Abstract
Generalised lymphatic anomaly (GLA), also known as lymphangiomatosis, is a rare disease caused by congenital abnormalities of lymphatic development. It usually presents in childhood but can also be diagnosed in adults. GLA encompasses a wide spectrum of clinical manifestations ranging from single-organ involvement to generalised disease. Given the rarity of the disease, most of the information regarding it comes from case reports. To date, no clinical trials concerning treatment are available. This review focuses on thoracic GLA and summarises possible diagnostic and therapeutic approaches.
Short abstract
Possible diagnostic and therapeutic approaches to generalised lymphatic anomaly (lymphangiomatosis) http://ow.ly/4n4pgU
Introduction
Generalised lymphatic anomaly (GLA) is an ultra-rare disorder characterised by the presence of multiple lymphangiomas infiltrating different tissues to various extents [1]. The disease, previously known as lymphangiomatosis, has been recently renamed by the International Society for the Study of Vascular Anomalies (ISSVA) in the updated classification of vascular anomalies [2]. Most of the information obtained about GLA comes from case reports or small case series. The condition presents a large spectrum of clinical manifestations and may involve a single organ system (e.g. diffuse pulmonary lymphangiomatosis in the lung) or, more frequently, multiple organs [1]. Within the thorax, GLA may involve the lungs, mediastinum, heart, pleura, thoracic duct and chest wall [3–7]. Although Gorham–Stout disease (GSD), a rare lymphatic disorder characterised by progressive osteolysis, shares many features with GLA, it is currently considered as a different entity [2, 8].
GLA appears to be caused by congenital abnormalities of lymphatic development [9–11]. It usually presents in childhood but can also be diagnosed in adults and seems to have no sex predilection [3, 12–15]. To date, no clinical trials have been conducted due to the rarity of the disease; treatment modalities have been reported, which require further investigation.
Pathology
GLA is characterised by the presence of lymphangiomas in different organs. As solitary lymphangiomas, they are likely due to a lymphatic developmental abnormality [9–11]; the influence of hormonal factors or a subtler defect requiring a longer growth period have been proposed to explain the presentation of GLA at an older age [1].
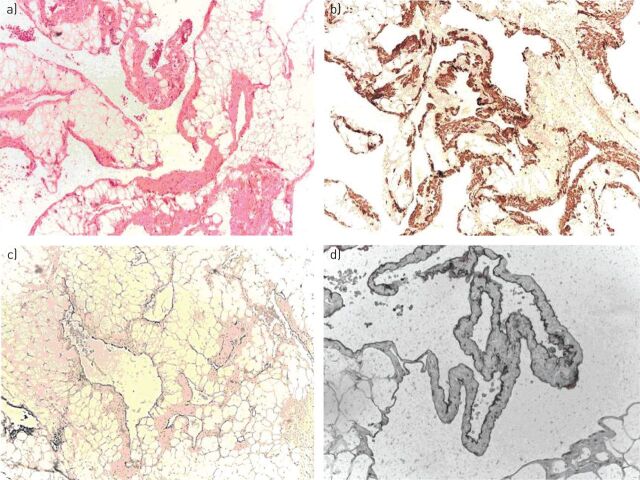
The histopathology of GLA resembles that of lymphangiomas, showing an increased number of dilated anastomosing lymphatic channels, lined by endothelial cells [4, 5]. The anastomosing spaces can be filled with chyle or eosinophilic material [1, 16, 17]. These lesions always have a benign appearance and are composed of mature cells, although they may infiltrate tissues [5] (figure 1a). Bone involvement is usually characterised by lytic lesions; bone biopsies of these lesions will show that they are lymphangiomas containing lymphatic fluid [1].
FIGURE 1.
a) Biopsy of the left parietal pleura in a case of generalised lymphatic anomaly showing complex proliferation of vascular spaces infiltrating fibroadipose tissue. Haematoxylin and eosin staining. b) In different areas, the walls of the lymphatic channels are formed by smooth muscle cells expressing desmin but not oestrogen and progesterone receptors. No reaction with the monoclonal antibody HMB-45 was found. c, d) The lymphatic endothelium is characterised by a diffuse and strong expression of D2-40, shown here at two different scales. Images courtesy of Barberis Massimo (Istituto Europeo di Oncologia, Milan, Italy).
Diffuse pulmonary lymphangiomatosis (DPL) usually involves both lungs and has no extrathoracic lymphatic involvement, although one case of a patient with single-lung involvement and histopathological findings compatible with DPL has recently been described [1, 18]. Histologically, DPL is characterised by a proliferation of complex anastomosing lymphatic channels with a significant expansion of pre-existing lymphatic routes within the lungs and mediastinum [3]. Compared with primary pulmonary lymphangiectasis, a substantial amount of collagen and spindle-shaped cells surround the endothelial-lined lymphatic channels. Haemosiderin-laden macrophages may be present in the adjacent lung parenchyma, which, in contrast to lymphangioleiomyomatosis (LAM), is always preserved with no evidence of cystic changes [19, 20].
The endothelial cells lining lymphatic channels in GLA react with anti-CD31 antibodies, which recognise platelet–endothelial cell adhesion molecule 1 [15, 17]. Lymphatic endothelial cells also react with the antibody D2-40, which recognises podoplanin, a more specific transmembrane protein regulated by the lymphatic transcription factor PROX1 [15, 21, 22] (figure 1c and d). Spindle cells variably express smooth muscle cell proteins, reflected in their positivity to antibodies that bind antigens commonly found in these cells such as actin, vimentin and desmin. However, in contrast to cells involved in LAM, they lack oestrogen receptor and glycoprotein gp100, which reacts with the monoclonal antibody HMB-45 [3, 23] (figure 1b). Usually, progesterone receptor is not found [24].
Clinical manifestations
Although GLA mainly presents in childhood [1, 12, 25], it has been diagnosed in patients in all age groups, up to even 80 years old [12, 13]. Males and females are affected similarly [1, 12, 13].
Thoracic GLA may occur as an isolated entity but it is more frequently a multisystem disorder [26] with bone lesions, splenic lesions, cervical involvement and skin involvement [27]. The bones are involved in >75% of patients with GLA [1]. Bone involvement, which can occur with or without lung involvement, can present with pain and pathological fractures but, in contrast to GSD, is characterised by lytic areas confined to the medullary cavity without extensive cortical destruction [28]. The prognosis for GLA is variable and depends on the extent of the disease and involvement of critical organs. Patients with liver, spleen and thoracic duct involvement usually have a poor prognosis because the lesions tend to be diffuse and are not accessible for surgical excision [11].
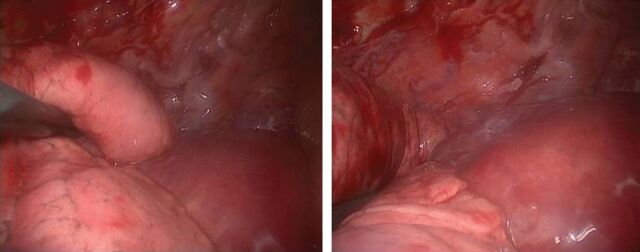
Within the thorax, single or multiple lymphangiomas may be discovered in the mediastinum, pleura, heart, lung and chest wall [1] (figure 2). Proliferation of lymphangiomas only within the lung leads to the rare syndrome of DPL [3].
FIGURE 2.
Thoracoscopic images showing two different views of a mass enveloping the thoracic aorta and lesions connected to the thoracic wall, diagnosed as lymphangiomas at histological examination of the biopsy. Images courtesy of Spaggiari Lorenzo and Gasparri Roberto (Istituto Europeo di Oncologia, Milan, Italy).
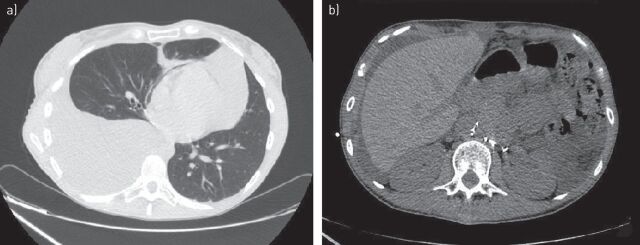
DPL, especially in paediatric populations, can often have a progressive evolution to respiratory insufficiency and death [15]. In a review of 53 cases of thoracic involvement in GLA from the literature, Alvarez et al. [27] reported that patients up to 16 years of age had a worse prognosis than older ones (39% versus 0% mortality). The clinical presentation in adults depends on the location and extent to which lymphangiomas have spread [15]. Nonspecific symptoms sometimes occur, such as mild wheezing, nonproductive cough, chest pain, chest tightness, shortness of breath, dyspnoea and the disease is often misdiagnosed as asthma or other respiratory illnesses [24]. Sometimes massive pleural effusion and progressive pulmonary infiltration cause respiratory failure [15]. Pleural effusions are often chylous, due to spontaneous rupture of diseased lymph vessels within the lymphangiomas [26], and patients may have associated chyloptysis [29], haemoptysis [6, 30, 31] or chylopericardium [32]. In some case reports, chylous ascites, protein wasting enteropathy, peripheral lymphoedema, hemihyperplasia and lymphopenia have been also described [1] (figure 3). DPL patients may rarely present with fever caused by a concurrent pulmonary infection [18]. Although the mechanism is not yet well understood [33], disseminated intravascular coagulation (DIC) has sometimes been associated with thoracic lymphangiomatosis [23, 27, 33]. It has been suggested that the coagulopathy may cause local fibrinolysin production by lymphangiomas [34] or that a mechanism similar to that of the coagulation abnormalities associated with venous malformations may be involved [27]. It has been reported that the presence of a coagulopathy is not always associated with a poor prognosis [27]. Recently, DIC has been associated with a new distinct lymphatic anomaly known as kaposiform lymphangiomatosis (KLA). KLA is characterised histologically by spindled lymphatic endothelial cells, and clinically by a worse progression with haemorrhagic effusions and haematological anomalies, particularly moderate thrombocytopenia (50–100 000 platelets per μL) [35–37].
FIGURE 3.
a) Massive chylous pleural effusion with contralateral shift of mediastinum and b) chylous ascites in a young patient with histological diagnosis of generalised lymphatic anomaly.
Diagnosis
Often, a definitive diagnosis of GLA is delayed because of the rarity of the disease and its different course in each patient [38]. Concomitant lytic bone lesions and chylothorax/pleural effusion could be indicative of this disease given that lymphangiomas are most commonly present in lung and bone [1]. In particular, because of the often slower course of GLA, making a diagnosis of DPL in adults is difficult [39].
Chest radiography can detect diffuse interstitial infiltrates and pleural effusions, which are nonspecific signs of DPL [15]. On high-resolution chest computed tomography, some common characteristics are suggestive, but are not pathognomonic, of DPL [15]. One such instance is the presence of bilateral smooth thickening of the interlobular septa and bronchovascular bundles, which indicates growth of anastomosing lymphatic vessels in the interlobular and central peribronchovascular interstitium [7, 40–42]. Frequent patchy ground-glass opacities may be found, which, according to some authors [3, 41], represent a recent haemorrhage and haemosiderin-laden macrophages in the air spaces [43]. Proliferation of lymphatic channels causes accumulation of lymphatic fluid in the mediastinal and hilar soft tissue; mediastinal soft tissue infiltration, however, seems to have no mass effect on mediastinal vessels [44]. Finally, bilateral pleural effusions are typical [17] and are sometimes chylous but often serosanguineous [41, 44]. These features are generally present in both lungs but Zhang et al. [18] reported a 24-year-old patient with DPL initially located in one lung.
DPL lesions are different from the pulmonary lesions of systemic disease. In systemic disease, Laverdiere et al. [45] described cystic lesions with linear opacities in both lungs. In another case of diffuse systemic lymphangiomatosis, massive chylothorax was reported without pulmonary parenchymal infiltrations [46].
A restrictive pattern is typical on pulmonary function testing although often, a mixed pattern is present [15].
Bronchoscopy is nonspecific, sometimes revealing airway mucosal erythema and oedema, as well as bronchial narrowing. In advanced cases, thin-walled vesicles containing chylous fluid have been found [17]. Although one case of DPL was diagnosed with transbronchial biopsy [7, 43], in most cases reported in the literature, the diagnosis was made with open-lung biopsy [3, 41, 47]. In cases of bone involvement, a bone biopsy may be considered. However, some authors advise not to perform rib biopsy because of the higher risk of developing refractory pleural effusions after this procedure [37].
To obviate the need for a surgical biopsy, other authors have relied on lymphography for a definitive diagnosis of GLA [48, 49]. Lymphangiography has helped differentiate lymphangiomas from haemangiomas and malignant tumours, and accurately evaluate the extension of lesions [1, 50]. However, it is a technique that may cause pulmonary complications since it requires the cannulation of a lymphatic vessel into which an oil-based dye is directly injected [51]. An alternative technique that has been used in children with chylous effusion is the intranodal lymphangiography, a less invasive method of lymphangiography with sonographically guided injection of contrast into a lymph node [52]. Some authors have described the efficacy of lymphoscintigraphy in evaluating disorders of the lymphatic system [53, 54]. Compared to lymphangiography, lymphoscintigraphy is minimally invasive and is not known to have any side-effects but lymphangiography provides more detailed images [33]. Magnetic resonance imaging (MRI) is also an accurate and safe modality to study lymphatic circulation and investigate the pathological lymphatic system of patients with minimal invasiveness and absence of radiation exposure [55]. In particular, lymphatic vessels can be clearly marked on heavily T2-weighted images because of the very slow lymphatic flow within lymphangiomatous malformations [55–57].
In the future, near-infrared fluorescence lymphatic imaging, nanotechnology-based MRI agents, and gene reporter technologies may represent new tools for studying the structure and function of the lymphatic system [58].
Differential diagnosis
Based on clinical and pathological features, GLA should be differentiated from other thoracic lymphatic disorders, some of which are similarly due to congenital errors of lymphatic development, including solitary lymphangiomas, lymphangiectasis and lymphatic dysplasia syndrome [1].
As in GLA, histologically, lymphangiomas are characterised by an increased number of dilated lymphatic channels, lined by endothelial cells. They usually present in early childhood; however, thoracic lymphangiomas may become clinically evident in adulthood or present as accidental findings because of their slow growth. Most thoracic lymphangiomas are found in the mediastinum, while intrapulmonary lesions are extremely rare [1]. Clinical manifestations generally become apparent due to compression of vital structures and/or are related to a secondary infection [59–61].
Primary pulmonary lymphangiectasis usually presents in early life with severe manifestations such as pulmonary hypoplasia and respiratory failure, and is often fatal [62–64]. Radiation therapy, surgery, infection, trauma or tumours impairing effective lymphatic drainage can cause secondary lymphangiectasis leading to respiratory distress at any age. Congenital heart disease in children and severe mitral valve disease in adults can also be associated with lymphangiectasis [65, 66]. Lymphangiectasis is characterised by a dilatation of lymphatic spaces, which in some cases can be cystic, with no evidence of proliferation. A small amount of smooth muscle cells and collagen may be found in the walls of vessels, especially in the secondary form of the disease [3]. The interlobular septa are widened and prominent, and the visceral pleura shows a network of dilated lymphatics.
The “lymphatic dysplasia syndrome” comprises lymphoedema, and effusions of the pericardium, pleura and peritoneum when there is no identifiable cause, and in the absence of lymphangiomas, lymphangiectasis or GLA [30, 67]. The syndrome includes primary lymphoedema, congenital chylothorax, idiopathic chylous effusions and the yellow nail syndrome (i.e. a triad of idiopathic pleural effusions, lymphoedema and dystrophic nails) [30, 67].
LAM is a rare disease of premenopausal women characterised by proliferation of abnormal smooth muscle-like cells (LAM cells) leading to cystic destruction of the lung parenchyma, lymphatic abnormalities and abdominal tumours. Cystic structures in the axial lymphatics (i.e. lymphangioleiomyomas) and chylous pleural effusions are common clinical manifestations of the disease. LAM cells usually express oestrogen receptor and glycoprotein gp100 [68].
Therapy
Treatment is mostly palliative and aimed at slowing the progression of the disease or relieving symptoms related to compression of adjacent structures and chylous fluid accumulation [24]. Different therapies have been tried in patients with GLA but there are no standardised treatment protocols or guidelines for these patients. Due to the rarity of the disease, various treatments have been reported in the literature as part of single case reports or small series of patients.
Dietary treatments, such as total parenteral nutrition, medium-chain triglycerides and high-protein diets, have generally proven to be ineffective [45, 69].
For small or localised lymphangiomas, a surgical approach for thoracoscopy or thoracotomy may be considered, especially if the lymphangiomas cause symptoms because of their rapid growth [70]. When surgery is the chosen option, it is often challenging to differentiate diseased lymphatic tissue from healthy tissue, and complete resection of the affected tissue may prove to be difficult given the proximity to vital structures and organs. Diseased tissue residues can thus proliferate leading to a return of symptoms [71]. Other surgical procedures have included parietal pleurectomy, pleurodesis and ligation of the thoracic duct to reduce recurrent pleural effusion [71–74]. Sclerotherapy has been tested in patients with few lymphangiomas: local injection of agents as Streptococcus antigen OK-432 results in sclerosis of the dilated lymphatic vessels [75]; however, it is a painful procedure that many patients tend to avoid [24]. Molitch et al. [76] treated five patients with percutaneous doxycycline as the sclerosing agent, an approach that proved to be effective for palliative treatment in patients with unresectable lymphangiomas.
Another treatment option is radiation therapy whereby radiation-induced fibrosis of the lymphatic endothelium causes destruction of the lymph vessels [71]. In patients with extensive disease, radiation therapy has resulted in a regression of lesions for several months, although the risk of radiation pneumonitis must always be considered [69].
Systemic chemotherapy [77] and systemic interferon treatment have been tried for patients with extensive and inoperable lymphangiomas, but with limited success [71]. Interferon, which has mild side-effects, seems to stop or reduce lesion growth, but at this time, no data confirm symptom resolution after discontinuation of therapy [45, 78, 79].
Treatment with propranolol, a nonselective β-blocker, may be effective in diffuse GLA. This drug may reduce the levels of vascular endothelial growth factor (VEGF), which is an angiogenic as well as a lymphangiogenic factor, as shown in several studies [80]. In a case report regarding a 13-year-old patient, propranolol was initiated at a dose of 0.5 mg·kg−1 body weight per day and then gradually increased to 4 mg·kg−1 per day. The therapy induced progressive reduction of pleural effusion [81].
In another case report, a 40-year-old woman with GLA was treated with intravenous bevacizumab (1 mg·kg−1 every 3 weeks), a monoclonal antibody that binds to VEGF-A. In this patient, immunohistochemical staining showed increased VEGF-A expression in affected lymphatic vessels compared to healthy vessels. The tumour size decreased with bevacizumab and was stable 10 months after therapy was discontinued due to development of hypertension [82].
Recently, some case reports showed successful treatment with the mTOR inhibitor sirolimus, which proved to be very effective in reducing the disease, with good tolerability and few side-effects, even in newborns [83–86]. The effect of sirolimus on the disease may be explained by our current understanding of the lymphangiogenesis pathway. Activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway occurs through signalling induced after ligand binging to VEGF receptor 3 on the surface of the lymphatic endothelium [87, 88].
Finally, one case of successful bilateral lung transplantation for DPL has been reported. This experience demonstrated that lung transplantation can be considered as a potential treatment for GLA with pulmonary involvement but accurate selection of patients according to their thoracic, skeletal and abdominal involvement is paramount [89].
The little knowledge we have about the aetiology and pathogenesis of this uncommon disease explains the difficulties in achieving real therapeutic advances. However, despite the absence of an ideal laboratory model for GLA, several promising in vivo models have recently been developed, as reported by Rockson [90]. These models include acquired lymphatic endothelial hyperplasia (attempting to reproduce the histological characteristics of human lesions in lymphangiomatosis), maldevelopment of dermal lymphatics in Wnt5a-knockout mice (showing the role this gene may have in the regulation of normal lymphangiogenesis), pulmonary lymphangiectasia induced by murine developmental VEGF-C overexpression and spheroid-based engineering of a human lymphatic vasculature in mice (a useful technique for the specific study of lymphatic development) [91–95].
Although several cases of thoracic involvement in GLA have been described clarifying the clinical manifestations and histopathology of the disease, further investigation is needed to better understand the pathogenesis of the disease and explore more effective therapeutic approaches. While mTOR inhibitors may represent a valid therapeutic option, clinical trials should be conducted to study the actual effectiveness of these agents for our management strategies. Finally, the development of new imaging techniques specific for the lymphatic system may be useful to examine the spread of the disease and facilitate follow-up.
Footnotes
Editorial comments in Eur Respir Rev 2016; 25: 101–103.
Conflict of interest: Disclosures can be found alongside this article at err.ersjournals.com
Provenance: Submitted article, peer reviewed.
References
- 1.Faul JL, Berry GJ, Colby TV, et al. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 2000; 161: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 2.Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 2015; 136: 203–214. [DOI] [PubMed] [Google Scholar]
- 3.Tazelaar HD, Kerr D, Yousem SA, et al. Diffuse pulmonary lymphangiomatosis. Hum Pathol 1993; 24: 1313–1322. [DOI] [PubMed] [Google Scholar]
- 4.Ramani P, Shah A. Lymphangiomatosis: histologic and immunohistochemical analysis of four cases. Am J Surg Pathol 1993; 17: 329–335. [PubMed] [Google Scholar]
- 5.Carlson KC, Parnassus WN, Klatt EC. Thoracic lymphangiomatosis. Arch Pathol Lab Med 1987; 111: 475–477. [PubMed] [Google Scholar]
- 6.Nair LG, Kurtz CP. Lymphangiomatosis presenting with bronchial cast formation. Thorax 1996; 51: 765–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi K, Takahashi H, Maeda K, et al. An adult case of lymphangiomatosis of the mediastinum, pulmonary interstitium and retroperitoneum complicated by chronic disseminated intravascular coagulation. Eur Respir J 1995; 8: 1799–1802. [DOI] [PubMed] [Google Scholar]
- 8.Lala S, Mulliken JB, Alomari AI, et al. Gorham–Stout disease and generalized lymphatic anomaly – clinical, radiologic, and histologic differentiation. Skeletal Radiol 2013; 42: 917–924. [DOI] [PubMed] [Google Scholar]
- 9.Zadvinskis DP, Benson MT, Kerr HH, et al. Congenital malformations of the cervicothoracic lymphatic system: embryology and pathogenesis. Radiographics 1992; 12: 1175–1189. [DOI] [PubMed] [Google Scholar]
- 10.Hilliard RI, McKendry JB, Phillips MJ. Congenital abnormalities of the lymphatic system: a new clinical classification. Pediatrics 1990; 86: 988–994. [PubMed] [Google Scholar]
- 11.Levine C. Primary disorders of the lymphatic vessels – a unified concept. J Pediatr Surg 1989; 24: 233–240. [DOI] [PubMed] [Google Scholar]
- 12.Kransdorf MJ. Benign soft tissue tumors in a large referral population: distribution of specific diagnoses by age, sex, and location. AJR Am J Roentgenol 1994; 164: 395–402. [DOI] [PubMed] [Google Scholar]
- 13.Moerman P, Van Geet C, Devlieger H. Lymphangiomatosis of the body wall: a report of two cases associated with chylothorax and fatal outcome. Pediatr Pathol Lab Med 1997; 17: 617–624. [PubMed] [Google Scholar]
- 14.Liu Y, Sui X, Chen K,et al. Thoracic lymphangiomatosis: report of 3 patients with different presentations. Ann Thorac Surg 2012; 94: 2111–2113. [DOI] [PubMed] [Google Scholar]
- 15.Kadakia CK, Patel SM, Yi ES, et al. Diffuse pulmonary lymphangiomatosis. Can Respir J 2013; 20: 52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lima AS, Martynychen MG, Florêncio RT, et al. Pulmonary lymphangiomatosis: a report of two cases. J Bras Pneumol 2007; 33: 229–233. [DOI] [PubMed] [Google Scholar]
- 17.Du MH, Ye RJ, Sun KK, et al. Diffuse pulmonary lymphangiomatosis: a case report with literature review. Chin Med J (Engl) 2011; 124: 797–800. [PubMed] [Google Scholar]
- 18.Zhang J, Jin H, Wang Y, et al. A case of diffuse pulmonary lymphangiomatosis with unilateral lung invasion. Oxf Med Case Rep 2015; 10: 346–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalassian KG, Doyle R, Kao P, et al. Lymphangioleiomyomatosis: new insights. Am J Respir Crit Care Med 1997; 55: 1183–1186. [DOI] [PubMed] [Google Scholar]
- 20.Harari S, Torre O, Cassandro R, et al. The changing face of a rare disease: lymphangioleiomyomatosis. Eur Respir J 2015; 46: 1471–1485. [DOI] [PubMed] [Google Scholar]
- 21.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 2005; 438: 946–953. [DOI] [PubMed] [Google Scholar]
- 22.Boland JM, Tazelaar HD, Colby TV, et al. Diffuse pulmonary lymphatic disease presenting as interstitial lung disease in adulthood. Am J Surg Pathol 2012; 36: 1548–1554. [DOI] [PubMed] [Google Scholar]
- 23.Tamay Z, Saribeyoglu E, Ones U, et al. Diffuse thoracic lymphangiomatosis with disseminated intravascular coagulation in a child. J Pediatr Hematol Oncol 2005; 27: 685–687. [DOI] [PubMed] [Google Scholar]
- 24.Satria MN, Pacheco-Rodrigues G, Moss J. Pulmonary lymphangiomatosis. Lymphat Res Biol 2011; 9: 191–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kransdorf MJ. Vascular and lymphatic tumors. In: Kransdorf MJ, Murphey MD, eds. Imaging of Soft Tissue Tumors. Philadelphia, WB Saunders, 1997; pp. 118–123. [Google Scholar]
- 26.Liu Yanguo, Sui Xizhao, Chen Kezhong, et al. Thoracic lymphangiomatosis: report of 3 patients with different presentations. Ann Thorac Surg 2012; 94: 2111–2113. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez OA, Kjellin I, Zuppan CW. Thoracic lymphangiomatosis in a child. J Pediatr Hematol Oncol 2004; 26: 136–141. [DOI] [PubMed] [Google Scholar]
- 28.Aviv RI, McHugh K, Hunt J. Angiomatosis of bone and soft tissue: a spectrum of disease from diffuse lymphangiomatosis to vanishing bone disease in young patients. Clin Radiol 2001; 56: 184–190. [DOI] [PubMed] [Google Scholar]
- 29.Sanders JS, Rosenow EC III, Piehler JM, et al. Chyloptysis (chylous sputum) due to thoracic lymphangiectasis with successful surgical correction. Arch Intern Med 1988; 148: 1465–1466. [PubMed] [Google Scholar]
- 30.Fox GF, Challis D, O'Brien KK, et al. Congenital chylothorax in siblings. Acta Pediatr 1998; 87: 1010–1012. [DOI] [PubMed] [Google Scholar]
- 31.Bresser P, Kromhout JG, Reekers JA, et al. Chylous pleural effusion associated with primary lymphedema and lymphangioma-like malformations. Chest 1993; 103: 1916–1918. [DOI] [PubMed] [Google Scholar]
- 32.Chen YL, Lee CC, Yeh ML, et al. Generalized lymphangiomatosis presenting as cardiomegaly. J Formos Med Assoc 2007; 106: 3 Suppl, S10–S14. [DOI] [PubMed] [Google Scholar]
- 33.Fukahori S, Tsuru T, Asagiri K, et al. Thoracic lymphangiomatosis with massive chylothorax after a tumor biopsy and with disseminated intravenous coagulation – lymphoscintigraphy, an alternative minimally invasive imaging technique: Report of a case. Surg Today 2011; 41: 978–982. [DOI] [PubMed] [Google Scholar]
- 34.Dietz WH Jr, Stuart MJ. Splenic consumptive coagulopathy in a patient with disseminated lymphangiomatosis. J Pediatr 1977; 90: 421–423. [DOI] [PubMed] [Google Scholar]
- 35.Safi F, Gupta A, Adams D, et al. Kaposiform lymphangiomatodid, a newly characterized vascular anomaly presenting with hemoptysis in an adult woman. Ann Am Thorac Soc 2014; 11: 92–95. [DOI] [PubMed] [Google Scholar]
- 36.Croteau SE, Kozakewich HP, Perez-Atayde AR, et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr 2014; 164: 383–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trenor CC III, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg 2014; 23: 186–190. [DOI] [PubMed] [Google Scholar]
- 38.Papagiannopoulos K, Van Raemdonck DE, De Boeck K, et al. Pediatric thoracic lymphangiomatosis: is chest wall resection too radical? Ann Thorac Surg 2004; 77: 695–697. [DOI] [PubMed] [Google Scholar]
- 39.Lim HJ, Han J, Kim HK, et al. A rare case of diffuse pulmonary lymphangiomatosis in a middle-aged woman. Korean J Radiol 2014; 15: 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frazer RS, Colman N, Müller NL, et al. Developmental anomalies affecting vessels. In: Frazer RS, Colman N, Müller NL, eds. Diagnosis of diseases of the chest. Philadelphia, PA, USA, Saunders, 1999; pp. 662–664. [Google Scholar]
- 41.Swensen SJ, Hartman TE, Mayo JR, et al. Diffuse pulmonary lymphangiomatosis: CT findings. J Comput Assist Tomogr 1995; 19: 348–352. [DOI] [PubMed] [Google Scholar]
- 42.Aquino SL, Hayman LA, Loomis SL, et al. Source and direction of thoracic lymphatics, part I. Upper thorax. J Catal 2003; 27: 292–296. [DOI] [PubMed] [Google Scholar]
- 43.El Hajj L, Mazieres J, Rouquette I, et al. Diagnostic value of bronchoscopy, CT and transbronchial biopsies in diffuse pulmonary lymphangiomatosis: Case report and review of the literature. Clin Radiol 2005; 60: 921–925. [DOI] [PubMed] [Google Scholar]
- 44.Yekeler E, Dursun M, Yıldırım A, et al. Diffuse pulmonary lymphangiomatosis: imaging findings. Diagn Interv Radiol 2005; 11: 31–34. [PubMed] [Google Scholar]
- 45.Laverdiere C, David M, Dubois J, et al. Improvement of disseminated lymphangiomatosis with recombinant interferon therapy. Pediatr Pulmonol 2000; 29: 321–324. [DOI] [PubMed] [Google Scholar]
- 46.Konez O, Pranav KV, Goyal M. Disseminated lymphangiomatosis presenting with massive chylothorax. Pediatr Radiol 2000; 30: 35–37. [DOI] [PubMed] [Google Scholar]
- 47.Swank DW, Hepper NG, Folkert KE, et al. Intrathoracic lymphangiomatosis mimicking lymphangioleiomatosis in a young woman. Mayo Clin Proc 1989; 64: 1264–1268. [DOI] [PubMed] [Google Scholar]
- 48.Canil K, Fitzgerald P, Lau G. Massive chylothorax associated with lymphangiomatosis of the bone. J Pediatr Surg 1994; 29: 1186–1188. [DOI] [PubMed] [Google Scholar]
- 49.Peh WC, Ngan H. Lymphography − still useful in the diagnosis of lymphangiomatosis. Br J Radiol 1993; 66: 28–31. [DOI] [PubMed] [Google Scholar]
- 50.Watts MA, Gibbons JA, Aaron BL. Mediastinal and osseous lymphangiomatosis: case report and review. Ann Thorac Surg 1982; 34: 324–328. [DOI] [PubMed] [Google Scholar]
- 51.Baulieu F, Baulieu JL, Mesny J, et al. Visualization of the thoracic duct by lymphoscintigraphy. Eur J Nucl Med 1987; 13: 264–265. [DOI] [PubMed] [Google Scholar]
- 52.Rajebi MR, Chaudry G, Padua HM, et al. Intranodal lymphangiography: feasibility and preliminary experience in children. J Vasc Interv Radiol 2011; 22: 1300–1305. [DOI] [PubMed] [Google Scholar]
- 53.Guzman AE, Rossi L, Witte CL, et al. Traumatic injury of the thoracic duct. Lymphology 2002; 35: 4–14. [PubMed] [Google Scholar]
- 54.Moadel-Sernick RM, Crooke GA, Freeman LM. Lymphoscintigraphy demonstrating thoracic duct injury in an infant with hypoplastic left heart syndrome. Clin Nucl Med 2000; 25: 335–336. [DOI] [PubMed] [Google Scholar]
- 55.Lohrmann C, Foeldi E, Langer M. Assessment of the lymphatic system in patients with diffuse lymphangiomatosis by magnetic resonance imaging. Eur J Radiol 2011; 80: 576–581. [DOI] [PubMed] [Google Scholar]
- 56.Wunderbaldinger P, Paya K, Partik B, et al. CT and MR imaging of generalized cystic lymphangiomatosis in pediatric patients. AJR 2000; 174: 827–832. [DOI] [PubMed] [Google Scholar]
- 57.Lu Q, Xu J, Liu N. Chronic lower extremity lymphedema: a comparative study high-resolution interstitial MR lymphangiography and heavily T2-weighted MRI. Eur J Radiol 2008; 73: 365–373. [DOI] [PubMed] [Google Scholar]
- 58.Sevick-Muraca EM, Kwon S, Rasmussen JC. Emerging lymphatic imaging technologies for mouse and man. J Clin Invest 2014; 124: 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holden WE, Morris JF, Antonovic R, et al. Adult intrapulmonary and mediastinal lymphangioma causing haemoptysis. Thorax 1987; 42: 635–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papsin BC, Evans JN. Isolated laryngeal lymphangioma: a rare cause of airway obstruction in infants. J Laryngol Otol 1996; 110: 969–972. [DOI] [PubMed] [Google Scholar]
- 61.Williams WT, Cole RR. Lymphangioma presenting as congenital stridor. Int J Pediatr Otorhinolaryngol 1993; 26: 185–191. [DOI] [PubMed] [Google Scholar]
- 62.Stocker JT. Congenital and developmental diseases. In: Dail DH, Hammar SP, eds. Pulmonary Pathology. New York, Springer-Verlag, 1988; pp. 41–57. [Google Scholar]
- 63.Moerman P, Vandenberghe K, Devlieger H, et al. Congenital pulmonary lymphangiectasis with chylothorax: a heterogeneous lymphatic vessel abnormality. Am J Med Genet 1993; 47: 54–58. [DOI] [PubMed] [Google Scholar]
- 64.Gilewski MK, Statler CC, Kohut G, et al. Congenital pulmonary lymphangiectasia and other anomalies in a child: provisionally unique syndrome? Am J Med Genet 1996; 66: 438–440. [DOI] [PubMed] [Google Scholar]
- 65.Felman AH, Rhatigan RM, Pierson KK. Pulmonary lymphangiectasia: observation in 17 patients and proposed classification. Am J Roentgenol Radium Ther Nucl Med 1972; 116: 548–558. [DOI] [PubMed] [Google Scholar]
- 66.Lloyd ES, Press HC Jr. Congenital pulmonary lymphangiectasis. South Med J 1979; 72: 1205–1206. [DOI] [PubMed] [Google Scholar]
- 67.Cooke JP, Rooke TW. Lymphedema. In: Loscalzo J, Creager MA, Dzau VJ, eds. Vascular Medicine: A Textbook of Vascular Biology and Diseases. Boston, Little, Brown and Co., 1996; pp. 1133–1146. [Google Scholar]
- 68.Harari S, Torre O, Moss J. Lymphangioleiomyomatosis: what do we know and what are we looking for? Eur Respir Rev 2011; 20: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kandil A, Rostom AY, Mourad WA, et al. Successful control of extensive thoracic lymphangiomatosis by irradiation. Clin Oncol (R Coll Radiol) 1997; 9: 407–411. [DOI] [PubMed] [Google Scholar]
- 70.Takemura T, Watanabe M, Takagi K, et al. Thoracoscopic resection of a solitary pulmonary lymphangioma: report of a case. Surg Today 1995; 25: 651–653. [DOI] [PubMed] [Google Scholar]
- 71.Rostom AY. Current topic: treatment of thoracic lymphangiomatosis. Arch Dis Child 2000; 83: 138–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Browse NL, Allen DR, Wilson NM. Management of chylothorax. Br J Surg 1997; 84: 1711–1716. [PubMed] [Google Scholar]
- 73.Hamada K, Ishii Y, Nakaya M, et al. Solitary lymphangioma of the lung. Histopathology 1995; 27: 482–483. [DOI] [PubMed] [Google Scholar]
- 74.Bhatti MAK, Ferrante JW, Gielchinsky I, et al. Pleuropulmonary and skeletal lymphangiomatosis with chylothorax and chylopericardium. Ann Thorac Surg 1985; 40: 398–401. [DOI] [PubMed] [Google Scholar]
- 75.Bermejo Casero EJ, Mongil Poce R, Arrabal Sánchez R, et al. Diffuse thoracic lymphangiomatosis: diagnosis and treatment. Arch Bronconeumol 2004; 40: 599–601. [PubMed] [Google Scholar]
- 76.Molitch HI, Unger EC, Witte CL, et al. Percutaneous sclerotherapy of lymphangiomas. Radiology 1995; 194: 343–347. [DOI] [PubMed] [Google Scholar]
- 77.Turner C, Gros S. Treatment of recurrent suprahyoid cervicofacial lymphngioma with intravenous cyclophosphamide. Am J Pediatr Hematol Oncol 1994; 16: 325–328. [PubMed] [Google Scholar]
- 78.Reinhardt MA, Nelson SC, Sencer SF, et al. Treatment of childhood lymphangiomas with interferon-α. J Pediatr Hematol Oncol 1997; 19: 232–236. [DOI] [PubMed] [Google Scholar]
- 79.Ozeki M, Funato M, Kanda K, et al. Clinical improvement of diffuse lymphangiomatosis with pegylated interferon alfa-2b therapy: case report and review of the literature. Pediatr Hematol Oncol 2007; 24: 513–524. [DOI] [PubMed] [Google Scholar]
- 80.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004; 25: 581–611. [DOI] [PubMed] [Google Scholar]
- 81.Ozeki M, Fukao T, Kondo N. Propranolol for intractable diffuse lymphangiomatosis. N Engl J Med 2011; 364: 1380–1382. [DOI] [PubMed] [Google Scholar]
- 82.Aman J, Thunnissen E, Paul MA, et al. Successful treatment of diffuse pulmonary lymphangiomatosis with bevacizumab. Ann Intern Med 2012; 156: 839–840. [DOI] [PubMed] [Google Scholar]
- 83.Laforgia N, Schettini F, De Mattia D, et al. Lymphatic malformation in newborns as the first sign of diffuse lymphangiomatosis: successful treatment with sirolimus. Neonatology 2016; 109: 52–55. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z, Li K, Yao W, et al. Successful treatment of kaposiform lymphangiomatosis with sirolimus. Pediatr Blood Cancer 2015; 62: 1291–1293. [DOI] [PubMed] [Google Scholar]
- 85.Bassi A, Syed S. Multifocal infiltrative lymphangiomatosis in a child and successful treatment with sirolimus. Mayo Clin Proc 2014; 89: e129. [DOI] [PubMed] [Google Scholar]
- 86.Reinglas J, Ramphal R, Bromwich M. The successful management of diffuse lymphangiomatosis using sirolimus: a case report. Laryngoscope 2011; 121: 1851–1854. [DOI] [PubMed] [Google Scholar]
- 87.Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer 2011; 57: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 88.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 2002; 8: 128–135. [DOI] [PubMed] [Google Scholar]
- 89.Kinnier CV, Eu JPC, Davis RD, et al. Successful bilateral lung transplantation for lymphangiomatosis. Am J Transplant 2008; 8: 1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rockson SG. Laboratory models for the investigation of lymphangiomatosis. Microvasc Res 2014; 96: 64–67. [DOI] [PubMed] [Google Scholar]
- 91.Bruyere F, Malen-Lamalle L, Blacher S, et al. Does plasminogen activator inhibitor-1 drive lymphangiogenesis? PLoS One 2010; 5: e9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mancardi S, Stanta G, Dusetti N, et al. Lymphatic endothelial tumors induced by intraperitoneal injection of incomplete Freund's adjuvant. Exp Cell Res 1999; 246: 368–375. [DOI] [PubMed] [Google Scholar]
- 93.Norgall S, Papoutsi M, Rossler J, et al. Elevated expression of VEGFR-3 in lymphatic endothelial cells from lymphangiomas. BMC Cancer 2007; 7: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao LC, Testini C, Tvorogov D, et al. Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period. Circ Res 2014; 114: 806–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alajati A, Laib AM, Weber H, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods 2008; 5: 439–445. [DOI] [PubMed] [Google Scholar]