Abstract
Worldwide, the COVID-19 pandemic disrupted healthcare services, including cervical cancer management, and an increased burden for this condition is expected. This systematic review synthetizes the available evidence on the impact of the pandemic on prevention, diagnosis and treatment of cervical cancer. Searches were performed on PubMed, Embase, and Scopus for relevant studies on these topics with the purpose of comparing service access and care delivery before and during COVID-19 pandemic. Due to the methodological heterogeneity among the studies, findings were narratively discussed. Of the 715 screened titles and abstracts, 33 articles were included, corresponding to 42 reports that covered the outcomes of interest: vaccination against human papillomavirus (HPV) (6 reports), cancer screening (19), diagnosis (8), and treatment (8). Seven studies observed reductions in HPV vaccination uptake and coverage during COVID-19. Reports on cervical screening and cancer diagnosis activities showed a substantial impact of the pandemic on access to screening services and diagnostic procedures. All but one study that investigated cervical cancer treatment reported changes in the number of women with cervical lesions who received treatments, as well as treatment delay and interruption. With a major impact during the first wave in 2020, COVID-19 and restriction measures resulted in a substantial disruption in cervical cancer prevention and management, with declines in screening and delays in treatment. Taken together, findings from this systematic review calls for urgent policy interventions for recovering cervical cancer prevention and care.
Keywords: Cervical cancer, COVID-19, Human papillomavirus, Cancer screening, Cancer treatment
1. Introduction
Cervical cancer is one of the most frequently diagnosed cancers, and a leading cause of cancer-related death in women (Zhao et al., 2021). The last iteration of the Global Cancer Statistics 2020–GLOBOCAN censused approximately 600,000 global cases and 340,000 deaths in 2020, and both statistics are expected to increase without broad interventions (Sung et al., 2021).
To contrast cervical cancer as a public health problem, the World Health Organization (WHO) steered a global health strategy, being the first time ever that the world has committed to eliminate a cancer (World Health Organization, 2020). Indeed, cervical cancer is both preventable and treatable, and the reduction of its burden includes tertiary interventions ranging from primary prevention strategies to screening campaigns, to effective treatment options (Peirson et al., 2013; Ferrara et al., 2020a).
Infection with high-risk types of human papillomavirus (HPV) is a necessary cause of cervical cancer, with 12 oncogenic HPV types classified as group 1 carcinogens by the International Agency for Research on Cancer (Sung et al., 2021; Ferrara et al., 2020a). Vaccination against HPV has proven to offer protective benefits in the reduction of neoplastic lesions' incidence (Signorelli et al., 2017). Again, robust evidence supports the importance of cervical screening for the early detection of cancerous lesions, which positively impacts on invasive cervical cancers' occurrence and mortality (Peirson et al., 2013; Lozano et al., 2020; Ferrara et al., 2020b). HPV vaccination and cervical screening are therefore essential part components of women's health (Acuti Martellucci et al., 2022), while timely and effective treatments for cervical precancerous lesions and cancers have been shortlisted among the most relevant indicators for an effective universal health coverage for women aged 20 years or older (Lozano et al., 2020).
The rapid spread of the coronavirus disease 2019 (COVID-19) caught unprepared healthcare systems (Ferrara and Albano, 2020), and hospitals and other healthcare facilities responded to the increased demand by internal reorganizations, which resulted in diversion of healthcare delivery for nonurgent conditions especially in the first epidemic months of 2020 (Voza et al., 2021; Matenge et al., 2021; Odone et al., 2020). This disruption of health services predominantly affected primary care services, leading to limitation in activities, reallocation of healthcare workers (HCW), and reduction of patients' access to facilities, as a consequence of the containment measures and fear of contagion (Ferrara and Albano, 2020; Matenge et al., 2021).
Along with broad analyses conducted to quantify the direct and indirect effects of the pandemic, some evidence has highlighted a worrisome impact on care of several cancers, including cervical cancer, but the majority of the studies focused on specific parts of prevention or clinical management (Acuti Martellucci et al., 2021; Saxena et al., 2021; Medenwald et al., 2022; Bonadio et al., 2021). Thus, it is crucial to extensively describe the extent of the COVID-19 impact on cervical cancer patients' and care, providing evidence-based support for the planning of flexible and integrated models of care for women.
In the light of the above and considering the importance of ensuring appropriate care for cervical cancer, we conducted the present systematic review with the aim of summarizing epidemiological research on the impact of COVID-19 pandemic on the prevention, diagnosis and treatment of this condition.
2. Methods
This literature revision was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 2020 (Page et al., 2021). Methods were published in advance in the Prospective Register of Systematic Reviews (PROSPERO) with number CRD42022311206.
2.1. Search strategy
Studies were identified surfing the electronic databases PubMed/MEDLINE, Scopus and Embase. We combined a search strategy of free-text terms and MeSH headings for the topics of HPV prevention, and cervical cancer diagnosis and treatment, as well as of the COVID-19 pandemic. The complete search strategy is presented in the Supplementary material (Appendix 1). The strategy was first developed for the PubMed database and subsequently adapted for the others. Efforts to include further possible relevant articles included cross-referencing of the citation lists of the retrieved articles. Searches were performed up to February 8, 2022, exploring evidence published from 2020 onwards. No restriction on publication status was applied.
2.2. Study selection and inclusion criteria
Selection criteria for screening titles and abstracts were as follows: (1) primary reports available in full-text (trial or observational studies such as case-control, cohort or cross-sectional studies); (2) reporting primary data on HPV vaccination coverage, as well as screening programs, diagnostic procedures and treatment of cervical cancer; (3) including data comparison before and after COVID-19 pandemic; (4) studies published in English or Italian. Records that met the following criteria were excluded: (1) studies without measures of the outcomes of interest; (2) not considering the impact of COVID-19; (3) published as narrative review, editorial, or letter to editor.
2.3. Data extraction, data synthesis and quality assessment
Two authors (GD and FA) independently evaluated the retrieved titles, abstracts, and full-texts for inclusion. Possible disagreements were solved through discussion and consultation of a senior author (PF). Data extraction was performed using a pre-piloted spreadsheet elaborated in Microsoft Excel® for Windows (Microsoft Corporation, Redmond, WA, USA). The following baseline characteristics were extracted for each article: first author's last name, year and country of publication, study design, population size and characteristics, source of information, type of outcome of interest (HPV vaccination, and/or cervical cancer screening, diagnosis and treatment) and measures, and main findings.
Due to the significant heterogeneity in methods and outcomes across the retrieved studies, results were not pooled in a meta-analysis but discussed according to the aim to analyze the impact of COVID-19 on cervical cancer care.
The two reviewers (GD and FA) also assessed the methodological quality of the body of found evidence through an adapted version of the Newcastle-Ottawa Scale (aNOS) available in literature (Supplementary Material, Appendix 2), in which reports achieving an aNOS score of 5 or greater were considered high-quality studies (Wells et al., 2014).
3. Results
3.1. Search results and articles overview
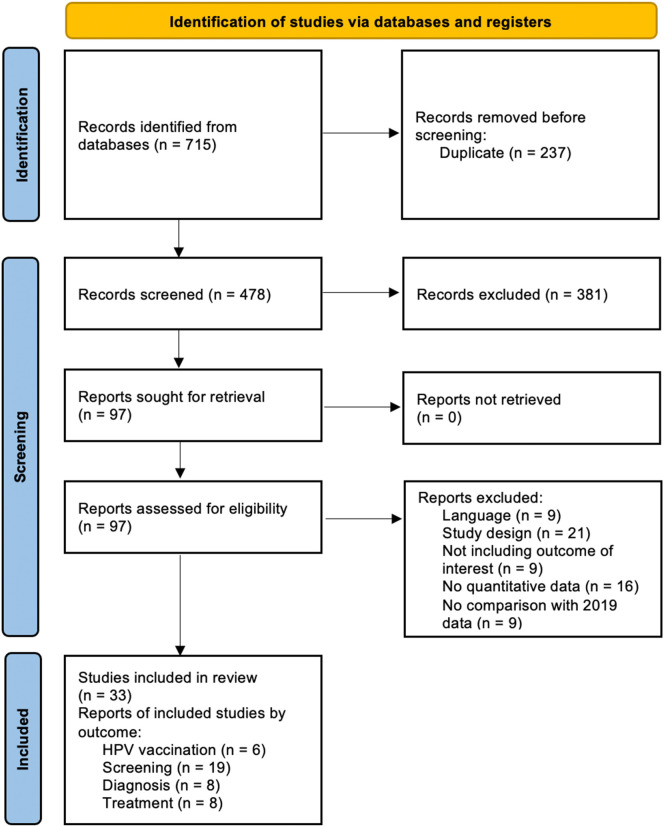
The flow chart of included studies and selection process is presented in Fig. 1 . Overall, the search strategy retrieved a total of 715 articles. After titles' and abstracts' screening, 33 articles met the inclusion criteria, corresponding to 41 reports that covered the different outcomes of interest (Fig. 1): HPV vaccination (6), cervical cancer screening (19) and diagnosis (8), treatment (8).
Fig. 1.
PRISMA flow chart of included studies and selection process.
The characteristics of included studies are presented in Table 1, Table 2, Table 3 . All had an observational design and were published after July 2020. Of the total, 16 studies were carried out in America, 13 in Europe, three in Asia, and two in Africa.
Table 1.
Main characteristics of studies including data on HPV vaccination.
| First author and year | Country | Data source (and target population) | Sample size | Outcome measure | Main findings | aNOS score (max 7) | |
|---|---|---|---|---|---|---|---|
| 1 | (Casey et al., 2022) | United States | Population-level healthcare administrative data (adolescents aged 10–18 years) | NR | Number of vaccine doses administered monthly | From March to May 2020, monthly HPV vaccine declined up to 15 doses (vs. 376/month in the period March 2016–December 2019), rebounding to 25 monthly doses in October 2020. | 7 |
| 2 | (Daniels et al., 2021) | United States | Individual-level healthcare claims data from insurers' database (population aged 9–26 years) | NR | Vaccination coverage | Coverage decreased through March and April 2020, reaching a low of 23% of the rate for the previous years (2018–2019). Coverage increased through May and June 2020 to a high of 79% of the previous rate and fell again in July and August. | 6 |
| 3 | (Gabutti et al., 2021) | Italy | Population-level healthcare administrative data (15-year-old girls) | NA | Vaccination coverage | Full-cycle coverage for 15-year-old girls has been equal 63.8%, with a decrease compared to the previous year (70.4%). | 6 |
| 4 | (Ramírez et al., 2022) | Spain | Doses of HPV vaccines distributed by pharmacies during 2008–2020 | NA | Temporal trend in vaccination coverage in women aged 15–55 years not included in vaccination programs during 2007–2020 | In 2020, it was estimated that 4.0% in women aged 15–55 years not included in vaccination programs were vaccinated against HPV, representing an absolute increase of 0.4% from 2019. | 6 |
| 5 | (Sabbatucci et al., 2022) | Italy | Population-level healthcare administrative data (13-year-old girls) | NR | Vaccination full-cycle coverage | Vaccination coverage decreased from 60.8% in 2019 (168,680/277,302) to 58.7% in 2020 (160,219/273,154), for a difference of −2.2 | 6 |
| 6 | (Saxena et al., 2021) | United States | Individual-level healthcare claims data from insurers' database (population aged 9–16 years) | NR | Total vaccine doses administered | HPV vaccine doses administered in 2019 were 446,431, with an average monthly number of doses 37,203. In 2020, these numbers were respectively 339,408 and 28,284, with a total reduction of 24% between the two years. | 6 |
Abbreviations: HPV, human papillomavirus; aNOS, adapted version of the Newcastle-Ottawa Scale checklist for assessing the quality of non-randomized studies; NR, not reported; NA, not applicable; 95% CI, 95% confidence interval.
Table 2.
Main characteristics of studies including data on cervical cancer screening.
| First author and year | Country | Data source (and target population) | Sample size | Outcome measure | Main findings | aNOS score (max 7) | |
|---|---|---|---|---|---|---|---|
| 1 | (Acuti Martellucci et al., 2021) | Italy | Population-level healthcare administrative data | NR | Number of obstetricians' worked hours; number of Pap smears performed; hourly rate of Pap smears | In the first semester of 2020, a 64.5% decrease of Pap smears was registered (compared with the same period of 2019, 12,415 vs. 4411 smears), with 0 tests performed from March 9 to June 30, 2020. Obstetricians' worked hours devoted to the screening almost doubled in the second semester of 2020 (+93.1% increase: 3445 vs 1784 h of 2019). In the second semester of 2020, 12,349 Pap smears were performed, against 7252 in 2019 (+70.3%). Overall, in 2020 screening participation was 14.8% lower than in 2019. | 7 |
| 2 | (de Pelsemaeker et al., 2021) | Belgium | Electronic histopathological reports of a single pathology laboratory | NA | Number of Pap smear samples received at pathology department | 43.3% reduction in the number of Pap smears in 2020: 5941 samples in Jan-Apr 2019 vs. 3370 in Jan-Apr 2020. | 6 |
| 3 | (DeGroff et al., 2021) | United States | Individual-level healthcare administrative data from the US National Breast and Cervical Cancer Early Detection Program screening services | 594,566 screening tests conducted between 2015 and 2020 (353,398 women) | Number of screening tests performed | Overall, the volume of screening tests in 2020 was well below that for the previous 5-year averages for the months March–June, with a sharp decline observed in March–April 2020. Screening test volumes for cervical cancer were lowest in April 2020, when those declined 84% from the 5-year average of 18,347 to 2880 in 2020. In June 2020, cervical cancer screening tests represented a 40% decline from the 5-year average (9413 vs. 15,681). Regarding race/ethnicity, the greatest decline in the number of breast cancer screening tests was during April among American Indian/ Alaskan Native women (98%) followed by Asian/Pacific Islander women (97%). | 7 |
| 4 | (Dema et al., 2022) | United Kingdom | Web-based survey in members of market research company (aged 18–59) | 6654 | Access to screening services | Only 2.5% of women aged 25–59 years reported using cervical screening services during the 4-month period following the start of a national lockdown in Britain (March 23, 2020), which is lower than the estimated use of the cervical cancer screening program among this age group for the same time period under normal circumstances (6%). | 7 |
| 5 | (Dennis et al., 2021) | United States | Nation-wide telephone surveys among participants in the US behavioral risk factor surveillance system | 2014–2019: 473,360; 2020: 121,640 | Pap tests reported | An 8.6% reduction in reported Pap tests in 2020 (38% of the total sample had a pap test) compared to 2019 (46.6%). | 7 |
| 6 | (Desta et al., 2021) | Ethiopia | Population-level healthcare administrative data | NR | Number of women 30 to 49 years of age screened for cervical cancer using VIA | The number of women 30 to 49 years of age who were screened for cervical cancer using visual inspection with acetic acid (VIA) during the second quarter of 2020 decreased by 54.8% compared to the same period of 2019 (695 vs. 314). But the Wilcoxon signed rank test has shown that there was no statistical difference between the two quarters (p-value = 0.15). | 6 |
| 7 | (Doubova et al., 2021) | Mexico | Population-level healthcare administrative data | NA | Number of women screened for cervical cancer with VIA | The average number of monthly screening tests decreased of −68% from 2019 to 2020 (216,808 vs 84,752). | 7 |
| 8 | (Gorin et al., 2021) | United States | Individual-level EMR of patients receiving routine cancer screening | 42,974 | Number of cervical cancer screening via ThinPrep and/or the human papillomavirus DNA high-risk profile | During the pandemic, analyses of cancer patterns screening as of April 25, 2020, revealed a precipitous drop in cervical cytology screening of 94% compared to the previous three years. Cervical cancer screening also decreased considerably during the shelter-in-place orders (4990 to 444 overall). | 6 |
| 9 | (Ivanuš et al., 2021) | Slovenia | Population-based cervical cancer screening registry (from the National Cervical Cancer Screening Registry – ZORA Registry), including women aged 20–64 years | NR | Number of screening tests (cervical cytopathology, histopathology and HPV tests) | Compared to the average of a three-year period (2017–2019), Slovenia entered the second wave of epidemics with a pandemic deficit of −19,460 (−23%) program screening smears, however with excess in follow-up smears (412, 4%) and HPV triage tests (523, 8%). Older women (aged 40 to 64) had significantly larger deficit of screening smears during the epidemic than younger (aged 20 to 39). Due to different intensities of scaling-up during the summer, the pandemic deficit of screening smears was significantly larger in age group 30 to 39 than in older groups and age group 20 to 29 was more similar to older groups than 30 to 39. Also, younger women had a smaller pandemic excess in follow-up smears and HPV triage tests. The opposite was observed for invasive diagnostics, for which the pandemic deficit was still larger in older women. The most affected were women in the 30–39 age group, who had the largest pandemic deficit of screening smears (−26%), the second-lowest scaling-up of FU tests (+31%), the lowest scaling-up of HPV tests during summer (+31%), and the highest epidemic (−25%) and pandemic (−18%) deficit in treatment. | 7 |
| 10 | (Kim et al., 2022) | United States | Individual-level EMR of women eligible for screening among the patients of the UCLA Health System | 113,125 women (pre-pandemic period); 116,540 (stay-at-home period); 119,324 (reopening period) | Odds ratio (OR) for screening completion | Stay-at-home vs. pre-pandemic: OR = 0.83 (95%CI, 0.76–0.91; p-value <0.001); phased reopening vs. pre-pandemic: OR = 0.69 (95% CI, 0.63–0.76; p-value <0.001); phased reopening vs stay-at-home: OR = 0.83 (95% CI, 0.76–0.92; p-value <0.001). | 6 |
| 11 | (Koczkodaj et al., 2021) | Poland | Population-level data from the National Health Fund | NR | Cytology coverage percentages from January to September in the years 2019 and 2020 | Cytology tests 16.34% (2019) vs 14.35% (2020). | 5 |
| 12 | (Laing and Johnston, 2021) | Canada | EMR of three urban primary care clinics | 6754 | Pap smear rates among patients eligible for cervical cancer screening. For reference the lockdown and re-opening stages have been identified. Lockdown = March 20, 2020, Stage 2 = June 12, 2020, Stage 3 = July 17, 2020, and Modified Stage 2 = October 13, 2020 | A total of 505 (95% CI, 20–993) patients would need to have Pap smear testing. Cervical cancer screening rates decreased by 0.23% per week. The mean percentage of patients appropriately screened for cervical cancer decreased by 7.5% (95% CI, −0.3 − −14.7%). | 5 |
| 13 | (Li et al., 2021) | China | Web-based survey among registered physicians who practice obstetrics and gynecology in public hospitals | 7434 | Reported reduction in activities between January and August 2020 | 37.3% (95%CI, 35.4–39.2) reported a complete shutdown (or > 50% reduction); 22.0% (95%CI, 20.6–23.4) reported a reduction by 25%–50%; 40.7% (95%CI, 39.0–42.5) reported a reduction <25% or no change in clinical activities. | 6 |
| 14 | (Mantellini et al., 2020) | Italia | Quantitative survey of aggregated regional healthcare data | NA | Number of screening tests | In January–May 2020, 371,273 screening tests were conducted in Italy, being −55,3% compared to compared to the same period of 2019. | 6 |
| 15 | (Meggetto et al., 2021) | Canada | Multiple population-based administrative databases | NR | Monthly cervical screening test | From March to August 2020, monthly cervical screening cytology test volumes were substantially reduced compared with the same months 1 year prior (average was 29,147 tests, compared with 81,877 in 2019). From March to August 2020, the average number of individuals with a high-grade cytology result identified per month was 280 (compared to an average of 572 individuals between November 2019 and February 2020). | 7 |
| 16 | (Miller et al., 2021) | United States | Individual-level EMR of approximately 1.5 million women served by Kaiser Permanente Southern California | 2,947,686 women 21–65 years | Screening rate | Among women aged 21–29 years, screening rates in 2020 were 8% lower before the stay-at-home order, 78% lower during the stay-at-home order, and 29% lower after the stay-at-home order compared with rates during 2019. Among women aged 30–65 years, screening rates in 2020 were 3% lower before the stay-at-home order, 82% lower during the stay-at-home order, and 24% lower after the stay at-home-order compared with rates during 2019. | 6 |
| 17 | (Nogami et al., 2022) | Japan | Web- and telephone- questionnaire in the municipalities of the metropolitan area of Tokyo | NA | Number of cancer screening | During the first wave when “the State of Emergency” was first declared (March–June 2020), all subject municipalities showed a marked decrease in the number of screenings compared with the previous year, but then showed a recovery, and with the aggregate up to where they could have complete data, 82.9% were implemented compared with 2019. | 6 |
| 18 | (Ortiz et al., 2021) | Puerto Rico | Patient-level claims data from insurance database of women aged 21–65 years | 352,520 | Time trends of Pap tests | A substantial decrease occurred in screening utilization from January 2016 (2.81 per 100 person-months) to July 2020 (0.72 per 100 person-months). Screening rates were particularly low after the COVID-19–related lockdown (April 2020: 0.37 per 100 person-months). Screening rates among women aged 21–29 years dropped from 2.90 per 100 person-months (95%CI, 2.83–2.97) in January to March 2016 to 1.00 (95%CI, 0.95–1.02) during April to June 2020 (table). Among women aged 30–65 years, rates for the same comparison periods decreased from 3.85 (95%CI, 3.80–3.90) to 1.10 per 100 person-months (95%CI, 1.08–1.12). | 6 |
| 19 | (Walker et al., 2021) | Canada | Population-level data on screening participation from healthcare administrative database | 761,891 in 2019 and 404,945 in 2020 | Number of cervical cytology screening | Relative to 2019, a higher percentage of cytology screening participants in the pandemic period were 21–29 years (19.9 vs. 17.3%) and 30–39 years (25.9 vs. 23.2%) and a lower percentage were 50–59 years (19.6 vs. 22.0%) and 60–69 years of age (13.9 vs. 15.8%). | 7 |
Abbreviations: aNOS, adapted version of the Newcastle-Ottawa Scale checklist for assessing the quality of non-randomized studies; NR, not reported; NA, not applicable; EMR, electronic medical record; 95% CI 95% confidence interval; CIN, cervical intraepithelial neoplasia; VIA, visual inspection of cervix with acetic acid.
Table 3.
Main characteristics of studies including data on cervical cancer diagnosis.
| First author and year | Country | Data source (and target population) | Sample size | Outcome measure | Main findings | aNOS score (max 7) | |
|---|---|---|---|---|---|---|---|
| 1 | (Bonadio et al., 2021) | Brazil | Hospital EMR of cancer patients | 60 (2019) 44 (2020) |
Stage of cancer at diagnosis; interval from tumor biopsy to the first cancer center visit. | The proportion of patients with more advanced disease stages was numerically higher in September/20–January 21, although the difference was not statistically significant (p-value = .328). Locally advanced disease (FIGO stages III–IVA) occurred in 56.8% (N = 25/44) of the cervical cancer patients in September/20–January/21, compared to 43.3% (N = 26/60) in September/19–January/20. Median time from tumor biopsy to first cancer center visit was 4 months in September/20–January/21 and 6.1 months in September/19–January/20 (p-value = .010). The proportion of stages III–IVA cervical cancer increased by 13.5%. | 7 |
| 2 | (Davies et al., 2022) | United Kingdom | Medical records from six cancer centers | 233 (2019) + 173 (2020) | New diagnoses | 25.7% (n = 60) reduction in the number of cases diagnosed between May–October 2019 and May–October 2020 (respectively, 233 vs. 173 cases). | 6 |
| 3 | (Istrate-Ofițeru et al., 2021) | Romania | Medical records from Obstetrics and Gynecology Clinics patients with pathological Pap smears | 396 | Number of patients hospitalized with pathological results at Pap test | During the first pandemic year (15.03.2020–14-03.2021), only 21.4% of total patients with pathological results on the Pap smear and colposcopy were admitted to the clinics. There is a significant decrease of 57.2% in the number of cases diagnosed with high-grade dysplasia in the first pandemic year (15.03.2020–14-03.2021) compared to the 12 months before (78.6% of patients admitted). The diagnosis rate decreased to about one-third for CIN 1, and about 80% for both CIN 2 and 3. | 5 |
| 4 | (Ivanuš et al., 2021) | Slovenia | Population-based cervical cancer screening registry (from the National Cervical Cancer Screening Registry – ZORA Registry), including women aged 20–64 years | NR | Number of newly diagnosed CIN2+ | In the period 2017–2019, on average, 1522 new cases of CIN2+ per year have been detected in the 20–64 age group. After the two-month screening lockdown, 71 (32%) CIN2+ fewer cases were detected than expected. The relative deficit of newly detected CIN2+ cases was the largest one week after the lockdown ended (100 cases, −18%); later, the deficit gradually decreased, and by the end of September 2020, there were 113 missing cases of CIN2+ (−10%), which is a significant difference. The only age group with a significant CIN2+ deficit at the end of September (−19%) was 30–39 years, in which almost one third of all new CIN2+ cases were detected in the previous years. | 7 |
| 5 | (Meggetto et al., 2021) | Canada | Multiple population-based administrative databases | NR | Monthly cervical screening test, colposcopy and cervical treatment volumes | Between March and August 2020, the average monthly decrease in colposcopy volumes was 3555 colposcopies or 39.7% compared with the same months in 2019. The average monthly decrease in cervical treatment volumes was 31.1% or 288 cervical treatments, compared with the same months in 2019. | 7 |
| 6 | (Morais et al., 2021) | Portugal | Hospital cancer registry and clinical EMR | 1430 cancer cases before (between February and July 2019) and 866 (between February and July 2020) | Number of cervical cancer diagnoses | Cervical cancer diagnosis in 2019 were 35 (2.4%), while in 2020 the number decreased to 9 (1.0%), with a difference of −74.3% (95% CI, −87.6 to −46.6). | 5 |
| 7 | (Van Wyk et al., 2021) | South Africa | Reports of a single pathology laboratory | NR | Number of newly diagnosed cervical cancer | The number of newly diagnosed cervical cancers decreased by 5 cases (−7%) in cervical cancer from 71 (−7%) in the period April–June 2019 to 66 in the corresponding period in 2020. The mean age at diagnosis for the six cancers in 2020 was 2 years younger than 2019 (p-value = 0.02). | 5 |
| 8 | (Walker et al., 2021) | Canada | Population-level data on screening participation from healthcare administrative database | 761,891 in 2019 and 404,945 in 2020 | Number of follow-up colposcopies | The percentage of participants for whom colposcopy was not performed was increased for participants with high-grade cervical cytology tests in April–June 2020 (range = 12.8%–21.1%) compared with the reference period. | 7 |
Abbreviations: aNOS, adapted version of the Newcastle-Ottawa Scale checklist for assessing the quality of non-randomized studies; NR, not reported; NA, not applicable; EMR, electronic medical record; 95% CI 95% confidence interval; CIN, cervical intraepithelial neoplasia.
Methodological quality varied across the 34 studies, of which 32 scored five or more stars on the aNOS quality assessment, while two were classified as low quality given the high risk of bias. Major reasons for bias across studies included lack of representativeness of the sampled participants, as well as substandard assessment of the outcomes as they were mostly self-reported through questionnaires. A more detailed description of the aNOS assessment can be found in Table S1 (Supplementary material).
3.2. HPV vaccination
Six large-scale studies investigated HPV vaccination uptake and coverage during the COVID-19 pandemic from healthcare administrative databases (Table 1) (Saxena et al., 2021; Casey et al., 2022; Daniels et al., 2021; Gabutti et al., 2021; Ramírez et al., 2022; Sabbatucci et al., 2022). Findings from five reports, which included adolescents and young girls aged 9–26 years, observed a decrease in vaccination coverage starting from March 2020. Of those, two measured the decrease in the number of vaccine doses administered monthly that dropped by the 96% in March–May 2020 in the research by Casey et al. (2022) Three evaluated the population-level vaccination coverage during the whole year, with slight decreases in 13- (Sabbatucci et al., 2022) and 15-year-old adolescents (Gabutti et al., 2021) (respectively −2.2 and −6.6% compared to 2019), as well as a −77% in girls aged 9–26 years during the stay-at-home period (Daniels et al., 2021) (Table 1). Conversely, Ramírez et al. did not find significant variations in vaccination coverage during the pandemic, describing a 0.4% absolute increase in the temporal trend of vaccination coverage in women aged 15–55 years not included in national vaccination programs (Ramírez et al., 2022).
3.3. Screening
The impact of COVID-19 and restrictive measures on cervical screening was identified in 20 reports, which varied considerably according to study periods and outcomes (Table 2), which sourced data from healthcare administrative databases, hospital medical records, cancer registries, pathology records, or web surveys (Acuti Martellucci et al., 2021; Bonadio et al., 2021; Davies et al., 2022; de Pelsemaeker et al., 2021; DeGroff et al., 2021; Dema et al., 2022; Dennis et al., 2021; Desta et al., 2021; Doubova et al., 2021; Gorin et al., 2021; Istrate-Ofițeru et al., 2021; Ivanuš et al., 2021; Kim et al., 2022; Koczkodaj et al., 2021; Laing and Johnston, 2021; Li et al., 2021; Mantellini et al., 2020; Meggetto et al., 2021; Miller et al., 2021; Morais et al., 2021; Nogami et al., 2022; Ortiz et al., 2021; Van Wyk et al., 2021; Walker et al., 2021). Seven studies evaluated the impact of COVID-19 on the number of Pap smears performed during the first half of 2020: of those, four were conducted in Europe and reported a reduction ranging from 43.3% and 91.5% (Acuti Martellucci et al., 2021; de Pelsemaeker et al., 2021; Ivanuš et al., 2021; Mantellini et al., 2020), while the other conducted in America observed a reduction between 84% and 91.5%, or of more than two-third during the pandemic, compared to the reference periods (DeGroff et al., 2021; Ortiz et al., 2021).
Meggetto et al. examined the average monthly screening tests in Canada, finding a decrease of 63.3% between March–August 2020 (Meggetto et al., 2021). Desta et al. highlighted a 54.8% reduction among 30–49-year-old women screened for cervical cancer using Visual Inspection with Acetic Acid during the second quarter of 2020 in Ethiopia (Desta et al., 2021). Four studies carried out their analysis over the whole 2020 year, in which the rate of Pap smears fell down by −7.5 to −68% (Doubova et al., 2021; Ivanuš et al., 2021; Laing and Johnston, 2021; Walker et al., 2021).
Access to screening services was evaluated in four studies. In the United Kingdom, the number of women using screening services dropped from 6% in 2019 to 2.5% in 2020 during the first four months of the year (Dema et al., 2022). In Poland, cytology coverage decreased by two points comparing data of 2019 and 2020 (from 16.34% to 14.35%, between January–September) (Koczkodaj et al., 2021). In a study conducted in USA, compared with the pre-pandemic period, the odds for screening completion among women decreased of 17% and 31% respectively during the stay-at-home and re-opening phases (Kim et al., 2022). A substantial decrease occurred in screening utilization from January 2016 (2.81 per 100 person-months) to July 2020 (0.72 per 100 person-months) in Puerto Rico (Ortiz et al., 2021).
Acuti Martellucci et al. also evaluated COVID-19-induced variations in screening ambulatory services in a province of central Italy, finding 70.3 and 93.1% increases respectively in the numbers of obstetricians' work hours and Pap smears in the second semester of 2020, compared with the same period of 2019 (Acuti Martellucci et al., 2021). Nogami et al. reported that full screening capacity took six months to recover up to pre-pandemic levels, after having reached values as low as 10% in May 2020 (Nogami et al., 2022).
Li et al. surveyed registered physicians who practiced obstetrics and gynecology in Chinese public hospitals, the 60% of which reported a reduction in cervical screening activities from 25% to 100% 2020 (January–August) due to COVID-19, with the most significant reductions observed in cities with more hospital beds and high-level hospitals (Li et al., 2021).
3.4. Diagnosis
Eight studies specifically analyzed the impact of COVID-19 on cervical cancer diagnosis and diagnostic procedures, comparing 2020 with the pre-pandemic period (Table 3). Among these, Davies et al. censused 25.7% less cancer cases between May and October 2020 in United Kingdom (Davies et al., 2022), and van Wyk et al. -7% in April–June 2020 in South Africa (Van Wyk et al., 2021), and Morais et al. -73.4% during the whole 2020 year in Portugal (Morais et al., 2021). Ivanuš highlighted a decrease of 13% in diagnostic invasive procedures in Slovenia in 2020 (Ivanuš et al., 2021). Istrate-Ofițeru et al. found that the number of biopsies and excisional procedures has been decreasing by more than a factor of three in Romania during the pandemic period (March 2020–March 2021) compared to the year before (Istrate-Ofițeru et al., 2021). A decrease in the number of follow-up colposcopy tests was also seen in the pandemic period in two Canadian studies (Meggetto et al., 2021; Walker et al., 2021), while in Brazil Bonadio et al. showed that patients had a more advanced-stage at diagnosis during the pandemic, with the proportion of stages III-IVA increased by 13.5% (Bonadio et al., 2021).
3.5. Treatment
Eight of the included reports considered the differences in practice between pre- and pandemic period (Table 4 ). Data included in the analyses were sourced from population-level healthcare administrative data (Desta et al., 2021; Koczkodaj et al., 2021; Meggetto et al., 2021), hospital medical records (Medenwald et al., 2022; Istrate-Ofițeru et al., 2021; Hathout et al., 2021), or cancer screening registry (Ivanuš et al., 2021) in six studies. Of those, all but one reported changes in the delivery of cervical cancer treatment, mostly in terms of decreased number of women with cervical lesion who received treatments, or treatment delays and interruption (Table 3): Altin er al. interviewed 70 gynecologic oncologists: 97.1% reported changes in changes of gynecological cancers due to pandemic situation, in terms of delayed surgery (33.3%) and shift to hypo-fractionated radiotherapy was preferred to standard dose (57.1 vs. 27.1%, respectively), in order to reduce the number of hospital visits (Altın et al., 2020).
Table 4.
Main characteristics of studies including data on cervical cancer treatment.
| First author and year | Country | Data source (and target population) | Sample size | Outcome measure | Main findings | aNOS score (max 7) | |
|---|---|---|---|---|---|---|---|
| 1 | (Altın et al., 2020) | Turkey | Web-based survey among gynecologic oncologists affiliated to the Turkish Society of Gynecologic Oncology | 70 | Number of standard surgeries, delay, referral to other hospitals, radiotherapy. Stratification for cancer stage | Overall, 97.1% of surveyees responded that cancer management changed during the pandemic (for all gynecological cancers). 58% of surgeons continued to operate microinvasive cervical cancer, while 33.3% delayed surgery. Standard surgery (67.1%) and delay (20%) were the two leading responses for early-stage cervical cancer. Primary RT or chemo-RT was applied without delay to most of the locally advanced cervical cancer patients, but hypo-fractional dose (57.1%) was preferred to standard dose (27.1%), in order to reduce the number of hospital visits. 67.1% of surgeons continued to perform surgery or administered CT/RT to metastatic or recurrent cervical cancer patients. | 4 |
| 2 | (Desta et al., 2021) | Ethiopia | Population-level healthcare administrative data | NR | Number of women with cervical lesions who received treatment | During the second quarter of 2020, there was a decrease in number of women aged 30–49 years with cervical lesion and received treatment (−85.0%; 20 vs. 3), compared to the same period of 2019. | 6 |
| 3 | (Hathout et al., 2021) | United States | Medical records from four cancer centers | 15 | Days of treatment delays | All patients with cervical cancer received their treatments as planned; however, four of 15 patients (26.7%) had a treatment interruption during their course. Two patients experienced significant delays (>20 days) owing to COVID-19 infection and the other two patients had treatment interruptions due to non-COVID medical problems. | 4 |
| 4 | (Istrate-Ofițeru et al., 2021) | Romania | Medical records from Obstetrics and Gynecology Clinics patients with pathological Pap smears | 396 | Number of patients with high-grade dysplasia treated and type of surgical intervention performed | Of the total patients who had been diagnosed with high-grade dysplasia, 21.4% were treated surgically in the pandemic year (15.03.2020–14-03.2021) and 78.6% in non-pandemic period (15.03.2019–14-03.2020). Before COVID-19, excisional biopsies were performed in 31.3% and LEETZ in 47.3% of the total surgical procedures. During the pandemic, excisional biopsy was performed in 6.2% and LEETZ in 15.2% of the total procedures. | 5 |
| 5 | (Ivanuš et al., 2021) | Slovenia | Population-based cervical cancer screening registry (from the National Cervical Cancer Screening Registry – ZORA registry), including women aged 20–64 years | NA | Number of women who underwent invasive procedures 120 days after high-grade screening diagnosis. | Compared to the average of a three-year period (2017–2019), a significantly higher deficit in cold-knife conizations (−91, −39%) compared to LLETZ (−4, −1%) was observed. | 7 |
| 6 | (Koczkodaj et al., 2021) | 2021 | Population-level data from the National Health Fund | Number of issued oncology diagnosis and treatment cards (ODaTCs) from January to September in the years 2019 and 2020 | Absolute number of issued ODaTCs: 651 (2019) vs. 705 (2020). | 5 | |
| 7 | (Medenwald et al., 2022) | Germany | Patient-level claims data from fourteen university hospitals | 9365 inpatient hospital admissions | Number of radiotherapy fractions (primary outcome) and inpatient hospital admissions (secondary outcome) |
The lockdown period (from March 16 to April 28, 2020), radiotherapeutic fractions decreased by 20.0% (1232 to 1539.5, p-value <0.001) in the study cohort compared to the control cohort (2018 and 2019). Megavoltage radiation therapy decreased by 29.5% (660 to 936.5, p-value <0.001), whereas no change was observed for brachytherapy-related fractions (164 to 163, p-value ≥0.05). Within the return-to-normal period (from May 4 to August 2, 2020), the reduction in overall radiotherapeutic fractions was 28.6% (829 to 1160.5, p-value <0.001), whereas megavoltage radiation therapy fractions decreased by 43.7% (411 to 730.5, p-value <0.001) and brachytherapy fractions even increased by 15.0% (123 to 107, p-value ≥0.05;) in 2020 in comparison with the control cohort. Within the lockdown period, overall hospital admissions for cervical cancer fell by 10.2% (352 to 392, p-value >0.05;) in the study cohort compared to the control cohort, whereas a reduction of 22.1% (218 to 280, p-value <0.01) was observed in the return-to-normal period. Radiotherapy admissions without brachytherapy were reduced by 23.9% (167 to 219.5, p-value <0.05), whereas admissions with chemotherapy procedures were reduced by 12.5% (126 to 144, p-value ≥ 0.05), and brachytherapy-related admissions were reduced by 5.0% (85 to 89.5, p-value ≥ 0.05) within the lockdown period in 2020 compared with the control cohort. In contrast, admissions with surgery-related procedures increased non-significantly by 20.5% (100 to 83, p-value ≥ 0.05) in the same period. For the return-to-normal period, radiotherapy admissions without brachytherapy decreased by 44.9% (92 to 167, p-value <0.001;), which was also true for admission with radio- and chemotherapy (37.1%; 66 to 105, p-value <0.01;). In contrast, radiotherapeutic admissions with brachytherapy increased by 14.8% (70 to 61;) and admissions with surgical procedures increased by 7.7% (56 to 52, p-value ≥0.05) in the same period compared with the control cohort. | 6 |
| 8 | (Meggetto et al., 2021) | Canada | Multiple population-based administrative databases | NR | Monthly cervical treatment volumes | The average monthly decrease in cervical treatment volumes was 31.1% or 288 cervical treatments, compared with the same months in 2019. | 7 |
Abbreviations: aNOS, adapted version of the Newcastle-Ottawa Scale checklist for assessing the quality of non-randomized studies; NR, not reported; NA, not applicable; 95% CI 95% confidence interval; CT, chemotherapy; RT, radiotherapy; LEETZ, Large Loop Excision of the Transformation Zone.
4. Discussion
This is, to the best of our knowledge, the first systematic review that summarizes the current body of evidence about the COVID-19 impact on the care of cervical cancer, with the main goal of comparing service access and care delivery before and during the pandemic.
Although differences in design and setting across the retrieved studies did not allow to provide synthesis measures of this impact, results revealed that COVID-19-related health service reductions have significantly inhibited cervical cancer prevention, diagnosis and treatment, particularly in the very first pandemic months of 2020, which coincided with an important interruption of non-urgent health services (Walker et al., 2021; Conti et al., 2020a; Balasco et al., 2021). More in general, the wide impact on healthcare systems and organizations due to the spread of SARS-CoV-2 have been well-described elsewhere (Ferrara and Albano, 2020; Voza et al., 2021; Sabbatucci et al., 2022; Conti et al., 2020a). Major reasons are excessive hospital overload and high shortage of healthcare resources, workload of professionals and their task-shifting to ensure the care of COVID-19 and acute life-threatening conditions, as well as internal rearrangements of their routine activities and closure of certain services, which resulted in a critical amount of patients referred during the pandemic (Ferrara and Albano, 2020; Voza et al., 2021; Acuti Martellucci et al., 2021; Viganò et al., 2020; Conti et al., 2020b; Della Valle et al., 2021), Yet, healthcare users' and patients' fear of contagion and long quarantine due to COVID-19 might have contributed to less usage of preventive health services and facilities (Wilson et al., 2021; Mantica et al., 2020; Antonazzo et al., 2022; Bittleston et al., 2022).
With regards to HPV vaccination, the routine immunization services had significant disruptions amid the COVID-19 pandemic and social distancing measures, with drops in immunization coverage that depended on vaccines, contexts and populations studied (Ramírez et al., 2022; Sabbatucci et al., 2022). This review found that the pandemic led to declines in the administration of HPV vaccines, although data vary greatly from context to context. Beyond the crude prevalence of vaccination uptake and coverage during COVID-19, it should be remarked that vaccination of adolescent girls (and other at-risk individuals) is the most effective long-term intervention for reducing the risk of cervical cancer (World Health Organization, 2020), and it is well-recognized that even small decrease in vaccination rates could have significant long-term public health and economic consequences attributable to burden of preventable diseases (Lo and Hotez, 2017). Continued organizational efforts are therefore required to reach at-risk population (particularly young people) to protect from HPV infection (World Health Organization, 2020; Gabutti et al., 2021; Sabbatucci et al., 2022). It is worth also noting that infection with HPV is a necessary – although not sufficient – cause of human cancer other than to cervix, including carcinoma of the larynx, oropharynx and oral cavity, as well as vulvar, vaginal, anal, and penile tumors (Ferrara et al., 2020a; Ferrara et al., 2020b).
Of particular concern is the lower percentage of screening and diagnosis preneoplastic/neoplastic lesions of the cervix during the COVID-19 pandemic highlighted in this literature review, along with worrisome decreases of screening test performed compared to non-pandemic periods. Results showed huge variations in the extent of the reported reductions, which were likely patterned by screening policies and intensity and types of COVID-19 responses across the different contexts, as well as by study's type, setting and population. In some cases, screening participation was found to remain low despite the efforts made to address the backlog attributable to lockdown measures in the late phase of the pandemic (Acuti Martellucci et al., 2021; Kim et al., 2022; Miller et al., 2021). This may be due either to a decrease in cervical screening addressability or to a tendency to access the medical system, explained by the fear of not being infected with SARS-CoV-2 virus (Istrate-Ofițeru et al., 2021; Wilson et al., 2021).
This systematic review also reveals an evident pandemic deficit in the number of diagnoses and diagnostic procedures, compared with pre-COVID-19 period, as well as a decreased number of patients admitted with cervical intraepithelial neoplasia (Istrate-Ofițeru et al., 2021; Ivanuš et al., 2021). Again, a higher number of advanced stages at the new diagnosis or particularly long intervals from tumor biopsy to the first cancer center were also seen in the post pandemic period (Bonadio et al., 2021). Of note, the fundamental aims of cervical screening and early diagnosis is to reduce the burden and subsequent mortality from invasive cervical cancer (Peirson et al., 2013). These came in addition to reduced adherence to surgical treatments and chemo- and radio-therapies for patients diagnosed with high-grade dysplasia (Davies et al., 2022; Ivanuš et al., 2021), even in those contexts without suspension of cancer screening during the corona lockdown (Medenwald et al., 2022). Indeed, the COVID-19 emergency and related responses have negatively impacted on several components of cervical cancer prevention and care, a type of tumor in which no delays of diagnosis and treatment can be accepted due to the rapidity of its proliferation (Medenwald et al., 2022).
In brief, our systematic review provides context to strengthen the health services response to meet cervical cancer patients' needs, as well as to promote health education initiatives tending to address women's awareness and attitudes towards HPV vaccination and cervical screening. In this frame, more research is needed to understand the exact extent of COVID-19 impact on cervical cancer diagnosis and management, including the potentially damaging effects of the screening program pause and delays in diagnosis on patients' survival (Sud et al., 2020; Smith et al., 2021; Burger et al., 2021). For instance, recent analyses fitted provisional models to predict the excess of cases caused by pauses in cervical cancer care over the next years, advocating the build of healthcare extra capacity to ensure patients' access to screening programs and cancer therapies before their disease progresses to advanced disease stages (Davies et al., 2022; Castanon et al., 2021). Furthermore, other modeling studies have suggested that 6- to 12-month disruptions to screening may result in only nominal changes in cervical cancer burden (Smith et al., 2021; Burger et al., 2021). It is also worth mentioning that reduction of new diagnoses, although temporary, results in shift towards higher stage at diagnosis and thus and an increased healthcare and social cancer burden in the next years (Maringe et al., 2020; Cantini et al., 2022).
The mentioned WHO strategy for the elimination cervical cancer as a public health problem fixed a 90–70–90 target, which specifically refers to 90% of girls fully vaccinated with HPV vaccine by age 15 years; 70% of women screened with a high-performance test by 35 years of age and again by 45 years of age; 90% of women identified with cervical disease receive treatment (90% of women with precancer treated, and 90% of women with invasive cancer managed) (World Health Organization, 2020). According to the studies here summarized, COVID-19 strongly inhibits the attainment of these goals – which were assumed to be achieved by 2030 –, and the mentioned global public health efforts and urgent policy interventions are needed to create an innovative pipeline for recovering cervical cancer care from prevention to treatment, particularly through additional HPV vaccination and cervical screening campaigns. In doing so, health services research should further analyze local experiences which have successfully addressed lockdown backlog by virtue of prompt adaptation of services and reorganization of obstetrician activities to minimize COVID-19 impact. These experiences may serve as reference model for the implementation of sustainable and effective changes in other on sexual and reproductive healthcare contexts (Acuti Martellucci et al., 2022; Acuti Martellucci et al., 2021; Campbell et al., 2021).
Some limitations must be considered in this systematic review. First, despite being systematic in nature, the search strategy was limited to literature databases and did not include surveillance reports. However, the assessment of the evidence was in line with the minimum requirements (at least two databases) set by the PRISMA guidelines (Page et al., 2021), and collected the most updated available studies daily on COVID-19. Moreover, cross-referencing of the citation list was also consulted in order to collect and analyze all the available evidence. Nevertheless, at the time of study, evidence about this topic is still relatively sparse and the literature so far available does not allow us to consider the exact extent of the pandemic-related service reduction and its consequences. For these reasons and due to limited quality of data and reporting, these findings should be interpreted with caution, and require further exploration in studies specifically designed to examine the long-term effects of COVID-19 on cervical cancer prevention, diagnosis and treatment. Lastly, disparity across the included reports regarding data types and sources (e.g., administrative data, survey-based self-reported data, individual-level medical data, etc.) makes difficult drawing definite conclusions of this qualitative analysis. Despite the listed limitations, the present study is a comprehensive synthesis of these topics, providing important insights for public health and policymakers.
5. Conclusion
In conclusion, this systematic review provides context to highlight how the prevention, diagnosis and treatment of cervical cancer suffered from the consequences of COVID-19 pandemic. Our results offer actionable metrics of this impact, which could be used to develop health services response, and call for more resilient and sustainable targeted interventions aimed at guaranteeing the access to quality healthcare and prevention to women, as well as at meeting the WHO targets for the elimination of cervical cancer among the major public health problems.
Funding
This research received no external funding.
CRediT authorship contribution statement
Pietro Ferrara: Conceptualization, Methodology, Data curation, Project administration, Validation, Writing – original draft, Writing – review & editing. Giulia Dallagiacoma: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing. Federica Alberti: Data curation, Formal analysis, Investigation, Writing – review & editing. Leandro Gentile: Investigation, Visualization, Writing – review & editing. Paola Bertuccio: Investigation, Validation, Visualization, Writing – review & editing. Anna Odone: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2022.107264.
Appendix A. Supplementary data
Supplementary material.
Data availability
Data will be made available on request.
References
- Acuti Martellucci C., Morettini M., Flacco M.E., et al. Delivering cervical cancer screening during the COVID-19 emergency. BMJ Sex Reprod. Health. 2021;47(4):296–299. doi: 10.1136/bmjsrh-2021-201099. [DOI] [PubMed] [Google Scholar]
- Acuti Martellucci C., Morettini M., Brotherton J.M., et al. Impact of a human papillomavirus vaccination programme within organized cervical cancer screening: cohort study. Cancer Epidemiol. Biomarkers Prev. 2022 doi: 10.1158/1055-9965.EPI-21-0895. Published online January 13. cebp.0895.2021. [DOI] [PubMed] [Google Scholar]
- Altın D., Yalçın İ., Khatib G., et al. Management of gynecological cancers in the COVID-19 era: a survey from Turkey. J. Turk. Ger. Gynecol. Assoc. 2020;21(4):265–271. doi: 10.4274/jtgga.galenos.2020.2020.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonazzo I.C., Fornari C., Maumus-Robert S., et al. Antidepressants drug use during COVID-19 waves in the Tuscan general population: an interrupted time-series analysis. J. Pers. Med. 2022;12(2):178. doi: 10.3390/jpm12020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasco N., d’Alessandro V., Ferrara P., Smaldone G., Vitagliano L. Analysis of the time evolution of COVID-19 lethality during the first epidemic wave in Italy. Acta Biomed. Atenei Parm. 2021;92(2) doi: 10.23750/abm.v92i2.11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittleston H., Goller J.L., Temple-Smith M., Hocking J.S., Coombe J. “I didn’t want to visit a doctor unless it was extremely necessary”: perspectives on delaying access to sexual and reproductive health care during the COVID-19 pandemic in Australia from an online survey. Aust. J. Prim. Health. 2022 doi: 10.1071/PY21239. Published online February 3. [DOI] [PubMed] [Google Scholar]
- Bonadio R.C., Messias A.P., Moreira O.A., et al. Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil. ecancermedicalscience. 2021;15 doi: 10.3332/ecancer.2021.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger E.A., Jansen E.E., Killen J., et al. Impact of COVID-19-related care disruptions on cervical cancer screening in the United States. J. Med. Screen. 2021;28(2):213–216. doi: 10.1177/09691413211001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.J., Barlow-Evans R., Jewell S., Woodhead N., Singh R., Jaffer K. ‘Our COVID-19 cloud silver lining’: the initiation and progress of postnatal contraception services during the COVID-19 pandemic in a UK maternity hospital. BMJ Sex Reprod. Health. 2021;47(3):224–227. doi: 10.1136/bmjsrh-2020-200764. [DOI] [PubMed] [Google Scholar]
- Cantini L., Mentrasti G., Russo G.L., et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): fewer cases and higher stages from a real-world scenario. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey S.M., Jansen E., Drainoni M.L., Schuch T.J., Leschly K.S., Perkins R.B. Long-term multilevel intervention impact on human papillomavirus vaccination rates spanning the COVID-19 pandemic. J. Low Genit. Tract Dis. 2022;26(1):13–19. doi: 10.1097/LGT.0000000000000648. [DOI] [PubMed] [Google Scholar]
- Castanon A., Rebolj M., Pesola F., Sasieni P. Recovery strategies following COVID-19 disruption to cervical cancer screening and their impact on excess diagnoses. Br. J. Cancer. 2021;124(8):1361–1365. doi: 10.1038/s41416-021-01275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S., Ferrara P., Mazzaglia G., et al. Magnitude and time-course of excess mortality during COVID-19 outbreak: population-based empirical evidence from highly impacted provinces in northern Italy. ERJ Open Res. 2020;6(3):00458–02020. doi: 10.1183/23120541.00458-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S., Ferrara P., Fornari C., et al. Estimates of the initial impact of the COVID-19 epidemic on overall mortality: evidence from Italy. ERJ Open Res. 2020;6(2):00179–02020. doi: 10.1183/23120541.00179-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels V., Saxena K., Roberts C., et al. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: a model based analysis. Vaccine. 2021;39(20):2731–2735. doi: 10.1016/j.vaccine.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J.M., Spencer A., Macdonald S., et al. Cervical cancer and COVID —an assessment of the initial effect of the pandemic and subsequent projection of impact for women in England: a cohort study. BJOG Int. J. Obstet. Gynaecol. 2022 doi: 10.1111/1471-0528.17098. Published online February 6. 1471-0528.17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pelsemaeker M.C., Guiot Y., Vanderveken J., Galant C., Van Bockstal M.R. The impact of the COVID-19 pandemic and the associated Belgian governmental measures on cancer screening, surgical pathology and cytopathology. Pathobiology. 2021;88(1):46–55. doi: 10.1159/000509546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroff A., Miller J., Sharma K., et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January–June 2020, in the United States. Prev. Med. 2021;151 doi: 10.1016/j.ypmed.2021.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Valle P., Fabbri M., Madotto F., et al. Occupational exposure in the Lombardy Region (Italy) to SARS-CoV-2 infection: results from the MUSTANG–OCCUPATION–COVID-19 study. Int. J. Environ. Res. Public Health. 2021;18(5):2567. doi: 10.3390/ijerph18052567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dema E., Gibbs J., Clifton S., et al. Initial impacts of the COVID-19 pandemic on sexual and reproductive health service use and unmet need in Britain: findings from a quasi-representative survey (Natsal-COVID) Lancet Public Health. 2022;7(1):e36–e47. doi: 10.1016/S2468-2667(21)00253-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis L.K., Hsu C.H., Arrington A.K. Reduction in standard cancer screening in 2020 throughout the U.S. Cancers. 2021;13(23) doi: 10.3390/cancers13235918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desta A.A., Woldearegay T.W., Gebremeskel E., et al. Impacts of COVID-19 on essential health services in Tigray, northern Ethiopia: a pre-post study. Pastakia SD, ed. PLoS ONE. 2021;16(8):e0256330. doi: 10.1371/journal.pone.0256330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubova S.V., Leslie H.H., Kruk M.E., Pérez-Cuevas R., Arsenault C. Disruption in essential health services in Mexico during COVID-19: an interrupted time series analysis of health information system data. BMJ Glob. Health. 2021;6(9) doi: 10.1136/bmjgh-2021-006204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara P., Albano L. COVID-19 and healthcare systems: what should we do next? Public Health. 2020;185:1–2. doi: 10.1016/j.puhe.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara P., Conti S., Agüero F., et al. Estimates of cancer mortality attributable to carcinogenic infections in Italy. Int. J. Environ. Res. Public Health. 2020;17(23):8723. doi: 10.3390/ijerph17238723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara P., Agüero F., Masuet-Aumatell C., Ramon-Torrell J.M. Burden of cancer mortality attributable to carcinogenic infections in Spain. Med. Clín. 2020;154(10):394–397. doi: 10.1016/j.medcli.2019.11.005. [DOI] [PubMed] [Google Scholar]
- Gabutti G., d’Anchera E., De Motoli F., Savio M., Stefanati A. Human Papilloma Virus vaccination: focus on the Italian situation. Vaccines. 2021;9(12):1374. doi: 10.3390/vaccines9121374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin S.N.S., Jimbo M., Heizelman R., Harmes K.M., Harper D.M. The future of cancer screening after COVID-19 may be at home. Cancer. 2021;127(4):498–503. doi: 10.1002/cncr.33274. [DOI] [PubMed] [Google Scholar]
- Hathout L., Ennis R.D., Mattes M.D., et al. The impact of COVID-19 on brachytherapy during the pandemic: a Rutgers-Robert Wood Johnson Barnabas health multisite experience. Adv. Radiat. Oncol. 2021;6(1) doi: 10.1016/j.adro.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istrate-Ofițeru A.M., Berbecaru E.I.A., Ruican D., et al. The influence of SARS-CoV-2 pandemic in the diagnosis and treatment of cervical dysplasia. Med. (Mex). 2021;57(10):1101. doi: 10.3390/medicina57101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanuš U., Jerman T., Gašper Oblak U., et al. The impact of the COVID-19 pandemic on organised cervical cancer screening: the first results of the Slovenian cervical screening programme and registry. Lancet Reg. Health - Eur. 2021;5 doi: 10.1016/j.lanepe.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Kojima N., Vangala S., et al. Impact of COVID-19 on primary care quality measures in an academic integrated health system. J. Gen. Intern. Med. 2022 doi: 10.1007/s11606-021-07193-7. Published online January 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczkodaj P., Sulkowsa U., Kaminski M. SARS-CoV-2 as a new possible long-lasting determining factor impacting cancer death numbers. Based on the example of breast, colorectal and cervical cancer in Poland. Nowotw. J Oncol. 2021;71(1):42–46. doi: 10.5603/NJO.2021.0007. [DOI] [Google Scholar]
- Laing S., Johnston S. Estimated impact of COVID-19 on preventive care service delivery: an observational cohort study. BMC Health Serv. Res. 2021;21(1):1107. doi: 10.1186/s12913-021-07131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Cao Y., Fan J., et al. Impact of COVID-19 pandemic on the clinical activities in obstetrics and gynecology: a national survey in China. Front. Med. 2021;8 doi: 10.3389/fmed.2021.633477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo N.C., Hotez P.J. Public health and economic consequences of vaccine hesitancy for measles in the United States. JAMA Pediatr. 2017;171(9):887. doi: 10.1001/jamapediatrics.2017.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Fullman N., Mumford J.E., et al. Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1250–1284. doi: 10.1016/S0140-6736(20)30750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantellini P., Battisti F., Armaroli P., et al. Ritardi maturati dai programmi di screening oncologici ai tempi del COVID-19 in Italia, velocita della ripartenza e stima dei possibili ritardi diagnostici. Epidemiol. Prev. 2020;44(5–6 Suppl 2):344–352. doi: 10.19191/EP20.5-6.S2.136. [DOI] [PubMed] [Google Scholar]
- Mantica G., Riccardi N., Terrone C., Gratarola A. Non-COVID-19 visits to emergency departments during the pandemic: the impact of fear. Public Health. 2020;183:40–41. doi: 10.1016/j.puhe.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matenge S., Sturgiss E., Desborough J., Hall Dykgraaf S., Dut G., Kidd M. Ensuring the continuation of routine primary care during the COVID-19 pandemic: a review of the international literature. Fam. Pract. 2021 doi: 10.1093/fampra/cmab115. Published online October 6. cmab115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medenwald D., Brunner T., Christiansen H., et al. Shift of radiotherapy use during the first wave of the COVID-19 pandemic? An analysis of German inpatient data. Strahlenther Onkol. 2022 doi: 10.1007/s00066-021-01883-1. Published online January 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggetto O., Jembere N., Gao J., et al. The impact of the COVID-19 pandemic on the Ontario Cervical Screening Program, colposcopy and treatment services in Ontario, Canada: a population-based study. BJOG Int. J. Obstet. Gynaecol. 2021;128(9):1503–1510. doi: 10.1111/1471-0528.16741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Xu L., Qin J., et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21–65 years in a large integrated health care system — Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70(4):109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais S., Antunes L., Rodrigues J., Fontes F., Bento M.J., Lunet N. The impact of the coronavirus disease 2019 pandemic on the diagnosis and treatment of cancer in Northern Portugal. Eur. J. Cancer Prev. 2021 doi: 10.1097/CEJ.0000000000000686. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogami Y., Makabe T., Komatsu H., et al. Impact of COVID-19 on cervical cancer screening in Japan: a survey of population-based screening in urban Japan by the Japan Society of Gynecologic Oncology. J. Obstet. Gynaecol. Res. 2022;48(3):757–765. doi: 10.1111/jog.15130. [DOI] [PubMed] [Google Scholar]
- Odone A., Delmonte D., Scognamiglio T., Signorelli C. COVID-19 deaths in Lombardy, Italy: data in context. Lancet Public Health. 2020;5(6) doi: 10.1016/S2468-2667(20)30099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A.P., Gierbolini-Bermúdez A., Ramos-Cartagena J.M., et al. Cervical cancer screening among medicaid patients during natural disasters and the COVID-19 pandemic in Puerto Rico, 2016 to 2020. JAMA Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. Published online March 29. n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson L., Fitzpatrick-Lewis D., Ciliska D., Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst. Rev. 2013;2(1):35. doi: 10.1186/2046-4053-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez M., Fuente J., Andía D., José Hernández J., Fiol G., Torné A. HPV vaccination coverage in women between 15 and 55 years old in Spain: temporal trend during the period 2007–2020. Int. J. Gynecol. Obstet. 2022 doi: 10.1002/ijgo.14067. Published online January 7. ijgo.14067. [DOI] [PubMed] [Google Scholar]
- Sabbatucci M., Odone A., Signorelli C., et al. Childhood immunisation coverage during the COVID-19 epidemic in Italy. Vaccines. 2022;10(1):120. doi: 10.3390/vaccines10010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena K., Marden J.R., Carias C., et al. Impact of the COVID-19 pandemic on adolescent vaccinations: projected time to reverse deficits in routine adolescent vaccination in the United States. Curr. Med. Res. Opin. 2021;37(12):2077–2087. doi: 10.1080/03007995.2021.1981842. [DOI] [PubMed] [Google Scholar]
- Signorelli C., Odone A., Ciorba V., et al. Human papillomavirus 9-valent vaccine for cancer prevention: a systematic review of the available evidence. Epidemiol. Infect. 2017;145(10):1962–1982. doi: 10.1017/S0950268817000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.A., Burger E.A., Castanon A., et al. Impact of disruptions and recovery for established cervical screening programs across a range of high-income country program designs, using COVID-19 as an example: a modelled analysis. Prev. Med. 2021;151 doi: 10.1016/j.ypmed.2021.106623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud A., Torr B., Jones M.E., et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Van Wyk A.C., De Jager L.J., Razack R., et al. The initial impact of the COVID-19 pandemic on the diagnosis of new cancers at a large pathology laboratory in the public health sector, Western Cape Province, South Africa. S. Afr. Med. J. Suid-Afr. Tydskr Vir Geneeskd. 2021;111(6):570–574. [PubMed] [Google Scholar]
- Viganò M., Voza A., Harari S., et al. Letter to the editor: clinical management of nonrespiratory diseases in the COVID-19 pandemic: what have we done and what needs to be done? Telemed. E-Health. 2020;26(10):1206–1208. doi: 10.1089/tmj.2020.0148. [DOI] [PubMed] [Google Scholar]
- Voza A., Desai A., Luzzi S., et al. Clinical outcomes in the second versus first pandemic wave in Italy: impact of hospital changes and reorganization. Appl. Sci. 2021;11(19):9342. doi: 10.3390/app11199342. [DOI] [Google Scholar]
- Walker M.J., Meggetto O., Gao J., et al. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: a provincial, population-based study. Prev. Med. 2021;151 doi: 10.1016/j.ypmed.2021.106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G.A., Shea B., O’Connell D., Paterson J., Welch V., Losos M. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Published online. 2014 http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- Wilson R., Quinn-Scoggins H., Moriarty Y., et al. Intentions to participate in cervical and colorectal cancer screening during the COVID-19 pandemic: a mixed-methods study. Prev. Med. 2021;153 doi: 10.1016/j.ypmed.2021.106826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem.https://apps.who.int/iris/handle/10665/336583 Accessed March 9, 2022. [Google Scholar]
- Zhao M., Wu Q., Hao Y., et al. Global, regional, and national burden of cervical cancer for 195 countries and territories, 2007–2017: findings from the Global Burden of Disease Study 2017. BMC Womens Health. 2021;21(1):419. doi: 10.1186/s12905-021-01571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.