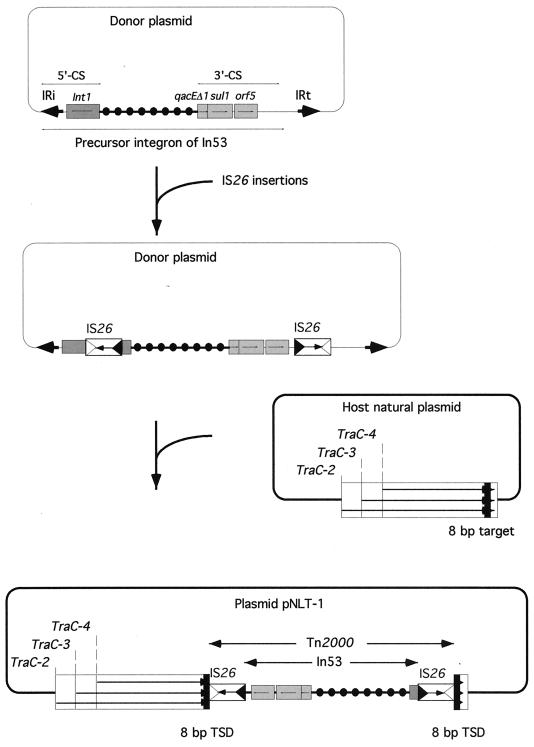
In53 is contained on a composite transposon, Tn2000.
On either side of In53, an IS26 element was found and in opposite orientations. The DNA sequence immediately next to these insertion sequences was identical to those of the traC genes of E. coli plasmid R751, which code for the conjugation proteins TRA-C-2, -3, and -4 (65). On both sides of the IS26 elements not facing the integron, a target site duplication of 8 bp (characteristic of IS26 transposition) was found, suggesting that the two IS26 elements may form a composite transposon along with the resistance genes. This transposon, named Tn2000, may be responsible for the integron movement and its insertion into plasmid pNLT-1. IS26 belongs to the IS6 family of insertion sequences. This family is characterized by the fact that it gives rise exclusively to replicon fusions (cointegrates) in which the donor and target replicons are separated by two directly repeated IS copies (37, 41). Two IS26 in direct repeat were found at both ends of the kanamycin resistance transposon Tn2680 (27). They were able to mediate cointegration in E. coli K-12 that contains no IS26 in its chromosome (27). Upon cointegration, mediated by either of the two IS26 elements, the IS element is duplicated in a direct repeat. However, this cannot be the case for Tn2000, where the two elements are in opposite orientations. Some composite transposons, such as Tn10, Tn5, and Tn9, are made of insertion sequences that are in opposite orientations with the 3′ ends of the IS elements facing outwards. For Tn2000, the 3′ ends of the elements are facing the integron sequence. This kind of structure may result by a so-called “inside-out,” or inverse, transposition as observed for Tn10 (30). In this kind of transposition, the 5′ ends of the elements are recognized, at a much lower frequency, rather than the 3′ ends (30). Figure 7 outlines a possible mechanism that led to the genesis of Tn2000. Whether Tn2000 is still active in transposition remains to be determined.
FIG. 7.
Schematic representation of the genesis of Tn2000, which contains In53 from E. coli MG-1. ORFs and genes are shown as boxes with an arrow indicating the orientation of the coding sequence and with the gene name above the corresponding box. IS26-related inverted right and left repeats are shown by empty and filled triangles, respectively. The coding orientation of the IS26 transposase is represented by an arrow. The Tn2000 and the In53 structures are indicated between two divergent arrows. IS26-related target site duplications (TSD) are displayed as filled squares at each end of Tn2000. IRi and IRt, integron-specific inverted repeats found at the ends of class 1 integrons (31, 56). The mechanism involved in Tn2000 transposition could be inverse transposition as was described for Tn10 (30).
Conclusion.
This work describes a novel integron, In53, which, instead of residing on a defective Tn402-based transposon, acquired mobility by the insertion of IS26 elements into the 5′ and 3′-CSs. In53 is a peculiar class 1 integron lacking a functional integrase. It is the largest class 1 integron, containing nine different antibiotic resistance genes of different classes including those for β-lactams, aminoglycosides, phenicol, rifampin, and sulfonamides and antiseptic resistance genes. Use of each class of antibiotic or/and antiseptic may result in the selection in vivo of such integron-containing enterobacterial strains. Additionally, its plasmid and transposon locations may provide an easy means of dissemination, as already exemplified by the isolation of other enterobacterial strains, such as Klebsiella pneumoniae MG-2 (51) and Proteus mirabilis Lil-1 (T. Naas, unpublished data), that carried the same resistance gene.
The sequences of the different cassettes revealed information on the origins of some of them. cmlA5 is closely related to the cmlA1 gene cassette, and they probably derive from a common ancestor. In contrast, the qacE and qacI genes, which are also closely related, are part of cassettes that contain distinct 59-base elements. This observation implies the independent genesis of two cassettes by acquisition of 59-base elements following an unexplained mechanism (56). Furthermore, our results indicate that promoter-containing gene cassettes may arise from existing cassettes that normally lack any promoter sequence.
Our results on arr-2, along with those on arr-1 from M. smegmatis, raise the questions of how these two genes have evolved and if they have been transferred between Mycobacterium spp., E. coli, and Pseudomonas spp. These bacterial species are found in soil, where genetic exchange may have occurred. In this respect, E. coli and P. aeruginosa strains that both contained arr-2/blaVEB-1/oxa10 gene cassettes have been isolated from clinical specimens from patients with the same geographical origin, Vietnam and Thailand, respectively (44, 51, 67). The identification of arr-2 on a plasmid and on different integrons in several gram-negative species of medical interest is of concern. While in P. aeruginosa pTH2 (67) the gene was chromosomal, we identified a plasmid- and transposon-borne gene in E. coli. Rifampin is currently used for treating infections such as meningitis due to gram-negative nosocomial pathogens and thus may favor selection of rifampin resistance genes.
The identification of blaOXA-10 in In53 is consistent with the identification of class D β-lactamase genes most often associated with class 1 integrons (46). This is the first description of an integron that carries two β-lactamase genes belonging to two structurally unrelated molecular classes (1). In addition, this is the third description of a blaVEB-1-containing integron that is different in size and structure from the blaVEB-1 integrons previously described (44, 51, 67). These findings confirm the ability of the blaVEB-1 gene to spread among clinically relevant species and highlight the considerable heterogeneity of the genetic environment in which the blaVEB-1 alleles can be found in different clinical isolates. A similar condition likely reflects the intervention of various mechanisms, such as horizontal plasmid transfer and cassette excision or integration, in the dissemination of the blaVEB-1 gene among different hosts and different replicons.
ACKNOWLEDGMENTS
This work was funded by the Ministère de l'Education Nationale et de la Recherche (grant UPRES, JE-2227), Université Paris XI, Paris-Sud, France.
REFERENCES
- 1.Ambler R P. The structure of β-lactamases. Philos Trans R Soc Lond B. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Arcangioli M A, Leroy-Setrin S, Martel J L, Chaslus-Dancla E. A new chloramphenicol and florfenicol resistance gene flanked by two integron structures in Salmonella typhimurium DT104. FEMS Microbiol Lett. 1999;174:327–332. doi: 10.1111/j.1574-6968.1999.tb13586.x. [DOI] [PubMed] [Google Scholar]
- 3.Bissonnette L, Champetier S, Buisson J P, Roy P H. Characterization of the nonenzymatic chloramphenicol resistance (cmlA) gene of the In4 integron of Tn1696: similarity of the product to transmembrane transport proteins. J Bacteriol. 1991;173:4493–4502. doi: 10.1128/jb.173.14.4493-4502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bissonnette L, Roy P H. Characterization on In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol. 1992;174:1248–1257. doi: 10.1128/jb.174.4.1248-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bito A, Susani M. Revised analysis of aadA2 gene of plasmid pSA. Antimicrob Agents Chemother. 1994;38:1172–1175. doi: 10.1128/aac.38.5.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolton L F, Kelley L C, Lee M D, Fedorka-Cray P J, Maurer J J. Detection of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 based on a gene which confers cross-resistance to florfenicol and chloramphenicol. J Clin Microbiol. 1999;37:1348–1351. doi: 10.1128/jcm.37.5.1348-1351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs C E, Fratamico P M. Molecular characterization of the antibiotic gene cluster of Salmonella typhimurium DT104. Antimicrob Agents Chemother. 1999;43:846–849. doi: 10.1128/aac.43.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunny K, Hall R M, Stokes H W. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob Agents Chemother. 1995;39:686–693. doi: 10.1128/AAC.39.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron F H, Groot Obbink D J, Ackennan V P, Hall R M. Nucleotide sequence of the AAD(2′) aminoglycoside adenylyltransferase determinant aadB. Evolutionary relationship of this region with those surrounding aadA in R538–1 and dfrII in R388. Nucleic Acids Res. 1986;14:8625–8635. doi: 10.1093/nar/14.21.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrol S F, Collier R J. NAD binding site of diphtheria toxin: identification of a residue within the nicotinamide subsite by photochemical modification with NAD. Proc Natl Acad Sci USA. 1984;81:3307–3311. doi: 10.1073/pnas.81.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrasekaran S, Lalithakumari D. Plasmid-mediated rifampicin resistance in Pseudomonas fluorescens. J Med Microbiol. 1998;47:197–200. doi: 10.1099/00222615-47-3-197. [DOI] [PubMed] [Google Scholar]
- 12.Collis C M, Hall R M. Site-specific deletion and rearrangement of integron insert genes catalyzed by the integron DNA integrase. J Bacteriol. 1992;174:1574–1585. doi: 10.1128/jb.174.5.1574-1585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collis C M, Hall R M. Gene cassettes from the insert region of integrons are excised as covalently closed circles. Mol Microbiol. 1992;6:2875–2885. doi: 10.1111/j.1365-2958.1992.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 14.Collis C M, Hall R M. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–162. doi: 10.1128/aac.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabbs E R, Yazawa K, Mikami Y, Miyaji M, Morisaki N, lwasaki S, Furihata K. Ribosylation by mycobacterial strains as a new mechanism of rifampin inactivation. Antimicrob Agents Chemother. 1995;39:1007–1009. doi: 10.1128/aac.39.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabbs E R, Yazawa K, Tanaka Y, Mikami Y, Miyaji M, Andersen S J, Morisaki N, Iwasaki S, Shida O, Takagi H, Furihata K. Rifampicin inactivation by Bacillus species. J Antibiot (Tokyo) 1995;48:815–819. doi: 10.7164/antibiotics.48.815. [DOI] [PubMed] [Google Scholar]
- 17.Dorman C J, Foster T J. Posttranscriptional regulation of the inducible nonenzymatic chloramphenicol resistance determinant of IncP plasmid R26. J Bacteriol. 1985;161:147–152. doi: 10.1128/jb.161.1.147-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu Z, Harrod R, Rogers E J, Loyett P. Anti-peptidyl transferase leader peptides of attenuation-regulated chloramphenicol-resistance genes. Proc Natl Acad Sci USA. 1994;91:5612–5616. doi: 10.1073/pnas.91.12.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerineau F, Brooks L, Mullineaux P. Expression of the sulfonamide resistance gene from plasmid R46. Plasmid. 1990;23:35–41. doi: 10.1016/0147-619x(90)90042-b. [DOI] [PubMed] [Google Scholar]
- 20.Hall L C M, Livermore D M, Gur D, Akova M, Akalin H E. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:1637–1644. doi: 10.1128/aac.37.8.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall R M, Brookes D E, Stokes H W. Site-specific insertion genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991;5:1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 22.Hall R M, Brown H J, Brookes D E, Stokes H W. Integrons found in different locations have identical 5′ ends but variable 3′ ends. J Bacteriol. 1994;176:6286–6294. doi: 10.1128/jb.176.20.6286-6294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi O, Ueda K. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- 25.Honoré N, Cole S T. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob Agents Chemother. 1993;37:414–418. doi: 10.1128/aac.37.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huovinen P, Huovinen S, Jacoby G A. Sequence of PSE-2 β-lactamase. Antimicrob Agents Chemother. 1988;32:134–136. doi: 10.1128/aac.32.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iida S, Mollet B, Meyer J, Arber W. Functional characterization of the prokaryotic mobile genetic element IS26. Mol Gen Genet. 1984;198:84–89. doi: 10.1007/BF00328705. [DOI] [PubMed] [Google Scholar]
- 28.Imai T, Watanabe K, Mikami Y, Yazawa K, Ando A, Nagata Y, Morisaki N, Hashimoto Y, Furihata K, Dabbs E R. Identification and characterization of a new intermediate in the ribosylative inactivation pathway of rifampin by Mycobacterium smegmatis. Microb Drug Resist. 1999;5:259–264. doi: 10.1089/mdr.1999.5.259. [DOI] [PubMed] [Google Scholar]
- 29.Innis A M, Gelfand D H, Sninsky J J, White T J. PCR protocols. New York, N.Y: Academic Press, Inc.; 1990. [Google Scholar]
- 30.Kleckner N. Transposon Tn10. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 227–268. [Google Scholar]
- 31.Laraki N, Galleni M, Thamm I, Riccio M L, Amicosante G, Frère J-M, Rossolini G M. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levesque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 33.Liebert C A, Hall R M, Summers A O. Transposon Tn21, flagship of the floating genome. Microbiol Mol Biol Rev. 1999;63:507–522. doi: 10.1128/mmbr.63.3.507-522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Littlejohn T G, Diberardino D, Messerotti L J, Spiers S J, Skurray R A. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene. 1992;101:59–66. doi: 10.1016/0378-1119(91)90224-y. [DOI] [PubMed] [Google Scholar]
- 35.Lovett P S. Translational attenuation as the regulator of inducible cat genes. J Bacteriol. 1990;172:1–6. doi: 10.1128/jb.172.1.1-6.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowery R G, Ludden P W. Endogenous ADP ribosylation in procaryotes. In: Moss J, Vaughan M, editors. ADP-ribosylating toxins and G proteins: insights into signal transduction. Washington, D.C.: American Society for Microbiology; 1990. pp. 459–468. [Google Scholar]
- 37.Mahillon J, Chandler M. Insertion sequences. Microbiol Mol Biol Rev. 1998;62:725–774. doi: 10.1128/mmbr.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Freijo P, Fluit A C, Schmitz F J, Verhoef J, Jones M E. Many class I integrons comprise distinct stable structures occurring in different species of Enterobacteriaceae isolated from widespread geographic regions in Europe. Antimicrob Agents Chemother. 1999;43:686–689. doi: 10.1128/aac.43.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazel D, Dychinco B, Webb V, Davies J. A distinctive class of integron in the Vibrio cholerae genome. Science. 1998;280:605–608. doi: 10.1126/science.280.5363.605. [DOI] [PubMed] [Google Scholar]
- 40.Middlebrook J L, Dorland R B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984;48:199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mollet B, Iida S, Shepherd J, Arber W. Nucleotide sequence of IS26, a new prokaryotic mobile genetic element. Nucleic Acids Res. 1983;11:6319–6330. doi: 10.1093/nar/11.18.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morisaki N, Hashimoto Y, Furihata K, Imai T, Watanabe K, Mikami Y, Yazawa K, Ando A, Nagata Y, Dabbs E R. Structures of ADP-ribosylated rifampicin and its metabolite: intermediates of rifampicin-ribosylation by Mycobacterium smegmatis DSM43756. J Antibiot (Tokyo) 2000;53:269–275. doi: 10.7164/antibiotics.53.269. [DOI] [PubMed] [Google Scholar]
- 43.Naas T, Philippon L, Poirel L, Ronco E, Nordmann P. An SHV-derived extended-spectrum β-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:1281–1284. doi: 10.1128/aac.43.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naas T, Poirel L, Karim A, Nordmann P. Molecular characterization of In50, a class 1 integron encoding the gene for the extended-spectrum β-lactamase VEB-1 in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1999;176:411–419. doi: 10.1111/j.1574-6968.1999.tb13691.x. [DOI] [PubMed] [Google Scholar]
- 45.Naas T, Poirel L, Nordmann P. Molecular characterization of In51, a class 1 integron containing a novel aminoglycoside adenylyltransferase gene cassette, aadA6, in Pseudomonas aeruginosa. Biochim Biophys Acta. 1999;1489:445–451. doi: 10.1016/s0167-4781(99)00202-x. [DOI] [PubMed] [Google Scholar]
- 46.Naas T, Nordmann P. OXA-type β-lactamases. Curr Pharm Design. 1999;5:865–879. [PubMed] [Google Scholar]
- 47.Ohno K, Koga H, Kohno S, Tashiro T, Hara K. Relationship between rifampin MICs and rpob mutations of Mycobacterium tuberculosis strains isolated in Japan. Antimicrob Agents Chemother. 1996;40:1053–1056. doi: 10.1128/aac.40.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paulsen I T, Littlejohn T G, Radström P, Sundström L, Sköld O, Swedberg G, Skurray R A. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother. 1993;37:761–768. doi: 10.1128/aac.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paulsen I T, Skurray R A, Tam R, Saier M H, Turner R J, Weiner J H, Goldberg E B, Grinius L L. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol. 1996;19:1167–1175. doi: 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 50.Ploy C M, Courvalin P, Lambert T. Characterisation of In40 of Enterobacter aerogenes BM2688, a class 1 integron with two novel gene cassettes, cmlA2 and qacF. Antimicrob Agents Chemother. 1998;42:2557–2563. doi: 10.1128/aac.42.10.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poirel L, Naas T, Guibert M, Chaibi E B, Labia R, Nordmann P. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob Agents Chemother. 1999;43:573–581. doi: 10.1128/aac.43.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poirel L, Le Thomas I, Naas T, Karim A, Nordmann P. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob Agents Chemother. 2000;44:622–632. doi: 10.1128/aac.44.3.622-632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purewal A S. Nucleotide sequence of the ethidium efflux gene from Escherichia coli. FEMS Microbiol Lett. 1991;82:229–232. doi: 10.1016/0378-1097(91)90338-b. [DOI] [PubMed] [Google Scholar]
- 54.Radström P, Sköld O, Swedberg G, Flensburg J, Roy P H, Sundström L. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J Bacteriol. 1994;176:3257–3268. doi: 10.1128/jb.176.11.3257-3268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quan S, Venter H, Dabbs E R. Ribosylative inactivation of rifampin by Mycobacterium smegmatis is a principal contributor to its low susceptibility to this antibiotic. Antimicrob Agents Chemother. 1997;41:2456–2460. doi: 10.1128/aac.41.11.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Recchia G D, Hall R M. Origins of the mobile gene cassettes found in integrons. Trends Microbiol. 1997;5:389–394. doi: 10.1016/S0966-842X(97)01123-2. [DOI] [PubMed] [Google Scholar]
- 57.Rendenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 58.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 59.Steers E, Foltz E I, Graves B S, Riden J. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot Chemother (Basel) 1959;9:307–311. [PubMed] [Google Scholar]
- 60.Stokes H W, Hall R M. Sequence analysis of the inducible chloramphenicol resistance determinant in the Tn1696 integron suggests regulation by translational attenuation. Plasmid. 1991;26:10–19. doi: 10.1016/0147-619x(91)90032-r. [DOI] [PubMed] [Google Scholar]
- 61.Stokes H W, O'Gorman D B, Recchia G D, Parsekhian L, Hall R M. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol. 1997;26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]
- 62.Sundström L, Radström P, Swedberg G, Sköld O. Site-specific recombination promotes linkage between trimethoprim and sulfonamide resistance genes. Sequence characterization of dfrV and sulI and a recombination active locus of Tn21. Mol Gen Genet. 1988;213:191–201. doi: 10.1007/BF00339581. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka Y, Yazawa K, Dabbs E R, Nishikawa K, Komaki H, Mikami Y, Miyaji M, Morisaki N, lwasaki S. Different rifampicin inactivation mechanisms in Nocardia and related taxa. Microbiol Immunol. 1996;40:1–4. doi: 10.1111/j.1348-0421.1996.tb03303.x. [DOI] [PubMed] [Google Scholar]
- 64.Tenover F C, Filpula D, Phillips K L, Plorde J J. Cloning and sequencing of a gene encoding an aminoglycoside 6′-N-acetyltransferase from an R factor of Citrobacter diversus. J Bacteriol. 1988;170:471–473. doi: 10.1128/jb.170.1.471-473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thorsted P B, Macartney N A, Akhtar P, Haines A S, Ali N, Davidson P, Stafford T, Pocklington M J, Pansegrau W, Wilkins B M, Lanka E, Thomas C M. Complete sequence of the IncP beta plasmid R751: implications for evolution and organisation of the IncP backbone. J Mol Biol. 1998;282:969–990. doi: 10.1006/jmbi.1998.2060. [DOI] [PubMed] [Google Scholar]
- 66.Tolmasky E M, Crosa J H. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa9) in the multiresistance transposon Tn1331. Plasmid. 1993;29:31–40. doi: 10.1006/plas.1993.1004. [DOI] [PubMed] [Google Scholar]
- 67.Tribuddharat C, Fennewald M A. Integron-mediated rifampin resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:960–962. doi: 10.1128/aac.43.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams D L, Waguespack C, Eisenach K, Crawford J T, Portaels F, Salfinger M, Nolan C M, Abe C, Sticht-Groh V, Gillis T P. Characterization of rifampin-resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380–2386. doi: 10.1128/aac.38.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yazawa K, Mikami Y, Maeda A, Morisaki N, Iwasaki S. Phosphorylative inactivation of rifampicin by Nocardia otitidiscaviarum. J Antimicrob Chemother. 1994;33:1127–1135. doi: 10.1093/jac/33.6.1127. [DOI] [PubMed] [Google Scholar]