Standfirst:
Important colorectal cancer (CRC) studies in 2021, including a new standard of care for first-line treatment of MSI-H–dMMR metastatic CRC, single-cell and spatial analysis of primary tumours and investigations of diet in preclinical models of cancer initiation, have provided novel insights into the CRC immune microenvironment.
Immune checkpoint inhibitor (ICI) therapy has been a game changer for the treatment of many solid tumours because it can achieve long-term durable remissions in some patients with heavily treated, advanced metastatic disease. Colorectal cancers (CRC) that accumulate mutations through somatic or germline loss of mismatch repair protein function (termed microsatellite high (MSI-H)–mismatch repair deficient (dMMR)) present neoantigens on their MHC class I molecules, enabling T cell recognition and activation, while microsatellite stable (MSS) or mismatch repair proficient (pMMR) tumours are immunologically cold1. Accordingly, immune checkpoint blockade with programmed cell death-1 (PD1) inhibitors, either alone or in combination with cytotoxic T-lymphocyte protein 4 (CTLA4) inhibitors, have demonstrated clinical benefit in MSI-H–dMMR CRC previously treated with the chemotherapy drugs 5-fluorouracil, oxaliplatin and irinotecan2,3.
KEYNOTE-177, a phase III, open label study of 307 patients with untreated metastatic MSI-H–dMMR CRC who were randomly assigned, in a 1:1 ratio, to receive pembrolizumab (anti PD-1 monoclonal antibody) or chemotherapy has now established ICI therapy as the standard of care for the first-line treatment of MSI-H–dMMR CRC4. With a median follow-up duration of 32.4 months, pembrolizumab achieved superior progression-free survival (16.5 vs. 8.2 months) and radiographic complete or partial response (43.8% vs. 33.1%) compared with chemotherapy. Importantly, 83% of pembrolizumab-treated patients with an overall response, as compared with 35% in the chemotherapy-treated group, had ongoing responses at 24 months.
While this study has changed clinical practice, 29.4% of patients in the pembrolizumab group and 12.3% in the chemotherapy group had progressive disease, highlighting the need for biomarkers and further optimization of immunotherapy in MSI-H–dMMR CRC. Dual immune checkpoint blockade with nivolumab (anti-PD1) and ipilimumab (anti-CTLA-4) had shown improved clinical benefit in indirect comparisons with nivolumab monotherapy in previously treated patients with advanced CRC, albeit with increased toxicity5. In the first-line metastatic setting, the ongoing phase II CheckMate 142 study in 2021 reported 69% objective response with nivolumab plus low dose ipilimumab, warranting further validation of the role of first-line dual ICI therapy in randomized studies6.
To date, ICI therapy in MSI-H–dMMR CRC is only approved for patients with metastatic disease, but there are indications that early stage tumours might offer a more receptive tumour microenvironment for pharmacological immune activation. Clinical trials in melanoma and non-small-cell lung cancer have shown markedly more effective responses to ICIs when administered in the adjuvant or neoadjuvant setting than in the metastatic setting, suggesting increasingly deeper immunosuppressive reprogramming of the tumour microenvironment during cancer progression, despite no significant difference in tumour mutation burden7. In support of this hypothesis, early results from the neoadjuvant phase II NICHE-2 study demonstrated major pathological response in 19/20 (95%) patients with MSI-H–dMMR CRC administered pre-operative ipilimumab plus nivolumab, with further studies of perioperative ICI therapy and longer follow-up forthcoming8. Beyond cancer therapy, ongoing clinical trials are also testing the use of ICI therapy for precision tumour prevention in high-risk individuals with hereditary loss of mismatch repair due to Lynch syndrome (NCT04711434, NCT03631641).
Despite these advances, the inescapable elephant in the room remains: MSI-H–dMMR CRC represents only a small proportion of CRC, whereas ICI therapy is largely ineffective for metastatic MSS–pMMR CRC, representing the majority of patients. To gain insight into the differences in the tumour microenvironment that underlie these distinct clinical phenotypes, Pelka et al. used single-cell RNA sequencing and spatial analyses to assemble a comprehensive atlas of 371,223 cells from 28 pMMR and 34 dMMR primary tumours and tumour-adjacent normal cells from patients9. They identified 88 cell populations encompassing normal and neoplastic epithelial cells, immune and stromal cells. Compared with normal colon samples, both pMMR and dMMR tumours were enriched in monocyte and macrophage populations. However, dMMR and pMMR tumours differed in several ways: dMMR tumours were infiltrated by myeloid cells expressing more immune-activating programmes and were enriched in T cells expressing CXCL13 and cytotoxic programmes. Among the malignant epithelial cells, in comparison to pMMR tumours, dMMR tumours expressed higher levels of interferon stimulated genes, MHC class II and to a lesser extent MHC class I programmes, and immune-attracting cytokines.
Using graph-based clustering of gene expression programmes, the researchers identified multicellular programmes of co-varying gene expression across all dMMR or pMMR samples termed immune hubs9. An inflammatory hub comprising tumour cells, neutrophils, myeloid cells and fibroblasts, located at the tumour:luminal interface of both pMMR and dMMR tumours, was associated with tumour necrosis. A second, immune stimulatory hub was identified exclusively in dMMR tumours, characterized by myeloid and malignant cells expressing interferon and MHC class II programmes, covarying with CXCL13-expressing T cells also expressing activation and exhaustion markers. Immunohistological analysis of patient tumour tissue sections confirmed the spatial colocalization of these immune hubs, and further identified spatial co-localization of CXCL13+ T cells with malignant cells expressing the CXCL13 receptor, CXCR3. This comprehensive dataset has, therefore, delineated extensive remodeling of the intestinal microenvironment in both pMMR and dMMR tumours, and identified divergent spatially organized networks of cancer-immune cell crosstalk. Future mechanistic interrogation of the multicellular interaction networks illuminated by this work might yield improved strategies for remodeling the pMMR microenvironment to become receptive to ICI therapy. An important unresolved question is to what extent the tumour-immune interactions observed in primary tumours are conserved in, and hence remain targetable in advanced metastatic disease.
Pelka et al. identified MHC class II gene expression programmes as a key feature of immune stimulatory hubs in human CRC. While neoantigen presentation on MHC class I molecules activates cytotoxic CD8+ T cells, MHC Class II recognition is crucial for CD4+ T helper cell activation and cytokine secretion to maintain effective anti-tumour immunity. In 2021, Beyaz et al. discovered that a high-fat diet (HFD) can modulate expression of MHC Class II on tumour-initiating LGR5+ intestinal stem cells (ISCs) in mouse models, thus enabling nascent adenomas to evade immune surveillance10. Because diet can influence the gut microbiome, Beyaz and colleagues treated mice with broad-spectrum antibiotics, and found that in HFD-fed mice reduction of bacterial diversity was accompanied by decreased MHC Class II expression by LGR5+ ISCs. Mice housed in rooms harboring multiple species of Helicobacter, a dominant bacterial genus in HFD-fed animals, had higher MHC Class II expression, while pharmacological or genetic perturbation of pattern recognition receptor and interferon gamma signalling inhibited MHC Class II expression. Since loss-of-function mutations in adenomatous polyposis coli (APC) often initiate CRC, the researchers used genetic tools to modulate MHC Class II expression levels in APC deficient adenoma mouse models fed high fat or control diets. Flow-sorted APCnull MHC II- LGR5+ ISCs demonstrated significantly (p<0.01) increased tumourigenicity when transplanted orthotopically in vivo in contrast to APCnull MHC II+ LGR5+ ISCs, a phenotype that was observed only in immunocompetent mice, but not in organoids cultured in vitro or when transplanted orthotopically into immunodeficient mice. Together, the results suggest that HFD-dependent intestinal microbial dysbiosis leads to downregulation of MHC Class II on LGR5+ ISCs, in turn enabling immune evasion and promoting tumour growth.
In sum, these three studies, a randomized clinical trial that has established a new standard of care for first line treatment of MSI-H–dMMR CRC, a comprehensive single cell and spatial analysis of dMMR and pMMR primary tumours, and investigations of diet, microbiota and antigen presentation in preclinical models of CRC initiation, have provided novel insights into the CRC immune microenvironment (Figure 1). Leveraging this work spanning the clinic and the laboratory might yield more effective immunotherapies for all patients with CRC.
Figure 1.
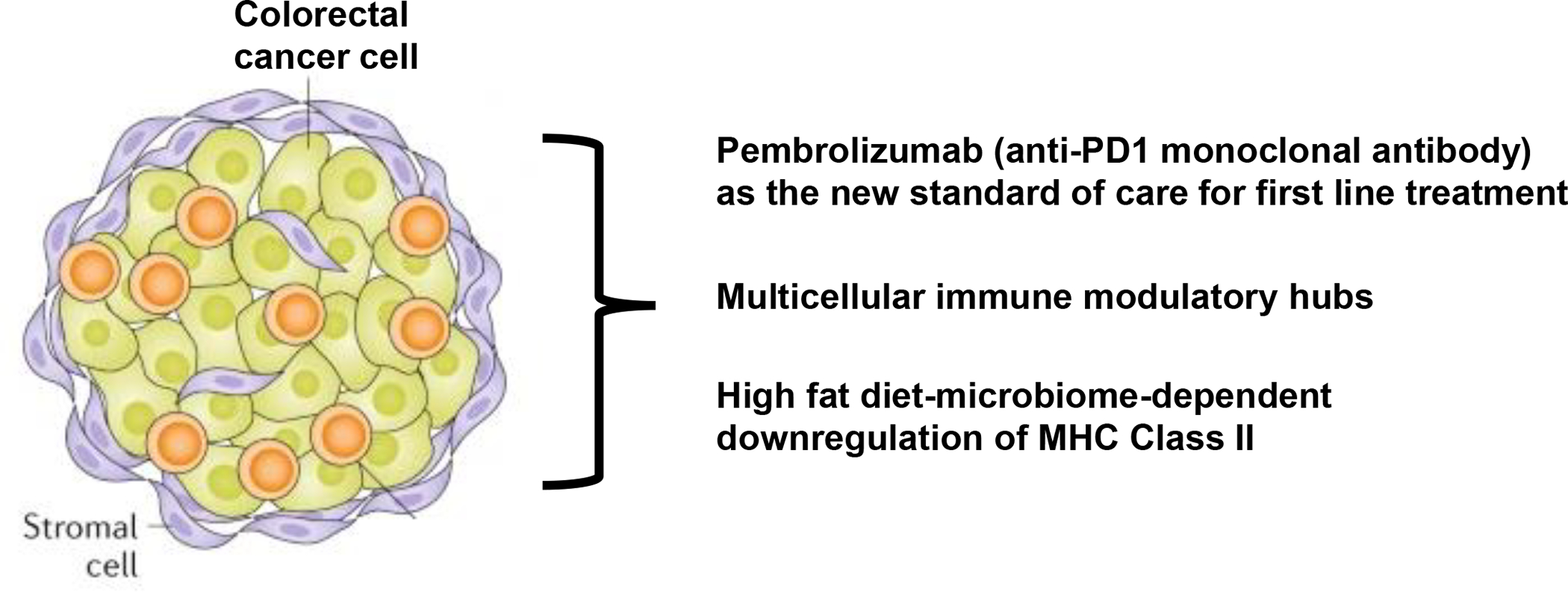
Modulating the local and systemic immune microenvironment of colorectal cancer. Three studies4, 9, 10 have advanced our understanding of colorectal cancer immune microenvironment.
Key advances.
Pembrolizumab (anti-PD1 monoclonal antibody) has been established as the new standard of care for first-line treatment of metastatic microsatellite-high (MSI-H)– mismatch repair deficient (dMMR) colorectal cancer4.
Comprehensive single-cell and spatial analyses of patient samples have identified distinct multicellular immune modulatory hubs in mismatch repair deficient and proficient primary colon tumours9.
High-fat diet can cause microbiome-dependent downregulation of MHC class II expression on LGR5+ intestinal stem cells in mice, in turn enabling immune evasion and promoting intestinal tumorigenesis10.
Acknowledgments:
K.G. is supported by NIH grants K08 CA230213, U2C CA233284 and P30 CA 008748, a Damon Runyon Clinical Investigator Award, Burroughs Wellcome Career Award for Medical Scientists, AACR NextGen Grant for Transformative Cancer Research, Josie Robertson Investigator Award and a Stand Up To Cancer Convergence Award.
Footnotes
Competing interests: The author declares no competing interests.
References
- 1.Ganesh K et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 16, 361–375, doi: 10.1038/s41575-019-0126-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le DT et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372, 2509–2520, doi: 10.1056/NEJMoa1500596 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overman MJ et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 18, 1182–1191, doi: 10.1016/S1470-2045(17)30422-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andre T et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 383, 2207–2218, doi: 10.1056/NEJMoa2017699 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Overman MJ et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 36, 773–779, doi: 10.1200/JCO.2017.76.9901 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Lenz HJ et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol, JCO2101015, doi: 10.1200/JCO.21.01015 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Ganesh K & Massague J Targeting metastatic cancer. Nat Med 27, 34–44, doi: 10.1038/s41591-020-01195-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalabi M et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 26, 566–576, doi: 10.1038/s41591-020-0805-8 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Pelka K et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752 e4720, doi: 10.1016/j.cell.2021.08.003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyaz S et al. Dietary suppression of MHC class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. Cell Stem Cell 28, 1922–1935 e1925, doi: 10.1016/j.stem.2021.08.007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


