Abstract
Objectives
To evaluate humoral responses to three doses of the inactivated SARS-CoV-2 vaccine (CoronaVac) in patients with spondyloarthritis (SpA) and the effect of therapy, compared with a control group (CG).
Methods
Prospective cohort of axial SpA/psoriatic arthritis patients and age/sex-balanced CG from the CoronavRheum phase 4 trial (NCT04754698). CoronaVac was given in two doses (28-days interval) with a booster at day 210. Blood samples were collected in the days 0/28 (D28)/69 (D69) and 240 (D240) to evaluate anti-SARS-CoV-2 IgG seropositivity (SP) and neutralising antibodies (NAb).
Results
One hundred and ninety-four SpA patients were enrolled and 183 patients were age/sex-balanced with 183 CG. At D69, SpA patients showed a high SP (80.2% vs. 95.7%, P < 0.001) and moderate NAb positivity (61.6% vs. 82.7%, P < 0.001), but lower than CG. In patients, older age prednisone (P < 0.001), methotrexate (MTX) (P < 0.001) and TNF inhibitors (TNFi) (P < 0.001) were independently associated with lower SP, while Caucasian ethnicity (P < 0.05) and prednisone (P < 0.01) were associated with diminished NAb. In contrast, sulfasalazine (SSZ) use was associated with NAb presence (P < 0.05). In monotherapy, only TNFi was also associated with absence of SP (P < 0.05). Further comparison with CG revealed that TNFi and/or MTX negatively impacted SP/NAb (P < 0.05). In contrast, patients under SSZ monotherapy achieved 100% SP (P > 0.999) and 83.3% NAb positivity (P > 0.999). SSZ + TNFi combination resulted in a similar response than CG [SP (P = 0.153) and NAb (P = 0.715)]. After third dose (D69–D240), a major increment occurred for SP (81.3% to 93.1%, P < 0.001) and NAb (63.2% to 86.1%, P < 0.001), but still lower than CG (P < 0.05), and only TNFi impaired both SP (P = 0.016)/NAb (P = 0.002).
Conclusions
We provided novel data demonstrating that TNFi attenuates immunogenicity in SpA patients while SSZ has a positive impact on vaccine antibody production. We also confirmed that MTX in combination with TNFi had a major negative impact in vaccine humoral response (CoronavRheum clinicaltrials.gov #NCT04754698).
Keywords: Ankylosing spondylitis, Psoriatic arthritis, COVID-19, Vaccination, Therapeutics and TNFi
1. Introduction
In the context of the severe acute respiratory syndrome–Coronavirus–2 (SARS-CoV-2) pandemic, which has already killed millions of people around the world in the last two years, the development of a safe and effective vaccine has been a global priority [1].
Autoimmune rheumatic diseases (ARD) patients have a high risk of infection and severe SARS-CoV-2 infection [2]. Several reports have demonstrated SARS-CoV-2 vaccine safety and effectiveness in ARD patients including different platforms [3], [4], [5]. With regard to the CoronaVac inactivated vaccine, it is currently recommended by the World Health Organization (WHO) and has been used in several countries around the world, including three of the six most populated ones (Brazil, China and Turkey) [1]. Its effectiveness was demonstrated in an elegant study from Chile with 10.2 million people [6]. Our group demonstrated in 910 ARD patients a moderate immunogenicity with a significant reduction in incident cases and an excellent safety profile [3]. We further observed in this population an immunogenicity decay of approximately 24% in seropositivity after 6 months of the regular 2-dose regimen [7], and a marked response after booster dose response [8].
Recently, many studies showed that anti-SARS-CoV-2 immunogenicity in ARD patients was affected by age and specific DMARD (disease modifying antirheumatic drugs) [3], [4], [7], [8], [9], [10], [11]. However, no study has specifically focused only on spondyloarthritis (SpA). Such patients more frequently use drugs in monotherapy or without association with corticosteroids, avoiding the interference of these confounding variables in immunogenicity [4], [9], [12], [13], [14], [15]. Moreover, the profile of DMARD in SpA population is unique. A significant amount of subjects are under sulfasalazine, that was previously reported as beneficial in inflammatory bowel diseases (IBD) patients vaccinated with anti-SARS-CoV-2 mRNA or viral-vector vaccines [16] and were underrepresented is ARD vaccine studies. Regarding biological DMARD (bDMARD), data on tumor necrosis factor inhibitors (TNFi) are still controversial, since some studies show an impairment of vaccine response in IBD [16], [17], while, in general ARD population, this effect is considered minor when compared to other bDMARD, such as rituximab and abatacept [3], [4], [18]. Moreover, anti-IL17 bDMARD was also underrepresented in vaccine studies.
Therefore, the objective of the present study was to evaluate humoral immune responses to three doses of the homologous schedule of the inactivated anti-SARS-CoV-2 vaccine (CoronaVac) in SpA patients taking synthetic DMARDs and commonly targeted biological therapy, compared with a control group (CG).
2. Methods
2.1. Study Design and vaccination protocol
This prospective observational cohort study was conducted at a single tertiary center in Brazil. Patients with SpA were paired by sex and age with a CG without ARD and both groups were vaccinated with inactivated SARS-CoV-2 vaccine (CoronaVac), which does not include recombinant mRNA technology, such as BNT162b2 (Pfizer-BioNtech) and CX-024414 (Moderna), or viral vectored platforms, such as ChAdOx1 nCoV-19 (AstraZeneca) or Ad.26.COV2.S (Janssen). Each dose consisted of 3 μg in 0.5 mL of β-propiolactone inactivated SARS-CoV-2 (derived from the CN02 strain of SARS-CoV-2 grown in African green monkey kidney cells—Vero 25 cells), with aluminum hydroxide as adjuvant.
The vaccination protocol consisted of a 28-day appart two-dose schedule of the inactivated SARS-CoV-2 vaccine CoronaVac (batch #20200412; Sinovac Life Sciences, Beijing, China). Both doses were applied at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. Vaccinated subjects were assigned to blood collection at baseline (D0), immediately before the second dose (D28), and 69 days (D69) from baseline.
Participants were also invited to a study extended phase and received a third dose of CoronaVac at D210 (210 days from baseline) and had their humoral response re-evaluated 240 days from baseline (D240).
Patients were invited to participate after the review of their electronic records.
2.2. Ethical statement
The study was performed in accordance with the principles of the Declaration of Helsinki and approved by the National (Comissão Nacional de Ética em Pesquisa – CONEP) and Institutional Ethical Committee (Comissão de Ética para Análise de Projetos de Pesquisa – CAPPesq) of Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Brazil (ID CAAE: 42566621.0.0000.0068). Written informed consent was obtained from all participants.
2.3. Inclusion criteria
Adult (age ≥ 18 years old) SpA patients, including axSpA (non-radiographic SpA and Ankylosing Spondylitis) and psoriatic arthritis, with regular follow-up at the Outpatient Clinics of Rheumatology Division (Hospital das Clinicas, Faculdade de Medicina, Universidade de São Paulo, Brazil).
The control group (CG) consisted of subjects without ARD or immunosupression among the maintenance/administrative hospital workers and relatives, selected in a 1:1 ratio (1 SpA patient:1 control) to have comparable sex and age (≤ 5 years difference), with groups assortment made by an Excel program for random selection of individuals.
2.4. Exclusion criteria
Patients with any of the following criteria were excluded: history of anaphylactic reaction to the vaccine components, acute febrile illness, Guillain–Barré syndrome, demyelinating disease, cardiac insufficiency (classes III/IV), history of live virus vaccination 4 weeks prior to the study, or inactivated virus vaccination 2 weeks prior to the study, history of hemoderivatives transfusion 6 months prior to the study, individuals that refused to participate, and hospitalized patients.
2.5. Immunogenicity assessment
The co-primary outcomes were the presence of anti-SARS-CoV-2 S1/S2 proteins IgG and the presence of NAb at D69 and D240. The secondary outcomes were geometric mean titres (GMT) of anti-S1/S2 IgG and neutralising activity of NAb at D69 and D240. The influence of demographic data, SpA diagnosis and current therapy was also assessed.
IgG antibodies against the SARS-CoV-2 S1/S2 proteins were checked by a chemiluminescent immunoassay (indirect ELISA; ETI-MAX 3000 equipment, LIAISON SARS-CoV-2 S1/S2 IgG Kit, DiaSorin, Italy). Seroprotection (SP) was defined as positive serology (≥ 15.0 UA/mL). GMT (95% CIs) were calculated attributing the value of 1.9 UA/mL (half of the lower limit of quantification 3.8 AU/mL) to undetectable levels (< 3.8 UA/mL).
Circulating NAb were detected by the SARS-CoV-2 sVNT Kit (GenScript, Piscataway, New Jersey, USA). Positivity was defined as ≥ 30% inhibition of the linkage between the receptor-binding domain of the viral spike glycoprotein with the ACE2 cell surface receptor. Mean (SD) of the percentage of neutralising activity were only calculated considering positive samples at D28, D69 and D240.
2.6. Medication and disease activity
Demographic data, disease activity status before vaccination, current medications and associated comorbidities of SpA patients were assessed by electronic chart review.
2.7. Safety assessment
Patients and controls were oriented about possible side effects of the vaccine. The standard diary of side effects was evaluated by the researchers after the first, second and third doses. This diary included orientations about phone communication in case of whichever effects that demanded medical care. If tertiary care was required, the participant was transferred to the Hospital das Clínicas by the vacancies control. Serious adverse effects were defined as those that resulted in hospitalization or death.
2.8. Sample size calculation and statistical analysis
Sample size calculation was based on the previous reduction of 16% of seroconversion rate after influenza A/H1N1 vaccination in a large cohort of patients [15]. Considering an alpha error of 5%, with power of 80%, and expecting a seropositivity rate of 58% in SpA patients, and 74% in CG, the minimum sample required was 136 SpA patients and 136 controls, paired by age and sex.
Categorical variables were presented as number (percentage) and compared using χ2 or Fisher's exact tests, as appropriated. Continuous general data were presented as mean (± standard deviation – SD) and compared using Student's t-test, Mann–Whitney U test (two groups) or one-way analysis of variance or Kruskal–Wallis test, as appropriated. For SpA patients, multivariate logistic regression analyses were performed with SP or the presence of NAb at D69 as dependent variables, and variables with P < 0.200 in each univariate analysis as independent variables. Subgroup analyses, including only patients with no DMARD or with DMARD monotherapy, were also performed with the same parameters. Comparisons of IgG titres were assessed as Napierian logarithm(ln)-transformed data, using generalized estimating equations (GEE) with normal marginal distribution and gamma distribution, respectively, and identity binding function, assuming first-order autoregressive correlation matrix between moments, followed by Bonferroni's multiple comparisons between groups (overall patients with SpA and CG) and time points (D0, D28 and D69 for the regular 2-dose schedule, or D69 and D240 for patients who received the 3rd dose). Statistical significance was defined as P < 0.05. All statistical analyses were performed using SPSS, V.20.0 (IBM-SPSS for Windows V.20.0).
3. Results
This was a subanalysis of a prospective, single center, controlled phase 4 study (CoronavRheum, clinicaltrials.gov). For the original study, a total of 315 SpA patients were invited to participate. Forty-eight patients were excluded: not accepted to participate (n = 34), previously vaccinated in national protocol (n = 9), patients with past history of anaphylaxis to vaccine components (n = 4), and hospitalisation (n = 1). The remaining 267 SpA patients were vaccinated in the study. Sixty-four patients were excluded from immunogenicity analysis due to baseline positive IgG and/or NAb against SARS-CoV-2. A CG with comparable sex frequency and age (≤ 5-year difference) was randomly selected from the original sample using an Excel program (1:1), comprising a total of 183 patients matched with 183 controls (Figure S1).
SpA patients and CG were balanced for age [51.2 (12.03) vs. 51.2 (12.3) years, non-significant] and female sex (40.4% vs. 40.4%, non-significant), but there were more Caucasian patients in SpA group compared with CG (62.3% vs. 43.7%, P < 0.001). Some comorbidities and risk factors for severe COVID-19 were more common in SpA patients than in CG (P < 0.05) (Table 1 ).
Table 1.
Baseline characteristics of spondyloarthritis (SpA) patients and control group (CG).
| SpA (n = 183) |
CG (n = 183) |
|
|---|---|---|
| Demographics | ||
| Current age, years | 51.2 (12.0) | 51.2 (12.3) |
| Female, sex | 74 (40.4) | 74 (40.4) |
| Caucasian ethnicity | 114 (62.3)b | 80 (43.7) |
| Comorbidities | ||
| Systemic arterial hypertension | 80 (43.7)a | 53 (29.0) |
| Diabetes mellitus | 27 (14.8) | 29 (15.8) |
| Dyslipidemia | 60 (32.8)b | 9 (4.9) |
| Obesity | 58 (31.7) | 44 (23.3) |
| Chronic cardiomyopathy | 6 (3.3) | 3 (1.6) |
| Chronic renal disease | 14 (7.7)b | 0 |
| Current smoking | 9 (4.9)a | 24 (13.1) |
| COPD | 1 (0.5) | 2 (1.1) |
| Asthma | 5 (2.7) | 6 (3.3) |
| Interstitial lung disease | 1 (0.5) | 0 |
| Pulmonary hypertension | 2 (1.1) | 0 |
| Hepatic disease | 15 (8.6)b | 1 (0.5) |
| Current cancer | 1 (0.5) | 0 |
| Stroke | 1 (0.5) | 0 |
| Current tuberculosis | 1 (0.5) | 0 |
| SpA | ||
| AxSpA | 100 (54.6) | – |
| PsA | 83 (45.4) | – |
| Disease activity | ||
| ASDAS-CRP | 2.0 (1.1) | – |
| BASDAI | 2.3 (2.0) | – |
| DAPSA | 13.9 (14.5) | – |
| PASI | 2.9 (4.5) | – |
| Current therapy | ||
| Prednisone | 15 (8.2) | – |
| Prednisone dose, mg | 8 (6.0) | – |
| NSAID | 74 (40.4) | – |
| Sulfasalazine | 41 (22.4) | – |
| Immunosuppressive drugs | 74 (40.4) | – |
| Methotrexate | 53 (29.0) | – |
| Leflunomide | 29 (15.8) | – |
| Biologic therapy | 106 (57.9) | – |
| TNFi | 73 (39.9) | – |
| Adalimumab | 29 (15.3) | – |
| Infliximab | 26 (14.2) | – |
| Etanercept | 12 (6.6) | – |
| Golimumab | 8 (4.4) | – |
| Certolizumab | 2 (1.1) | – |
| Secukinumab | 27 (14.8) | – |
| Ustekinumab | 3 (1.6) | – |
Results are expressed in mean (SD) and n (%). COPD: chronic obstructive pulmonary disease; ASDAS-CRP: ankylosing spondylitis disease activity score-C reactive protein; BASDAI: Bath ankylosing spondylitis disease activity index; DAPSA: disease activity in psoriatic arthritis; PASI: psoriasis area severity index; NSAID: non-steroidal anti-inflammatory drugs; TNFi: tumor necrosis factor inhibitor; AxSpA: axial spondyloarthritis (non-radiographic spondyloarthritis and ankylosing spondylitis); PsA: psoriatic arthritis.
For comparisons between SpA patients and CG: P < 0.01.
For comparisons between SpA patients and CG: P < 0.001.
3.1. Immunogenicity in patients with SpA and CG after two-doses of homologous schedule inactivated SARS-CoV-2 vaccine
From 183 patients in each group, 7 SpA patients and 21 CG were excluded from immunogenicity analysis for RT-PCR confirmed COVID-19 between the two vaccine doses (7 patients/5 controls) or for not attending the final visit (16 controls). The remaining 176 SpA patients and 162 CG persisted comparable regarding age distribution (51.2 ± 12.1 vs. 51.6 ± 12.4 years, non-significant) and female sex frequency (40.3% vs. 41.3%, non-significant). Immunogenicity analysis of these patients after the first vaccine dose (D28), demonstrated that both IgG SP rate (19.9% vs. 30.2%, P = 0.032) and NAb positivity (21.0% vs. 35.2%, P = 0.005) were reduced in SpA patients compared to CG. At D69, SpA patients showed a high IgG SP (80.2% vs. 95.7%, P < 0.001) and a moderate positive NAb rate (61.6% vs. 82.7%, P < 0.001) but lower than CG. In addition, analysis of the anti-S1/S2 IgG GMT (95% CI) evidenced lower titres in SpA [34.4 (29.4–40.2) AU/mL vs. 68.9 (60.9–78.0) AU/mL, P < 0.001] compared to CG. Mean NAb activity (SD) was moderate, but also reduced among SpA patients compared to CG [61.4 (15.2) % vs. 66.7 (16.1) %, P < 0.05] (Table 2 ).
Table 2.
Data regarding anti-S1/S2 IgG seropositivity (SP) rates, anti-SARS-CoV-2 S1/S2 IgG titres, frequency of neutralising antibodies (NAb) and mean percentage of neutralising activity in spondyloarthritis (SpA) patients and controls (CG) after the first (D28) and second doses (D69) of CoronaVac vaccination.
| After 1st dose |
After 2 doses |
|||
|---|---|---|---|---|
| SpA (n = 176) | CG (n = 162) | SpA (n = 176) | CG (n = 162) | |
| Anti-S1/S2 IgG | ||||
| SP, n (%) | 35 (19.9)a | 49 (30.2) | 141 (80.2)c | 155 (95.7) |
| GMT (95% CI) | 5.6 (4.7–6.6)c | 9.7 (7.9–12.0) | 34.4 (29.4–40.2)c | 68.9 (60.9–78.0) |
| Neutralising antibodies (NAb) | ||||
| NAb presence, n (%) | 37 (21.0)b | 57 (35.2) | 108 (61.6)c | 134 (82.7) |
| Mean neutralising activity, (SD)% | 51.1 (16.4) | 57.4 (21.4) | 61.4 (15.2)a | 66.7 (16.1) |
Seropositivity (SP) is defined as post-vaccination titre ≥ 15 AU/mL by indirect ELISA, LIAISON SARS-CoV-2 S1/S2 IgG. Frequencies of SP are presented as number (%), and were compared using a two-sided Chi2 test between SpA patients and CG at pre-specified time points (D28 and D69). IgG antibody titres are expressed as geometric means (GMT) with 95% confidence interval (CI). Data regarding IgG titres were analysed using generalised estimating equations (GEE) with normal marginal distribution and gamma distribution respectively and identity binding function assuming first order autoregressive correlation matrix between moments (D0, D28 and D69) in the comparison of the 2 groups (SpA vs. CG), followed by Bonferroni's multiple comparisons. The behavior of IgG titres was different for SpA and CG groups between D28 and D69: mean titres increased at each time point for SpA and CG (P < 0.001). All analyses were two-sided. Frequencies of subjects with positive NAb are expressed as number (%). Positivity for NAb was defined as neutralising activity ≥ 30% (cPass sVNT Kit). Data were compared using a two-sided Chi2 test between SpA patients and CG at pre-specified time points (D28 and D69). Percentages of neutralising activity among subjects with positive NAb are expressed as mean (± standard deviation – SD). Data were compared using a two-sided Mann–Whitney U-test for comparison between SpA patients and CG, at pre-specified time points (D28 and D69).
For comparisons between SpA patients and CG: P < 0.05.
For comparisons between SpA patients and CG: P < 0.01.
For comparisons between SpA patients and CG: P < 0.001.
3.2. Assessment of factors associated with immunogenicity in patients with SpA after two-doses of homologous schedule inactivated SARS-CoV-2 vaccine
Among all 267 SpA patients who were vaccinated, 194 completed the protocol at D69 (64 patients were excluded from immunogenicity analysis for IgG and/or NAb positivity at baseline and 9 patients were excluded for RT-PCR confirmed SARS-CoV-2 infection between the two doses) and were evaluated in regression models. Univariate analysis demonstrated that older age (OR = 0.82, 95% CI: 0.70–0.97, P < 0.05), Caucasian ethnicity (OR = 0.42, 95% CI: 0.18–0.98, P < 0.05), prednisone use (OR = 0.16, 95% CI: 0.06–0.45, P < 0.001), methotrexate use (OR = 0.25, 95% CI: 0.12–0.54, P < 0.001), leflunomide use (OR = 0.40, 95% CI: 0.17–0.94, P < 0.05) and TNFi use (OR = 0.31, 95% CI: 0.15–0.66, P < 0.01) were associated with non-SP for anti-S1/S2 IgG. For NAb, only Caucasian ethnicity (OR = 0.48, 95% CI: 0.26–0.91, P < 0.05), prednisone use (OR = 0.21, 95% CI: 0.07–0.62, P < 0.01), methotrexate use (OR = 0.51, 95% CI: 0.27–0.97, P < 0.05) and TNFi use (OR = 0.52, 95% CI: 0.29–0.95, P < 0.05) were associated with absence of NAb. The use of sulfasalazine was associated with IgG SP (OR = 3.98, 95% CI: 1.16–13.67, P < 0.05) and NAb positivity (OR = 2.85, 95% CI: 1.28–6.34, P < 0.05). No difference for SP and NAb positivity was observed between AxSpA patients and PsA (Table 3 ).
Table 3.
Unadjusted logistic regression models examining the factors associated with positive anti-S1/S2 IgG antibodies and NAb after two doses of CoronaVac (at D69) in spondylarthritis (SpA) patients (n = 194).
| Anti-SARS-CoV-2 S1/S2 IgG SC |
Neutralising antibodies (NAb) |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Demographic data | ||||
| Current age (each 5 years interval) | 0.82 | (0.70–0.97)* | 0.92 | (0.82–1.05) |
| Current age ≥ 60 years, n = 59 | 0.63 | (0.29–1.33) | 0.63 | (0.34–1.19) |
| Female sex, n = 71 | 0.89 | (0.42–1.88) | 0.82 | (0.45–1.51) |
| Caucasian ethnicity, n = 122 | 0.42 | (0.18–0.98)* | 0.48 | (0.26–0.91)* |
| SpA diagnosis | ||||
| AxSpA, n = 108 | 2.00 | (0.96–4.17) | 1.64 | (0.91–2.96) |
| PsA, n = 86 | 0.50 | (0.24–1.04) | 0.61 | (0.34–1.10) |
| Disease activity | ||||
| ASDAS-CRP | 0.81 | (0.52–1.26) | 0.96 | (0.66–1.38) |
| BASDAI | 1.09 | (0.82–1.45) | 1.02 | (0.82–1.27) |
| DAPSA | 1.03 | (0.98–1.08) | 1.02 | (0.98–1.06) |
| PASI | 1.13 | (0.90–1.42) | 0.96 | (0.85–1.09) |
| Current therapies | ||||
| No drugs, n = 28 | 15.94 | (0.95–267.48) | 2.36 | (0.91–6.13) |
| Prednisone, n = 17 | 0.16 | (0.06–0.45)*** | 0.21 | (0.07–0.62)** |
| Prednisone dose, mg/day | 1.06 | (0.88–1.28) | 1.07 | (0.89–1.30) |
| Sulfasalazine, n = 45 | 3.98 | (1.16–13.67)* | 2.85 | (1.28–6.34)* |
| Immunosuppressive drugs | ||||
| Methotrexate, n = 54 | 0.25 | (0.12–0.54)*** | 0.51 | (0.27–0.97)* |
| Leflunomide, n = 31 | 0.40 | (0.17–0.94)* | 0.90 | (0.41–1.98) |
| Biologic therapy | ||||
| TNFi, n = 74 | 0.31 | (0.15–0.66)** | 0.52 | (0.29–0.95)* |
| Secukinumab, n = 28 | 3.35 | (0.76–14.81) | 1.88 | (0.76–4.68) |
| Ustequinumab, n = 3 | 1.64 | (0.08–32.51) | 1.75 | (0.18–17.15) |
Results are expressed in OR (95% CI) regarding the positivity for anti-S1/S2 IgG and for NAb. Seropositivity defined as a positive serology (IgG titre ≥ 15 AU/mL) for anti-SARS-CoV-2 S1/S2 IgG antibodies after vaccination (Indirect ELISA, LIAISON® SARS-CoV-2 S1/S2 IgG, DiaSorin, Italy). Positivity for NAb defined as a neutralising activity ≥ 30% (cPass sVNT Kit, GenScript, Piscataway, USA); AxSpA: axial spondyloarthritis (non-radiographic spondyloarthritis and ankylosing apondylitis); PsA: psoriatic arthritis; ASDAS-CRP: ankylosing spondylitis disease activity score-C reactive protein; BASDAI: Bath ankylosing spondylitis disease activity index; DAPSA: disease activity in psoriatic arthritis; PASI: psoriasis area severity index; NSAID: non-steroidal anti-inflammatory drugs; TNFi: tumor necrosis factor inhibitor.
P < 0.05.
P < 0.01.
P < 0.001.
Further multivariate logistic regression analyses of SpA patients confirmed that older age (OR = 0.80, 95% CI: 0.65–0.99, P < 0.05), prednisone (OR = 0.06; 95% CI: 0.01–0.29; P < 0.001), methotrexate (OR = 0.06, 95% CI: 0.01–0.26, P < 0.001) and TNFi (OR = 0.05, 95% CI: 0.01–0.25, P < 0.001) were independently associated with lower IgG SP. Caucasian ethnicity (OR = 0.49; 95% CI: 0.24–0.99; P < 0.05), prednisone (OR = 0.19, 95% CI: 0.06–0.65, P < 0.01) were independently associated with lower NAb positivity. The use of SSZ increased NAb positivity (OR = 2.86, 95% CI: 1.04–7.86, P < 0.05).
3.3. Assessment of factors associated with immunogenicity in SpA patients under monotherapy or with no DMARD or prednisone
A subgroup analysis including only patients under monotherapy (n = 93) or with no DMARD or prednisone (n = 28) was performed and pointed TNFi use (P < 0.01) as negatively associated to SP and to absence of Nab (P < 0.05) (Table 4 ).
Table 4.
Unadjusted logistic regression models examining the factors associated with positive anti-S1/S2 IgG antibodies and NAb after 2 doses of CoronaVac (at D69) in spondylarthritis (SpA) patients with no DMARD and no prednisone (n = 28) or with DMARD monotherapy (n = 86).
| Anti-SARS-CoV-2 S1/S2 IgG SC |
Neutralising antibodies (NAb) |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Demographic data | ||||
| Current age (each 5 years interval) | 0.75 | (0.54–1.04) | 0.93 | (0.78–1.12) |
| Current age > 60 years, n = 41 | 1.91 | (0.57–6.38) | 0.77 | (0.35–1.69) |
| Female sex, n = 36 | 0.42 | (0.12–1.40) | 0.66 | (0.29–1.50) |
| Caucasian ethnicity, n = 69 | 0.74 | (0.21–2.63) | 0.63 | (0.28–1.41) |
| SpA | ||||
| AS, n = 75 | 2.09 | (0.63–6.98) | 1.75 | (0.78–3.89) |
| PsA, n = 39 | 0.48 | (0.14–1.60) | 0.57 | (0.26–1.28) |
| Disease activity | ||||
| ASDAS-CRP | 0.96 | (0.43–2.12) | 1.18 | (0.65–2.12) |
| BASDAI | 1.12 | (0.65–1.94) | 0.86 | (0.58–1.25) |
| DAPSA | 1.02 | (0.89–1.17) | 1.02 | (0.92–1.13) |
| PASI | 1.95 | (0.46–8.28) | 0.86 | (0.62–1.19) |
| Current therapies | ||||
| No DMARD or prednisone, n = 28 | 9.56 | (0.55–166.92) | 2.40 | (0.88–6.52) |
| Sulfasalazine, n = 19 | 5.84 | (0.33–102.91) | 3.40 | (0.93–12.49) |
| Immunosuppressive drugs | ||||
| Methotrexate, n = 16 | 0.44 | (0.10–1.83) | 0.65 | (0.22–1.91) |
| Leflunomide, n = 2 | 0.62 | (0.03–13.71) | 0.53 | (0.03–8.78) |
| Biologic therapy | ||||
| TNFi, n = 40 | 0.15 | (0.04–0.57)** | 0.37 | (0.17–0.83)* |
| Secukinumab, n = 8 | 2.25 | (0.12–41.39) | 0.89 | (0.20–3.95) |
Results are expressed in OR (95% CI) regarding the positivity for anti-S1/S2 IgG and for Nab. Seropositivity defined as a positive serology (IgG titre ≥ 15 AU/mL) for anti-SARS-CoV-2 S1/S2 IgG antibodies after vaccination (Indirect ELISA, LIAISON® SARS-CoV-2 S1/S2 IgG, DiaSorin, Italy). Positivity for NAb defined as a neutralising activity ≥ 30% (cPass sVNT Kit, GenScript, Piscataway, USA); AxSpA: axial spondyloarthritis (non-radiographic spondyloarthritis and ankylosing apondylitis); PsA: psoriatic arthritis; ASDAS-CRP: ankylosing spondylitis disease activity score-C reactive protein; BASDAI: Bath ankylosing spondylitis disease activity index; DAPSA: disease activity in psoriatic arthritis; PASI: psoriasis area severity index; NSAID: Non-steroidal anti-inflammatory drugs; TNFi: tumor necrosis factor inhibitor.
P < 0.05.
P < 0.01.
After multivariate analysis, TNFi use persisted associated only with absence of IgG SP (OR = 0.21, 95% CI: 0.05–0.90, P < 0.05).
3.4. Factors associated with immunogenicity in patients with SpA on combination or monotherapy after two-doses of homologous schedule inactivated SARS-CoV-2 vaccine compared to CG
Comparisons of SpA patients under various therapies subgroups and CG were also performed and are presented in Fig. 1 , Figure S2 and Table S1.
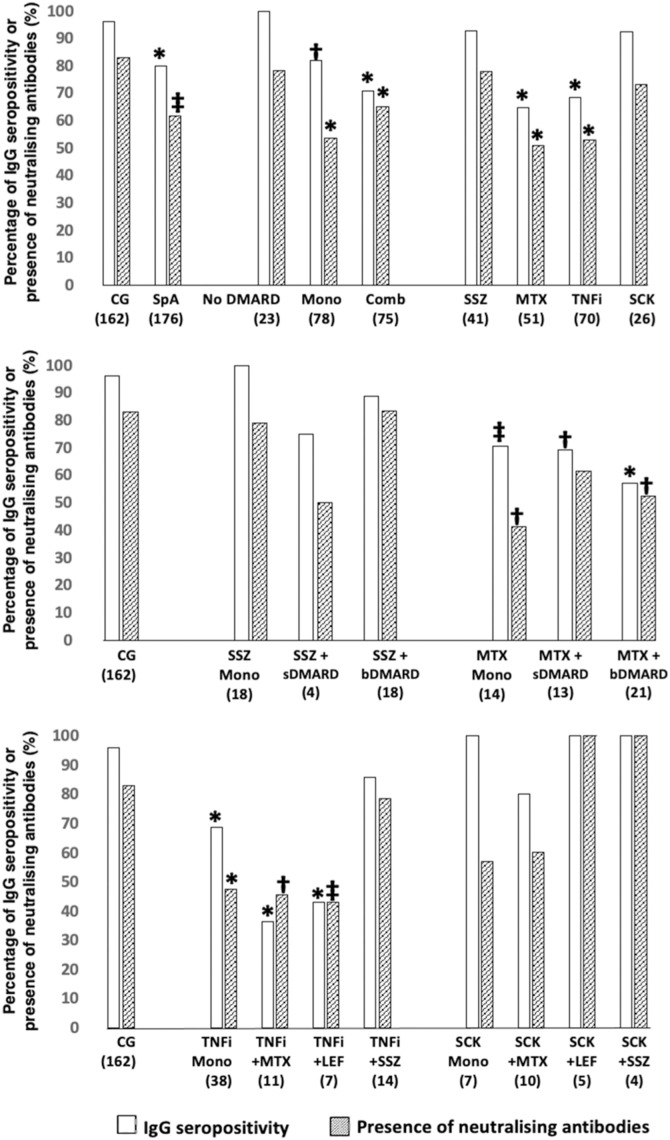
Fig. 1.
Immunogenicity at D69, according to SpA treatments in comparison to CG. Two-sided Chi2 or Fisher's exact test was used as appropriate. All the analyses are two-sided. Data are shown as percentages. CG: control group; SpA: spondyloarthritis; DMARD: disease modifying antirheumatic drugs; Mono: DMARD monotherapy and without corticosteroids; Comb: combination therapy of DMARD; SSZ: sulfasalazine; MTX: methotrexate; TNFi: tumor necrosis factor inhibitors; SCK: secukinumab; sDMARD: synthetic disease modifying antirheumatic drugs; bDMARD: biological disease modifying antirheumatic drugs; LEF: leflunomide; * P < 0.001 vs. CG; † P < 0.01 vs. CG; ‡ P < 0.05 vs. CG. The number of patients in groups is depicted under their designations.
SpA patients without current DMARD or prednisone presented similar IgG SP (100% vs. 96%, non-significant) and NAb positivity (78.3% vs. 83%, non-significant) compared to CG. Methotrexate in monotherapy or in combination with bDMARD impacted in both IgG SP and NAb (P < 0.05). TNFi also reduced IgG SP and NAb positivity in monotherapy (SP: 76.3% vs. 96%; P < 0.001; NAb: 47.4% vs. 83%; P < 0.001) or in combination with methotrexate (SP: 36.4% vs. 96%; P < 0.001; NAb: 45.5% vs. 83%, P < 0.01) or leflunomide (SP: 42.9% vs. 96%, P < 0.001; NAb: 42.9% vs. 83%, P < 0.05), but not in combination with sulfasalazine (SP: 85.7% vs. 96%, non-significant; NAb: 78.6% vs. 83%, non-significant). In contrast, sulfasalazine and secukinumab did not impact the humoral response either in monotherapy or in combination.
The same pattern was observed for GMT of anti-SARS-CoV-2 IgG (Figure S2).
3.5. Comparative immunogenicity in patients with SpA in comparison to CG after third dose (D69 to D240)
In the extended phase, 160 SpA patients and 107 CG received the third dose of homologous CoronaVac vaccine, and 145 SpA and 89 CG were included in the immunogenicity analysis (15 SpA patients and 18 CG were excluded for not attending the last blood collection). The remaining patients were comparable to controls regarding age (52.0 ± 12.2 vs. 53.0 ± 11.5 years, non-significant) and female sex frequency (41.0% vs. 44.9%, non-significant). From D69 to D240, an increment of response was observed among SpA patients with an increase in anti-S1/S2 IgG SP (81.3% to 93.1%, P < 0.001) and in NAb positivity (63.2% to 86.1%, P < 0.001). Among CG a significant increase was not found for anti-S1/S2 IgG positivity (97.8% to 100%, non-significant) but was for NAb positivity (83.1% to 97.8%, P < 0.01). Despite the increment after the third dose, the response among SpA patients remained inferior in comparison with the CG for anti-S1/S2 IgG at D240 (93.1% vs. 100%, P < 0.05) and for NAb (86.1% vs. 97.8%, P < 0.01). At D240, SpA patients compared to CG had lower GMT titres (95% CI) [145.5 (119.4–177.2) AU/mL vs. 231.1 (204.4–261.2) AU/mL; P < 0.001] and similar mean (SD) NAb activity [78.0 (21.0) % vs. 80.9 (17.9) %; non-significant] (Table 5 ).
Table 5.
Comparison of anti-SARS-CoV-2 S1/S2 IgG seropositivity, geometric mean titres, neutralising antibodies (NAb) seropositivity and mean percentage activity after second dose (D69) and 30 days after the third dose (D240) of Sinovac-CoronaVac vaccination in spondyloarthritis patients (SpA) and control group (CG).
| Groups | Anti S1/S2 seropositivity |
Anti-S1/S2 IgG GMT (95% CI) AU/mL |
NAb positivity |
Mean neutralising activity (SD) % |
||||
|---|---|---|---|---|---|---|---|---|
| D69 | D240 | D69 | D240 | D69 | D240 | D69 | D240 | |
| SpA- (n = 145) | 117 (81.3)c |
135 (93.1)b,e |
35.7 (29.9–42.7)c |
145.5 (119.4–177.2)c,e |
91 (63.2)c |
124 (86.1)b,e |
61.8 (18.7)a |
78.0 (21.0)e |
| CG- (n = 89) | 87 (97.8) |
89 (100) |
78.8 (65.5–94.4) |
231.1 (204.4–261.2)e |
74 (83.1) |
87 (97.8)d |
68.2 (18.6) |
80.9 (17.9)e |
Seropositivity (SP) is defined as post-vaccination titre ≥ 15 AU/mL by indirect ELISA, LIAISON SARS-CoV-2 S1/S2 IgG. Frequencies of SP are presented as number (%), and were compared using a two-sided Chi2 test between SpA patients and CG at pre-specified time points (D69 and D240). IgG antibody titres are expressed as geometric means (GMT) with 95% confidence interval (CI). Data regarding IgG titres were analysed using generalised estimating equations (GEE) with normal marginal distribution and gamma distribution respectively and identity binding function assuming first order autoregressive correlation matrix between moments (D69 and D240) in the comparison of the 2 groups (SpA vs. CG), followed by Bonferroni's multiple comparisons. All analyses were two-sided. Frequencies of subjects with positive NAb are expressed as number (%). Positivity for NAb was defined as neutralising activity ≥ 30% (cPass sVNT Kit). Data were compared using a two-sided Chi2 test or Fisher's exact test between SpA and CG at pre-specified time points (D28 and D69). Percentages of neutralising activity among subjects with positive NAb are expressed as mean (± standard deviation – SD). Data were compared using a two-sided Mann–Whitney U-test for comparison between SpA patients and CG, at pre-specified time points (D69 and D240).
For comparisons between SpA patients and CG: P < 0.05.
For comparisons between SpA patients and CG: P < 0.01.
For comparisons between SpA patients and CG: P < 0.001.
For within longitudinal comparisons of subjects at D69 vs. D240: P < 0.01.
For within longitudinal comparisons of subjects at D69 vs. D240: P < 0.001.
Regression models analysis of SpA patients after the third dose showed that the use of TNFi impaired anti-SARS-CoV-2 IgG (OR = 0.14, 95% CI: 0.03–0.70; P < 0.05) and Nab (OR = 0.08, 95% CI: 0.02–0.40, P < 0.01) response. NAb positivity was also impaired by female sex (OR = 0.24, 95% CI: 0.08–0.76, P < 0.05).
3.6. Disease activity
Disease activity status pre-vaccination did not influence humoral response (Table 3, Table 4).
3.7. Safety analysis
Regarding safety analysis, only mild AE were reported and no difference in number of AE between SpA patients and CG (139 vs. 127, non-significant) was observed. Local reactions had similar frequencies in SpA patients and CG (15.8% vs. 16.9%, non-significant). Pain at the injection site was the most common in both groups (12.6% vs. 13.7%, non-significant). We found differences in the frequency of systemic reactions among SpA and CG with more reactions among SpA (37.7% vs. 25.7%, P < 0.05). Headache was the most common in both groups (15.8% vs. 7.1%, P < 0.01). No major adverse events were identified in this study.
4. Discussion
The present study is the first to evaluate three doses with homologous schedule of inactivated SARS-CoV-2 vaccine (CoronaVac) in SpA patients. We demonstrated that TNFi reduces immunogenicity in SpA patients while sulfasalazine has a positive impact on vaccine antibody response.
The large number of SpA patients under TNFi in our study is a major strength to evaluate the impact of TNFi in the vaccine humoral response in SpA patients. The small sample size of this population under biological therapy in previous studies precludes a definitive conclusion about the effect of these drugs [4], [15]. A CG population balanced for age was also relevant since this parameter is known to influence vaccine response [19]. Another advantage of the present report is the evaluation of NAb antibodies, not included in previous studies [4], [15]. In fact, NAb levels were reported to be predictive of COVID-19 protection [20], [21]. Our study had some limitations such as the absence of cellular immunity assessment and lack of prospective measures of disease activity.
We confirmed previous observations that SpA patients have a lower immunogenicity to IgG and NAb two doses of SARS-CoV-2 vaccines than CG [4], [15]. Reinforcing our finding, a lower humoral response after influenza A/H1N1 vaccine was found in SpA patients under TNFi therapy [14]. In addition, the deleterious effect of TNFi in SARS-CoV-2 vaccine-induced antibody response was reported in other autoimmune diseases, including patients with IBD and psoriasis [3], [16], [18], [22].
We extended this finding addressing specifically AS and PSA and we were able to identify that specific drugs in combination and even in monotherapy impaired immunogenicity. Supporting this assertion, patients without any therapy in the present study had comparable immune responses to CG, suggesting that disease itself is not a major factor to influence this parameter.
Among patients, prednisone reduced vaccine-induced antibody response, as also reported for other autoimmune diseases with the same vaccine platform [3], [10] and for mRNA vaccine [4]. MTX also impaired immunogenicity as previously described for CoronaVac in chronic inflammatory arthritis [23], primary Sjogren's syndrome [24] and RA [9], for mRNA vaccines [11] and for a pool of SARS-CoV-2 vaccines [24] even in monotherapy [9], [25].
With regard to TNFi, a recent review from EULAR did not include this target therapy among the drugs that impair humoral immune response in overall patients with ARD [26]. On the contrary, we demonstrated with a large representation of SpA patients that TNFi significantly decreased vaccine response in this population. Reinforcing this novel finding, TNFi in monotherapy evaluated herein also had the same effect in these patients. The isolated effect of TNFi in RA [9] and in ARD [4], [25] was not reported previously, probably due to the fact that the regression models used in these studies included drugs that had a greater impact in immunogenicity, such as abatacept [9] and rituximab [4], [18]. In addition, the underrepresentation of TNFi monotherapy in previous studies hampered the interpretation of their findings [18], [27]. Other target therapies, such as IL-17 inhibitors and IL-12/23 did not seem to impact immunogenicity consistent with other reports in IBD and psoriasis [16], [28].
Of note, SSZ combination was capable of counterbalancing the impairment in vaccine humoral response verified with TNFi and MTX, whereas combination therapy between TNFi and MTX or LEF deteriorated the response to vaccine in consonance with other reports [11], [12], [13]. Supporting the possible beneficial effect of SSZ, all patients under this drug in monotherapy had seroconversion and high frequency of NAb, comparable to the CG. In fact, SSZ was reported to have a relevant immunomodulatory action with a low immunosuppressive effect, which may account for the observed benefit of this therapy [29]. Accordingly, SSZ was associated with reduced odds of inadequate antibody response [16].
The recommendation for the third dose was approved in Brazil [30] and it was particularly relevant for patients under TNFi therapy monotherapy or combination with MTX or LEF taking into consideration that less than half of these patients attained SP with complete vaccine scheme. The overall response to the booster dose was remarkable for IgG and NAb in SpA patients as also reported by our group for several autoimmune rheumatic diseases [31] and small sample population rheumatoid arthritis [32]. But TNFi remained as the only drug to impair immunogenicity after the additional dose.
No major adverse events were reported in this study. Local and systemic adverse events were mild and did not compromise the well-being of the subjects regardless of group or disease. This safety profile was comparable with other vaccine platforms against SARS-CoV-2 in ARD patients [4], [31], [33].
We provided novel data demonstrating that TNFi attenuates immunogenicity in SpA patients while SSZ has a positive impact on vaccine antibody production. We also confirmed that MTX in combination with TNFi had a major negative impact in vaccine humoral response.
Disclosure of interest
The authors declare that they have no competing interest.
Funding statement
This study was sponsored by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (#2015/03756–4 to N.E.A., C.A.S., S.G.P. and E.B; #2019/17272-0 to L.V.K.K), Conselho Nacional de Desenvolvimento Científico e Tecnológico (#304984/2020-5 to C.A.S. and #305242/2019-9 to E.B.), B3-Bolsa de Valores do Brasil and Instituto Todos pela Saúde (ITPS 01/2021, C1313 to N.E.A., C.A.S. S.G.P and E.B.). Instituto Butantan supplied the study product and had no other role in the trial.
Author contribution
C.G.S.S., M.S.R.S., A.C.M.-R., N.E.A., E.F.N.Y., S.G.P., C.A.S. and E.B. conceived and designed the study, participated in data collection and analysis and supervised clinical data management, writing of the manuscript and revision of the manuscript. E.B. is responsible for the overall content as the guarantor. E.B., and L.V.K.K. organized and supervised blood collection and vaccination protocol. S.G.P. supervised serum processing, SARS-CoV-2-specific antibody ELISA/neutralisation assays and SARS-CoV-2 RT–PCR. C.G.S.S., M.S.R.S., P.D.S.B., J.C.B.d.M., C.G.S., C.R.G., A.Y.S., N.E.A., E.F.N.Y., L.V.K.K., S.G.P., R.K.A., C.S.R.A., C.A.S., A.C.M.-R. and E.B. collected epidemiological and clinical data and assisted with the identification of SARS-CoV-2 infection and follow-up of patients. C.G.S.S., M.S.R.S., A.C.M.-R., E.F.N.Y., S.G.P., C.A.S., L.V.K.K., N.E.A. and E.B. were responsible for writing and revision of the manuscript. All authors helped to edit the manuscript.
Acknowledgements
We thank the contribution of the undergraduate students, post-graduation students for collecting epidemiological and clinical data; Central Laboratory Division, Registry Division, Security Division, IT Division, Superintendence, Pharmacy Division and Vaccination Center for their technical support. We also thank the volunteers for participating in the in-person visits of the protocol and for handling the biological material, and those responsible for the follow-up of all participants.
Footnotes
Supplementary data (Figure S1–S2, Table S1) associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jbspin.2022.105464.
Appendix A. Supplementary data
References
- 1.World Health Organization . World Health Organization; 2022. Interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac: interim guidance, first issued 24 May 2021, updated 21 October 2021, updated 15 March 2022. [ https://apps.who.int/iris/handle/10665/352472 (accessed Aug 10, 2022)] [Google Scholar]
- 2.Strangfeld A., Schäfer M., Gianfrancesco M.A., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medeiros-Ribeiro A.C., Aikawa N.E., Saad C.G.S., et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 4.Furer V., Eviatar T., Zisman D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 5.Geisen U.M., Berner D.K., Tran F., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jara A., Undurraga E.A., González C., et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva CA, Medeiros-Ribeiro AC, Kupa LVK. Anti-SARS-CoV-2 immunogenicity decay and incident cases six months after Sinovac-CoronaVac inactivated vaccine in autoimmune rheumatic disease patients: phase 4 prospective trial, 23 November 2021, PREPRINT (version 1) available at research square: doi: 10.21203/rs.3.rs-1054476/v1.
- 8.Aikawa N.E., Kupa L.D.V.K., Medeiros-Ribeiro A.C., et al. Increment of immunogenicity after third dose of a homologous inactivated SARS-CoV-2 vaccine in a large population of patients with autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81:1036–1043. doi: 10.1136/annrheumdis-2021-222096. [Published Online First: 11 March 2022] [DOI] [PubMed] [Google Scholar]
- 9.Medeiros-Ribeiro A.C., Bonfiglioli K.R., Domiciano D.S., et al. Distinct impact of DMARD combination and monotherapy in immunogenicity of an inactivated SARS-CoV-2 vaccine in rheumatoid arthritis. Ann Rheum Dis. 2022;81:710–719. doi: 10.1136/annrheumdis-2021-221735. [Published Online First: 08 February 2022] [DOI] [PubMed] [Google Scholar]
- 10.Yuki EFN, Borba EF, Pasoto, SG, et al. Impact of distinct therapies on antibody response to SARS-CoV-2 vaccine in systemic lupus erythematosus. Arthritis Care Res;74:562–571. [DOI] [PMC free article] [PubMed]
- 11.Haberman R.H., Herati R., Simon D., et al. Methotrexate hampers immunogenicity to BNT162b2 DimRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman M.A., Curtis J.R., Winthrop K.L. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1255–1265. doi: 10.1136/annrheumdis-2021-221244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahil S.K., Bechman K., Raharja A., et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.França I., Ribeiro A., Aikawa N., et al. TNF blockers show distinct patterns of immune response to the pandemic influenza A H1N1 vaccine in inflammatory arthritis patients. Rheumatology (Oxford) 2012;51:2091–2098. doi: 10.1093/rheumatology/kes202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simader E., Tobudic S., Mandl P., et al. Importance of the second SARS-CoV-2 vaccination dose for achieving serological response in patients with rheumatoid arthritis and seronegative spondyloarthritis. Ann Rheum Dis. 2022;81:416–421. doi: 10.1136/annrheumdis-2021-221347. [DOI] [PubMed] [Google Scholar]
- 16.Kappelman M.D., Weaver K.N., Zhang X., et al. Factors affecting initial humoral immune response to SARS-CoV-2 vaccines among patients with inflammatory bowel diseases. Am J Gastroenterol. 2022;117:462–469. doi: 10.14309/ajg.0000000000001619. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy N.A., Goodhand J.R., Bewshea C., et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 18.Wieske L., van Dam K.P.J., Steenhuis M., et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022;4:e338–e350. doi: 10.1016/S2665-9913(22)00034-0. [published online ahead of print, 2022 Mar 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerqueira-Silva T., Oliveira V.A., Boaventura V.S., et al. Influence of age on the effectiveness and duration of protection of Vaxzevria and CoronaVac vaccines: a population-based study. Lancet Reg Health Am. 2022;6:100154. doi: 10.1016/j.lana.2021.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury D., Cromer D., Reynaldi A., et al. Neutralising antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 21.Earle K.A., Ambrosino D.M., Fiore-Gartland A., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geisen U.M., Berner D.K., Tran F., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bugatti S., De Stefano L., Balduzzi S., et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis. 2021;80:1635–1638. doi: 10.1136/annrheumdis-2021-220862. [DOI] [PubMed] [Google Scholar]
- 24.Pasoto S.G., Halpern A.S.R., Guedes L.K.N., et al. Inactivated SARS-CoV-2 vaccine in primary Sjögren's syndrome: humoral response, safety, and effects on disease activity. Clin Rheumatol. 2022;1–11 doi: 10.1007/s10067-022-06134-x. [published online ahead of print, 2022 Mar 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boekel L., Steenhuis M., Hooijberg F., et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furer V., Rondaan C., Heijstek M.W., et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 27.Simon D., Tascilar K., Fagni F., et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021;80:1312–1316. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benucci M., Damiani A., Infantino M., et al. Vaccination for SARS-CoV-2 in patients with psoriatic arthritis: can therapy affect the immunological response? Front Med (Lausanne) 2022;9:811829. doi: 10.3389/fmed.2022.811829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghasemnejad-Berenji M. Can sulfasalazine as an old drug with immunomodulatory and anti-inflammatory effects be effective in COVID-19? J Basic Clin Physiol Pharmacol. 2021;33:113–115. doi: 10.1515/jbcpp-2021-0349. [DOI] [PubMed] [Google Scholar]
- 30.Meiruze SF, National Health Vigilance Agency (ANVISA), Brazil. Process 25351.931900/2021-84. 2021.
- 31.Aikawa N.E., Kupa L.V.K., Medeiros-Ribeiro A.C., et al. Increment of immunogenicity after third dose of a homologous inactivated SARS-CoV-2 vaccine in a large population of patients with autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81:1036–1043. doi: 10.1136/annrheumdis-2021-222096. [published online ahead of print 2022 Mar 11. annrheumdis-2021-222096] [DOI] [PubMed] [Google Scholar]
- 32.Schmiedeberg K., Vuilleumier N., Pagano S., et al. Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses. Lancet Rheumatol. 2022;4:e11–e13. doi: 10.1016/S2665-9913(21)00328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machado P.M., Lawson-Tovey S., Strangfeld A., et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis. 2022;81:695–709. doi: 10.1136/annrheumdis-2021-221490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.