Abstract
Pre-employment examination is considered to be an important practice and is commonly performed in several countries within the European Union. The benefits of medical surveillance programmes are not generally accepted and their structure is often inconsistent.
The aim of this review was to evaluate, on the basis of the available literature, the usefulness of medical screening and surveillance. MEDLINE was searched from its inception up to March 2010. Retrieved literature was evaluated in a peer-review process and relevant data was collected following a systematic extraction schema.
Pre-placement screening identifies subjects who are at an increased risk for developing work-related allergic disease, but pre-employment screening is too low to be used as exclusion criteria. Medical surveillance programmes can identify workers who have, or who are developing, work-related asthma. These programmes can also be used to avoid worsening of symptoms by implementing preventive measures. A combination of different tools within the surveillance programme, adjusted for the risk of the individual worker, improves the predictive value.
Medical surveillance programmes provide medical as well as socioeconomic benefits. However, pre-employment screening cannot be used to exclude workers. They may act as a starting point for surveillance strategies. A stratified approach can increase the effectiveness and reduce the costs for such programmes.
Keywords: Nonspecific bronchial hyperresponsiveness, pre-employment examination, sensitisation, skin-prick test, work-related asthma
Due to heterogenous usage of the terms “medical screening” and “medical surveillance”, we will first describe these terms as they are referred to in this article.
Medical screening, in the strictest sense, is a method for detecting disease or body dysfunction before an individual would normally seek medical care. The fundamental purpose of screening is early diagnosis and treatment of the individual and, thus, it has a clinical focus. Screening tests are usually administered to individuals in a larger population who have not yet sought medical care, but who may be at high risk for certain adverse health outcomes. In essence, it involves detection of individuals with an elevated probability of having the disorder in question. In the occupational asthma field, the term “screening” is often used more loosely for detecting individuals with existing disease (secondary or tertiary prevention) to avoid worsening. Related to screening is the activity of screening not for the presence of disease, but for the presence of risk factors of disease. This activity has been suggested for use in pre-employment or pre-placement evaluations. At the request of the World Health Organization, criteria for screening have been formulated to help decide whether screening in a particular situation should be recommended [1]. Among other criteria, the screening programme should involve a major health problem, should lead to improved prognosis, the test should be acceptable for individuals undergoing the test, there should be clear criteria for referral, the costs of screening must be compared to costs and benefits for the healthcare system, and screening should be a long-term service. The evidence potentially underpinning screening is explicitly considered in this article.
Medical surveillance is the analysis of health information in order to identify problems that may be occurring in the workplace that require targeted prevention. Surveillance can include both population or group-based activities and individual-based activities. The individual-oriented activities are often referred to as worker screening and monitoring functions. However, medical surveillance is usually used in a broader context than screening because it is followed by intervention steps, aimed at improving the work environment, to prevent further exacerbation or development of disease.
Medical screening is usually performed by applying a highly sensitive test in all workers followed by further clinical evaluation in workers identified to be at risk. Preferably, screening tests are simple, rapid and inexpensive and should, in principle, have high positive and negative predictive values.
The rationale of medical surveillance is reducing the burden of work-related asthma by targeted interventions. In other words, the best prognosis and greatest chance of eradicating work-related asthma is associated with a shorter duration of symptoms, milder asthma before diagnosis and complete removal from further exposure to the causative work agent [2]. Being a preventive strategy medical surveillance combines screening, case finding, monitoring and intervention.
Both medical screening and surveillance are secondary preventative strategies. Medical screening differs from surveillance, in that the goal of screening is detection of cases with an elevated probability of having the disorder of interest, whereas medical surveillance is a preventive strategy to improve workers safety and health. Surveillance is often used interchangeably with monitoring and both are used as generic terms. Screening and monitoring might, in specific situations, be difficult to separate in the case of occupational asthma and allergy. A potential early sign (sensitisation) is usually followed quite rapidly by development of symptoms and, sometimes, even clinical disease. So, the symptom-free early stage of disease, to which ideally screening applies, might be too short to distinguish from early symptomatic disease. Moreover, in practice, medical instruments proposed for screening and monitoring are often similar in the case of asthma and allergy, which contributes to confusion.
Medical surveillance programmes are only effective when detection of work-related asthma is followed by appropriate preventive measures in terms of exposure reduction, job change support, compensation and pharmacological therapy in individual workers with work-related asthma. This implies a multidisciplinary approach. Effective prevention measures aimed to improve the prognosis of occupational asthma and work-related asthma require both surveillance and exposure assessment [3].
Medical surveillance of the number/incidence of filed, acknowledged or compensated compensation claims can be a valuable tool for monitoring time trends in the frequency of work-related asthma when work-related asthma is a registered occupational disease and when a, preferably compulsory, reporting and notification system is available. In some countries, health surveillance is required in certain circumstances (e.g. Germany, UK and France) and should be conducted to a high clinical and quality standard.
In summary, by covering all elements related to prevention, medical surveillance provides insight into the occupational healthcare supply chain, but is less useful as a single outcome parameter. In the present context, medical surveillance is regarded as disease surveillance (including early detection of work-related asthma), treatment and monitoring of affected workers, as well as interventions to prevent progression of the disease, reduce its duration and prevent other workers becoming affected.
METHODS
The statements and recommendations formulated in this review are based on a systematic evaluation of the international literature according to the methods extensively described by Baur et al. [4]. The statements are graded according to the Royal College of General Practitioner’s (RCGP) three-star system: ***: strong evidence, provided by generally consistent findings in multiple high quality scientific studies; **: moderate evidence, provided by generally consistent findings in fewer, smaller or lower quality scientific studies; *: limited or contradictory evidence, provided by one scientific study or inconsistent findings in multiple scientific studies, no scientific evidence based on clinical studies, theoretical considerations and/or clinical consensus [5].
Search results, as well as a list of considered articles, are included in evidence tables and presented in the supplementary material (table O3) in the article by Baur et al. [4].
RESULTS
As a result of the systematic literature search, 225 titles were identified. After full evaluation we selected 72 papers which were relevant for the key question: “What are the benefits of medical screening and surveillance?”
Pre-employment screening
Several studies found an increased risk of developing work-related respiratory symptoms in subjects with pre-existing sensitisation to allergens structurally related to occupational allergens, e.g. domestic animals and laboratory animals [6–8] or grass pollen and flour [9], due to cross-reactivity of allergens. Longitudinal studies have been equivocal with respect to a relationship between occupational sensitisation and asthma like-symptoms [10]. Several authors have found such a relationship [11–14], whereas others have been unable [15]. Exposure and sensitisation to occupational allergens are probably not the only cause of asthma in this group of young adults. Allergy and asthma may actually develop through separate pathways [16]. Pre-existing nonspecific bronchial hyperresponsiveness (NSBHR) is associated with an increased risk for the development of work-related chest symptoms, work-related asthma and sensitisation to work-related allergens and isocyanates. In a longitudinal study of 408 apprentices exposed to high-molecular-weight (HMW) allergens (laboratory animals, latex and flour) [17], NSBHR at baseline and pre-existing, physician-diagnosed asthma were found to be significantly associated with an increased risk for occupational asthma. Two studies by Kongerud and co-workers [18, 19] in workers exposed to aluminium fluoride described a significant association and a trend between family history of asthma and work-related asthma-like symptoms, respectively. A history of allergies or atopy was not associated with an increased risk for asthma like-symptoms in both studies. Even though a true relationship existed, exclusion of subjects with a family history of asthma would reduce the incidence of respiratory symptoms by no more than ∼5%.
Despite the fact that many variables at baseline determine the risk for developing work-related symptoms and asthma at follow-up, positive predictive values of these risk factors are generally too low for a meaningful prediction and for screening out potentially susceptible individuals [20, 21]. This is particularly true in the case of atopy, which is a highly prevalent disease in the general population. Excluding atopic individuals from jobs involving exposure to HMW allergens would dramatically reduce the number of potential new employees and would be unduly discriminatory. There were only a few longitudinal investigations on the effectiveness (predicted value) of pre-exposure screening tests. Most information was gained from medical surveillance programmes in groups already under exposure. Most of these programmes considered sensitisation to work-related agents as the outcome of interest (and not work-related asthma). A higher rate of work-related asthma was observed among atopic workers exposed to HMW allergens such as those from laboratory animals [22, 23], flour dust [8, 9], citrus red mites [24] and alcalase enzymes [25]. For papain enzyme [26] and aluminium pot room fume-exposed workers [19], no increased risk for atopics of developing occupational asthma was found.
Due to the lack of epidemiological genetic studies, the yield and benefit of genetic screening cannot be evaluated. A negative association was found between a positive skin-prick test (SPT) result with mouse allergens and human leukocyte antigen (HLA)-DRW6 in two studies [27, 28]. No other publication investigated the relationships between HLA patterns and sensitisations or other work-related outcomes. Due to the unknown positive and negative predicted value and the cost-intensive analysis, HLA typification is not an option for mass medical screening.
Screening and medical surveillance of workers during exposure
The sequential use of different screening tools according to evaluated algorithms increases the accuracy and effectiveness of detecting work-related asthma compared to using one screening method alone. Pre-selection in high- and low-risk groups based on questionnaire items increases the predicted probability of having occupational asthma or the probability of a strongly related preliminary characteristic, such as sensitisation to the associated allergen [29–31].
Studies of subjects exposed to HMW allergens showed that the latency period may vary considerably before development of work-related asthma and that the first signs of disease may occur after weeks or up to several years after first exposure [32, 33]. Therefore, surveillance of exposed workers from the start of exposure is necessary and should start with vocational training.
Medical surveillance has been implemented in workplaces where there is exposure to known asthma-inducing agents [2] or after a single case or a sentinel event [34–39].
Individual studies have different designs and effect evaluations using a broad spectrum of measures, interventions and outcome parameters. Few systematic evaluations of surveillance programmes have been undertaken. The available evidence, mainly consisting of retrospective studies, is indicative of beneficial effects of medical surveillance including intervention directed to reduce exposure [39–44].
Prospective studies of medical surveillance in work-related asthma are scarce. Wild et al. [45] used a mathematical simulation model of isocyanate asthma to compare annual outcome of surveillance by symptom questionnaire and spirometry with passive case findings in 100,000 isocyanate workers. Similar findings were reported by Warren et al. [46].
The content of medical surveillance varies widely among studies depending on the setting and on the study objective and design, making a detailed comparison impossible. In most studies the effectiveness of a surveillance programme was not the subject of the study; rather, medical surveillance represented the diagnostic approach. Medical surveillance programmes may include the following components [20, 47, 48]: 1) pre-placement and periodic administration of a questionnaire aimed at detecting work-related symptoms; 2) detection of sensitisation to occupational agents by means of SPTs or serum specific immunoglobulin (Ig)E antibodies when there is exposure to a respiratory allergen and respective tests are available and standardised; 3) early referral of symptomatic and/or sensitised workers for specialised medical assessment, including specific inhalation challenge in the laboratory and/or in the workplace [49]; and 4) investigation of possible asthma in all workers with confirmed occupational rhinitis and/or NSBHR.
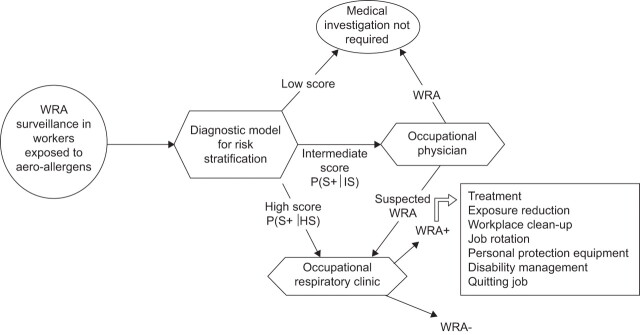
A relatively new approach to medical surveillance is based on diagnostic models developed to predict the probability of sensitisation in workers exposed to HMW allergens. Used as a screening tool, the questionnaire-based prediction model enables risk stratification of workers. This makes it possible to select or exclude workers for further clinical evaluation [50–54]. Figure 1 shows the use of a questionnaire-derived prediction model in a medical surveillance programme for work-related asthma.
Figure 1.
Flow chart for medical surveillance to detect work-related asthma (WRA) due to aero-allergens. P(S+): probability of sensitisation assessed by a regular questionnaire; HS: high score, i.e. high probability; IS: intermediate score, i.e. intermediate probability. Adapted from [54] with permission from the publisher.
DISCUSSION
The following statements, graded according to the RCGP three-star system, summarise the results of the systematic analysis (table O3 in the supplementary material in Baur et al. [4]).
1) Workers (pre-)sensitised to allergens that they will be exposed to in their future work environment have an increased risk of developing occupational asthma or NSBHR soon after exposure (***) [6–8, 10, 11, 13, 14, 24].
2) Specific sensitisation can, for this purpose, be assessed by SPTs with work-associated allergens and IgE serology (***) [6, 13, 23, 26, 55].
3) The positive predictive value of atopy screening results is not sufficiently predictive for future occupational sensitisation, work-related asthma or respiratory occupational allergy (***) [8, 12, 13, 22, 25, 56].
4) SPTs with standardised high-quality allergen extracts are a suitable screening method for the identification of occupational sensitisation as a work-related risk factor in workers exposed to HMW sensitisers, such as laboratory animals, latex, enzymes or flour (***) [6, 23, 24, 55].
5) A questionnaire-based diagnostic model separates individuals at low risk who do not need further examination from those who need further clinical investigation and management (*) [30, 31, 51].
6) The positive predictive value of NSBHR (observed pre- as well as during current employment) is not sufficiently reliable to be used as a predictive tool for occupational asthma (**) [7, 10, 17, 57, 58].
7) Genetic makers measured at pre-employment are not useful in predicting future occupational asthma as genetic markers identified to date are too weakly associated with development of work-related asthma, symptoms or other signs of work-related asthma (*) [27, 59, 60].
8) A combination of different tests (questionnaires as well as physiological and immunological tests, etc.) can improve the predictive value of individual screening methods (***) [29–31].
9) Early detection of work-related respiratory symptoms, sensitisation and work-related asthma is possible by medical surveillance, including a questionnaire in combination with at least one of the following options: detection of specific sensitisation, NSBHR testing, specific inhalation challenge and diagnostic work-up in a referral centre (**) [20, 47, 49, 51, 54, 61–63].
10) Evaluations of surveillance programmes consisting of medical surveillance (case finding), employers' feedback and exposure control measures indicate that the incidence of work-related asthma may decline following the introduction of a surveillance programme (**) [2, 39–41, 43].
11) Medical surveillance may reduce the occurrence of disability and socioeconomic costs (*) [45].
12) Diagnostic models used for prediction in medical surveillance programmes should be carefully designed and clearly state the context of where and how it can be used. Such diagnostic models have limited accuracy and need validation and calibration and, in many cases, an occupational agent tailored approach (*) [50, 51].
The effectiveness of the individual screening tools is discussed in the article by Baur et al. [4]. The predictive value of risk factors such as atopy or smoking is extensively explained in the study on risk factors for work-related asthma by Maestrelli et al. [61].
Different and overlapping definitions exist for work-related asthma, occupational asthma, screening and surveillance. The differences in the outcome measures and the heterogeneity of the studies complicated the use of sophisticated meta-analytical methods. Nonetheless, some statements and recommendations can be made (table 1).
Table 1. Recommendations.
| Recommendations | Strength of recommendation | Level of evidence |
| Questionnaire-based identification of all workers at risk of developing work-related asthma is recommended as basis for surveillance | Strong | High |
| Pre-placement screening for specific cross-reacting, work-associated sensitisation among potentially HMW allergen-exposed subjects is recommended in order to identify those at higher risk for work-related asthma | Strong | Moderate |
| Detection of sensitisation either by specific IgE or SPT should be included in surveillance (not only pre-placement) for identification of subjects at risk of work-related asthma with foreseeable regular exposure to HMW agents (such as laboratory animals, bakery dust, enzymes or latex) | Strong | Moderate |
|
In atopics and subjects with pre-existing asthma or sensitisation, pre-employment investigation should be performed in order to inform them about their increased work-related asthma risk
Because of the low PPV, exclusion of asymptomatic atopics or sensitised subjects from exposure to potential occupational allergens or irritant agents cannot be recommended |
Weak | Moderate |
|
In all workers with confirmed occupational rhinitis and/or NSBHR, medical surveillance programmes should be performed
They should include periodic administration of a questionnaire, detection of sensitisation by standardised SPT or serum specific IgE antibodies, early referral of symptomatic and/or sensitised subjects for specialised medical assessment and assessment of asthma Surveillance programmes should already be implemented during vocational training of individuals at risk |
Strong | Moderate |
| Identification of symptoms or sensitisation during surveillance should result in an investigation to confirm or exclude occupational asthma, work-related asthma, rhinitis and COPD | Strong | High |
| Risk stratification by diagnostic models can be used in medical surveillance to select exposed workers for further medical evaluation | Strong | Moderate |
| As a secondary prevention measure, a comprehensive medical surveillance programme should, in addition to early detection of sensitisation, allergic symptoms and occupational asthma, comprise exposure assessment and intervention targeted both at workers and exposure | Strong | Moderate |
HMW: high molecular weight; Ig: immunoglobulin; SPT: skin-prick test; PPV: positive predictive value; NSBHR: nonspecific bronchial hyperresponsiveness; COPD: chronic obstructive pulmonary disease.
Pre-placement screening can identify those workers at a higher risk of developing work-related symptoms. The positive predictive value of these tests is too low to be used as a selection criterion as many workers who would never develop work-related asthma would be excluded. Nevertheless, pre-placement examinations provide an opportunity to inform workers about their increased risk and should encourage the uptake of preventive measures; pre-employment results can be taken as a baseline for targeted medical surveillance programmes. This is in accordance with the British Occupational Health Research Foundation [60, 63] and American College of Chest Physicians guidelines [64].
Recent publications [65–67] have shown that surveillance programmes for work-related asthma in occupationally high-risk populations are beneficial and may lead to a reduction in cases of occupational asthma. Early identification followed by removal from exposure increases the chance of a better health outcome, including reduction of NSBHR and reduced costs for medical treatment.
It is important to evaluate the independent and additional predictive value of diagnostic tests given the presence of earlier information. Prediction research offers a solution for this by using a multivariate approach in design and analysis that accounts for mutual dependence between different test results. The information of every item can then be translated into a predicted probability of the chosen outcome. This technique allows weights to be given to each independent predictor in the probability equation, and provides estimations of the probability of an outcome at present (diagnosis) or in the future (prognosis). Prediction models applied in occupational health practice may, therefore, enable an occupational physician to deal with uncertainties in considering workers at risk of having occupational diseases. The main goal is to optimise risk estimation at low costs, and it may be the first step in the clinical evaluation and management of work-related asthma [29, 30, 50]. The models may initiate counselling and interventions (such as the replacement of powdered allergenic latex gloves [5, 42]) and, thus, are potentially useful for the identification of specific groups at risk. A new approach to medical surveillance is the use of diagnostic models that have been developed to predict the probability of sensitisation in workers exposed to HMW allergens. Used as a screening tool, the questionnaire-based prediction model enables stratification of workers in low-, medium- and high-risk groups. This makes it possible to select or exclude workers for further clinical evaluation.
Future research
Although its role in disease management is not disputed, when and how to set up medical surveillance, which tests to use, test frequency and which outcome parameters should be used in different occupational groups are important questions still awaiting answer. As direct evidence for the benefit of medical surveillance is limited there is a need for prospective studies using clearly defined instruments and outcome.
Acknowledgments
The members of the Task Force are as follows. X. Baur (Chair: Institute for Occupational and Maritime Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany); T. Sigsgaard (Co-chair: Århus University, Dept of Environmental and Occupational Medicine, School of Public Health, Århus, Denmark); T.B. Aasen (Haukeland University Hospital, Bergen, Norway); P.S. Burge (Heart of England NHS Foundation Trust, Dept of Respiratory Medicine, Birmingham, UK); H. Dressel (Städt. Klinikum München GmbH Klinikum Neuperlach Zentrum für Akutgeratrie und Frührehabilitation, Munich, Germany); D. Heederik (Environmental Epidemiology Division, Institute for Risk Assessment Sciences (IRAS), University of Utrecht, Utrecht, the Netherlands); P.K. Henneberger (National Institute for Occupational Safety and Health, Centres for Disease Control and Prevention, Morgantown, WV, USA); P. Maestrelli (Dept of Environmental Medicine and Public Health, University of Padova, Padova, Italy); C. Redlich (Yale Occupational/Environmental, New Haven, CT, USA); J. Rooyackers (Netherlands Expertise Center for Occupational Respiratory Disorders – NECORD IRAS NKAL, Utrecht, the Netherlands); V. Schlünssen (Århus University, Århus, Denmark); O. Vandenplas (Dept of Chest Medicine, Mont-Grodinne Hospital, Université Catholique de Louvain, Yvoir, Belgium); D. Wilken (Institute for Occupational and Maritime Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany).
Footnotes
Support Statement
The work of the Task Force on the Management of Work-related Asthma was funded by the European Respiratory Society.
Statement of Interest
None declared.
Provenance
Submitted article, peer reviewed.
REFERENCES
- 1.Wilson JMG, Junger G. Principles and Practice of Screening for Disease. Printed Health Papers No. 34. Geneva, World Health Organization, 1968. Available from: http://whqlibdoc.who.int/php/WHO_PHP_34.pdf. [Google Scholar]
- 2.Tarlo SM, Liss GM. Can medical surveillance measures improve the outcome of occupational asthma? J Allergy Clin Immunol 2001; 107: 583–585. [DOI] [PubMed] [Google Scholar]
- 3.Heederik D, van Rooy F. Exposure assessment should be integrated in studies on the prevention and management of occupational asthma. Occup Environ Med 2008; 65: 149–150. [DOI] [PubMed] [Google Scholar]
- 4.Baur X, Sigsgaard T, Aasen TB, et al. Guidelines for the management of work-related asthma. Eur Respir J 2012; 39: 529–545. [DOI] [PubMed] [Google Scholar]
- 5.The Royal College of General Practitioners. The Development and Implementation of Clinical Guidelines. Report of the Clinical Guidelines Working Group. London, RCGP, 1995. [Google Scholar]
- 6.Bryant DH, Boscato LM, Mboloi PN, et al. Allergy to laboratory animals among animal handlers. Med J Aust 1995; 163: 415–418. [DOI] [PubMed] [Google Scholar]
- 7.Gautrin D, Infante-Rivard C, Ghezzo H, et al. Incidence and host determinants of probable occupational asthma in apprentices exposed to laboratory animals. Am J Respir Crit Care Med 2001; 163: 899–904. [DOI] [PubMed] [Google Scholar]
- 8.Venables KM, Tee RD, Hawkins ER, et al. Laboratory animal allergy in a pharmaceutical company. Br J Ind Med 1988; 45: 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baur X, Degens PO, Sander I. Baker's asthma: still among the most frequent occupational respiratory disorders. J Allergy Clin Immunol 1998; 102: 984–997. [DOI] [PubMed] [Google Scholar]
- 10.Skjold T, Dahl R, Juhl B, et al. The incidence of respiratory symptoms and sensitisation in baker apprentices. Eur Respir J 2008; 32: 452–459. [DOI] [PubMed] [Google Scholar]
- 11.Walusiak J, Hanke W, Gorski P, et al. Respiratory allergy in apprentice bakers: do occupational allergies follow the allergic march? Allergy 2004; 59: 442–450. [DOI] [PubMed] [Google Scholar]
- 12.De Zotti R, Bovenzi M. Prospective study of work related respiratory symptoms in trainee bakers. Occup Environ Med 2000; 57: 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renstrom A, Malmberg P, Larsson K, et al. Prospective study of laboratory-animal allergy: factors predisposing to sensitization and development of allergic symptoms. Allergy 1994; 49: 548–552. [DOI] [PubMed] [Google Scholar]
- 14.Portengen L, Hollander A, Doekes G, et al. Lung function decline in laboratory animal workers: the role of sensitisation and exposure. Occup Environ Med 2003; 60: 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullinan P, Cook A, Nieuwenhuijsen MJ, et al. Allergen and dust exposure as determinants of work-related symptoms and sensitization in a cohort of flour-exposed workers; a case-control analysis. Ann Occup Hyg 2001; 45: 97–103. [DOI] [PubMed] [Google Scholar]
- 16.Lau S, Illi S, Sommerfeld C, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet 2000; 356: 1392–1397. [DOI] [PubMed] [Google Scholar]
- 17.Gautrin D, Ghezzo H, Infante-Rivard C, et al. Long-term outcomes in a prospective cohort of apprentices exposed to high-molecular-weight agents. Am J Respir Crit Care Med 2008; 177: 871–879. [DOI] [PubMed] [Google Scholar]
- 18.Kongerud J, Samuelsen SO. A longitudinal study of respiratory symptoms in aluminum potroom workers. Am Rev Respir Dis 1991; 144: 10–16. [DOI] [PubMed] [Google Scholar]
- 19.Kongerud J, Gronnesby JK, Magnus P. Respiratory symptoms and lung function of aluminum potroom workers. Scand J Work Environ Health 1990; 16: 270–277. [DOI] [PubMed] [Google Scholar]
- 20.Cullinan P, Tarlo S, Nemery B. The prevention of occupational asthma. Eur Respir J 2003; 22: 853–860. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson PJ, Cullinan P, Taylor AJ, et al. Evidence based guidelines for the prevention, identification, and management of occupational asthma. Occup Environ Med 2005; 62: 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cockcroft A, Edwards J, McCarthy P, et al. Allergy in laboratory animal workers. Lancet 1981; 1: 827–830. [DOI] [PubMed] [Google Scholar]
- 23.Agrup G, Belin L, Sjostedt L, et al. Allergy to laboratory animals in laboratory technicians and animal keepers. Br J Ind Med 1986; 43: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YK, Son JW, Kim HY, et al. Citrus red mite (Panonychus citri) is the most common sensitizing allergen of asthma and rhinitis in citrus farmers. Clin Exp Allergy 1999; 29: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 25.Juniper CP, Roberts DM. Enzyme asthma: fourteen years' clinical experience of a recently prescribed disease. J Soc Occup Med 1984; 34: 127–132. [DOI] [PubMed] [Google Scholar]
- 26.Baur X, Konig G, Bencze K, et al. Clinical symptoms and results of skin test, RAST and bronchial provocation test in thirty-three papain workers: evidence for strong immunogenic potency and clinically relevant “proteolytic effects of airborne papain”. Clin Allergy 1982; 12: 9–17. [DOI] [PubMed] [Google Scholar]
- 27.Schumacher MJ, Tait BD, Holmes MC. Allergy to murine antigens in a biological research institute. J Allergy Clinical Immunol 1981; 68: 310–318. [DOI] [PubMed] [Google Scholar]
- 28.Jeal H, Draper A, Jones M, et al. HLA associations with occupational sensitization to rat lipocalin allergens: a model for other animal allergies? J Allergy Clin Immunol 2003; 111: 795–799. [DOI] [PubMed] [Google Scholar]
- 29.Meijer E, Grobbee DE, Heederik D. Detection of workers sensitised to high molecular weight allergens: a diagnostic study in laboratory animal workers. Occup Environ Med 2002; 59: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer E, Grobbee DE, Heederik D. A strategy for health surveillance in laboratory animal workers exposed to high molecular weight allergens. Occup Environ Med 2004; 61: 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarthana E. Predicting occupational lung diseases. PhD thesis. Utrecht University, Utrecht, the Netherlands, 2008; p. 127. [Google Scholar]
- 32.Kruize H, Post W, Heederik D, et al. Respiratory allergy in laboratory animal workers: a retrospective cohort study using pre-employment screening data. Occup Environ Med 1997; 54: 830–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott L, Heederik D, Marshall S, et al. Progression of self-reported symptoms in laboratory animal allergy. J Allergy Clinical Immunol 2005; 116: 127–132. [DOI] [PubMed] [Google Scholar]
- 34.Bakke PS, Baste V, Hanoa R, et al. Prevalence of obstructive lung disease in a general population: relation to occupational title and exposure to some airborne agents. Thorax 1991; 46: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kogevinas M, Anto JM, Sunyer J, et al. Occupational asthma in Europe and other industrialised areas: a population-based study. European Community Respiratory Health Survey Study Group. Lancet 1999; 353: 1750–1754. [DOI] [PubMed] [Google Scholar]
- 36.Carlsen KH, Anderson SD, Bjermer L, et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy 2008; 63: 387–403. [DOI] [PubMed] [Google Scholar]
- 37.Karjalainen EM, Laitinen A, Sue-Chu M, et al. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med 2000; 161: 2086–2091. [DOI] [PubMed] [Google Scholar]
- 38.Hansson SO. Critical effects and exposure limits. Risk Anal 1997; 17: 227–236. [DOI] [PubMed] [Google Scholar]
- 39.Merget R, Caspari C, Dierkes-Globisch A, et al. Effectiveness of a medical surveillance program for the prevention of occupational asthma caused by platinum salts: a nested case-control study. 2001; 107: 707–712. [DOI] [PubMed] [Google Scholar]
- 40.Grammer LC, Shaughnessy MA, Henderson J, et al. A clinical and immunologic study of workers with trimellitic-anhydride-induced immunologic lung disease after transfer to low exposure jobs. Am Rev Respir Dis 1993; 148: 54–57. [DOI] [PubMed] [Google Scholar]
- 41.Liss GM, Tarlo SM. Natural rubber latex-related occupational asthma: association with interventions and glove changes over time. Am J Ind Med 2001; 40: 347–353. [DOI] [PubMed] [Google Scholar]
- 42.Tarlo SM, Easty A, Eubanks K, et al. Outcomes of a natural rubber latex control program in an Ontario teaching hospital. J Allergy Clin Immunol 2001; 108: 628–633. [DOI] [PubMed] [Google Scholar]
- 43.Tarlo SM, Liss GM, Yeung KS. Changes in rates and severity of compensation claims for asthma due to diisocyanates: a possible effect of medical surveillance measures. Occup Environ Med 2002; 59: 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amital H, Glikson M, Burstein M, et al. Clinical characteristics of unexpected death among young enlisted military personnel: results of a three-decade retrospective surveillance. Chest 2004; 126: 528–533. [DOI] [PubMed] [Google Scholar]
- 45.Wild DM, Redlich CA, Paltiel AD. Surveillance for isocyanate asthma: a model based cost effectiveness analysis. Occup Environ Med 2005; 62: 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren N, Meijster T, Heederik D, et al. A dynamic population-based model for the development of work-related respiratory health effects among bakery workers. Occup Environ Med 2009; 66: 810–817. [DOI] [PubMed] [Google Scholar]
- 47.Gannon PF, Berg AS, Gayosso R, et al. Occupational asthma prevention and management in industry – an example of a global programme. Occup Med (Lond) 2005; 55: 600–605. [DOI] [PubMed] [Google Scholar]
- 48.Moscato G, Vandenplas O, Gerth Van Wijk R, et al. Occupational rhinitis. Allergy 2008; 63: 969–980. [DOI] [PubMed] [Google Scholar]
- 49.Ruoppi P, Koistinen T, Susitaival P, et al. Frequency of allergic rhinitis to laboratory animals in university employees as confirmed by chamber challenges. Allergy 2004; 59: 295–301. [DOI] [PubMed] [Google Scholar]
- 50.Suarthana E, Vergouwe Y, Nieuwenhuijsen M, et al. Diagnostic model for sensitization in workers exposed to occupational high molecular weight allergens. Am J Ind Med 2005; 48: 168–174. [DOI] [PubMed] [Google Scholar]
- 51.Suarthana E, Meijer E, Grobbee DE, et al. Predicting occupational diseases. Occup Environ Med 2009; 66: 713–714. [DOI] [PubMed] [Google Scholar]
- 52.Suarthana E, Meijer E, Heederik D, et al. The Dutch diagnostic model for laboratory animal allergen sensitization was generalizable in Canadian apprentices. J Clin Epidemiol 2009; 62: 542–549. [DOI] [PubMed] [Google Scholar]
- 53.Suarthana E, Vergouwe Y, Moons KG, et al. A diagnostic model for the detection of sensitization to wheat allergens was developed and validated in bakery workers. J Clin Epidemiol 2010; 63: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 54.Meijer E, Suarthana E, Rooijackers J, et al. Application of a prediction model for work-related sensitisation in bakery workers. Eur Respir J 2010; 36: 735–742. [DOI] [PubMed] [Google Scholar]
- 55.Gautrin D, Ghezzo H, Infante-Rivard C, et al. Natural history of sensitization, symptoms and occupational diseases in apprentices exposed to laboratory animals. Eur Respir J 2001; 17: 904–908. [DOI] [PubMed] [Google Scholar]
- 56.Slovak AJ, Hill RN. Does atopy have any predictive value for laboratory animal allergy? A comparison of different concepts of atopy. Br J Ind Med 1987; 44: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baur X, Huber H, Degens PO, et al. Relation between occupational asthma case history, bronchial methacholine challenge, and specific challenge test in patients with suspected occupational asthma. Am J Ind Med 1998; 33: 114–122. [DOI] [PubMed] [Google Scholar]
- 58.Mapp CE, Dal Vecchio L, Boschetto P, et al. Toluene diisocyanate-induced asthma without airway hyperresponsiveness. Eur J Respir Dis 1986; 68: 89–95. [PubMed] [Google Scholar]
- 59.Balboni A, Baricordi OR, Fabbri LM, et al. Association between toluene diisocyanate-induced asthma and DQB1 markers: a possible role for aspartic acid at position 57. Eur Respir J 1996; 9: 207–210. [DOI] [PubMed] [Google Scholar]
- 60.Horne C, Quintana PJ, Keown PA, et al. Distribution of DRB1 and DQB1 HLA class II alleles in occupational asthma due to western red cedar. Eur Respir J 2000; 15: 911–914. [DOI] [PubMed] [Google Scholar]
- 61.Maestrelli P, Schlünssen V, Mason P, et al. Contribution of host factors and workplace exposure to the outcome of occupational asthma. Eur Respir Rev 2012; 21: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newman Taylor A, Nicholson PJ, Cullinan Pet al. Guidelines for the Prevention, Identification, and Management of Occupational Asthma: Evidence Review and Recommendations. London, British Occupational Health Research Foundation, 2004. Available from: www.bohrf.org.uk/downloads/asthevre.pdf. [Google Scholar]
- 63.Newman Taylor AJ, Cullinan P, Burge PS, et al. BOHRF guidelines for occupational asthma. Thorax 2005; 60: 364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarlo SM, Balmes J, Balkissoon R, et al. Diagnosis and management of work-related asthma: American College of Chest Physicians Consensus Statement. Chest 2008; 134: 1–41. [DOI] [PubMed] [Google Scholar]
- 65.Buyantseva LV, Liss GM, Ribeiro M, et al. Reduction in diisocyanate and non-diisocyanate sensitizer-induced occupational asthma in ontario. J Occup Environ Med 2011; 53: 420–426. [DOI] [PubMed] [Google Scholar]
- 66.Vandenplas O, Dressel H, Wilken D, et al. Management of occupational asthma: cessation or reduction of exposure? A systematic review of available evidence. Eur Respir J 2011; 38: 804–811. [DOI] [PubMed] [Google Scholar]
- 67.Labrecque M, Malo JL, Alaoui KM, et al. Medical surveillance programme for diisocyanate exposure. Occup Environ Med 2011; 68: 302–307. [DOI] [PubMed] [Google Scholar]