Abstract
In 9–20% of cases, Sjögren's syndrome is associated with various respiratory symptoms. The most typical manifestations are chronic interstitial lung disease (ILD) and tracheobronchial disease. The most common manifestation of ILD is nonspecific interstitial pneumonia in its fibrosing variant. Other types of ILD, such as organising pneumonia, usual interstitial pneumonia and lymphocytic interstitial pneumonitis, are rare. Their radiological presentation is less distinctive, and definitive diagnosis may require the use of transbronchial or surgical lung biopsy. Corticosteroid therapy is the mainstay of ILD treatment in Sjögren's syndrome, but the use of other immunosuppressive drugs needs to be determined. ILD is a significant cause of death in Sjögren's syndrome. Tracheobronchial disease is common in Sjögren's syndrome, characterised by diffuse lymphocytic infiltration of the airway. It is sometimes responsible for a crippling chronic cough. It can also present in the form of bronchial hyperresponsiveness, bronchiectasis, bronchiolitis or recurrent respiratory infections. The management of these manifestations may require treatment for dryness and/or inflammation of the airways. Airway disease has little effect on respiratory function and is rarely the cause of death in Sjögren's syndrome patients. Rare respiratory complications such as amyloidosis, lymphoma or pulmonary hypertension should not be disregarded in Sjögren's syndrome patients.
Short abstract
We present the features, diagnostic tests and treatments of thoracic manifestations of Sjögren's syndrome http://ow.ly/10m8vd
Introduction
Primary Sjögren's syndrome was originally described in 1926 by Gougerot [1]. Its prevalence is estimated at 0.5% with a female preponderance [2]. It is the second most common multisystem autoimmune disease after rheumatoid arthritis, and is characterised by eye and mouth dryness and lymphocytic infiltration of the salivary glands, which are easily accessible and thus facilitate diagnosis. New classification criteria were endorsed by the American College of Rheumatology in 2012 [3]. Like other autoimmune diseases, it is associated with a specific genetic background, impaired immune response and environmental factors. These factors are believed to trigger an innate immune response leading to activation of glandular cells, followed by activation of B and T lymphocytes within the glands.
In addition to dryness, clinical presentation of Sjögren's syndrome generally includes asthenia and arthralgia. The disease can extend beyond the exocrine glands, and systemic manifestations including vasculitis, lung, renal or neurological involvement can occur. Patients with Sjögren's syndrome also have an increased risk of lymphoma [4].
The pulmonary manifestations of Sjögren's syndrome include airway abnormalities, interstitial lung disease (ILD) and lymphoproliferative disorders (table 1). Lung involvement occurs in ∼9–20% of patients. Subclinical lung disease is even more frequent, including small airway disease and airway inflammation [5]. The presence of ILD is associated with impaired respiratory function. Importantly, pulmonary involvement leads to increased risk of mortality [6]. Herein, we summarise the literature about Sjögren's syndrome pulmonary manifestations. To exclude pulmonary manifestations of conditions associated with Sjögren's syndrome, we only focused on pulmonary involvement of primary Sjögren's syndrome.
TABLE 1.
Thoracic manifestations of Sjögren's syndrome
| Thoracic manifestations | Prevalence | Peculiar aspects in Sjögren's syndrome | Treatment |
| Airway disease | |||
| Cough | 41–61%# | Secretagogues (pilocarpine) Nebulised saline solution |
|
| BHR | 42–60%# | Inhaled corticosteroids | |
| Bronchiolitis | 12–24%# | Mainly follicular bronchiolitis | Steroids Rituximab Macrolides |
| Bronchiectasis | 7–54%# | Mainly cylindrical bronchiectasis | |
| Pulmonary infections | 10–35%# | ||
| Interstitial lung disease | |||
| Nonspecific interstitial pneumonia |
45%¶ | Steroids Hydroxychloroquine Azathioprine Cyclophosphamide Rituximab |
|
| Usual interstitial pneumonia | 16%¶ | No benefit of immunosuppressive drugs | |
| Lymphocytic interstitial pneumonitis |
15%¶ | Steroids Azathioprine Cyclophosphamide Chlorambucil Rituximab |
|
| Organising pneumonitis | 11%# | Steroids Azathioprine Cyclosporine Infliximab Rituximab Tocilizumab |
|
| Others pulmonary manifestations in Sjögren's syndrome | |||
| Pulmonary amyloidosis | Rare | 96.5% female | Steroids |
| Pulmonary lymphoma | 2%¶ | Specific haematological treatment | |
| Pulmonary embolism and pulmonary hypertension |
Rare | Risk of venous thrombosis or pulmonary embolism in Sjögren's syndrome patients is greater than in the general population |
BHR: bronchial hyperresponsiveness. #: in Sjögren's syndrome patients; ¶: of interstitial lung disease in Sjögren's syndrome.
Background
Epidemiology of Sjögren's syndrome
Sjögren's syndrome is the second most common autoimmune disease after rheumatoid arthritis. Incidence has recently been estimated at between 3.9 and 5.3 cases per 100 000 person-years in Europe [7]. Population-based and sample-based studies estimate its prevalence at 43 and 282 per 100 000 inhabitants respectively, the former probably being more representative of the actual prevalence [7]. Sjögren's syndrome mostly affects middle-aged women, with a peak at 56 years of age; men are affected less frequently and later in life, mostly after the age of 65 years [7]. There is a clear sex ratio, ranging from 9 to 13 women to one man [8]. Sjögren's syndrome is likely to be associated with other autoimmune diseases such as thyroiditis.
Pathophysiology of Sjögren's syndrome
Like other autoimmune diseases, the aetiopathogenesis of Sjögren's syndrome combines environmental factors such as viruses (cytomegalovirus, HIV, Human T-cell leukaemia virus, and hepatitis C virus) [9] or solvents [10], a genetic predisposition as reported by the large 2013 genome-wide association study [11], and hormonal deregulation, which leads to an initial glandular inflammation called autoimmune epithelitis and a deregulated immune response [12].
Recent studies have sought to characterise the immune response that occurs in Sjögren's syndrome. They confirm that B and T cells play a major role in this immune response, but also highlight the important role of innate immunity. B-cell demethylation [13], activation and proliferation [14] are abnormal, and activated B-cells promote plasma cell secretion of anti-Ro (SSA) and anti-La (SSB) autoantibodies directed to the small cytoplasmic RNP-bound peptides SSA-60kD, SSA-52kD and SSB-48kD [15]. It is noteworthy that anti-Ro52 autoantibodies have only recently been proven to be able to directly cause gland dysfunction in mice [16]. The role of T-cells in Sjögren's syndrome has been extensively studied and reviewed [10] with divergent conclusions, especially regarding the roles of regulatory T-cells and T-helper 17 cells [17], but T-cell activation and cytotoxicity are undeniable. Innate immunity in Sjögren's syndrome is an area of growing interest, with promising results, such as the role of the interferon signature [18]. New findings regarding innate immunity also include apoptotic cell clearance defect [19], the interaction between P2X7 receptors and inflammasome [20], and the role of epithelial cells, like other pro-inflammatory elements, in the immune response [21].
This immune response, combined with local inflammation, gland infiltration and pathogenic anti-Ro52 autoantibodies, could cause the bronchopulmonary damage observed in some Sjögren's syndrome patients.
Extrathoracic involvement
While glandular involvement is by far the most frequent manifestation, with sicca symptoms affecting up to 95% of patients, numerous extra-glandular symptoms have be encountered in up to one-third of patients with Sjögren's syndrome [22].
Sicca symptoms mostly concern the mouth; xerostomia, periodontal disease, caries, angular cheilitis or endobuccal ulcers are frequently encountered. Eyes are the next most involved organ; xerophtalmia is often incapacitating and causes photophobia and impaired night vision, while cornea dryness can be complicated by keratitis, ulcers or even perforations. Other ocular complications such as conjunctivitis, uveitis, scleritis and episcleritis, orbital inflammation, retinal vasculitis or optic neuritis have also been reported [23]. The ear, nose and throat area is also affected, with symptoms such as smell and taste disorders, hoarseness and itching of the external ear canal, while parotitis or salivary gland swelling is also common (30% of patients). Genital sicca can significantly impair quality of life, especially in women [24], with dyspareunia and loss of desire and arousal. Skin involvement includes xeroderma, hypohydrosis, itching, cutaneous shedding, annular erythema, atypical folliculitis and Raynaud's phenomenon. Purpura or leukocytoclastic vasculitis, sometimes mimicking urticaria, can also be encountered, especially in association with cryoglobulinaemia [25].
Extraglandular, extrathoracic involvement includes psychological manifestations, such as asthenia (70–80%), sleep disorder (15%), anxiety (20%) or depression (40%) [26]. Arthralgia and/or myalgia are estimated to be present in half the cases of Sjögren's syndrome, and symmetrical non-erosive arthritis can be observed. Neurological involvement is common (20%) [27]. The central nervous system can be affected (2%); vasculitis and cranial nerve palsy have been described, as well as transverse myelitis and optic neuritis. The peripheral nervous system is more frequently affected (5–15% of Sjögren's syndrome); sensitive neuropathy, mostly dorsal root ganglionopathy (5%), and painful small-fibre neuropathy (5–10%) are the most common, but sensorimotor polyneuropathy, mononeuritis multiplex, polyradiculoneuropathies, autonomic neuropathies, etc. are also found. Digestive involvement has been reported in Sjögren's syndrome, particularly dysphagia and dyspepsia, probably due to xerostomia (24%). Sjögren's syndrome can also be associated with primary biliary sclerosis [28], autoimmune hepatitis or inflammatory bowel disease [23]. Renal and urological involvement are not unusual; tubulointestitial nephritis, interstitial cystitis, kidney stones and glomerulonephritis have been described, as well as vasculitis and distal renal tubular acidosis [29, 30].
Haematological involvement is amongst the more serious complications of Sjögren's syndrome; even if polyclonal hypergammaglobulinaemia is mostly asymptomatic, it can evolve towards oligoclonal or monoclonal gammapathy. Cryoglobulinaemia (mostly type 2 and 3) can occur and cause vasculitis, which is mostly cutaneous but likely to be responsible for multi-organ involvement. Cryoglobulinaemic vasculitis can also affect peripheral nerves. Lymphadenopathy is also frequently reported, possibly associated with autoimmune cytopenias. Finally, the most serious haematological complication is B-cell mantle lymphoma, with a frequency that is six to 44 times higher in Sjögren's syndrome than in the general population [31].
The SSA/Ro antigen consists of two polypeptide components of 52 and 60 kDa. The clinical associations of anti-SSA60 autoantibodies include Sjögren's syndrome, systemic lupus erythematosus and foetal–maternal autoimmune syndromes. Anti-Ro/SSA52 autoantibodies are the most prevalent extractable nuclear antigen specificity identified. Their specific diagnostic value is mostly based on the simultaneous presence of other autoantibodies whose diagnostic weight is more relevant. Anti-Ro/SSA52 autoantibodies are expressed in systemic lupus erythematosus (53%), myositis (35%), systemic sclerosis (19%), Sjögren's syndrome (63%) and primary biliary cirrhosis (28%) [32]. Finally, babies of mothers with SSA antibodies can develop neonatal lupus, with cutaneous involvement and/or congenital atrioventricular heart block [33].
Classification criteria
The involvement of the lacrimal and salivary glands results in the typical features of dry eyes and salivary dysfunction, which are the most common subjective manifestations of Sjögren's syndrome. Evaluation of this impairment is based on four objective criteria: 1) Shirmer's test <5 mm in 5 min; 2) histopathology of minor salivary glands exhibiting focal lymphocytic sialadenitis with a focus score (the number of mononuclear cell infiltrates containing at least 50 inflammatory cells in a 4 mm2 glandular section) of >1; 3) salivary gland involvement (unstimulated whole salivary flow, parotid sialography showing the presence of diffuse sialectasia without evidence of obstruction in the major ducts, or salivary scintigraphy showing delayed uptake, reduced concentration and/or delayed excretion of tracer, salivary gland ultrasonography or magnetic resonance imaging is an alternative); and 4) autoantibodies found in the serum with SSA or SSB.
The American College of Rheumatology proposed new classification criteria in 2012 based on at least two of the three following objective features [3]: 1) positive serum anti-SSA/Ro and/or anti-SSB/La (or positive rheumatoid factor and anti-nuclear antibodies at a dilution >1/320); 2) minor salivary gland biopsy exhibiting focal lymphocytic sialadenitis with a focus score >1/4 mm2; and 3) keratoconjunctivitis sicca with ocular staining score >3 (assuming that the individual is not currently using daily eye drops for glaucoma and has not had corneal surgery or cosmetic eyelid surgery in the past 5 years).
The European League Against Rheumatism Sjögren Syndrome Disease Activity Index (ESSDAI) is a clinical score that measures disease activity in Sjögren's syndrome, including evaluation of pulmonary involvement. It is used as a measure of disease activity in clinical studies or as a primary outcome measure in clinical trials [34].
Sjögren's syndrome patients with pulmonary involvement
Prevalence of lung disease in Sjögren's syndrome
The pulmonary manifestations of primary Sjögren's syndrome include airway abnormalities, interstitial pneumonia and lymphoproliferative disorders (table 1). Interstitial pneumonia and airway abnormalities often coexist. Infections and drug-induced pneumonia should always be considered and ruled out first. Pulmonary involvement could already be present before diagnosis but it can sometimes begin at the same time as other extrathoracic signs indicative of Sjögren's syndrome (10% of cases) [8]. The respiratory manifestations of Sjögren's syndrome are polymorphic and vary in severity, explaining the wide variability of prevalence reported in different studies. The prevalence of clinically significant lung disease in Sjögren's syndrome is 9–20% [35] with a female predominance [36]. When investigated systematically, prevalence increases to between 43% and 75% [37]. Computed tomography (CT) scan abnormalities are found in 34–50% of patients [38]. The annual incidence of respiratory manifestations is estimated at 10% (±3%) 1 year after diagnosis of Sjögren's syndrome and increases to 20% (±4%) by 5 years [6].
Clinical and biological profile of patients with pulmonary involvement
Studies suggest that the main risk factors of lung involvement are being male, smoking, late onset and long evolution of disease [8, 39–41]. Patients with pulmonary involvement in Sjögren's syndrome have a lower health-related quality of life [42]. A lower physical functioning score and a higher risk of death are found in Sjögren's syndrome patients with lung disease [40].
Pulmonary involvement is mostly associated with systemic manifestations, hypergammaglobulinaemia and anti-SSA and anti-SSB antibodies [37, 39, 43, 44]. Other biological markers proposed as risk factors for pulmonary involvement include the presence of anti-nuclear antibodies or rheumatoid factors [39, 45].
Pulmonary function test in Sjögren's syndrome
Pulmonary function test (PFT) results appear to reflect impairment of either the lung (restrictive syndrome) or airways (obstructive syndrome) [46]. The majority of Sjögren's syndrome patients have preserved lung function [37, 40, 47]. When it is abnormal, the PFT generally shows a restrictive rather than an obstructive pattern. However, Sjögren's syndrome patients with lung involvement revealed by CT scan generally have abnormal PFT results [36]. Reduced diffusing capacity of the lung for carbon monoxide (DLCO) seems to be the most common abnormality [40, 43, 48–51]. Nevertheless, authors have suggested that DLCO is a relatively insensitive technique for detecting subclinical pulmonary disease [44]. Changes in PFT results vary between studies. Davidson et al. [44] reported that DLCO in Sjögren's syndrome patients decreased during the first 4 years and then remained stable for 6 years.
Airway disease in Sjögren's syndrome
Airway disease is the most frequent pulmonary involvement in Sjögren's syndrome and is manifested mainly by coughing [52]. In Sjögren's syndrome, airway lesions can be related either to destruction of exocrine glands (sicca syndrome) or to cell infiltration. They may affect the trachea, bronchi or bronchioles. Several studies have characterised the histopathological abnormalities [47]. Even patients who are asymptomatic and have no radiographic abnormality have extraglandular cell infiltration of bronchial and bronchiolar submucosa. Infiltration cells are CD4-positive T lymphocytes [49, 53]. Bronchoalveolar lavage (BAL) reveals CD4 lymphocytic alveolitis in 55% of patients with Sjögren's syndrome [53, 54].
Frequent abnormalities are observed on CT, but frequency varies across studies. Damage to central and peripheral bronchi and bronchioles includes bronchiectasis (5–54%), mosaic attenuation (22%) (figure 1c), bronchial wall thickening (8–68%) (figure 1a), and centrilobular nodules and branching nodular opacities (6–29%) (figure 1b) [40, 47, 55–59]. Mild thickening of interlobular septa and air cysts can be observed in follicular bronchiolitis making a true continuum with lymphocytic interstitial pneumonia [60]. In most cases, these abnormalities have little impact on PFT. The estimated frequency of distal airway disease in Sjögren's syndrome patients, assessed by PFT, varies from 22% to 46% [47–49, 56, 61]. Obstructive ventilatory syndrome is rare (11–14%) [37, 47] and seems to be related to severity of the disease. Severe forms are rarely observed [62].
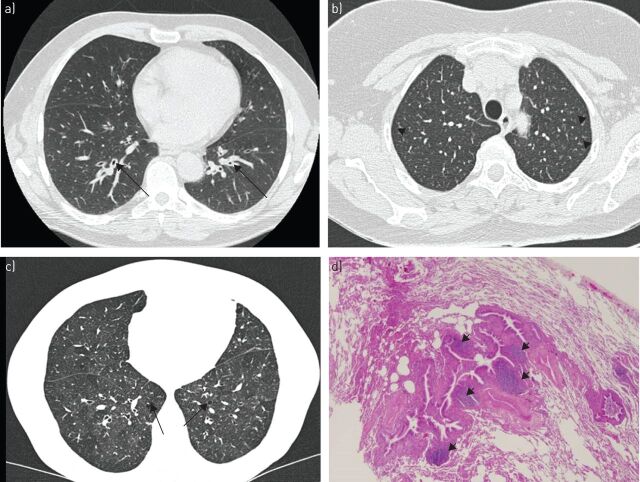
FIGURE 1.
a) Axial computed tomography (CT) showing mild thickening of bronchial walls (arrows) in a woman with Sjögren's syndrome. b) Chronic cough and recurrent pulmonary infections associated with bronchiolitis in a 60-year-old woman with primary Sjögren's syndrome. High-resolution CT showing multiple ill-defined centrilobular nodules (arrowheads) with sparing of the subpleural region. c, d) Follicular bronchiolitis in a 54-year-old woman with primary Sjögren's syndrome. c) High-resolution CT showing an area of heterogeneous attenuation in the left lower lobe of the lung (a finding referred to as mosaic attenuation) and the presence of well-defined, round, thin-walled air cysts (arrows). d) Photomicrograph (haematoxylin–eosin stain) showing lymphoplasmacytic infiltration and lymphoid follicles in the bronchiolar wall (arrowheads) and mild inflammation in the adjacent alveolar area. These findings are indicative of follicular bronchiolitis. Original magnification ×40.
Cough
Cough is observed in 41–61% of Sjögren's syndrome patients [47]. Dry cough may precede Sjögren's syndrome diagnosis by several years and affects quality of life in 50% of Sjögren's syndrome patients [63]. Several hypotheses have been put forward to explain coughing, including airway dryness, abnormal mucociliary clearance [64, 65], bronchial or bronchiolar inflammation, bronchial hyperresponsiveness, and gastro-oesophageal reflux. Cough severity could be correlated with tracheal dryness (local sicca syndrome) [66]. There is no codified treatment protocol, but secretagogues (pilocarpine) or nebulised saline solution may be required.
Bronchial hyperresponsiveness
In contrast to other systemic diseases (except systemic scleroderma), bronchial hyperresponsiveness is frequently reported in Sjögren's syndrome (42–60% of patients) [66–69]. Clinically, patients report increased coughing after exposure to airway irritants (tobacco, pollution, etc.). Sjögren's syndrome patients show greater bronchial reactivity on the methacholine challenge test, but a lower response to adenosine monophosphate, cold and hyperventilation [70]. The mechanisms involved are unknown. Unlike asthma, eosinophils do not seem to act on hyperresponsiveness in Sjögren's syndrome. The intensity of hyperresponsiveness is not correlated with salivary gland infiltration [69] or with exhaled nitric oxide level [71]. No difference in bronchial gland atrophy was observed between Sjögren's syndrome patients and controls [72]. Tracheobronchial dryness has been indirectly measured by mucociliary clearance [65]. These data suggest that local sicca syndrome is a consequence of a functional secretion defect rather than glandular destruction. Bronchial hyperresponsiveness seems to be insensitive to inhaled corticosteroids in 40–60% of cases [66].
Bronchiolitis
Bronchiolitis is the most frequent airway disease in Sjögren's syndrome patients. It may be isolated or associated with interstitial pneumonitis [46]. Lung biopsy reveals bronchiolitis in 12% of Sjögren's syndrome patients. Frequency increases to 24% when based on new anatomical and radiological criteria [46]. Lung biopsy reveals different types of bronchiolitis, mainly follicular bronchiolitis (29%) [51]. Follicular bronchiolitis is characterised by the presence of hyperplastic lymphoid follicles with reactive germinal centres distributed along bronchovascular bundles (figure 1d) [73, 74]. Less frequently, other bronchiolitis types have been found, including chronic bronchiolitis [51], obliterative bronchiolitis [51], lymphocytic bronchiolitis [75, 76], constrictive bronchiolitis associated with bronchiolar destruction [77], and panbronchiolitis [78].
The course of bronchiolitis in Sjögren's syndrome is typically described as being fairly mild. However, cases of severe bronchiolitis have been described [62]. Few studies have described treatment for bronchiolitis in Sjögren's syndrome: follicular bronchiolitis could be treated with steroids [41, 46], rituximab [79–82], or macrolides [62] though with a low level of scientific evidence.
Bronchiectasis
The frequency of bronchiectasis in Sjögren's syndrome patients, as assessed by CT, varies from 7% to 54% [43, 49, 56, 59, 83, 84]. In most cases, it concerns cylindrical bronchiectasis. Sjögren's syndrome patients with bronchiectasis are older at the time of diagnosis, are more likely to have hiatal hernia, have a higher frequency of anti-smooth muscle antibody and a lower frequency of anti-SSA antibody than those without bronchiectasis [85].
Pulmonary infections
Recurrent pulmonary infections (particularly pneumonia) are reported in 10–35% of Sjögren's syndrome patients [45, 64, 75]. Suspected mechanisms areabnormalities of mucociliary clearance [65], abnormal sputum, deficit of local immunity, gastro-oesophageal reflux, bronchiectasis, parodontopathy [85] and use of immunosuppressive drugs.
ILD in Sjögren's syndrome
The first association between Sjögren's syndrome and ILD, described in 1973, was lymphocytic interstitial pneumonitis (LIP) [86]. However, nonspecific interstitial pneumonitis (NSIP) seems to be the most common pathological subtype revealed by lung biopsy [36, 46, 51, 87–89]. However, these studies are based on small samples. A recent review of 146 histological cases in the literature found 45% NSIP, 16% usual interstitial pneumonitis (UIP), 7% organising pneumonia, 15% LIP and 17% other pathologies [89]. The main symptoms of these ILDs are dyspnoea and cough. Clubbing is rare [36]. ILD is associated with premature mortality [6]. The presence of anti-SSA is a predisposing factor of ILD [90].
Chest radiographs can show bilateral lung infiltrates with linear and reticular opacities (10–30%) [36, 38, 46]. Radiological abnormalities are not correlated with PFT and respiratory symptoms [91]. CT is the most sensitive method of detecting lung abnormalities (31–90% of Sjögren's syndrome patients). The main observed interstitial abnormalities (figures 2 and 3) are ground-glass opacities (92%) (figure 3a and d), nonseptal linear opacities (75%) (figure 2c), interlobular septal thickening (55%) (figure 2c) and cysts (30%) (figure 4b), reticulation and fibrosis (figure 2a and 3b) [5, 41, 46, 57]. It is often associated with airway disease (centrilobular nodules in 78% of Sjögren's syndrome ILD). Multiple air cysts are rare in other disorders and may be a crucial factor in diagnosing Sjögren's syndrome (figure 4b). Cysts vary in size from 0.5 cm to 7 cm. They have thin walls and are distributed randomly in the lung, often downstream of thickened bronchi. The suspected mechanisms are the trapping of a check-valve” mechanism upstream and destruction of the alveolar wall. The presence of cysts is not always linked to LIP but can be seen in amyloidosis or lymphoma, particularly when they are associated with nodules. Honeycombing and fibrosis are less common (figure 2a) [92]. The CT pattern seems to be correlated with the histological pattern, particularly for NSIP [36, 93].
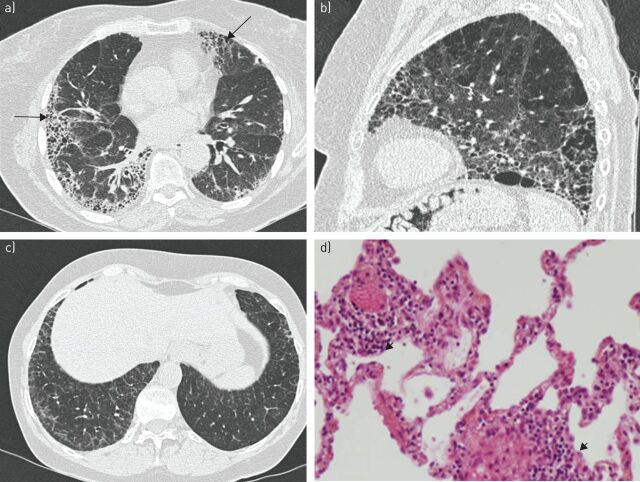
FIGURE 2.
a) Usual interstitial pneumonia in a 69-year-old woman with primary Sjögren's syndrome. High-resolution computed tomography (CT) showing bilateral reticular areas and honeycombing with peripheral and basal predominance (arrows). b) Combined pulmonary fibrosis and emphysema syndrome in a 68-year-old smoker with primary Sjögren's syndrome complicated by pulmonary hypertension. High-resolution CT shows bilateral reticular areas and honeycombing with posterior and basal predominance. Emphysema is predominant in apical areas. c, d) Lymphocytic interstitial pneumonia in a 59-year-old woman with primary Sjögren's syndrome and lymphocytic alveolitis. c) High-resolution CT shows thickening of interlobular septa with superimposition of intralobular reticulation. d) Photomicrograph (haematoxylin–eosin stain) shows diffuse thickening of alveolar septa and peribronchiolar infiltration with lymphocytes and plasma cells (arrowheads). Original magnification ×100.
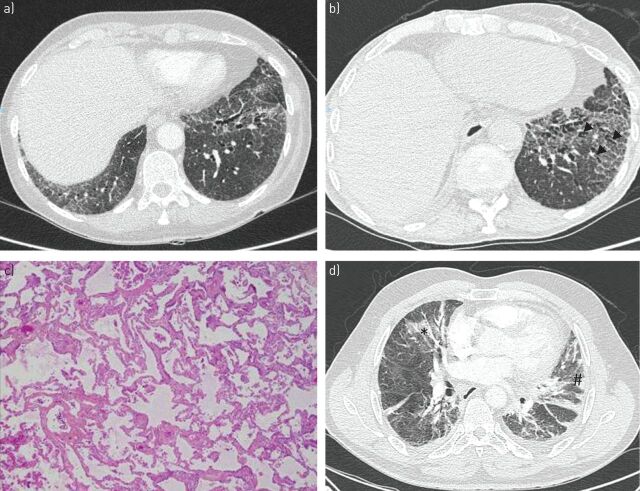
FIGURE 3.
Nonspecific interstitial pneumonia in a woman a, c) at the time of Sjögren's syndrome diagnosis and b) after 3 years. a, b) High-resolution computed tomography (CT) show bilateral areas of ground-glass attenuation and traction bronchiectasis (arrowheads) with peripheral intralobular reticulation. c) Photomicrograph (haematoxylin–eosin stain) shows diffuse and homogenous collagenous fibrosis in the alveolar area. d) Organising pneumonia and primary Sjögren's syndrome revealed by acute onset of pleuropneumonia in a 64-year-old man. High-resolution CT images show bilateral patchy areas of consolidation (#) and areas of ground-glass opacity (*). The patient was diagnosed with nonspecific interstitial pneumonia 2 years later.
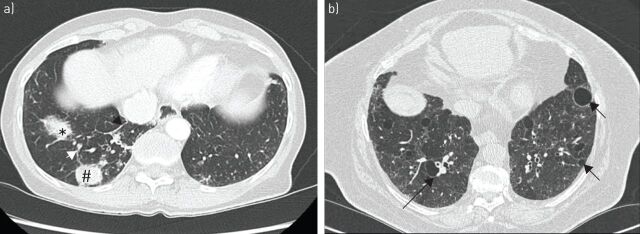
FIGURE 4.
a) Mucosa-associated lymphoid tissue lymphoma in a 45-year-old woman with primary Sjögren's syndrome. High-resolution computed tomography (CT) shows air space consolidation (*), mass (#) and interlobular septal thickening (arrowheads) in the right lower lobe. b) High-resolution CT obtained in a 64-year-old woman with lymphocytic interstitial pneumonitis and primary Sjögren's syndrome shows well-defined, round, thin-walled air cysts in the peribronchovascular regions (arrows), and areas of ground-glass and reticular attenuation.
Many BAL studies have found lymphocytic alveolitis in the majority of Sjögren's syndrome patients (64%) (essentially with T-cells), even among those who are asymptomatic [46, 49, 54, 94]. Patients with lymphocytic alveolitis have a higher level of gammaglobulins, higher frequency of rheumatoid factor and anti-nuclear antibodies, greater need for therapy and higher mortality, without systematically developing respiratory disease [94–96].
PFTs are usually abnormal in Sjögren's syndrome patients when ILD is discovered [36]. They show restrictive abnormalities and a decrease in DLCO [43, 46, 51]. The lung function of the majority of patients does not seem to be affected over time [44]. ILD is less serious in Sjögren's syndrome patients than idiopathic pulmonary fibrosis [46]. However, there can be acute exacerbations of ILD [97]. During a 38-month follow-up period, Parambil et al. [36] reported that 39% of patients died and 16% had acute exacerbation of ILD. New and extensive areas of airspace consolidation or ground-glass attenuation on CT should be considered as probable signs of acute exacerbation of interstitial pneumonia.
Nonspecific interstitial pneumonia
NSIP is the most common subtype of ILD observed in Sjögren's syndrome patients (45% of patients) [89]. The histological features of the NSIP pattern consist of varying amounts of interstitial inflammation and fibrosis with a uniform appearance (figure 3c). Lung architecture is frequently preserved. Honeycombing is rarely seen, but areas of interstitial fibrosis with enlarged air spaces may induce traction bronchiectasis. This fibrosing stage is the most common NSIP variant in Sjögren's syndrome patients [98]. These histological abnormalities result in a particular CT pattern: bibasal and symmetrically predominant reticular abnormalities with traction bronchiectasis, peri-bronchovascular extension (frequently associated with ground-glass attenuation) and uncommon features including sub-pleural sparing and pulmonary consolidations (figure 3a and b) [92]. These CT abnormalities help distinguish NSIP from UIP.
The outcome of NSIP in Sjögren's syndrome is unpredictable, it can be: 1) reversible with a risk of progression; 2) stable with residual disease; 3) progressive and irreversible with potential stabilisation; or 4) irreversible without respite, despite therapy [99]. The 5-year survival rate of patients with NSIP is 83% [46].
Corticosteroids (ranging 0.5–1 mg·kg−1·day−1 with azathioprine or cyclophosphamide) are usually prescribed for NSIP patients, but with no evidence of efficacy [26, 100]. In a cohort of 18 Sjögren's syndrome-ILD patients, Parambil et al. [36] described five patients with NSIP treated with corticosteroids associated with other drugs (hydroxychloroquine, azathioprine or cyclophosphamide). Three of these patients showed significant improvements in forced vital capacity (FVC) and/or DLCO. However, the PFT results of one patient worsened (<10% increase in FVC or <15% increase in DLCO) [36]. Although these therapies have been shown to be effective in case studies, clinical trials are needed.
Studies of rituximab have not distinguished between different forms of ILD in Sjögren's syndrome. A French review reported nine Sjögren's syndrome patients with pulmonary involvement treated by rituximab. Eight patients had ILD (with no distinction between different forms), of whom six improved after beginning treatment with rituximab, and two were persistent responders 30 months after a single cycle of rituximab [79]. Rituximab appears to be well tolerated, but a recent randomised, blinded, parallel-group trial found a significant increase in adverse events (mainly respiratory infections) for patients with a respiratory disorder treated with rituximab [101].
Usual interstitial pneumonia
UIP appears on CT scans as bilateral areas of intralobular reticular attenuation accompanied by traction bronchiectasis and small cystic changes, with both basal and peripheral predominance and temporal heterogeneity [99]. Honeycombing visible on CT scans (figure 2a) or in lung biopsies is characteristic of UIP. Other histopathological features of UIP are: 1) a patchy pattern of interstitial fibrosis mixed with normal parenchyma (abrupt shifts between fibrotic and normal parenchyma); 2) lesions that are worse directly under the pleura and in the periphery of the lobule; 3) scattered fibroblast foci; and 4) minimal interstitial inflammation.
In a study of 343 Sjögren's syndrome patients, Strimlan et al. [45] reported two cases with a histological diagnosis of UIP. UIP represents ∼16% of cases of ILD in Sjögren's syndrome [89]. It is regarded as an irreversible lung disease with a high risk of progression, despite therapy. The prognosis could be worse than NSIP.
Sjögren's syndrome patients with UIP are generally older and are more often women compared to those with idiopathic pulmonary fibrosis. In addition to the pathological pattern of UIP, Sjögren's syndrome patients have more interstitial inflammation, lymphoid follicles with germinal centres and cysts [102]. In that study, Sjögren's syndrome patients with UIP were younger with higher FVC at diagnosis and had a better prognosis than those with idiopathic pulmonary fibrosis [102]. Four out of ten Sjögren's syndrome–UIP patients died during the follow-up period (4–157 months). Immunosuppressive drugs do not seem to benefit Sjögren's syndrome patients with UIP [36]. However, no clinical trial has yet been performed to confirm this observation.
Lymphocytic interstitial pneumonitis
LIP is a benign lymphoproliferative disorder in Sjögren's syndrome. Probably, a continuum exits between LIP and follicular bronchiolitis. It is characterised by diffuse proliferation of polyclonal lymphocytes and plasma cells in the pulmonary parenchymal interstitium, with lymphoid follicles and germinal centres (figure 2d). Liebow and Carrington [86] found that in a cohort of patients with LIP, 28% had Sjögren's syndrome. LIP seems to account for 15% of cases of Sjögren's syndrome patients with ILD [89]. Most patients have respiratory symptoms (particularly dyspnoea and cough), and bilateral inspiratory crackles” may be heard [103]. Classically, PFT shows a restrictive syndrome depending on the progression of the disease [86]. The most frequent abnormalities shown on CT scans are thickened bronchovascular bundles (evoking association with follicular bronchiolitis), nodules and ground-glass opacities, and thickening of interlobular septa (figure 2b) [103, 104]. Studies have reported cysts in 68–82% of patients with LIP (figure 4b) [93, 103, 105]. Surgical lung biopsy and assessment of clonality are essential to exclude the diagnosis of lymphoma.
LIP seems to be a reversible lung disease with a potential risk of progression [99]. The majority of patients treated with corticosteroids remain clinically stable or show improvement [86, 103]. LIP can worsen with the development of honeycombing [106]. Other immunosuppressive agents (azathioprine, cyclophosphamide and chlorambucil) have been used but with variable response [107, 108]. Rituximab has been described as effective in one patient [82].
Organising pneumonitis
Histological diagnosis of organising pneumonia has been reported in 11% of Sjögren's syndrome patients [36, 46, 89]. Organising pneumonia could even reveal Sjögren's syndrome [109–112]. Clinical presentation is similar to other organising pneumonia aetiologies. CT scans show multiple patchy areas of consolidation with a subpleural or peribronchovascular distribution, often bilateral, accompanied by areas of ground-glass attenuation or fine centrilobular nodules (figure 3d). Histological analyses frequently reveal a pattern of NSIP overlapping with organising pneumonia [51]. Ito et al. [46] reported a good initial response to corticosteroid therapy, but all patients relapsed and developed an NSIP pattern.
Corticosteroids are the reference therapy for organising pneumonitis. In refractory organising pneumonia, immunosuppressive agents such as azathioprine, cyclosporine, infliximab and rituximab have been used [113]. Recently, one case of refractory organising pneumonia was treated by tocilizumab with good efficacy [114].
Uncommon causes of interstitial pneumonia in Sjögren's syndrome
Other causes of interstitial pneumonia in Sjögren's syndrome patients include: Langerhans’ histiocytosis [115], cavitary lung disease and granulomatosis with polyangiitis [116], and combined pulmonary fibrosis and emphysema syndrome (figure 2b) [117]. Alveolar haemorrhage has been reported in two cases, but in association with cryoglobulinaemia and with rapidly progressive pulmonary fibrosis [118, 119].
Other pulmonary manifestations
Pulmonary amyloidosis
Pulmonary amyloidosis associated with Sjögren's syndrome is rare. A review of the literature reported 37 cases; 96.5% of patients were women presenting with cough, dyspnoea, weakness, haemoptysis and pleuritic chest pain. Radiographically, nodules with or without calcification were the most common abnormalities (78.8%). It can be associated with LIP and cystic lesions. Transbronchial biopsy could lead to a high risk of haemoptysis. Surgical lung biopsy is usually required to establish the diagnosis and to rule out lymphoma [120]. No specific therapy has been reported, but corticosteroids could be used for some patients.
Pulmonary lymphoma
Sjögren's syndrome is associated with a 16- to 44-fold increased risk of non-Hodgkin lymphoma [121]. Marginal zone B-cell lymphoma and mucosa-associated lymphoid tissue are the most common subtypes. The prevalence of primary pulmonary lymphoma is 1–2% in patients with primary Sjögren's syndrome, with pulmonary localisation in 20% of cases [122]. It is rarely associated with an infectious agent (human herpesvirus-6 or Epstein–Barr virus). Non-Hodgkin lymphoma is associated with parotidomegaly, hypocomplementemia, type II cryoglobulinaemia, cutaneous vasculitis, CD4 cytopenia and a low CD4/CD8 ratio [123]. Radiological abnormalities are solitary or multiple nodules or masses (figure 4a), with areas of airspace consolidation or ground-glass attenuation, mediastinal lymphadenopathy and pleural effusions. The average 5-year survival rate is 65–90% [31, 122–125]. Lymphomas require specific haematological treatment.
Pulmonary embolism and pulmonary hypertension
A case series combined with a review of the literature described 28 cases of pulmonary hypertension in Sjögren's syndrome patients associated with a mean pulmonary artery pressure of 44 mmHg and a worsened prognosis with low survival rates (73% and 66% at 1 and 3 years, respectively) [126]. Clinical trials are needed to evaluate immunosuppressive drugs and pulmonary antihypertensive in Sjögren's syndrome patients with pulmonary hypertension.
There is an increased risk of thromboembolism in systemic autoimmune diseases. Some studies have found that the risk of venous thrombosis or pulmonary embolism in Sjögren's syndrome patients is significantly greater than in the general population [127, 128].
In practice
When Sjögren's syndrome is known, ILD screening should be systematic and when ILD is known, Sjögren's syndrome screening should likewise be systematic (table 2).
TABLE 2.
Screening pulmonary manifestations of Sjögren's syndrome
| When Sjögren's syndrome is known, ILD screening should be systematic | When ILD is known, Sjögren's syndrome screening must be systematic |
| Investigate respiratory symptoms (dyspnoea, cough, crackles and squeaks, clubbing) Exclude an infectious, medication (www.pneumotox.com) or cardiovascular cause Confirm ILD by performing chest computed tomography Consultation with a lung specialist to discuss bronchoscopy with BAL, histological sampling (transbronchial and surgical biopsy) and therapeutic care |
Investigate extra-respiratory symptoms suggestive of Sjögren's syndrome (keratoconjunctivitis sicca, swelling of the salivary glands or hyposialia, arthritis, arthralgia), perform a minor salivary gland biopsy and do a urine analysis Investigate antinuclear antibodies, rheumatoid factor, SSA/Ro and SSB/La, and perform serum protein electrophoresis Exclude another connective tissue disease Confirm dry eyes and dry mouth |
| Consult a connective tissue disease expert to discuss therapeutic care |
ILD: interstitial lung disease; BAL: bronchoalveolar lavage.
Not all pulmonary manifestations in Sjögren's syndrome should be treated. Those that are well tolerated and stable must be monitored. Owing to the lack of large, randomised clinical trials, there is no conclusive standard therapy for Sjögren's syndrome with pulmonary involvement. Active immune therapy (corticosteroids and/or immunosuppressive drugs and/or biological treatment) is indicated when the patient has progressive chest symptoms, impaired respiratory function or prominent abnormal chest or high-resolution CT findings [129]. Relevant biological therapies in Sjögren's syndrome could be those that target T-cells (anti-CD11a: efalizumab; anti-CD2: alefacept; CTLA-4 Ig: abatacept) and B-cells (anti-CD20: rituximab, ocrelizumab and ofatumumab; anti-CD22: epratuzumab; anti-BAFF: belimumab).
Supplementary Material
Acknowledgements
T. Flament, E. Diot and S. Marchand-Adam were responsible for the conception and design. All authors were responsible for analysis and interpretation, and drafting the manuscript and revision for important intellectual content.
Footnotes
Conflict of interest: Disclosures can be found alongside the online version of this article at err.ersjournals.com
Provenance: Submitted article, peer reviewed.
References
- 1.Gougerot H.. Insuffisance progressive et atrophie des glandes salivaires et muqueuses de la bouche, des conjonctives (et parfois des muqueuses nasale, laryngée, vulvaire) sécheresse de la bouche, des conjonctives, etc). Bull Méd 1926; 40: 360–368. [Google Scholar]
- 2.Bowman SJ, Ibrahim GH, Holmes G, et al. Estimating the prevalence among Caucasian women of primary Sjögren's syndrome in two general practices in Birmingham, UK. Scand J Rheumatol 2004; 33: 39–43. [DOI] [PubMed] [Google Scholar]
- 3.Shiboski SC, Shiboski CH, Criswell LA, et al. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res 2012; 64: 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quartuccio L, Isola M, Baldini C, et al. Biomarkers of lymphoma in Sjögren's syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J Autoimmun 2014; 51: 75–80. [DOI] [PubMed] [Google Scholar]
- 5.Kreider M, Highland K. Pulmonary involvement in Sjögren syndrome. Semin Respir Crit Care Med 2014; 35: 255–264. [DOI] [PubMed] [Google Scholar]
- 6.Nannini C, Jebakumar AJ, Crowson CS, et al. Primary Sjogren's syndrome 1976–2005 and associated interstitial lung disease: a population-based study of incidence and mortality. BMJ Open 2013; 3: e003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta- analysis. Ann Rheum Dis 2015; 74: 1983–1989. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Casals M, Solans R, Rosas J, et al. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore) 2008; 87: 210–219. [DOI] [PubMed] [Google Scholar]
- 9.Martel C, Jauberteau M-O, Vidal E, et al. [Pathophysiology of primary Sjögren's syndrome.]. Rev Med Interne 2014; 35: 524–530. [DOI] [PubMed] [Google Scholar]
- 10.Chaigne B, Lasfargues G, Marie I, et al. Primary Sjögren's syndrome and occupational risk factors: a case-control study. J Autoimmun 2015; 60: 80–85. [DOI] [PubMed] [Google Scholar]
- 11.Lessard CJ, Li H, Adrianto I, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nat Genet 2013; 45: 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapinos NI, Polihronis M, Tzioufas AG, et al. Sjögren's syndrome. Autoimmune epithelitis. Adv Exp Med Biol 1999; 455: 127–134. [PubMed] [Google Scholar]
- 13.Miceli-Richard C, Wang-Renault S-F, Boudaoud S, et al. Overlap between differentially methylated DNA regions in blood B lymphocytes and genetic at-risk loci in primary Sjögren's syndrome. Ann Rheum Dis 2016; 75: 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavie F, Miceli-Richard C, Ittah M, et al. B-cell activating factor of the tumour necrosis factor family expression in blood monocytes and T cells from patients with primary Sjögren's syndrome. Scand J Immunol 2008; 67: 185–192. [DOI] [PubMed] [Google Scholar]
- 15.Cornec D, Devauchelle-Pensec V, Tobón GJ, et al. B cells in Sjögren's syndrome: from pathophysiology to diagnosis and treatment. J Autoimmun 2012; 39: 161–167. [DOI] [PubMed] [Google Scholar]
- 16.Szczerba BM, Kaplonek P, Wolska N, et al. Interaction between innate immunity and Ro52-induced antibody causes Sjögren's syndrome-like disorder in mice. Ann Rheum Dis 2016; 75: 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alunno A, Carubbi F, Bistoni O, et al. T regulatory and T helper 17 cells in primary Sjögren's syndrome: facts and perspectives. Mediators Inflamm 2015; 2015: 243723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nezos A, Gravani F, Tassidou A, et al. Type I and II interferon signatures in Sjogren's syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjogren's related lymphomagenesis. J Autoimmun 2015; 63: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fragoulis GE, Vakrakou AG, Papadopoulou A, et al. Impaired degradation and aberrant phagocytosis of necrotic cell debris in the peripheral blood of patients with primary Sjögren's syndrome. J Autoimmun 2015; 56: 12–22. [DOI] [PubMed] [Google Scholar]
- 20.Baldini C, Rossi C, Ferro F, et al. The P2X7 receptor-inflammasome complex has a role in modulating the inflammatory response in primary Sjögren's syndrome. J Intern Med 2013; 274: 480–489. [DOI] [PubMed] [Google Scholar]
- 21.Luciano N, Valentini V, Calabrò A, et al. One year in review 2015: Sjögren's syndrome. Clin Exp Rheumatol 2015; 33: 259–271. [PubMed] [Google Scholar]
- 22.Ramos-Casals M, Font J, Garcia-Carrasco M, et al. Primary Sjögren syndrome: hematologic patterns of disease expression. Medicine (Baltimore) 2002; 81: 281–292. [DOI] [PubMed] [Google Scholar]
- 23.Mathews PM, Hahn S, Hessen M, et al. Ocular complications of primary Sjögren syndrome in men. Am J Ophthalmol 2015; 160: 447–452. [DOI] [PubMed] [Google Scholar]
- 24.Priori R, Minniti A, Derme M, et al. Quality of sexual life in women with primary Sjögren syndrome. J Rheumatol 2015; 42: 1427–1431. [DOI] [PubMed] [Google Scholar]
- 25.Roguedas A-M, Youinou P, Lemasson G, et al. Primary Gougerot–Sjögren syndrome: a dermatological approach. J Eur Acad Dermatol Venereol 2006; 20: 243–247. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, et al. Primary Sjogren syndrome. BMJ 2012; 344: e3821. [DOI] [PubMed] [Google Scholar]
- 27.Berkowitz AL, Samuels MA. The neurology of Sjögren's syndrome and the rheumatology of peripheral neuropathy and myelitis. Pract Neurol 2014; 14: 14–22. [DOI] [PubMed] [Google Scholar]
- 28.Ebert EC. Gastrointestinal and hepatic manifestations of Sjogren syndrome. J Clin Gastroenterol 2012; 46: 25–30. [DOI] [PubMed] [Google Scholar]
- 29.Duffles Amarante GB, Zotin MC, Rocha E, et al. Renal tubular dysfunction in patients with primary Sjögren syndrome. Clin Nephrol 2014; 81: 185–191. [DOI] [PubMed] [Google Scholar]
- 30.Khandelwal D, Bhattacharya S, Gadodia A, et al. Metabolic bone disease as a presenting manifestation of primary Sjögren's syndrome: three cases and review of literature. Indian J Endocrinol Metab 2011; 15: 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baimpa E, Dahabreh IJ, Voulgarelis M, et al. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: clinical and pathophysiologic aspects. Medicine (Baltimore) 2009; 88: 284–293. [DOI] [PubMed] [Google Scholar]
- 32.Defendenti C, Atzeni F, Spina MF, et al. Clinical and laboratory aspects of Ro/SSA-52 autoantibodies. Autoimmun Rev 2011; 10: 150–154. [DOI] [PubMed] [Google Scholar]
- 33.Lee LA. The clinical spectrum of neonatal lupus. Arch Dermatol Res 2009; 301: 107–110. [DOI] [PubMed] [Google Scholar]
- 34.Seror R, Ravaud P, Bowman SJ, et al. EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis 2010; 69: 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fauchais AL, Martel C, Gondran G, et al. Immunological profile in primary Sjögren syndrome: clinical significance, prognosis and long-term evolution to other auto-immune disease. Autoimmun Rev 2010; 9: 595–599. [DOI] [PubMed] [Google Scholar]
- 36.Parambil JG, Myers JL, Lindell RM, et al. Interstitial lung disease in primary Sjögren syndrome. Chest 2006; 130: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 37.Kelly C, Gardiner P, Pal B, et al. Lung function in primary Sjögren's syndrome: a cross sectional and longitudinal study. Thorax 1991; 46: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuyama N, Ashizawa K, Okimoto T, et al. Pulmonary lesions associated with Sjögren's syndrome: radiographic and CT findings. Br J Radiol 2003; 76: 880–884. [DOI] [PubMed] [Google Scholar]
- 39.García-Carrasco M, Ramos-Casals M, Rosas J, et al. Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine (Baltimore) 2002; 81: 270–280. [DOI] [PubMed] [Google Scholar]
- 40.Palm O, Garen T, Berge Enger T, et al. Clinical pulmonary involvement in primary Sjogren's syndrome: prevalence, quality of life and mortality – a retrospective study based on registry data. Rheumatology 2013; 52: 173–179. [DOI] [PubMed] [Google Scholar]
- 41.Aerni MR, Vassallo R, Myers JL, et al. Follicular bronchiolitis in surgical lung biopsies: clinical implications in 12 patients. Respir Med 2008; 102: 307–312. [DOI] [PubMed] [Google Scholar]
- 42.Belenguer R, Ramos-Casals M, Brito-Zerón P, et al. Influence of clinical and immunological parameters on the health-related quality of life of patients with primary Sjögren's syndrome. Clin Exp Rheumatol 2005; 23: 351–356. [PubMed] [Google Scholar]
- 43.Yazisiz V, Ozbudak IH, Nizam I, et al. A case of primary Sjögren's syndrome with pulmonary-limited Wegener's granulomatosis. Rheumatol Int 2010; 30: 1235–1238. [DOI] [PubMed] [Google Scholar]
- 44.Davidson BK, Kelly CA, Griffiths ID. Ten year follow up of pulmonary function in patients with primary Sjögren's syndrome. Ann Rheum Dis 2000; 59: 709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strimlan CV, Rosenow EC, Divertie MB, et al. Pulmonary manifestations of Sjögren's syndrome. Chest 1976; 70: 354–361. [DOI] [PubMed] [Google Scholar]
- 46.Ito I, Nagai S, Kitaichi M, et al. Pulmonary manifestations of primary Sjogren's syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med 2005; 171: 632–638. [DOI] [PubMed] [Google Scholar]
- 47.Papiris SA, Maniati M, Constantopoulos SH, et al. Lung involvement in primary Sjögren's syndrome is mainly related to the small airway disease. Ann Rheum Dis 1999; 58: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Constantopoulos SH, Papadimitriou CS, Moutsopoulos HM. Respiratory manifestations in primary Sjögren's syndrome. A clinical, functional, and histologic study. Chest 1985; 88: 226–229. [DOI] [PubMed] [Google Scholar]
- 49.Gardiner P, Ward C, Allison A, et al. Pleuropulmonary abnormalities in primary Sjögren's syndrome. J Rheumatol 1993; 20: 831–837. [PubMed] [Google Scholar]
- 50.Oxholm P, Bundgaard A, Birk Madsen E, et al. Pulmonary function in patients with primary Sjögren's syndrome. Rheumatol Int 1982; 2: 179–181. [DOI] [PubMed] [Google Scholar]
- 51.Shi J-H, Liu H-R, Xu W-B, et al. Pulmonary manifestations of Sjögren's syndrome. Respir Int Rev Thorac Dis 2009; 78: 377–386. [DOI] [PubMed] [Google Scholar]
- 52.Papadimitraki ED, Kyrmizakis DE, Kritikos I, et al. Ear-nose-throat manifestations of autoimmune rheumatic diseases. Clin Exp Rheumatol 2004; 22: 485–494. [PubMed] [Google Scholar]
- 53.Papiris SA, Saetta M, Turato G, et al. CD4-positive T-lymphocytes infiltrate the bronchial mucosa of patients with Sjögren's syndrome. Am J Respir Crit Care Med 1997; 156: 637–641. [DOI] [PubMed] [Google Scholar]
- 54.Hatron PY, Wallaert B, Gosset D, et al. Subclinical lung inflammation in primary Sjögren's syndrome. Relationship between bronchoalveolar lavage cellular analysis findings and characteristics of the disease. Arthritis Rheum 1987; 30: 1226–1231. [DOI] [PubMed] [Google Scholar]
- 55.Franquet T, Giménez A, Monill JM, et al. Primary Sjögren's syndrome and associated lung disease: CT findings in 50 patients. AJR Am J Roentgenol 1997; 169: 655–658. [DOI] [PubMed] [Google Scholar]
- 56.Uffmann M, Kiener HP, Bankier AA, et al. Lung manifestation in asymptomatic patients with primary Sjögren syndrome: assessment with high resolution CT and pulmonary function tests. J Thorac Imaging 2001; 16: 282–289. [DOI] [PubMed] [Google Scholar]
- 57.Koyama M, Johkoh T, Honda O, et al. Pulmonary involvement in primary Sjögren's syndrome: spectrum of pulmonary abnormalities and computed tomography findings in 60 patients. J Thorac Imaging 2001; 16: 290–296. [DOI] [PubMed] [Google Scholar]
- 58.Taouli B, Brauner MW, Mourey I, et al. Thin-section chest CT findings of primary Sjögren's syndrome: correlation with pulmonary function. Eur Radiol 2002; 12: 1504–1511. [DOI] [PubMed] [Google Scholar]
- 59.Lohrmann C, Uhl M, Warnatz K, et al. High-resolution CT imaging of the lung for patients with primary Sjogren's syndrome. Eur J Radiol 2004; 52: 137–143. [DOI] [PubMed] [Google Scholar]
- 60.Howling SJ, Hansell DM, Wells AU, et al. Follicular bronchiolitis: thin-section CT and histologic findings. Radiology 1999; 212: 637–642. [DOI] [PubMed] [Google Scholar]
- 61.Segal I, Fink G, Machtey I, et al. Pulmonary function abnormalities in Sjögren's syndrome and the sicca complex. Thorax 1981; 36: 286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borie R, Schneider S, Debray M-P, et al. Severe chronic bronchiolitis as the presenting feature of primary Sjögren's syndrome. Respir Med 2011; 105: 130–136. [DOI] [PubMed] [Google Scholar]
- 63.Mialon P, Barthélémy L, Sébert P, et al. A longitudinal study of lung impairment in patients with primary Sjögren's syndrome. Clin Exp Rheumatol 1997; 15: 349–354. [PubMed] [Google Scholar]
- 64.Fairfax AJ, Haslam PL, Pavia D, et al. Pulmonary disorders associated with Sjögren's syndrome. Q J Med 1981; 50: 279–295. [PubMed] [Google Scholar]
- 65.Mathieu A, Cauli A, Pala R, et al. Tracheo-bronchial mucociliary clearance in patients with primary and secondary Sjögren's syndrome. Scand J Rheumatol 1995; 24: 300–304. [DOI] [PubMed] [Google Scholar]
- 66.Gudbjörnsson B, Hedenström H, Stålenheim G, et al. Bronchial hyperresponsiveness to methacholine in patients with primary Sjögren's syndrome. Ann Rheum Dis 1991; 50: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.La Corte R, Bajocchi G, Potena A, et al. Bronchial hyperreactivity in systemic sclerosis patients: influence of associated Sjögren's syndrome. Ann Rheum Dis 1995; 54: 636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.La Corte R, Potena A, Bajocchi G, et al. Increased bronchial responsiveness in primary Sjögren's syndrome. A sign of tracheobronchial involvement. Clin Exp Rheumatol 1991; 9: 125–130. [PubMed] [Google Scholar]
- 69.Potena A, La Corte R, Fabbri LM, et al. Increased bronchial responsiveness in primary and secondary Sjögren's syndrome. Eur Respir J 1990; 3: 548–553. [PubMed] [Google Scholar]
- 70.Stålenheim G, Gudbjörnsson B. Anti-inflammatory drugs do not alleviate bronchial hyperreactivity in Sjögren's syndrome. Allergy 1997; 52: 423–427. [DOI] [PubMed] [Google Scholar]
- 71.Lúdvíksdóttir D, Janson C, Björnsson E, et al. Different airway responsiveness profiles in atopic asthma, nonatopic asthma, and Sjögren's syndrome. BHR Study Group. Bronchial hyperresponsiveness. Allergy 2000; 55: 259–265. [DOI] [PubMed] [Google Scholar]
- 72.Andoh Y, Shimura S, Sawai T, et al. Morphometric analysis of airways in Sjögren's syndrome. Am Rev Respir Dis 1993; 148: 1358–1362. [DOI] [PubMed] [Google Scholar]
- 73.Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med 2003; 168: 1277–1292. [DOI] [PubMed] [Google Scholar]
- 74.Yousem SA, Colby TV, Carrington CB. Follicular bronchitis/bronchiolitis. Hum Pathol 1985; 16: 700–706. [DOI] [PubMed] [Google Scholar]
- 75.Newball HH, Brahim SA. Chronic obstructive airway disease in patients with Sjögren's syndrome. Am Rev Respir Dis 1977; 115: 295–304. [DOI] [PubMed] [Google Scholar]
- 76.Wells AU, du Bois RM. Bronchiolitis in association with connective tissue disorders. Clin Chest Med 1993; 14: 655–666. [PubMed] [Google Scholar]
- 77.White ES, Tazelaar HD, Lynch JP. Bronchiolar complications of connective tissue diseases. Semin Respir Crit Care Med 2003; 24: 543–566. [DOI] [PubMed] [Google Scholar]
- 78.Okano A, Sato A, Suda T, et al. [A case of diffuse panbronchiolitis complicated by malignant thymoma and Sjögren's syndrome]. Nihon Kyōbu Shikkan Gakkai Zasshi 1991; 29: 263–268. [PubMed] [Google Scholar]
- 79.Gottenberg J-E, Cinquetti G, Larroche C, et al. Efficacy of rituximab in systemic manifestations of primary Sjogren's syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann Rheum Dis 2013; 72: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 80.Devauchelle-Pensec V, Pennec Y, Morvan J, et al. Improvement of Sjögren's syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum 2007; 57: 310–317. [DOI] [PubMed] [Google Scholar]
- 81.Meijer JM, Pijpe J, Vissink A, et al. Treatment of primary Sjogren syndrome with rituximab: extended follow-up, safety and efficacy of retreatment. Ann Rheum Dis 2009; 68: 284–285. [DOI] [PubMed] [Google Scholar]
- 82.Seror R, Sordet C, Guillevin L, et al. Tolerance and efficacy of rituximab and changes in serum B cell biomarkers in patients with systemic complications of primary Sjögren's syndrome. Ann Rheum Dis 2007; 66: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mandl T, Diaz S, Ekberg O, et al. Frequent development of chronic obstructive pulmonary disease in primary SS – results of a longitudinal follow-up. Rheumatol Oxf Engl 2012; 51: 941–946. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe M, Naniwa T, Hara M, et al. Pulmonary manifestations in Sjogren's syndrome: correlation analysis between chest computed tomographic findings and clinical subsets with poor prognosis in 80 patients. J Rheumatol 2010; 37: 365–373. [DOI] [PubMed] [Google Scholar]
- 85.Soto-Cardenas M-J, Perez-De-Lis M, Bove A, et al. Bronchiectasis in primary Sjögren's syndrome: prevalence and clinical significance. Clin Exp Rheumatol 2010; 28: 647–653. [PubMed] [Google Scholar]
- 86.Liebow AA, Carrington CB. Diffuse pulmonary lymphoreticular infiltrations associated with dysproteinemia. Med Clin North Am 1973; 57: 809–843. [DOI] [PubMed] [Google Scholar]
- 87.Yamadori I, Fujita J, Bandoh S, et al. Nonspecific interstitial pneumonia as pulmonary involvement of primary Sjögren's syndrome. Rheumatol Int 2002; 22: 89–92. [DOI] [PubMed] [Google Scholar]
- 88.Reina D, Roig Vilaseca D, Torrente-Segarra V, et al. Sjögren's syndrome-associated interstitial lung disease: a multicenter study. Reumatol Clin 2015; [in press DOI: 10.1016/j.reuma.2015.09.003]. [DOI] [PubMed] [Google Scholar]
- 89.Ramos-Casals M, Brito-Zerón P, Seror R, et al. Characterization of systemic disease in primary Sjögren's syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology 2015; 54: 2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X, Xu B, Ma Y, et al. Clinical and laboratory profiles of primary Sjogren's syndrome in a Chinese population: a retrospective analysis of 315 patients. Int J Rheum Dis 2015; 18: 439–446. [DOI] [PubMed] [Google Scholar]
- 91.Papathanasiou MP, Constantopoulos SH, Tsampoulas C, et al. Reappraisal of respiratory abnormalities in primary and secondary Sjögren's syndrome. A controlled study. Chest 1986; 90: 370–374. [DOI] [PubMed] [Google Scholar]
- 92.Desai SR, Veeraraghavan S, Hansell DM, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004; 232: 560–567. [DOI] [PubMed] [Google Scholar]
- 93.Honda O, Johkoh T, Ichikado K, et al. Differential diagnosis of lymphocytic interstitial pneumonia and malignant lymphoma on high-resolution CT. AJR Am J Roentgenol 1999; 173: 71–74. [DOI] [PubMed] [Google Scholar]
- 94.Dalavanga YA, Voulgari PV, Georgiadis AN, et al. Lymphocytic alveolitis: a surprising index of poor prognosis in patients with primary Sjogren's syndrome. Rheumatol Int 2006; 26: 799–804. [DOI] [PubMed] [Google Scholar]
- 95.Wallaert B, Hatron PY, Grosbois JM, et al. Subclinical pulmonary involvement in collagen-vascular diseases assessed by bronchoalveolar lavage. Relationship between alveolitis and subsequent changes in lung function. Am Rev Respir Dis 1986; 133: 574–580. [DOI] [PubMed] [Google Scholar]
- 96.Wallaert B, Dugas M, Dansin E, et al. Subclinical alveolitis in immunological systemic disorders. Transition between health and disease? Eur Respir J 1990; 3: 1206–1216. [PubMed] [Google Scholar]
- 97.Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med 2009; 103: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enomoto Y, Takemura T, Hagiwara E, et al. Prognostic factors in interstitial lung disease associated with primary Sjögren's syndrome: a retrospective analysis of 33 pathologically-proven cases. PloS One 2013; 8: e73774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manoussakis MN, Moutsopoulos HM. Sjögren's syndrome: current concepts. Adv Intern Med 2001; 47: 191–217. [PubMed] [Google Scholar]
- 101.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med 2014; 160: 233–242. [DOI] [PubMed] [Google Scholar]
- 102.Enomoto Y, Takemura T, Hagiwara E, et al. Features of usual interstitial pneumonia in patients with primary Sjögren′s syndrome compared with idiopathic pulmonary fibrosis. Respir Investig 2014; 52: 227–235. [DOI] [PubMed] [Google Scholar]
- 103.Swigris JJ, Berry GJ, Raffin TA, et al. Lymphoid interstitial pneumonia: a narrative review. Chest 2002; 122: 2150–2164. [DOI] [PubMed] [Google Scholar]
- 104.Johkoh T, Müller NL, Pickford HA, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1999; 212: 567–572. [DOI] [PubMed] [Google Scholar]
- 105.Carignan S, Staples CA, Müller NL. Intrathoracic lymphoproliferative disorders in the immunocompromised patient: CT findings. Radiology 1995; 197: 53–58. [DOI] [PubMed] [Google Scholar]
- 106.Johkoh T, Ichikado K, Akira M, et al. Lymphocytic interstitial pneumonia: follow-up CT findings in 14 patients. J Thorac Imaging 2000; 15: 162–167. [DOI] [PubMed] [Google Scholar]
- 107.Deheinzelin D, Capelozzi VL, Kairalla RA, et al. Interstitial lung disease in primary Sjögren's syndrome. Clinical-pathological evaluation and response to treatment. Am J Respir Crit Care Med 1996; 154: 794–799. [DOI] [PubMed] [Google Scholar]
- 108.Cha SI, Fessler MB, Cool CD, et al. Lymphoid interstitial pneumonia: clinical features, associations and prognosis. Eur Respir J 2006; 28: 364–369. [DOI] [PubMed] [Google Scholar]
- 109.Ioannou S, Toya SP, Tomos P, et al. Cryptogenic organizing pneumonia associated with primary Sjogren's syndrome. Rheumatol Int 2008; 28: 1053–1055. [DOI] [PubMed] [Google Scholar]
- 110.Matteson EL, Ike RW. Bronchiolitis obliterans organizing pneumonia and Sjögren's syndrome. J Rheumatol 1990; 17: 676–679. [PubMed] [Google Scholar]
- 111.Usui Y, Kimula Y, Miura H, et al. A case of bronchiolitis obliterans organizing pneumonia associated with primary Sjögren's syndrome who died of superimposed diffuse alveolar damage. Respir Int Rev Thorac Dis 1992; 59: 122–124. [DOI] [PubMed] [Google Scholar]
- 112.Henriet AC, Diot E, Marchand-Adam S, et al. Organising pneumonia can be the inaugural manifestation in connective tissue diseases, including Sjogren's syndrome. Eur Respir Rev 2010; 19: 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, et al. Topical and systemic medications for the treatment of primary Sjögren's syndrome. Nat Rev Rheumatol 2012; 8: 399–411. [DOI] [PubMed] [Google Scholar]
- 114.Justet A, Ottaviani S, Dieudé P, et al. Tocilizumab for refractory organising pneumonia associated with Sjögren's disease. BMJ Case Rep 2015; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.González García A, Callejas Rubio JL, Ríos Fernández R, et al. Pulmonary Langerhans histiocytosis: an uncommon cause of interstitial pneumonia in a patient with Sjögren syndrome. Clin Rheumatol 2016; 35: 825–828. [DOI] [PubMed] [Google Scholar]
- 116.Young C, Hunt S, Watkinson A, et al. Sjögren's syndrome, cavitating lung disease and high sustained levels of antibodies to serine proteinase 3. Scand J Rheumatol 2000; 29: 267–269. [DOI] [PubMed] [Google Scholar]
- 117.Cottin V, Nunes H, Mouthon L, et al. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum 2011; 63: 295–304. [DOI] [PubMed] [Google Scholar]
- 118.Johnston SL, Dudley CRK, Unsworth DJ, et al. Life-threatening acute pulmonary haemorrhage in primary Sjögren's syndrome with cryoglobulinaemia. Scand J Rheumatol 2005; 34: 404–407. [DOI] [PubMed] [Google Scholar]
- 119.Tomita Y, Mori S, Arima N, et al. Rapidly progressive pulmonary fibrosis following the onset of diffuse alveolar hemorrhage in Sjögren's syndrome: an autopsy case report. Intern Med Tokyo Jpn 2012; 51: 295–299. [DOI] [PubMed] [Google Scholar]
- 120.Rajagopala S, Singh N, Gupta K, et al. Pulmonary amyloidosis in Sjogren's syndrome: a case report and systematic review of the literature. Respirol Carlton Vic 2010; 15: 860–866. [DOI] [PubMed] [Google Scholar]
- 121.Kauppi M, Pukkala E, Isomäki H. Elevated incidence of hematologic malignancies in patients with Sjögren's syndrome compared with patients with rheumatoid arthritis (Finland). Cancer Causes Control 1997; 8: 201–204. [DOI] [PubMed] [Google Scholar]
- 122.Hansen LA, Prakash UB, Colby TV. Pulmonary lymphoma in Sjögren's syndrome. Mayo Clin Proc 1989; 64: 920–931. [DOI] [PubMed] [Google Scholar]
- 123.Theander E, Henriksson G, Ljungberg O, et al. Lymphoma and other malignancies in primary Sjögren's syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 2006; 65: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kojima M, Tsukamoto N, Yokohama A, et al. B-cell lymphoma associated with Sjögren's syndrome among Japanese patients: a clinicopathologic and immunohistochemical study of 15 cases. J Clin Exp Hematop 2009; 49: 89–95. [DOI] [PubMed] [Google Scholar]
- 125.Graham BB, Mathisen DJ, Mark EJ, et al. Primary pulmonary lymphoma. Ann Thorac Surg 2005; 80: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 126.Launay D, Hachulla E, Hatron P-Y, et al. Pulmonary arterial hypertension: a rare complication of primary Sjögren syndrome: report of 9 new cases and review of the literature. Medicine (Baltimore) 2007; 86: 299–315. [DOI] [PubMed] [Google Scholar]
- 127.Ramagopalan SV, Wotton CJ, Handel AE, et al. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med 2011; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chung W-S, Lin C-L, Sung F-C, et al. Increased risks of deep vein thrombosis and pulmonary embolism in Sjögren syndrome: a nationwide cohort study. J Rheumatol 2014; 41: 909–915. [DOI] [PubMed] [Google Scholar]
- 129.Sada PR, Isenberg D, Ciurtin C. Biologic treatment in Sjögren's syndrome. Rheumatology 2015; 54: 219–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.