Abstract
Background
In patients with advanced malignant obstructive jaundice (MOJ), it remains an intractable problem to maintain biliary patency, because repeated stent occlusion and poor immune condition can lead to serious infection. The aim of this study was to investigate the effect of endobiliary ablation combined with immune nutrition (IN) on advanced MOJ.
Material/Methods
A prospective randomized pilot study of patients undergoing percutaneous transhepatic biliary drainage (PTBD) for advanced MOJ was conducted. From January 2018 to December 2020, patients fulfilling eligibility criteria were enrolled and randomized into 2 groups: patients who received only PTBD and standard early enteral nutrition were defined as the control group, and those who underwent additional endobiliary ablation and early IN on basis of the standard therapy were defined as the study group. Primary outcome was assessment of the quality of life based on time to resuming normal daily activities, duration of stent patency, and the overall survival (OS). Secondary outcomes included time before relief of jaundice, hospital stay, inflammation responses, and related complications.
Results
We included 59 patients: 28 in the study group and 31 in the study group. Baseline characteristics were well balanced between the 2 groups. No statistically significant difference was found in time to resuming normal daily activities between the 2 groups. However, the study group presented statistically longer median duration of stent patency and survival time compared to the control group (stent patency 10.2 months vs 6.8 months, survival 9.6 months vs 7.1 months). The median time for relief of jaundice and the incidence of infection were similar between the 2 groups, but values of inflammatory response markers 3 days after the operation were significantly lower in the study group. No significant difference was found between the 2 groups in overall incidence of complications.
Conclusions
For patients at the advanced stage of MOJ, endobiliary ablation combined with postoperative IN therapy can significantly improve the quality of life.
Keywords: Jaundice, Obstructive; Catheter Ablation; Quality of Life
Background
Malignant obstructive jaundice (MOJ) is usually secondary to biliary stricture caused by malignancies originating from the pancreatic head, Vater ampulla, gallbladder, bile duct, or adjacent metastasis of lymph nodes. Because of the unresectability at the time of diagnosis and insensitivity to chemo-radiotherapy or adjuvant systemic treatment in most patients, MOJ usually predicts a poor prognosis [1,2]. If MOJ is not handled in time, it can lead to severe adverse events, resulting in life-threatening dysfunctions of multiple organs, delaying anti-tumor treatment, and reducing the quality of life [3]. Although percutaneous transhepatic biliary drainage (PTBD) combined with stent implantation is becoming the first-choice non-operative treatment for unresectable MOJ, how to overcome tumor overgrowth, biofilm deposition, or epithelial hyperplasia on the stent just to maintain biliary patency is always an intractable problem [4]. Furthermore, due to poor immune condition, damage of intestinal mucosal barrier, intestinal fluid reflux, and other adverse factors connected with MOJ or PTBD, the risk of serious infection should receive special attention [5].
During the course of searching for solutions to stent occlusion, convincing progress has been made by use of endobiliary ablation, which causes coagulative necrosis of the tumor. However, studies have shown that endobiliary ablation can aggravate malnutrition, caused by increased catabolic requirements as a consequence of advanced carcinoma, while a damaged immune system could increase the risk of systemic inflammatory response syndrome (SIRS) [6]. In recent years, great progress has been made in immune nutrition (IN) therapy, which refers to the application of specific nutrients that can improve the nutritional status of patients with advanced malignancies and regulate the inflammatory response so as to reduce complications [7]. Data about the effect of endobiliary ablation combined with IN therapy in patients with unresectable MOJ are sparse. Therefore, the aim of this pilot study was to investigate whether endobiliary ablation combined with early IN after PTBD could improve the quality of life.
Material and Methods
Protocol Design
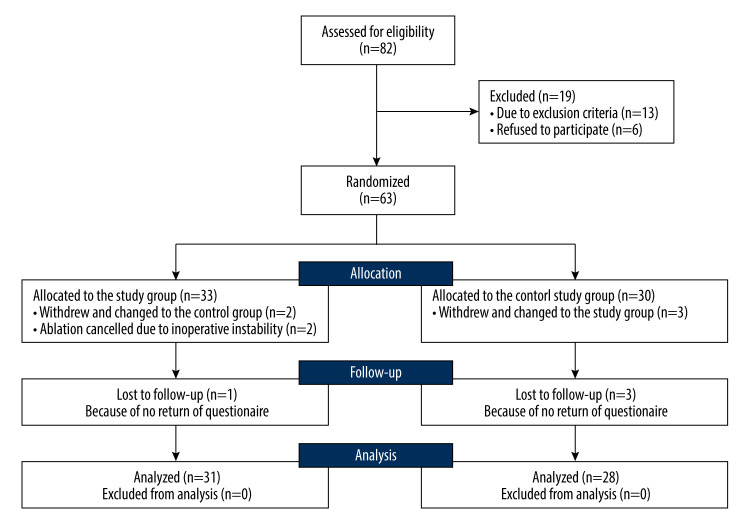
This pilot prospective, randomized, open-label study was approved by the Ethics Committee of Fu Xing Hospital affiliated to Capitol University. From January 2018 to December 2020, patients with advanced MOJ were randomized into the control group (patients received the standard therapy of biliary stent implantation through the PTBD pathway) and the study group (patients received endobiliary ablation and early IN therapy on basis of the standard therapy). Patients were assessed for eligibility immediately after being admitted to the hospital, and the simple randomization sequence was electronically generated for those eligible for this study. All enrolled patients were given a numbered, opaque, sealed envelope and were allocated after opening the envelope. Written informed consents were obtained from all participants and the study was conducted according to the Declaration of Helsinki. Figure 1 outlines the details of the recruitment process.
Figure 1.
The CONSORT flow diagram for this study.
Patient Eligibility
Patients were included into this study only if all listed criteria were met: 1) pathological confirmation of malignant biliary stricture by CT or MR; 2) no chance for radical resection concluded by the multidisciplinary board; 3) serum bilirubin level ≥35 μmol/L or cholangitis; 4) age ≥20 years; and 5) estimated lifetime ≥3 months. The exclusion criteria were the following: 1) severe dysfunction of vital organs; 2) surgical history of extrahepatic bile duct or duodenum; 3) refractory ascites; 4) uncorrectable coagulation disorders; 5) immune or blood system dysfunction; or 6) presence of cachexia.
Interventional Treatment
Endobiliary ablation was performed using Habib™ EndoHPB (EMcision Ltd, UK) for patients in the study group. Habib™ EndoHPB is a bipolar radiofrequency catheter of 8-F (2.6 mm) in diameter and 1.8 m long. Two radiologically marked electrodes, 8 mm in length and apart, are at its tip. After percutaneous transhepatic cholangiography (PTC) under digital subtraction angiography (DSA) was performed, the location and length of a biliary stricture could be accurately assessed, and then a 0.035-inch guide-wire passed through the stricture via a PTC catheter (Bard Navarre, Japan). Habib™ EndoHPB was placed exactly at the location of the biliary stricture guided by the wire. For patients with segmental strictures in the biliary tract, ablation could be performed section by section, and the parameters were set as 8–10 Watts for 90 s, 2 min each time, to reach the longest ablation length of 30 mm. After ablation, a self-expandable mental stent (SEMS) was placed at the location of biliary stricture and external biliary drainage was routinely performed. For patients in the control group, SEMS was placed through the PTC pathway but without endobiliary ablation performed [6]. Each SEMS was selected according to an interventional radiologist’s advice and manufacturer’s protocol, and every procedure was at the strict discretion of the interventional radiologist. The external drainage tube was usually removed 2 weeks later after the bile flow remained unobstructed after assessment of the bile drainage under DSA.
Nutrition Intervention
Enteral nutrition (EN) was initiated at a speed of 30 ml/h via a nasogastric tube in 48 h after PTBD procedures. Patients in the control group were given standard isocaloric isonitrogenic early EN, while patients in the study group were given added IN on the basis of standard EN (details of the formula are presented in Table 1). Tolerance to EN was assessed according to the residual gastric volume measured every 4–6 h. The rate of EN could be increased 30–50% every 10–12 h for patients exhibiting good tolerability. For patients with intolerability, defined as more than 300 mL in residual gastric volume, mosapride or similar gastrointestinal motility drugs could be administered. When EN could not cover 60% of the resting energy expenditure (REE) until 72 h after the interventional therapy, parenteral nutrition (PN) was started, with the 3-chamber bag containing lipid emulsion and amino acids used as suboptimal to EN [8,9]. EN was not discontinued until a normal diet was restored.
Table 1.
Composition of standard and added immune formulas for EN.
| Per 100 ml | Standard EN (control group) | Immunonutrition EN (study group) |
|---|---|---|
| Energy (Kcal) | 110 | 120 |
| Protein (g) | 3.8 | 6.5 |
| Glutamine included (g) | 0 | 2.0 |
| Carbohydrates (g) | 13.8 | 14.0 |
| Total Lipids (g) | 3.3 | 4.2 |
| Saturated fatty acids (g) | 1.0 | 1.6 |
| Essential fatty acids (g) | 1.5 | 1.4 |
| Omega-3 included (g) | 0 | 0.8 |
| Sodium (mg) | 100.0 | 135.0 |
| Potassium (mg) | 150.0 | 200.0 |
| Calcium (mg) | 75.0 | 100.0 |
| Magnesium (mg) | 20.0 | 27.0 |
| Phosphorus (mg) | 65.0 | 88.0 |
| Chloride (mg) | 100.0 | 135.0 |
| Iron (mg) | 1.2 | 1.6 |
| Zink (mg) | 1.2 | 2.0 |
| Copper (μg) | 150.0 | 200.0 |
| Iodine (μg) | 13.0 | 17.5 |
| Chromium (μg) | 7.0 | 9.5 |
| Fluorine (mg) | 0.1 | 0.1 |
| Manganese (mg) | 0.2 | 0.2 |
| Molybdenum (μg) | 10.0 | 13.5 |
| Selenium (μg) | 7.0 | 13.0 |
| Vitamin A (μg) | 90.0 | 120.0 |
| Vitamin D (μg) | 1.0 | 1.3 |
| Vitamin E (mg) | 1.5 | 4.0 |
| Vitamin K (μg) | 7.0 | 9.3 |
| Vitamin B1 (mg) | 0.2 | 0.2 |
| Vitamin B2 (mg) | 0.2 | 0.2 |
| Vitamin B6 (mg) | 0.2 | 0.2 |
| Vitamin B12 (μg) | 0.3 | 0.3 |
| Vitamin C (mg) | 10.0 | 26.6 |
| Niacin (mg) | 1.8 | 2.4 |
| Folic acid (μg) | 30.0 | 30.0 |
| Pantothenic acid (mg) | 0.6 | 0.6 |
| Biotin (μg) | 5.0 | 5.0 |
| Choline (mg) | 30.0 | 40.0 |
| Beta-Carotene (mg) | 0.1 | 0.1 |
Outcome
The duration before resuming normal daily activities, time span of keeping stent patent, and the overall survival (OS) were selected as the most relevant to assess the quality of life for patients with advanced and unresectable MOJ, and defined as the primary outcome. The Medical Outcomes Study 36-Item Short-Form Health Survey (MOS SF-36), including 8 scales to assess physical functioning, role limitation, bodily pain, general health perceptions, vitality, and social functioning, was used to measure the daily activities [9]. Patients were evaluated by MOS SF-36 once the day before and every 2 days after PTBD until discharge from hospital. The time to resuming normal daily activities was calculated by the postoperative days before the RP score returning to the norm-based 50 [10]. Stent patency was confirmed not only by normal direct bilirubin levels, but also absence of bile duct expansion on CT/MR. The duration of patency was calculated from stent placement until recurrent obstruction of the drained bile duct confirmed at any time. Duration before relief of jaundice, days of hospital stay, inflammatory responses, and related complications were defined as the secondary outcomes.
Statistical Analysis
Quantitative data in this exploratory pilot study are presented as median (interquartile range). Frequencies are presented as the number (%) within the relevant category. Comparison between or within groups were made using the mixed-effects model (with group as fixed and time and patient as random effects) with incorporated correction for multiplicity (Tukey’s method). Based on Levene’s test, the assumption of sphericity was considered as valid. Qualitative characteristics were compared using the χ2 test or Fisher’s exact test where appropriate. All statistical analyses were performed using SPSS 19.0 software, and P<0.05 (two-tailed) was considered statistically significant.
Results
Clinical Characteristics
From January 2018 to December 2020, 82 adult patients with advanced MOJ and no chance of radical resection were admitted to our institutions; 63 of them were enrolled, with 33 patients assigned to the study group and 30 to the control group. Just after randomization, 5 patients withdrew their consents and changed to the opposite group. Anticipated ablation was cancelled for 2 patients who had been allocated to the study group because of instability of cardiac rhythm and intolerance to pain, and they were then reallocated to the control group. Because the questionnaires of 4 patients were not able to be collected during the follow-up, all of them were ruled out of this study. Consequently, a total of 59 patients, 31 in the study group and 28 in the control group, were available for the following analyses. Table 2 presents the baseline characteristics. The 2 groups were well balanced (P>0.05).
Table 2.
Demographic characteristic of studied cohort.
| Category | Control group (n=28) | Study group (n=31) | P value |
|---|---|---|---|
| Median age (range), yr. | 52.6 (34–69) | 54.3 (33–67) | 0.714 |
| Sex | 0.781 | ||
| Male | 18 (64.3%) | 22 (71.0%) | |
| Female | 10 (35.7%) | 9 (29.0%) | |
| Type of tumor | 0.819 | ||
| Pancreatic carcinoma | 12 (42.9%) | 13 (41.9%) | |
| Hilar cholangiocarcinoma | 13 (46.4%) | 16 (51.6%) | |
| LNM | 3 (10.7%) | 2 (6.5%) | |
| Obstruction classification | 0.872 | ||
| Bismuth Type I* | 6 (21.4%) | 7 (22.6%) | |
| Type II* | 7 (25.0%) | 8 (25.8%) | |
| Type IIIa* | 7 (25.0%) | 5 (16.1%) | |
| Type IIIb* | 2 (7.1%) | 3 (9.7%) | |
| Common bile duct | 6 (21.4%) | 8 (25.8%) | |
| History of radical resection | 9 (32.1%) | 12 (38.7%) | 0.802 |
| Previous cholangitis | 5 (17.9%) | 6 (19.4%) | 0.583 |
| Distant Metastasis | 13 (46.4%) | 15 (48.4%) | 0.556 |
| BMI | 21.6 (14.5–25.1) | 21.8 (14.8–25.8) | 0.844 |
According to the Bismuth classification of hilar cholangiocarcinoma;
LNM, lymph node metastasis; BMI. Body mass index.
Primary Outcome
In all these censored cases, 1 patient from the study group and 3 from the control group did not resume normal daily activities throughout the entire follow-up period, and they were included in the data collection of resuming daily activities. The mean time to resuming daily activities was 16.3±6.7 days in the study group and 15.8±6.3 days in the control group, without significant difference between the 2 groups. Analyses of sensitivity also showed no statistically significant difference after excluding censored cases: 1.1 days (CI of 95%: −0.6 to 2.7, P=0.21) on the per-protocol set (PPS) analysis vs 1.6 days (95% CI: −0.3 to 2.5, P=0.13). All patients survived the first 30 days after the operation. Seven patients in the study group (22.6%) and 5 patients in the control group (17.9%) were still alive at the time of data analysis. The median and mean duration for keeping stent patent in the study group were 10.2 months and 10.6 months separately (range 4.6–16.7, 95% confidence interval [CI]: 7.8–11.4), significantly longer than that of the control group (median of 6.8 months and mean of 7.0 months, range: 1.4–13.5, P=0.015). The median and mean survival times, calculated from PTBD until death or last follow-up, were 9.6 months and 10.1 months separately in the study group (range 2.4–17.1, CI of 95%: 8.6–10.7), and 7.1 months and 7.4 months correspondingly in the control group (range 2.6–16.4, CI of 95%: 6.2–8.5), presenting a significant difference (P=0.017). OS analysis is shown in Figure 2. For patients treated in the study group, 2 died of hemorrhagic shock because of gastrointestinal (GI) bleeding, 1 died of cardiovascular accident, and the other 21 died of tumor progression. For patients treated in the control group, 2 died of septic shock, 3 died of GI bleeding, 2 died of cardiovascular or cerebrovascular accident, and the other 16 died of tumor progression. Details are presented in Table 3.
Figure 2.
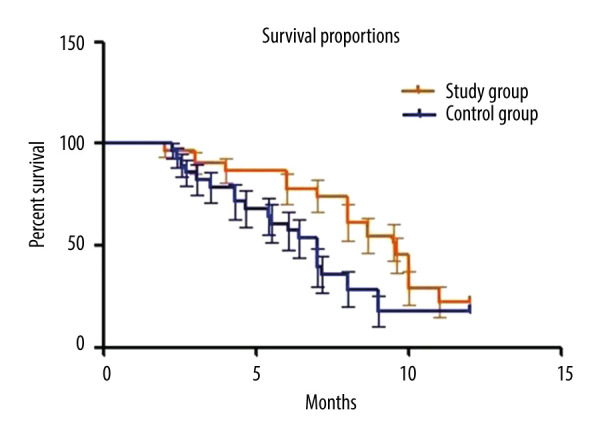
Overall survival analysis of the study and control groups.
Table 3.
Primary outcome of the studied groups.
| Variables | Control group (n=28) | Study group (n=31) | P value |
|---|---|---|---|
| Resume daily activities (day) | 15.8±6.3 | 16.9±6.7 | 0.741 |
| Excluding censored cases (day) | 12.1±4.1 | 13.7±5.4 | 0.263 |
| Duration of stent patency (month) | 7.0±3.7 | 10.6±4.1 | 0.015* |
| Death (n) | 23 (82.1%) | 24 (77.4%) | 0.168 |
| Tumor progression | 21 (75.0%) | 16 (45.7%) | 0.249 |
| Non-tumor progression | 3 (9.3%) | 7 (31.5%) | 0.012 |
| Overall survival (month) | 7.4±2.6 | 10.1±3.4 | 0.017* |
P<0.05.
Secondary Outcomes
Other clinical outcomes are summarized in Table 4. In the study group, the mean baseline of total bilirubin was 204.8 (172±58.4) μmol/L (direct bilirubin level of 164.7 and 133±49.2 μmol/L), and the median time for relief of jaundice was 12.3 days. As a comparison, total bilirubin in the control group was 197.5 (164±53.9) μmol/L, (direct bilirubin level of 153.2 and 118±49.6 μmol/L), and the median time for relief of jaundice was 12.7 days, without a significant difference between the 2 groups (P>0.05). The median and mean durations of hospital stay after PTBD were also similar (7.3 vs 7.1 days and 7.7 vs 7.5 days) between the 2 groups. As for the inflammatory response, the incidence of infection (body temperature ≥38.5°C, white blood cells, and neutral percentage exceeding the normal levels) of the study group was 22.6% (7/31), similar to the 21.4% (6/28) of the control group. Values of C-reactive protein (CRP), IL-1, IL-6, and TNF-α as inflammatory response markers 3 days after the operation were significantly lower in the study group (P<0.05). Other complications related to the interventional procedures happened in 13 patients in the functioning, was study group and 9 patients in the control group, but severe complications demanding emergent intervention or surgery did not occur. As for the overall incidence of various related complications, no significant difference was found (P=0.550). In the study group, biliary bleeding happened in 5 cases, including mild bleeding in 4 (no need for blood transfusion) and moderate bleeding in 1 (blood transfusion of more than 2 units) [10]. Acute pancreatitis, diagnosed because of epigastric pain within 24 h and elevated pancreatic enzyme levels more than 3 times the normal upper limit, occurred in 1 patient after ablation. Moderate pain that required intravenous analgesic and extended hospital stay occurred in 6 patients. As comparison, biliary bleeding occurred in 4 patients of the control group (mild bleeding in 3 and moderate bleeding in 1), acute pancreatitis happened in 2 patients, and moderate pain happened in 4 patients. Details are shown in Table 4. No significant difference was found between the groups (P>0.05).
Table 4.
Secondary outcomes of the studied groups.
| Variable | Study group (n=31) | Control group (n=28) | P value |
|---|---|---|---|
| Relief of jaundice (day) | 11.3±4.7 | 12.6±4.2 | 0.268 |
| Postoperative stay in hospital (day) | 7.7±3.1 | 7.5±2.8 | 0.562 |
| Inflammatory marker | |||
| CRP (mg/L) | 15.2±6.2 | 38.6±11.4 | 0.027* |
| IL-1 (pg/ml) | 64.3±12.4 | 103.6±18.6 | 0.016* |
| IL-6 (pg/ml) | 132.4±43.7 | 288.6±33.6 | <0.01* |
| TNF-α (pg/mL) | 1638±76.4 | 2168±87.8 | 0.036* |
| Complication | 14 (45.2%) | 12 (42.9%) | 0.550 |
| Infection | 7 (22.6%) | 6 (21.4%) | 0.589 |
| Biliary bleeding | 5 (16.1%) | 4 (14.3%) | 0.577 |
| Acute pancreatitis | 1 (3.2%) | 2 (7.1%) | 0.607 |
| Moderate pain | 6 (19.4%) | 4 (14.3%) | 0.742 |
P<0.05.
Discussion
Advanced MOJ indicates a very poor prognosis. Implantation of biliary stents has become a widely accepted palliative treatment for patients in which radical resection could not be performed. Endobiliary ablation delivers a large amount of thermal energy in local tumors and results in localized tumor necrosis, and can, in theory, prevent stent occlusion. In a systematic study containing 150 cases conducted by our center, long-term outcome of endobiliary ablation of prolonging the duration of stent patency have been reported by destroying the malignant biliary stricture just inside the biliary tract, and this proved the efficacy of endobiliary ablation for unresectable MOJ [11]. However, an inevitable situation that always accompanied the procedure was the increased incidence of complications after ablation, and the incidence of severe infections can reach as high as 30–50%, leading to prolonged hospital stay and increased costs [12]. The low immunity caused by MOJ, intestinal bacterial translocation due to the destruction of intestinal mucosal barrier, and retrograde infection related to stent implantation ultimately leads to release of enterogenous endotoxins into the blood, resulting in severe sepsis, multiple organ dysfunction syndrome (MODS), or even death [13]. From our experience, ablation can destroy the integrity of the mucosa layer and increase the risk of biliary infection. As the interventional therapy of endobiliary ablation combined with PTBD can affect multiple systems, it is very difficult to identify a satisfying therapy that could adequately benefit all organs. Although there is no solid evidence or specific recommendations, isocaloric and isonitrogenic feeding may reduce the risk of fluid overloading, and may be an option for nutrition therapy. In addition, advanced MOJ patients are frequently prone to severe systemic inflammation, and immune-modulating properties could be especially significant. There is little information on the effects of interventional therapy with immune-modulating properties in advanced MOJ, and only a few retrospective studies exist. Therefore, this pilot study is a pioneer randomized trial to investigate the effect of early immune-modulating nutritional support in patients with advanced MOJ after endobiliary ablation and to test this hypothesis.
The safety of early EN in patients with advanced MOJ with unstable hemodynamics has been corroborated in many studies. The ESPEN guidelines clarified the amount and type of dietary fat which could significantly affect adipose tissue function and systemic metabolism, and had an important impact on postoperative recovery [14]. The main components of ω-3pufas are eicosatetraenoic acid (EPA) and docosahexaenoic acid (DHA). EPA and DHA can compete for the synthesis pathway from arachidonic acid to eicosane and regulate the level of a series of cytokines in the body to enhance immune function, so as to increase the phospholipid (I)-3 fatty acid composition of cell membrane, reduce the production of inflammatory eicosane, and increase the production of non-inflammatory eicosane [15]. As an important substrate of intrahepatic gluconeogenesis, glutamine (Gln) can promote the regeneration and repair of mucosal epithelial cells, and plays an important role in maintaining the morphological and functional integrity of the intestinal mucosal barrier [16]. Studies have shown that Gln can reverse intestinal mucosal atrophy, repair intestinal mucosa, enhance intestinal immune function, and prevent intestinal bacterial translocation in various enteral or parenteral nutrition [17,18]. Our results showed that the incidence of infection in the study group was not increased and the hospital stay was not prolonged, indicating that IN can reduce the incidence of infection after endobiliary ablation and promote recovery. In fact, excessive pro-inflammatory reaction after stent implantation or endobiliary ablation is an important cause of systemic inflammatory response syndrome, which can even lead to MODS. Secondary anti-inflammatory reaction after excessive pro-inflammatory reaction can lead to immune function depression, which is also an important reason for poor prognosis. Therefore, the goal of IN support is to adjust the balance of inflammatory response, regulate immune dysfunction, and prevent bacterial translocation. In addition to the conventional nutrition support, ω-3pufas and Gln can enhance the humoral and cellular immune responses, regulate the production and release of cytokines, reduce harmful or excessive inflammatory reaction, and maintain the structure of intestinal mucosal barrier [19]. Our results showed that the serum values of inflammatory markers in the study group, including CRP, IL-1, IL-6, and TNF-α, were significantly lower than in the control group 3 days after interventional therapy, suggesting that IN could block the excessive inflammatory reaction and protect the immune function of those patients with MOJ. Studies have shown that ω-3pufas have certain anti-tumor and anti-inflammatory effects because they bind to the phospholipid layer of cell membrane and affect cell membrane structure and fluidity, inhibiting the expression of related signal pathways, and then inhibiting the over-expression of inflammatory cells. In addition, ω-3pufas can affect the over-expression of Cox, induce the transformation of PGE2 into PGE3, and influence the Hippo pathway, thus regulating the inflammatory responses [20].
Although endobiliary ablation was not planned for improving the short-term patency of the stent, because blockage of the SEMS rarely happened in 6 months, during which most patients with unresectable MOJ had died of tumor progression, the development of various subsequential therapy has significantly improved the OS, making it very important to keep the stent patent for a long time. As we found in our study, the percentage of patients who died of tumor progression rather than indirect complications was increased after endobiliary ablation. Combined endobiliary ablation and IN therapy improved the quality of life for patients with unresectable MOJ by alleviating clinical symptoms, providing opportunity for other potential therapy targeting tumors, without increasing additional complications and delaying recovery. Because the SEMS remained patent at the time of last follow-up, the median OS, even for the study group, who presented the longest OS between the 2 groups, was shorter than the duration of SEMS patency. This pilot study supports the feasibility of early initiation and acceptable tolerance to IN among MOJ patients after endobiliary ablation and PTBD intervention. Although the relatively small number of patients is a limitation of this study, the randomized and prospective design make up for this limitation to a large extent, and the preliminary results are satisfactory and desirable. The results will be useful for exploring different immune-modulating effects of IN in the future studies, as these topics are among the top nutritional questions that need to be systematically addressed in the near future [21]. In the next 5 years, with the development of adjuvant or related translational therapies, the preplanned sensitivity analysis may highlight a survival benefit, leading to the wide adoption of such treatments, and it is becoming more and more critical for patients with advanced MOJ to keep biliary stent patency and improve the quality of life [22].
Conclusions
Endobiliary ablation combined with postoperative application of IN therapy can significantly improve the quality of life for unresectable MOJ patients, being a safe and effective method for late-stage MOJ as an adjunct therapy for PTBD and stent implantation. Of course, more randomized trials containing more patients will be needed to reach more reliable and convincing conclusions.
Footnotes
Conflict of interest: None declared
Statement of Ethics
The study complied with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Ho CS, Warkentin AE. Evidence-based decompression in malignant biliary obstruction. Korean J Radiol. 2012;13(Suppl 1):S56–61. doi: 10.3348/kjr.2012.13.S1.S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessandro R, Giovanni B. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15:547–54. doi: 10.1080/17474124.2021.1890031. [DOI] [PubMed] [Google Scholar]
- 3.Alessandro R, Angela DR, Giorgio F, et al. How to choose between percutaneous transhepatic and endoscopic biliary drainage in malignant obstructive jaundice: An updated systematic review and meta-analysis. In Vivo. 2020;34:1701–14. doi: 10.21873/invivo.11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaroop VS, Dhir V, Mohandas KM, et al. Endoscopic palliation of malignant obstructive jaundice using resterilized accessories: An audit of success, complications, mortality and cost. Indian J Gastroenterol. 1997;16:91–93. [PubMed] [Google Scholar]
- 5.Marui S, Uza N, Yamazaki H, et al. Utility of laser-cut covered self-expandable metal stents for unresectable malignant distal biliary obstruction: A single-center experience. Endoscopy. 2020;52:664–68. doi: 10.1055/a-1149-1700. [DOI] [PubMed] [Google Scholar]
- 6.Hirata K, Kuwatani M, Hirata H, et al. Side-by-side placement of fully covered self-expandable metal stents for malignant distal biliary obstruction. Clin J Gastroenterol. 2020;13:455–58. doi: 10.1007/s12328-019-01056-9. [DOI] [PubMed] [Google Scholar]
- 7.Kawashima H, Hashimoto S, Ohno E, et al. Comparison of 8- and 10-mm diameter fully covered self-expandable metal stents: A multicenter prospective study in patients with distal malignant biliary obstruction. Dig Endosc. 2019;31:439–47. doi: 10.1111/den.13366. [DOI] [PubMed] [Google Scholar]
- 8.Tringali A, Hassan C, Rota M, et al. Correction: Covered vs. uncovered self-expandable metal stents for malignant distal biliary strictures: A systematic review and meta-analysis. Endoscopy. 2018;50:C5. doi: 10.1055/s-0044-102160. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH. Self-expandable metal stents for malignant distal biliary strictures. Gastrointest Endosc Clin N Am. 2011;21:463–80. doi: 10.1016/j.giec.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Li Y, Li TF, et al. [Clinical efficacy of malignant obstructive jaundice treated by domestic biliary metallic stent insertion]. Zhonghua Gan Zang Bing Za Zhi. 2012;20:843–47. doi: 10.3760/cma.j.issn.1007-3418.2012.11.009. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 11.Kong YL, Zhang HY, Liu CL, et al. Improving biliary stent patency for malignant obstructive jaundice using endobiliary radiofrequency ablation: experience in 150 patients. Surg Endosc. 2022;36:1789–98. doi: 10.1007/s00464-021-08457-3. [DOI] [PubMed] [Google Scholar]
- 12.Skipworth RJ, Fearon KC. The scientific rationale for optimizing nutritional support in cancer. Eur J Gastroenterol Hepatol. 2007;19:371–77. doi: 10.1097/MEG.0b013e3280bdbf87. [DOI] [PubMed] [Google Scholar]
- 13.Ghanaati H, Firouznia K, Vaziri Bozorg SM, et al. Nintinol self-expandable metallic stenting in management of malignant obstructive jaundice: A case series. Hepat Mon. 2010;10:57–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Feng GH, Cai Y, Jia Z, et al. Interventional therapy of malignant obstructive jaundice. Hepatobiliary Pancreat Dis Int. 2003;2:300–2. [PubMed] [Google Scholar]
- 15.Yilmaz A, Yildirim O, Tamer L, et al. Effects of caffeic acid phenethyl ester on endotoxin-induced uveitis in rats. Curr Eye Res. 2005;30:755–62. doi: 10.1080/02713680590967962. [DOI] [PubMed] [Google Scholar]
- 16.Asai Y, Bajotto G, Yoshizato H, et al. The effects of endotoxin on plasma free amino acid concentrations in rats. J Nutr Sci Vitaminol (Tokyo) 2008;54:460–66. doi: 10.3177/jnsv.54.460. [DOI] [PubMed] [Google Scholar]
- 17.Wittmann G, Mohacsik P, Balkhi MY, et al. Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice. Fluids Barriers CNS. 2015;12:21–25. doi: 10.1186/s12987-015-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gal’perin EI, Kotovskii AE, Momunova ON. [Rate of biliary ducts’ decompression by the tumorous obstructive jaundice]. Khirurgiia (Mosk) 2011;(8):33–40. [in Russian] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang W, Li T, et al. [Efficacy of short-term catheter-directed thrombolysis used with rt-PA combined with endovascular interventional therapy in patients with lower limb ischemia]. Zhonghua Yi Xue Za Zhi. 2014;94:1017–20. [in Chinese] [PubMed] [Google Scholar]
- 20.Li Q. [Interventional therapy in the treatment of avascular necrosis of femoral head and short-term efficacy]. Zhongguo Gu Shang. 2009;22:789–90. [in Chinese] [PubMed] [Google Scholar]
- 21.Yu G, Chen G, Huang B, et al. Effect of early enteral nutrition on postoperative nutritional status and immune function in elderly patients with esophageal cancer or cardiac cancer. Chin J Cancer Res. 2013;25:299–305. doi: 10.3978/j.issn.1000-9604.2013.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alessandro R, Giovanni B. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: Reflections on a standard of care. Expert Rev Gastroenterol Hepatol. 2021;15:483–85. doi: 10.1080/17474124.2021.1864325. [DOI] [PubMed] [Google Scholar]