Abstract
Positron emission tomography (PET) combined with computed tomography (CT) is an established diagnostic modality that has become an essential imaging tool in oncological practice. However, thanks to its noninvasive nature and its capability to provide physiological information, the main applications of this technique have significantly expanded.
18F-labelled fluorodeoxyglucose (FDG) is the most commonly used radiopharmaceutical for PET scanning and demonstrates metabolic activity in various tissues. Since activated inflammatory cells, like malignant cells, predominantly metabolise glucose as a source of energy and increase expression of glucose transporters when activated, FDG-PET/CT can be successfully used to detect and monitor a variety of lung diseases, such as infections and several inflammatory conditions.
The added value of FDG-PET/CT as a molecular imaging technique relies on its capability to identify disease in very early stages, long before the appearance of structural changes detectable by conventional imaging. Furthermore, by detecting the active phase of infectious or inflammatory processes, disease progression and treatment efficacy can be monitored.
This review will focus on the clinical use of FDG-PET/CT in nonmalignant pulmonary diseases.
Short abstract
PET/CT is an imaging modality that could play a role in evaluation of inflammatory and infectious lung diseases http://ow.ly/8YGT302aVlS
Introduction
Positron emission tomography (PET) is a medical imaging modality that enables in vivo imaging of the distribution of picomolar concentrations of labelled compounds in the body. The addition of transmission computed tomography (CT) to PET instruments results in images that provide both functional/molecular information and structural details. 18F-labelled fluorodeoxyglucose (FDG) is a positron emitter with an ∼110-min half-life. It permits external imaging using PET and represents the most widely used PET tracer.
FDG-PET was initially introduced to determine the state of physiological and pathological brain functions [1, 2]. Since then, it has become an established imaging modality in the workup for thoracic masses [3, 4], especially primary and secondary lung, pleural and mediastinal tumours. In malignant thoracic conditions, uncontrolled mitotic division of dysplastic tissue is known to be part of its long development process, and can be attributed to chronic irritation and inflammation, including that caused by noxious chemicals, parasites or chronic infection.
Following the discovery of FDG as PET tracer, the strength and importance of PET imaging escalated exponentially. Being a glucose analogue, FDG has an affinity towards glucose receptors on cell surface. Overexpression of glucose transporter 1 receptors on cancer tissue surfaces enhances glycolysis and attracts more FDG uptake, where in vivo conversion into FDG-6-phosphate by glucose-6-phosphatase takes place. The process essentially reflects the increased metabolic rate of cancer cells [5–9]. This process can be translated using a PET system as an intense uptake of FDG, called metabolic imaging.
In inflammatory and infective conditions, following exposure to aseptic or septic stimulus, a cascade of response will be initiated by the release of local chemokines, interleukins (ILs) and prostaglandins, which are well-known pro-inflammatory mediators. This in turn will regulate the function of “gate keepers” in the venular walls for the migration of neutrophils, monocytes and effector T-cells, facilitated by perivascular macrophages and mast cells. Many authors have elucidated in detail relationship between the inflammatory response, local hyperaemia and hypervascularisation, and uptake of FDG in previous studies [10, 11], supporting the use of FDG-PET/CT in infection and inflammatory conditions [12–15].
In chronic conditions, the polymorphs, granulocytes and macrophages are predominantly involved, and the quantity of energy generated is possibly greater. In these situations, multiple cytokines and growth factors have been shown to facilitate glucose transport without increasing the number of glucose transporters by a mechanism that has not been described in malignant conditions.
Kubota et al. [11] demonstrated a high accumulation of macrophages and granulation tissues surrounding an abscess in an animal model. Through autoradiography, this group displayed that increased FDG uptake could be found not only in tumour cells but also in the inflammatory cell elements associated with growth or tumour necrosis. Increased capillary permeability at the initial stage of infection could lead to the aggregation of inflammatory cells, like granulocytes, leukocytes and macrophages, at the entry point. This evidence proves that FDG uptake is not specific to malignancy but is also present in infection and inflammation.
In acute inflammation or infection of the chest, FDG uptake occurs primarily by activated neutrophils, whose metabolism (especially during the respiratory burst triggered by the rolling and adhesion phase) is heavily dependent on anaerobic glycolysis, requiring elevated uptake of glucose [16].
PET/CT permits quantification of radioactivity throughout the lungs, and in airspaces and the interstitium, enabling study of the behaviour of inflammatory cells in their native microenvironment. FDG activity has been reported in the course of several experimental and clinical inflammatory or infectious processes in the lungs.
Jones et al. [17], for example, used positron emission imaging following injection of FDG to monitor neutrophil metabolic activity in vivo, and showed a dissociation between migration and activation of these cells in pneumonia and bronchiectasis. The uptake of deoxyglucose by neutrophils reflected both processes of priming and activation [18]. This supported that neutrophil presence and activation are likely to be orchestrated by pulmonary alveolar and interstitial macrophages.
Thus, it is important for nuclear medicine physicians and radiologists to have a thorough understanding of the cellular mechanisms in relation to the pathophysiology in different clinical settings, including malignancy, infection and inflammatory conditions, to avoid misinterpretation of FDG-PET/CT imaging results that may lead to an inaccurate treatment delivery.
This review article will focus on PET/CT applications in nononcological lung diseases. As the number of clinical applications of FDG-PET/CT grows, it is necessary for nuclear physicians and clinicians to improve their understanding of the difference between inflammatory reaction, infection and malignancy, which is a real challenge.
Acute infectious processes
Tuberculosis and nontuberculous mycobacteria
Assessment of metabolic activity in tuberculosis lesions has been well described with the application of FDG-PET [19–22], although there is still insufficient literature describing evidence-based indications [23].
As a caseating granulomatous disease, tuberculosis can involve any organ by haematogenous spread, lymphatic spread or contiguity. Soussan et al. [24] identified two different patterns of tuberculosis on FDG PET scanning: a pulmonary type with a more localised type of infection, and a lymphatic pattern associated with a more intense, systemic infection. However, the most common presentation of active tuberculous lesions involves the lung parenchyma [25, 26].
In particular, tuberculoma typically appears as a fairly discrete nodule or mass with a central caseous necrosis surrounded by a mantle of epithelioid cells and collagen with peripheral inflammatory cell infiltration [27]. There is now growing interest in the potential role of FDG-PET in the functional assessment of inflammatory and infectious pulmonary diseases, including those caused by mycobacteria.
Due to the large number of activated inflammatory cells with high glycolytic rates, active tuberculous lesions usually display an intense FDG uptake, as previously also documented in animals [28].
PET accuracy is very high in identifying active granulomatous foci. In a pilot study, Sathekge et al. [22] demonstrated that in HIV-negative patients affected by tuberculosis, FDG revealed more extensive involvement when compared with diagnostic contrast-enhanced CT scanning also considering lymphonodal involvement.
However, this infectious process could present different uptake patterns according to the grade of inflammatory activity [20]. Yago et al. [29], for example, reported the case of a “cold” abscess due to Mycobacterium tuberculosis with only moderate peripheral and low central FDG activity because it is not accompanied by an inflammatory reaction.
Another important role of this widespread imaging technique is its ability to assess early treatment response. Morphological changes often take significantly longer to be detectable than molecular changes. Thus, the ability to identify active tuberculosis lesions earlier than by conventional radiology has an important impact on patient management. This is particularly relevant in certain clinical settings such as in severely immunosuppressed patients and those co-infected with HIV [30, 31].
FDG-PET allows the rapid assessment of pulmonary and extrapulmonary tuberculosis simultaneously, leading to time saving and cost-effectiveness. However, PET images should be interpreted with caution because of the lack of specificity and inability to clearly distinguish granulomatous disease from malignancy based on standardised uptake values (SUVs). Thus, in view of increasing prevalence, especially in cases of extrathoracic concomitant malignancies, tuberculosis should be considered in the differential diagnosis of FDG-avid thoracic findings, and histopathological confirmation is needed in most cases [32].
Like tuberculosis, recently, an increase in nontuberculous mycobacterial (NTM) infections, especially those by Mycobacterium avium–intracellulare complex (MAC), has been recorded. NTM infection is caused by a group of opportunistic bacterial pathogens that are hard to isolate and characterised by nonspecific clinical signs that, thus, could remain underdiagnosed.
Similar to tuberculosis, NTM could have a broad range of radiological patterns including nodular or pseudo-nodular lesions, parenchymal consolidation, cavitary lesions, pleural thickenings, and pleural effusions, or a mixed pattern [33–35].
FDG-PET/CT could reflect the activity and extent of disease by monitoring metabolic activity not only in nodular lesions but also within a broad range of radiologically visible lung lesions in NTM and M. tuberculosis infections.
Some authors [36] also propose a greater maximum SUV (SUVmax) cut-off of 4.0 to define highly active mycobacterial granuloma lesions and found that average uptake was higher in patients with MAC than those with tuberculosis. Conversely, other authors [37] demonstrated average values of lung lesions were less in NTM than in tuberculosis.
With these findings, it is clear that further investigations are required to identify specific SUV cut-offs, if possible, in order to define potential indications and limitations of PET/CT scanning in both pulmonary NTM and M. tuberculosis infections.
Despite these uncertainties, an important aspect of PET/CT imaging is the potential role in assessing not only extent of disease but also evolution and follow-up in both NTM and M. tuberculosis patients. Following Davis et al. [38], who showed the power of this technique to detect and to monitor response to chemotherapy in animals with tuberculous lesions, Namkoong et al. [39] recently published a case report on the role of FDG-PET/CT in assessing appropriate duration for antimycobacterial therapy in a patient with HIV infection. If confirmed, this application could offer a valuable tool for patient management, especially in complex cases such as AIDS, in which tuberculosis and NTM infections often cause disease dissemination and tend to relapse after treatments.
Other acute lung conditions
Like active granulomatous processes, other infectious diseases (figure 1) and active fibrotic lesions can mimic malignancy, leading to false-positive PET scans; thus, the role of this imaging technique in this setting is limited. However, thanks to its ability to quantitate FDG uptake, FDG-PET could be potentially useful in monitoring the infectious or inflammatory processes, and in determining treatment efficacy [40, 41]. As reported by several authors, FDG-PET imaging has been used to monitor the course of disease after treatment in aspergillosis [42, 43] and to evaluate the efficacy of chemotherapy in echinococcosis infection [44]. Conventional imaging techniques are not able to assess parasitic viability in alveolar echinococcosis and the long-term treatment of this pathological condition is very costly. By contrast, FDG-PET could show the disappearance of metabolic activity after treatment and may be useful for timely detection of relapses [45–47].
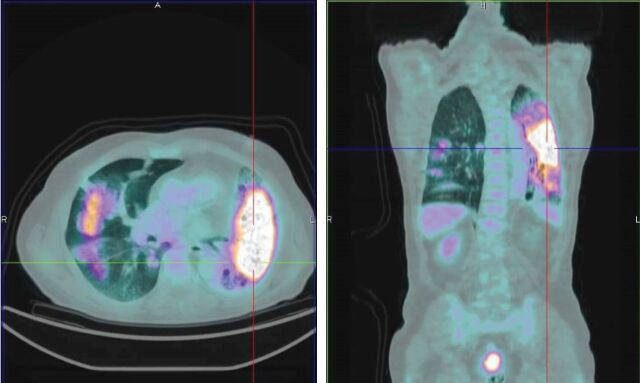
FIGURE 1.
Diffuse pulmonary infection in a 71-year-old male before antibiotic therapy. Infectious parenchymal alterations show an intense fluorodeoxyglucose uptake.
Similarly, in a clinical study, FDG uptake returned to normal levels after successful antifungal therapy for a lung abscess caused by candidal infection [48] and after therapy for Pneumocystis jiroveci pneumonia [49].
Recently, some authors proposed a role of FDG-PET in organising pneumonia (OP), a large group of lung diseases including infectious pneumonia, connective tissue diseases, consequences of solid-organ transplantation, drugs and radiotherapy, or aspiration diseases [50]. OP is an inflammatory disease that can mimic lung cancer by causing a positive FDG-PET. However, this imaging technique may be helpful in predicting prognosis in OP, although histopathological evaluation remains necessary in differential diagnosis [51–53].
Acute or chronic phase of noninfectious inflammatory processes
Besides infectious conditions, PET/CT could also be helpful in the evaluation of noninfectious lung disease. Preliminary studies by Jones et al. [16] used FDG-PET to assess neutrophil activity and 11C-PK11195 uptake to assess macrophage accumulation in six patients with COPD, six chronic asthmatics and five age-matched normal control subjects. They found that FDG uptake was greater in COPD than in normal subjects, with no increase in asthmatics.
Thus, they supported PET as a tool to provide an assessment of lung inflammatory cell activity in vivo, differentiation of patients with smoking-related COPD from those with asthma, and a potential marker of persistent lung inflammation. This last point is particularly relevant in studies that aim to prevent disease progression in COPD or testing anti-inflammatory treatments.
Pneumoconiosis
Pneumoconiosis is a parenchymal lung reaction to the inhalation and accumulation of noxious dust, such as iron, tin or barium, generally resulting from occupational exposure. Pneumoconioses include coal worker's silicosis, asbestosis and berylliosis. As shown by a number of reports, FDG-PET studies reveal increased uptake in this kind of pneumopathy [54–56]. Inhaled mineral dusts and fibres can cause chronic pulmonary inflammation, often leading to permanent scarring with loss of function by mechanisms not yet fully understood. From a clinicopathological point of view, this reaction could be mainly characterised by fibrosis or by aggregates of particle-laden macrophages with minimal or absent fibrosis, which is typically seen with inert dusts.
PET could represent a valuable tool for monitoring inflammatory processes in situ, and quantifying the biochemical and cellular responses involved. This point is particularly relevant in those conditions usually diagnosed in late stages, when therapeutic intervention may not be effective.
As reported in studies performed in animal models of fibrotic and nonfibrotic pulmonary responses to particulate instillation reproducing lung histological alterations typical of pneumoconiosis, PET could investigate different components of the inflammatory response through the use of different tracers.
In particular, FDG could track glucose uptake by neutrophils, which is responsible for an increased signal during pulmonary inflammation associated with lung scarring in cases of persistent high uptake. The radio-ligand 11C-R-PK11195 binds to benzodiazepine-like receptors abundant in macrophages and could point out poor macrophage clearance areas with a higher risk of developing fibrosis [57].
However, for the evaluation of specific kinds of pneumoconiosis, such as silicosis, that are mainly characterised by active fibrosis, a radiotracer that may selectively localise to fibroblasts and not inflammatory cells would be more appropriate. In this setting, as demonstrated by a preliminary study in a rabbit model of induced silicosis [58], PET imaging with labelled fluoroproline could represent the answer to this question, offering the possibility of providing an early and specific diagnosis.
Autoradiographic analysis in animal models showed tritiated or 14C-labelled proline uptake at the alveolar interstitial sites of fibroblast collagen synthesis, suggesting that a major fraction of the proline is taken up as a result of collagen synthesis. Obviously, further analyses are needed to better clarify whether the compound is trapped by the pulmonary interstitial fibroblasts or by the alveolar inflammatory cells as a consequence of inflammatory reaction. A biochemical assay of the fractional amount of fluoroproline sequestered in the lungs as procollagen or an autoradiographic study to evaluate the location of the accumulated fluoroproline at a microanatomic level could be useful to solve this question.
Finally, the increased FDG uptake in nononcological conditions also has to be considered whenever FDG-PET/CT is used in the diagnosis of malignancy in patients with underlying disease such as pneumoconiosis. In this setting, CT imaging probably remains the most appropriate approach.
Sarcoidosis
Sarcoidosis is a multisystem, noncaseating granulomatous disease with unknown aetiology that can affect virtually any organ in the body. It usually starts with mediastinal and hilar lymphadenopathy, followed by lung parenchymal involvement, which is the most frequently affected anatomic site.
Assessment of granulomatous inflammatory activity and extent of chronic sarcoidosis remains a challenge. For this purpose, FDG-PET could contribute significantly.
Due to the presence of activated leukocytes, macrophages and CD4+ T-lymphocytes together with epithelioid and multinucleated giant cells [59], both pathological lungs and thoracic lymph nodes can show increased FDG uptake (figure 2) [60, 61], and it has been suggested that intensity of FDG uptake may reflect disease activity [62]. In patients with sarcoidosis, FDG-PET has been demonstrated to be superior to conventional nuclear 67Ga-citrate scintigraphy, showing higher sensitivity (90–100%), minimal radiation exposure and shorter acquisition time [62–65]. It was also able to identify occult disease sites that were not detected clinically or by conventional imaging and CT in 15% of patients [59].
FIGURE 2.
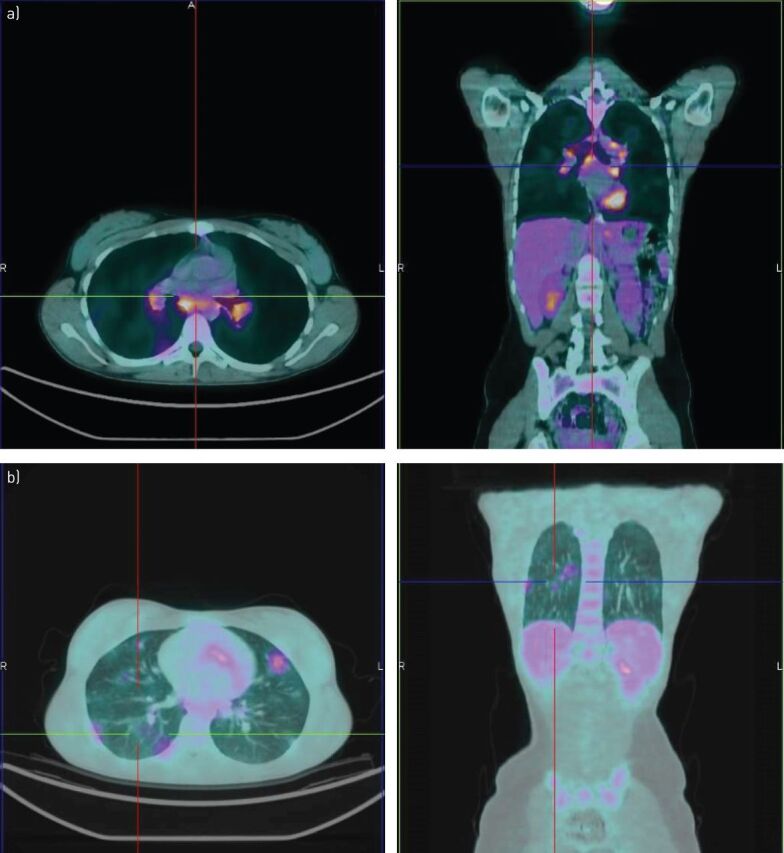
Intense fluorodeoxyglucose uptake within a) mediastinal lymph node and b) pulmonary localisations of sarcoidosis in a 45-year-old female.
Furthermore, in the diagnostic phase, FDG-PET/CT may be a substitute for invasive bronchoalveolar lavage (BAL) [66]. As reported in several studies [61], the extent of metabolic activity in the mediastinum or hila, expressed as SUVmax, correlates with the lymphocytosis and CD4/CD8 ratio, whereas the degree of metabolic activity in the lung parenchyma correlates with the number of neutrophils.
FDG-PET/CT was found to be more sensitive for assessing active sarcoidosis in comparison with serological markers of disease activity (i.e. angiotensin-converting enzyme, soluble IL-2 receptor and chitotriosidase), even if the latter correlate well with positive FDG-PET findings [61].
Thus, serological inflammatory markers should be measured first to assess the disease activity in patients with persistent symptoms. However, since negative serological findings do not exclude the presence of inflammatory activity, FDG-PET/CT may be recommended for use as in symptomatic patients [60].
Despite these well described applications (assessment of inflammatory active sarcoidosis in patients with prolonged symptoms, detecting extrathoracic sites of active sarcoidosis, and identifying occult sites and sites suitable for biopsy), FDG-PET/CT could be used for other clinical indications such as planning therapy and monitoring treatment response, and for the follow-up of patients with chronic persistent sarcoidosis.
In fact, the natural course of sarcoidosis in each patient is quite unpredictable and assessment of the degree of inflammatory activity plays a critical role in treatment planning. Identification of patients who need corticosteroid therapy or in whom the dose of the drug should be modified remains of crucial importance in order to ensure an adequate treatment in those patients in whom progression of the disease may be fatal, but also to reduce significant side-effects related to the inappropriate use of steroids and dosage level.
Furthermore, positive PET findings in patients with thoracic sarcoidosis seem to be associated with impaired lung function [67]: FDG uptake has been shown to correlate significantly with functional markers of disease severity, such as diffusing capacity for carbon monoxide and forced vital capacity [61]. This could give this technique potential prognostic value.
Finally, FDG-PET is also a valuable tool for assessing residual activity in patients with fibrotic pulmonary sarcoidosis [68, 69], contributing to disease monitoring and treatment response evaluation. Interestingly, FDG-PET is also able to detect recurrent sarcoidosis in transplanted lungs [70].
Beyond FDG, other PET tracers could be useful for assessing disease activity in sarcoidosis. Yamada et al. [71] evaluated 11C-methionine (Met) along with FDG in 31 patients with sarcoidosis in a prospective study. These authors noted that the ratio of FDG to Met before therapy could predict the post-treatment course of the disease. In particular, the response rate was higher in the FDG-dominant group (FDG/Met uptake ratio ≥2) with respect to the Met-dominant group (FDG/Met uptake ratio <2) (78% versus 38%, respectively). They concluded that the FDG/Met uptake ratio by PET studies may reflect the differential granulomatous state, suggesting a possible role of this parameter in pre-treatment evaluation of these patients.
In conclusion, FDG-PET/CT imaging allows complete morpho-functional mapping of active inflammatory sites and detection of treatment response in patients with sarcoidosis, especially in certain conditions such as in atypical, complex and multisystem forms of the disease [72].
Obviously, the mandatory prerequisite for all FDG-PET evaluations is an adequate and detailed knowledge about medical history to avoid that sarcoidosis is mistakenly interpreted as malignancy.
Cystic fibrosis
The inappropriate activity of macrophages and neutrophils is known to be involved in some common lung diseases. Cystic fibrosis (CF) represents another example of a pulmonary disease in which neutrophils are the predominant inflammatory cells in the lungs, and contribute to the progression of the illness [73, 74] by secreting various pro-inflammatory mediators, releasing potent proteases and producing reactive oxygen species. Thus, the optimal therapy for CF should be able to counteract the deleterious effects of neutrophil activation without impairing host defences against infection.
Chen and Schuster[75] showed significant differences between the rate of FDG uptake by the lungs of 20 adult patients with CF versus seven adult normal volunteers and, similarly to Labiris et al. [76], suggested that FDG-PET may discriminate patients with more rapidly declining lung function. In particular, they found that patients with CF and higher rates of decline in pulmonary function have higher numbers of neutrophils in BAL and higher PET-measured rates of FDG uptake.
In patients with CF, they also observed an increased FDG uptake in the upper lung zones compared to the lower lung zones. This regional variation of FDG lung uptake may reflect a greater inflammation in the more apical portions of the lungs, consistent with studies using chest radiography or high-resolution chest CT showing greater involvement in the upper lung zones of patients with CF [77, 78]. Therefore, also in this setting, PET imaging could be used as a noninvasive alternative to identify, quantify and monitor airway inflammation, and thus recognise patients who may benefit from more aggressive anti-inflammatory treatment (i.e. patients with rapidly deteriorating lung function).
In fact, the ability to identify and quantify local pulmonary inflammation could be a major advantage of PET imaging techniques over currently available methods of monitoring airway inflammation, potentially detecting anti-inflammatory effects of therapy intervention, as recently demonstrated in mice [79] and in humans [80]. Individual measurements of forced expiratory volume in 1 s, which may be highly variable depending on acute and chronic factors, may not be useful as a surrogates for testing therapies intended to modify the inflammatory burden in patients with CF. Nevertheless, PET can reveal inflammatory changes in the early phase of the disease preceding structural changes typical of the later stages that are well identifiable on CT scanning.
Acute lung injury and acute respiratory distress syndrome
Acute lung injury (ALI), associated with the acute respiratory distress syndrome (ARDS), is an inflammatory process of the lungs that causes significant morbidity and mortality in critically ill patients [81], with mechanisms that are not fully understood. However, neutrophilic inflammation is a key feature in the majority of cases of ALI. Accordingly, some authors support the hypothesis that tissue injury results from neutrophil activation after an initiating stimulus, and subsequent recruitment of these cells to the lungs, oxidant production and protease release [82]. Differently from nonspecific and sometimes invasive current methods (chest radiography, X-ray CT scans and BAL), FDG-PET imaging could potentially give quantitative information about neutrophil trafficking and kinetics [75], and contribute to our understanding of the pathophysiology of ALI.
Simon et al. [83] first recognised in 1947 that the injured lung is metabolically distinct from the normal lung. Later, FDG-PET was used to evaluate the inflammatory response in some experimental models of lung injury [84–87]. More recently, there has been growing interest in the use of FDG-PET to measure local lung inflammation in ALI with PET [88].
In particular, De Prost et al. [89] tried to determine whether local FDG phosphorylation rate and volume of distribution were sensitive to the initial regional inflammatory response during early ALI. 12 sheep were subjected to homogeneous unilateral surfactant depletion and were mechanically ventilated for 4 h while receiving intravenous endotoxin or not. FDG-PET emission scans were then acquired, and FDG phosphorylation rate and distribution volume were calculated with a four-compartment model. They showed that FDG uptake increased in the endotoxin group and surfactant-depleted sheep, with a topographically heterogeneous distribution. The increase in FDG uptake in the endotoxin group was related both to increases in the FDG phosphorylation rate and to distribution volume. FDG distribution volume increased with infiltrating neutrophils, and phosphorylation rate with the regional expression of inflammatory cytokines such as IL-1β, IL-8 and IL-10. Thus, they concluded that pulmonary glucidic metabolism depended on the mechanism of injury, and appeared to be additive to endotoxaemia and surfactant depletion. Analogous studies in patients also confirmed the role of FDG-PET as a valuable imaging biomarker of early lung injury.
The first available case report concerning FDG-PET imaging during ARDS in humans reported an intense metabolic activity in the lungs [90]. FDG uptake rate was increased across the entire lung density spectrum, suggesting that inflammatory cell activation also involves the normally aerated parenchyma [91]. However, the magnitude of FDG uptake and its topographical distribution varied considerably, with some ARDS patients showing activity predominantly in consolidated regions and others in normally aerated regions. Rodrigues et al. [92] studied eight patients with pulmonary contusion. He found a “diffuse” uptake pattern of FDG at 24–72 h after blunt thoracic trauma or pulmonary contusion (i.e. involving both nonaerated or poorly aerated, and normally aerated areas on CT) was related to the development of ARDS. However, patients with uptake confined to areas of weak or absent aeration did not subsequently develop ALI. This observation supports once again the predictive value of FDG-PET during the early stages of ALI.
Another radiopharmaceutical that could provide a useful contribution in this setting is represented by 68Ga-citrate. In particular, this radiopharmaceutical could explore vascular permeability, which is typically increased in ARDS and not easily detectable by conventional techniques [93, 94].
In conclusion, PET/CT can explore the pathophysiology of ALI/ARDS, allowing noninvasive quantitative assessment of regional pulmonary function and anti-inflammatory therapies effects, and providing a functional evaluation complementary to the morphological changes detectable with conventional radiological imaging techniques. However, whether PET will be used only as a research tool or as part of clinical patient management is still unclear.
Pulmonary Langerhans' cell histiocytosis
Langerhans's cell histiocytosis (LCH) is a rare disease. The aetiology is unknown but LCH is associated with abnormal function of regulatory T-cell, macrophage and/or cytokine-mediated process [95, 96].
Past studies demonstrated the role of immune dysfunction in the pathogenesis of LCH development. Although pro-inflammatory cytokines IL-17A was found in LCH lesions, the association has yet to be validated [97]. Studies suggest the involvement of vascular endothelial growth factor in LCH development while others found evidence of abnormal apoptosis proteins [98–100].
Due to the inflammatory base reactivity involved in LCH development, FDG-PET/CT can be helpful in its clinical assessment, including monitoring response to therapy [101].
Organ transplantation
Clinical evidence on the role of PET/CT in after surgical organ transplantation is lacking, but previous publications have pointed out that FDG-PET/CT could be useful and potentially reduce the number of biopsies required during the follow-up periods of patients after organ transplantation, including transplanted lungs, in distinguishing infection and rejection. In patients with lung transplants, FDG-PET is useful in differentiating pulmonary infection from acute rejection as the latter does not elicit as high a neutrophilic response to infection [102].
Future directions
Differentiating infection from malignancy using FDG-PET/CT could be a hard task. SUV is the most commonly used semiquantitative parameter in routine practice for assessing the degree of FDG accumulation within normal tissues and abnormal lesions. This value represents the radiopharmaceutical activity concentration in a region of interest drawn around a particular structure (in megabecquerels per millilitre) divided by the injected activity (in megabecquerels), in proportion to lean body weight [103]. Due to the numerous factors that can influence SUV (i.e. patient size, plasma glucose levels, type of PET camera, duration of uptake time, injected activity and method of image reconstruction), this parameter should be used with caution.
Although SUV measurement is usually used as a semiquantitative method for distinguishing between malignant and benign lesions, it is clear that there is quite often an overlap between SUVs of active inflammatory processes (both of infectious and noninfectious origin) with those of malignant lesions. In the majority of cases, visual assessment of the uptake pattern combined with semiquantitative analysis, clinical presentation and radiological findings allow a more accurate discrimination of benign and malignant findings. Furthermore, in specific clinical settings where there is suspicion of an infectious process, the possibility of supplementing the high sensitivity of FDG-PET with the upper specificity of other nuclear medicine techniques, such as scintigraphy with labelled leukocytes, should be considered.
Dual time-point imaging (DTPI) is recommended as a useful method for distinguishing acute inflammatory reaction from malignancy [104–106]. The rate of dephosphorylation of FDG-6-phosphate is responsible for the different behaviour of FDG uptake between malignant and benign lesions in dual-time FDG-PET imaging [107]: in malignancy, FDG activity will be persistently high after peak uptake in a time activity curve that is unlike that in inflammatory and infective conditions.
However, the real adjunct value of this approach has not been fully clarified yet. Differences in FDG uptake may be related to the type of inflammatory cells within the lesion and DTPI could be less accurate in discriminating chronic granulomatous infection like tuberculosis from cancer. Furthermore, the technique is time-consuming and is not applicable to all patients in the daily routine.
Another important field of investigation is the discovery of new biological radiopharmaceuticals. There are several PET radiopharmaceuticals that have been investigated targeting membrane markers, inflammatory cells, cytokines and vascular reactions in inflammation. Translocator protein, a potential imaging target that is found in macrophages, neutrophils and lymphocytes [108–110], has been used to image inflammatory lung diseases in previous experiments [111].
Besides that, somatostatin receptors (SSTRs) can also be found in abundance in activated lymphocytes and macrophages. Thus, SSTR analogues labelled with 68Ga-citrate, a positron emitter, are ideal candidate radiopharmaceuticals for imaging infectious diseases [112, 113].
Another radiopharmaceutical of interest in this setting is 11C-acetate, which accumulates in tumours but not in inflammatory lesions, thus potentially contributing to differentiating inflammation from neoplasms in patients with tuberculosis. Finally, inflammatory cytokines like cyclo-oxygenase, matrix metalloproteinases, IL-2 and tumour necrosis factor-α are being validated as a potential inflammatory and infective imaging targets in animal models.
In conclusion, PET/CT is a relatively new imaging modality that could play a significant role in the evaluation of inflammatory and infectious lung disease, improving patient management. However, the full potential of this imaging modality remains to be fully exploited and emerging new tracers will be useful for this purpose.
Footnotes
Conflict of interest: None declared.
Provenance: Submitted article, peer reviewed
References
- 1.Phelps ME, Mazziotta JC. Positron emission tomography: human brain function and biochemistry. Science 1985; 228: 799–809. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson DG, Greenberg JH. Positron emission tomography of the brain. Comput Med Imaging Graph 1989; 13: 61–79. [DOI] [PubMed] [Google Scholar]
- 3.Nolop KB, Rhodes CC, Brudin LH, et al. . Glucose utilization in vivo by human pulmonary neoplasm. Cancer 1987; 60: 2682–2689. [DOI] [PubMed] [Google Scholar]
- 4.Patz EF Jr, Goodman PC. Positron emission tomography imaging of the thorax. Radiol Clin North Am 1994; 32: 811–823. [PubMed] [Google Scholar]
- 5.Pauwels EK, Ribeiro MJ, Stoot JH, et al. . FDG accumulation and tumor biology. Nucl Med Biol 1998; 25: 317–322. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher BM, Fowler JS, Gutterson NI, et al. . Metabolic trapping as a principle of radiopharmaceutical design: some factors responsible for the biodistribution of [18F] 2-deoxy-2-fluoro-d-glucose. J Nucl Med 1978; 19: 1154–1161. [PubMed] [Google Scholar]
- 7.Wahi RL, Hutchins CD, Buchsbaum DJ, et al. . 18F-2-deoxy-2-fluoro-d-glucose uptake into human tumor xenografts: feasibility studies for cancer imaging with positron emission tomography. Cancer 1991; 67: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 8.Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta 1974; 355: 77–104. [DOI] [PubMed] [Google Scholar]
- 9.Bell GI, Burant CF, Takeda J, et al. . Structure and function of mammalian facilitative sugar transporters. J Biol Chem 1993; 268: 19161–19164. [PubMed] [Google Scholar]
- 10.Mochizuki T, Tsukamoto E, Kuge Y, et al. . FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med 2001; 42: 1551–1555. [PubMed] [Google Scholar]
- 11.Kubota R, Yamada S, Kubota K, et al. . Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992; 33: 1972–1980. [PubMed] [Google Scholar]
- 12.Zhuang H, Alavi A. 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med 2002; 32: 47–59. [DOI] [PubMed] [Google Scholar]
- 13.Mamede M, Higashi T, Kitaichi M, et al. . [18F]FDG uptake and PCNA, Glut-1, and hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia 2005; 7: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahara T, Ichiya Y, Kuwabara Y, et al. . High [18F]fluorodeoxyglucose uptake in abdominal abscesses: a PET study. J Comput Assisi Tomogr 1989; 13: 829–831. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki M, Ichiya Y, Kuwabara Y, et al. . Ringlike uptake of [18F]FDG in brain abscess: a PET study. J Comput Assist Tomogr 1990; 14: 486–487. [PubMed] [Google Scholar]
- 16.Jones HA, Marino PS, Shakur BH, et al. . In vivo assessment of lung inflammatory cell activity in patients with COPD and asthma. Eur Respir J 2003; 21: 567–573. [DOI] [PubMed] [Google Scholar]
- 17.Jones HA, Sriskandan S, Peters AM, et al. . Dissociation of neutrophil emigration and metabolic activity in lobar pneumonia and bronchiectasis. Eur Respir J 1997; 10: 795–803. [PubMed] [Google Scholar]
- 18.Jones HA, Cadwallader KA, White JF, et al. . Dissociation between respiratory burst activity and deoxyglucose uptake in human neutrophil granulocytes: Implications for interpretation of 18F-FDG PET images. J Nucl Med 2002; 43: 652–657. [PubMed] [Google Scholar]
- 19.Kosterink JG. Positron emission tomography in the diagnosis and treatment management of tuberculosis. Curr Pharm Des 2011; 17: 2875–2880. [DOI] [PubMed] [Google Scholar]
- 20.Kim IJ, Lee JS, Kim SJ, et al. . Double-phase 18F-FDG PET-CT for determination of pulmonary tuberculoma activity. Eur J Nucl Med Mol Imaging 2008; 35: 808–814. [DOI] [PubMed] [Google Scholar]
- 21.Ichiya Y, Kuwabara Y, Sasaki M, et al. . FDG-PET in infectious lesions: the detection and assessment of lesion activity. Ann Nucl Med 1996; 10: 185–191. . [DOI] [PubMed] [Google Scholar]
- 22.Sathekge M, Maes A, Kgomo M, et al. . Impact of FDG PET on the management of TBC treatment: a pilot study. Nuklearmedizin 2010; 49: 35–40. [DOI] [PubMed] [Google Scholar]
- 23.Jamar F, Buscombe J, Chiti A, et al. . EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med 2013; 54: 647–658. [DOI] [PubMed] [Google Scholar]
- 24.Soussan M, Brillet PY, Mekinian A, et al. . Patterns of pulmonary tuberculosis on FDG-PET/CT. Eur J Radiol 2012; 81: 2872–2876. [DOI] [PubMed] [Google Scholar]
- 25.Yang CM, Hsu CH, Lee CM, et al. . Intense uptake of [F-18]-fluoro-2 deoxy-d-glucose in active pulmonary tuberculosis. Ann Nucl Med 2003; 17: 407–410. [DOI] [PubMed] [Google Scholar]
- 26.Bakheet SM, Powe J, Ezzat A, et al. . F-18-FDG uptake in tuberculosis. Clin Nucl Med 1998; 23: 739–742. [DOI] [PubMed] [Google Scholar]
- 27.Goo JM, Im JG, Do KH, et al. . Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology 2000; 216: 117–121. [DOI] [PubMed] [Google Scholar]
- 28.Murawski AM, Gurbani S, Harper JS, et al. . Imaging the evolution of reactivation pulmonary tuberculosis in mice using 18F-FDG PET. J Nucl Med 2014; 55: 1726–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yago Y, Yukihiro M, Kuroki H, et al. . Cold tuberculous abscess identified by FDG PET. Ann Nucl Med 2005; 19: 515–518. [DOI] [PubMed] [Google Scholar]
- 30.Scharko AM, Perlman SB, Pyzalski RW, et al. . Whole-body positron emission tomography in patients with HIV-1 infection. Lancet 2003; 362: 959–961. [DOI] [PubMed] [Google Scholar]
- 31.Sathekge M, Maes A, Kgomo M, et al. . Fluorodeoxyglucose uptake by lymph nodes of HIV patients is inversely related to CD4 cell count. Nucl Med Commun 2010; 31: 137–140. [DOI] [PubMed] [Google Scholar]
- 32.Aziz MA, Wright A, Laszlo A, et al. . Epidemiology of antituberculosis drug resistance (the Global Project on Antituberculosis Drug Resistance Surveillance): an updated analysis. Lancet 2006; 368: 2142–2154. [DOI] [PubMed] [Google Scholar]
- 33.Abe M, Kobashi Y, Mouri K, et al. . Solitary pulmonary nodule due to Mycobacterium kansasii. Intern Med 2011; 50: 775–778. [DOI] [PubMed] [Google Scholar]
- 34.Spiliopoulou I, Foka A, Bounas A, et al. . Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg 2014; 69: 229–231. [DOI] [PubMed] [Google Scholar]
- 35.Malkin J, Shrimpton A, Wiselka M, et al. . Olecranon bursitis secondary to Mycobacterium kansasii infection in a patient receiving infliximab for Behçet's disease. J Med Microbiol 2009; 58: 371–373. [DOI] [PubMed] [Google Scholar]
- 36.Demura Y, Tsuchida T, Uesaka D, et al. . Usefulness of 18F-fluorodeoxyglucose positron emission tomography for diagnosing disease activity and monitoring therapeutic response in patients with pulmonary mycobacteriosis. Eur J Nucl Med Mol Imaging 2009; 36: 632–639. [DOI] [PubMed] [Google Scholar]
- 37.Del Giudice G, Bianco A, Cennamo A, et al. . Lung and nodal involvement in nontuberculous mycobacterial disease: PET/CT Role. Biomed Res Int 2015; 2015: 353202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis SL, Nuermberger EL, Um PK, et al. . Noninvasive pulmonary [18F]-2-fluoro-deoxy-d-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob Agents Chemother 2009; 53: 4879–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Namkoong H, Fujiwara H, Ishii M, et al. . Immune reconstitution inflammatory syndrome due to Mycobacterium avium complex successfully followed up using 18F-fluorodeoxyglucose positron emission tomography-computed tomography in a patient with human immunodeficiency virus infection: a case report. BMC Med Imaging 2015; 18: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichiya Y, Kuwabara Y, Sasaki M, et al. . FDG-PET in infectious lesions: the detection and assessment of lesion activity. Ann Nucl Med 1996; 10: 185–191. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe S, Nakamura Y, Kariatsumari K, et al. . Pulmonary paragonimiasis mimicking lung cancer on FDG-PET imaging. Anticancer Res 2003; 23: 3437–3440. [PubMed] [Google Scholar]
- 42.Ozsahin H, von Planta M, Müller I, et al. . Successful treatment of invasive aspergillosis in chronic granulomatous disease by bone marrow transplantation, granulocyte colony-stimulating factor-mobilized granulocytes, and liposomal amphotericin-B. Blood 1998; 92: 2719–2724. [PubMed] [Google Scholar]
- 43.Franzius C, Biermann M, Hülskamp G, et al. . Therapy monitoring in aspergillosis using F-18 FDG positron emission tomography. Clin Nucl Med 2001; 26: 232–233. [DOI] [PubMed] [Google Scholar]
- 44.Reuter S, Schirrmeister H, Kratzer W, et al. . Pericystic metabolic activity in alveolar echinococcosis: assessment and follow-up by positron emission tomography. Clin Infect Dis 1999; 29: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 45.Reuter S, Buck A, Manfras B, et al. . Structured treatment interruption in patients with alveolar echinococcosis. Hepatology 2004; 39: 509–517. [DOI] [PubMed] [Google Scholar]
- 46.Ehrhardt AR, Reuter S, Buck AK, et al. . Assessment of disease activity in alveolar echinococcosis: a comparison of contrast enhanced ultrasound, three-phase helical CT and [18F] fluorodeoxyglucose positron emission tomography. Abdom Imaging 2007; 32: 730–736. [DOI] [PubMed] [Google Scholar]
- 47.Stumpe KD, Renner-Schneiter EC, Kuenzle AK, et al. . F-18-fluorodeoxyglucose (FDG) positron-emission tomography of Echinococcus multilocularis liver lesions: prospective evaluation of its value for diagnosis and follow-up during benzimidazole therapy. Infection 2007; 35: 11–18. [DOI] [PubMed] [Google Scholar]
- 48.Bleeker-Rovers CP, Warris A, Drenth JP, et al. . Diagnosis of Candida lung abscesses by 18F-fluorodeoxyglucose positron emission tomography. Clin Microbiol Infect 2005; 11: 493–495. [DOI] [PubMed] [Google Scholar]
- 49.Win Z, Todd J, Al-Nahhas A. FDG-PET imaging in Pneumocystis carinii pneumonia. Clin Nucl Med 2005; 30: 690–691. [DOI] [PubMed] [Google Scholar]
- 50.Maldonado F, Daniels CE, Hoffman EA, et al. . Focal organizing pneumonia on surgical lung biopsy: causes, clinicoradiologic features, and outcomes. Chest 2007; 132: 1579–1583. [DOI] [PubMed] [Google Scholar]
- 51.Shin L, Katz DS, Yung E. Hypermetabolism on F-18 FDG PET of multiple pulmonary nodules resulting from bronchiolitis obliterans organizing pneumonia. Clin Nucl Med 2004; 29: 654–656. [DOI] [PubMed] [Google Scholar]
- 52.Orino K, Kawamura M, Hatazawa J, et al. . Efficacy of F-18 fluorodeoxyglucose positron emission tomography (FDG-PET) scans in diagnosis of pulmonary nodules. Jpn J Thorac Cardiovasc Surg 1998; 46: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 53.Marques G, Annweiler T, Raoux D, et al. . Nodular presentation of a cryptogenic organizing pneumonia. Rev Pneumol Clin 2011; 67: 314–317. [DOI] [PubMed] [Google Scholar]
- 54.Bakheet SM, Powe J. Benign causes of 18-FDG uptake on whole body imaging. Semin Nucl Med 1998; 28: 352–358. [DOI] [PubMed] [Google Scholar]
- 55.Strauss L. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med 1996; 23: 1409–1415. [DOI] [PubMed] [Google Scholar]
- 56.Reichert M, Bensadoun ES. PET imaging in patients with coal workers pneumoconiosis and suspected malignancy. J Thorac Oncol 2009; 4: 649–651. [DOI] [PubMed] [Google Scholar]
- 57.Jonesa H, Hamacherb K, Clarkc J, et al. . Positron emission tomography in the quantification of cellular and biochemical responses to intrapulmonary particulates. Toxicol Appl Pharmacol 2005; 207: 230–236. [DOI] [PubMed] [Google Scholar]
- 58.Wallace WE, Gupta NC, Hubbs AF, et al. . Cis-4-[18F]fluoro-l-proline PET imaging of pulmonary fibrosis in a rabbit model. J Nucl Med 2002; 43: 413–420. [PubMed] [Google Scholar]
- 59.Teirstein AS, Machac J, Almeida O, et al. . Results of 18 whole-body fluorodeoxyglucose positron emission tomography scansin137 patients with sarcoidosis. Chest 2007; 132: 1949–1953. [DOI] [PubMed] [Google Scholar]
- 60.Mostard RLM, Vöö S, van Kroonenburgh MJ, et al. . Inflammatory activity assessment by F18FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med 2011; 105: 1917–1924. [DOI] [PubMed] [Google Scholar]
- 61.Keijsers RG, Verzijlbergen FJ, van den Bosch JM, et al. . 18F-FDG PET as a predictor of pulmonary function in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2011; 28: 123–129. [PubMed] [Google Scholar]
- 62.Brudin LH, Valind SO, Rhodes CG, et al. . Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. Eur J Nucl Med 1994; 21: 297–305. [DOI] [PubMed] [Google Scholar]
- 63.Nishiyama Y, Yamamoto Y, Fukunaga K, et al. . Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med 2006; 47: 1571–1576. [PubMed] [Google Scholar]
- 64.Prager E, Wehrschuetz M, Bisail B, et al. . Comparison of 18F-FDG and 67Ga-citrate in sarcoidosis imaging. Nuklearmedizin 2008; 47: 18–23. [DOI] [PubMed] [Google Scholar]
- 65.Keijsers RG, Grutters JC, Thomeer M, et al. . Imaging the inflammatory activity of sarcoidosis: sensitivity and inter observer agreement of 67Ga imaging and 18F-FDG PET. Q J Nucl Med Mol Imaging 2011; 55: 66–71. [PubMed] [Google Scholar]
- 66.Drent M, Jacobs JA, de Vries J, et al. . Does the cellular bronchoalveolar lavage fluid profile reflect the severity of sarcoidosis? Eur Respir J 1999; 13: 1338–1344. [DOI] [PubMed] [Google Scholar]
- 67.Keijsers RG, Verzijlbergen FJ, Oyen WJ, et al. . 18F-FDG PET, genotype-corrected ACE and sIL-2R in newly diagnosed sarcoidosis. Eur J Nucl Med Mol Imaging 2009; 36: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 68.Mostard RL, Verschakelen JA, van Kroonenburgh MJ, et al. . Severity of pulmonary involvement and 18F-FDG PET activity in sarcoidosis. Respir Med 2013; 107: 439–447. [DOI] [PubMed] [Google Scholar]
- 69.Teirstein AT, Morgenthau AS. “End-stage” pulmonary fibrosis in sarcoidosis. Mt Sinai J Med 2009; 76: 30–36. [DOI] [PubMed] [Google Scholar]
- 70.Kiatboonsri C, Resnick SC, Chan KM, et al. . The detection of recurrent sarcoidosis by FDG-PET in a lung transplant recipient. West J Med 1998; 168: 130–132. [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada Y, Uchida Y, Tatsumi K, et al. . Fluorine-18-fluorodeoxyglucose and carbon-11-methionine evaluation of lymphadenopathy in sarcoidosis. J Nucl Med 1998; 39: 1160–1166. [PubMed] [Google Scholar]
- 72.Braun JJ, Kessler R, Constantinesco A, et al. . 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging 2008; 35: 1537–1543. [DOI] [PubMed] [Google Scholar]
- 73.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol 1997; 24: 137–142. [DOI] [PubMed] [Google Scholar]
- 74.Döring G, Worlitzsch D. Inflammation in cystic fibrosis and its management. Paediatr Respir Rev 2000; 1: 101–106. [DOI] [PubMed] [Google Scholar]
- 75.Chen D, Schuster D. Positron emission tomography with [18F]fluorodeoxyglucose to evaluate neutrophil kinetics during acute lung injury. Am J Physiol Lung Cell Mol Physiol 2004; 286: L834–L840. [DOI] [PubMed] [Google Scholar]
- 76.Labiris NR, Nahmias C, Freitag AP, et al. . Uptake of 18fluorodeoxyglucose in the cystic fibrosis lung: a measure of lung inflammation? Eur Respir J 2003; 21: 848–854. [DOI] [PubMed] [Google Scholar]
- 77.Bonnel AS, Song SM, Kesavarju K, et al. . Quantitative air-trapping analysis in children with mild cystic fibrosis lung disease. Pediatr Pulmonol 2004; 38: 396–405. [DOI] [PubMed] [Google Scholar]
- 78.Maffessanti M, Candusso M, Brizzi F, et al. . Cystic fibrosis in children: HRCT findings and distribution of disease. J Thorac Imaging 1996; 11: 27–38. . [DOI] [PubMed] [Google Scholar]
- 79.Zhou Z, Kozlowski J, Schuster D. Physiologic, biochemical, and imaging characterization of acute lung injury in mice. Am J Respir Crit Care Med 2005; 172: 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maffessanti M, Candusso M, Brizzi F, et al. . Cystic fibrosis in children: HRCT findings and distribution of disease. J Thorac Imaging 1996; 11: 27–38. [DOI] [PubMed] [Google Scholar]
- 81.Hudson LD, Steinberg KP. Epidemiology of acute lung injury and ARDS. Chest 1999; 116: 74S–82S. [DOI] [PubMed] [Google Scholar]
- 82.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care 2001; 7: 1–7. [DOI] [PubMed] [Google Scholar]
- 83.Simon FP, Potts AM, Gerard RW. Metabolism of isolated lung tissue: normal and in phosgene poisoning. J Biol Chem 1947; 167: 303–311. [PubMed] [Google Scholar]
- 84.Jones HA, Clark RJ, Rhodes CG, et al. . In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Respir Crit Care Med 1994; 149: 1635–1639. [DOI] [PubMed] [Google Scholar]
- 85.Schroeder T, Vidal Melo MF, Musch G, et al. . PET imaging of regional 18F-FDG uptake and lung function after cigarette smoke inhalation. J Nucl Med 2007; 48: 413–419. [PubMed] [Google Scholar]
- 86.Monkman SL, Andersen CC, Nahmias C, et al. . Positive end-expiratory pressure above lower inflection point minimizes influx of activated neutrophils into lung. Crit Care Med 2004; 32: 2471–2475. [DOI] [PubMed] [Google Scholar]
- 87.Musch G, Venegas JG, Bellani G, et al. . Regional gas exchange and cellular metabolic activity in ventilator-induced lung injury. Anesthesiology 2007; 106: 723–735. [DOI] [PubMed] [Google Scholar]
- 88.Musch G, Harris RS, Vidal Melo MF, et al. . Mechanism by which a sustained inflation can worsen oxygenation in acute lung injury. Anesthesiology 2004; 100: 323–330. [DOI] [PubMed] [Google Scholar]
- 89.de Prost N, Feng Y, Wellman T, et al. . 18F-FDG kinetics parameters depend on the mechanism of injury in early experimental acute respiratory distress syndrome. J Nucl Med 2014; 55: 1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacene HA, Cohade C, Wahl RL. F-18 FDG PET/CT in acute respiratory distress syndrome: a case report. Clin Nucl Med 2004; 29: 786–788. [DOI] [PubMed] [Google Scholar]
- 91.Richter T, Bellani G, Harris RS, et al. . Effect of prone position on regional shunt, aeration and perfusion in experimental acute lung injury. Am J Respir Crit Care Med 2005; 172: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodrigues RS, Miller PR, Bozza FA, et al. . FDG-PET in patients at risk for acute respiratory distress syndrome: a preliminary report. Intensive Care Med 2008; 34: 2273–2278. [DOI] [PubMed] [Google Scholar]
- 93.Sandiford P, Province MA, Schuster DP. Distribution of regional density and vascular permeability in the adult respiratory distress syndrome. Am J Respir Crit Care Med 1995; 151: 737–742. [DOI] [PubMed] [Google Scholar]
- 94.Kaplan JD, Calandrino FS, Schuster DP. A positron emission tomographic comparison of pulmonary vascular permeability during the adult respiratory distress syndrome and pneumonia. Am Rev Respir Dis 1991; 143: 150–154. [DOI] [PubMed] [Google Scholar]
- 95.Kannourakis G, Abbas A. The role of cytokines in the pathogenesis of Langerhans cell histiocytosis. Br J Cancer Suppl 1994; 23: S37–S40. [PMC free article] [PubMed] [Google Scholar]
- 96.Senechal B, Elain G, Jeziorski E, et al. . Expansion of regulatory T cells in patients with Langerhans cell histiocytosis. PLoS Med 2007; 4: e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen CE, McClain KL. Interleukin-17A is not expressed by CD207+ cells in Langerhans cell histiocytosis lesions. Nat Med 2009; 15: 483–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dina A, Zahava V, Iness M. The role of vascular endothelial growth factor in Langerhans cell histiocytosis. J Pediatr Hematol Oncol 2005; 27: 62–66. [DOI] [PubMed] [Google Scholar]
- 99.Bank MI, Gudbrand C, Rengtved P, et al. . Immunohistochemical detection of the apoptosis-related proteins FADD, FLICE, and FLIP in Langerhans cell histiocytosis. J Pediatr Hematol Oncol 2005; 27: 301–306. [DOI] [PubMed] [Google Scholar]
- 100.Marchal J, Kambouchner M, Tazi A, et al. . Expression of apoptosis-regulatory proteins in lesions of pulmonary Langerhans cell histiocytosis. Histopathology 2004; 45: 20–28. [DOI] [PubMed] [Google Scholar]
- 101.Phillips M, Allen C, Gerson P, et al. . Comparison of FDG-PET scans to conventional radiography and bone scans in management of Langerhans cell histiocytosis. Pediatr Blood Cancer 2009; 52: 97–101. [DOI] [PubMed] [Google Scholar]
- 102.Jones H, Donovan T, Goddard M, et al. . Use of 18FDG-PET to discriminate between infection and rejection in lung transplant recipients. Transplantation 2004; 77: 1462–1464. [DOI] [PubMed] [Google Scholar]
- 103.de Langen AJ, Vincent A, Velasquez LM, et al. . Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med 2012; 53: 701–708. [DOI] [PubMed] [Google Scholar]
- 104.Matthies A, Hickeson M, Cuchiara A, et al. . Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med 2002; 43: 871–875. [PubMed] [Google Scholar]
- 105.Zhuang H, Pourdehnad M, Lambright ES, et al. . Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 2001; 42: 1412–1417. [PubMed] [Google Scholar]
- 106.Hustinx R, Smith IL, Benard F, et al. . Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from infammation and normal tissue in the head and neck. Eur J Nucl Med 1999; 26: 1345–1348. [DOI] [PubMed] [Google Scholar]
- 107.Kubota K, Furumoto S, Iwata R, et al. . Comparison of 18F-fluoromethylcholine and 2-deoxy-d-glucose in the distribution of tumor and inflammation. Ann Nucl Med 2006; 20: 527–533. [DOI] [PubMed] [Google Scholar]
- 108.Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (translocator protein 18 kDa) in microglia: from pathology to imaging. Prog Neurobiol 2006; 80: 308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van der Laken CJ, Elzinga EH, Kropholler MA, et al. . Noninvasive imaging of macrophages in rheumatoid synovitis using 11C-(R)-PK11195 and positron emission tomography. Arthritis Rheum 2008; 58: 3350–3355. [DOI] [PubMed] [Google Scholar]
- 110.Fujimura Y, Hwang PM, Trout Iii H, et al. . Increased peripheral benzodiazepine receptors in arterial plaque of patients with atherosclerosis: an autoradiographic study with [3H]PK 11195. Atherosclerosis 2008; 201: 108–111. [DOI] [PubMed] [Google Scholar]
- 111.Hatori A, Yui J, Yamasaki T, et al. . PET imaging of lung inflammation with [18F]FEDAC, a radioligand for translocator protein (18 kDa). PLoS One 2012; 7: 4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Petrik M, Haas H, Laverman P, et al. . 68Ga-triacetylfusarinine C and 68Ga-ferrioxamine E for Aspergillus infection imaging: uptake specificity in various microorganisms. Mol Imaging Biol 2014; 16: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petrik M, Franssen GM, Haas H, et al. . Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur J Nucl Med Mol Imaging 2012; 39: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]