Abstract
Idiopathic pulmonary fibrosis (IPF) is marked by a very disappointing survival rate and still represents a clinical dilemma. According to the current pathogenic hypothesis, chronic damage of the alveolar epithelium is followed by abnormal tissue repair and impairment of the alveolar structure. This process is driven by pathogenic events very similar to cancer, including epigenetic and genetic changes, altered response to regulatory signals, abnormal expression of microRNAs and activation of specific signalling pathways. IPF also resembles cancer with regard to its poor response to medical treatment and prognosis, which is very often worse than many cancers. We have hypothesised that IPF might be assimilated to a neoproliferative disorder of the lung. Viewing IPF as a cancer-like disease may satisfy the need for a better understanding of the pathogenesis of IPF by exploiting the large amount of knowledge that cancer biology evokes. The recognition of common pathogenic pathways between the two diseases may stimulate new clinical trials with cancer drugs, different drug combinations and different lines of drugs, as already experimented in oncology. Moreover, the concept of IPF as a cancer-like disorder may improve the attention given to this dreadful disease on a public, political and healthcare level.
Introduction
Despite the increasing number of studies investigating the pathogenesis of idiopathic pulmonary fibrosis (IPF) and the simultaneous increase in clinical trials dedicated to this disease, IPF is still marked by a very disappointing survival rate and remains, in many ways, a clinical and therapeutic dilemma. According to the commonly accepted pathogenic hypothesis, unknown environmental and/or occupational factors, cigarette smoking, viral infection or even tractional injury to the peripheral lung may cause, in susceptible individuals [1, 2], chronic damage of the alveolar epithelium. This triggers a series of events leading to abnormal tissue repair and, ultimately, to severe derangement of the alveolar structure. This altered “wound healing” process is driven by a variety of pathogenic events, which are for the most part described in other degenerative diseases and, interestingly, also in cancer. It is no coincidence that cancer is defined by some authors as a wound that does not heal [3–5], with an often unknown aetiology, risk factors similar to IPF and the presence of a specific genetic background considered important for the insurgence of the disease. IPF also resembles cancer with regard to its poor response to medical treatment and prognosis, which is very often worse than many cancers [6]. In addition to these obvious and circumstantial similarities, IPF and cancer also share a number of cellular and molecular aberrances, including epigenetic and genetic changes, delayed apoptosis, altered response to regulatory signals, abnormal expression of microRNAs (mRNAs), reduced cell-to-cell communication and activation of specific signalling pathways. Based on this evidence, we have hypothesised that IPF might be assimilated to a neo-proliferative disorder of the lung. Approaching IPF as a cancer-like disease may have some practical advantages, although it may be open to some criticism. The main purpose of this review is to analyse the main arguments either in favour of or against the intriguing hypothesis of the cancer-like nature of IPF.
Why we should not consider IPF a cancer-like disease
Over time, many IPF experts have generically compared IPF with cancer, although this concept has always remained rather vague and based mainly on the low-survival rate that characterises both diseases. There are three main arguments against the hypothesis of the cancer-like nature of IPF, as follows. 1) Monoclonality is a distinctive feature of cancer cells, and myofibroblasts within fibroblast foci are instead characterised by cytogenetic heterogeneity; 2) cancer is a disease that metastasises by invading other organs, whereas IPF is a disease limited to the lungs; and 3) cancer is always unilateral, whereas IPF is by definition bilateral. These arguments apparently exclude the cancer-like nature of IPF. However, these assertions about cancer are based largely on concurring opinions and often represent myths that need to be dispelled.
Monoclonality is a distinctive feature of cancer cells, and myofibroblasts within fibroblast foci are instead characterised by cytogenetic heterogeneity
A few years ago, Cool et al. [7] were the first to observe that “fibroblast foci” are not isolated areas of fibrosis, but are instead interconnected, infiltrating the tissue as a cancer. Based on this observation, they hypothesised that myofibroblasts within fibroblast foci could have a malignant nature. However, the absence of monoclonality of the fibroblasts infiltrating lung tissue led them to conclude that the origin of fibroblast foci is reactive and nonmalignant, abandoning the idea of the cancer-like nature of IPF [7]. Although it is common opinion that cancers are always of monoclonal origin, more recently, a number of studies have shown that only some cancers are monoclonal; instead, others are characterised by cytogenetic heterogeneity. Moreover, modifications of the clonal status of cancer during the course of the disease are anything but rare. Some cancers are initially monoclonal before acquiring clonal heterogeneity, others have a polyclonal origin becoming monoclonal over time, and some are first polyclonal, temporarily assuming a monoclonal pattern, that later reverts to polyclonality [8]. The existence of polyclonal cancers and occurrence of clonal convergence and clonal divergence brings into question the “dogma” of monoclonality as a distinctive feature of cancer, casting new light on the real nature of the fibroblast reticulum infiltrating the lung in IPF.
Cancer is a disease that metastasises by invading other organs, whereas IPF is a disease limited to the lungs
Despite what is generally thought, not all cancers metastasise; desmoid tumours, for instance, are fibroblastic/myofibroblastic cancers marked by aggressive local invasiveness and a lack of metastatic ability. This cancer, of unknown origin, is usually sporadic, sometimes familiar and, interestingly, few cases of bilateral involvement or recurrent multicentric, synchronous lesions of the same part of the body have been described. In desmoid tumours, similarly to other cancers, and in the same way as IPF, transforming growth factor (TGF)-β expression, fibroblast differentiation into myofibroblasts, collagen production, activation of the β-catenin/Wnt pathway and tyrosine kinase receptors deregulation are all events strictly related to the pathogenesis of this disease. Moreover, the diagnosis, based on clinical, radiological and histological criteria, may not be able to distinguish this disease from other fibrotic processes such as scars or other benign fibroblastic disorders [9]. The pathogenic and behavioural pattern of desmoid tumours, and the number of analogies between this fibroblastic cancer and IPF, lends further support to the possibility of considering IPF as a disease with cancer-like features.
Cancer is always unilateral, whereas IPF is by definition bilateral
Another firm belief about cancer is that this disease involves first one organ and, only at a later stage by metastasising, invades other tissues and organs. Several studies have instead demonstrated that the synchronous appearance of cancer in both lungs, breasts, kidneys, testicles, tonsils, ovary and parathyroids [10–13], although not frequent, is well described in 2–6% of cancers. This interesting observation confirms that bilateral and synchronous involvement of “twin organs”, such as the lungs, may occur in both IPF and cancer.
Why we should consider IPF a cancer-like disease
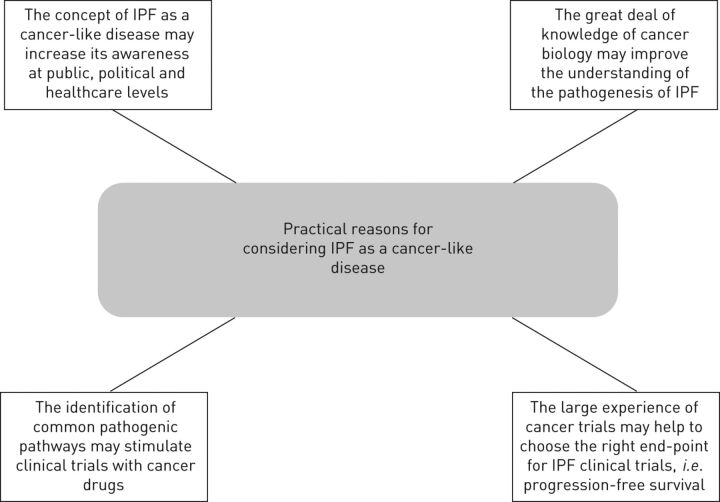
The cytogenetic heterogeneity observed within myofibroblasts, the incapacity to metastasise and the contemporary involvement of both lungs are all arguments against the cancer-like nature of IPF, as these features are not believed to be exhibited in cancer. However, this is primarily based on the general belief that cancer is always monoclonal, involving only one of the “twin organs” before metastasising and invading other organs. Although this does not confirm in any way the cancer-like attitude of IPF, it does not preclude “per se” the concept that IPF may exhibit a cancer-like trait. In addition, a number of pathogenic features, such as epigenetic and genetic abnormalities, altered cell-to-cell communications, uncontrolled proliferation and abnormal activation of specific signal transduction pathways, are all biological hallmarks that characterise the pathogenesis of both IPF and cancer. These mechanisms have already been reported in detail in a previous review [6], and will be updated and summarised here (figs 1 and 2).
Figure 1.
Scientific reasons for considering idiopathic pulmonary fibrosis (IPF) as a cancer-like disease.
Figure 2.
Practical reasons for considering idiopathic pulmonary fibrosis (IPF) as a cancer-like disease.
Epigenetic and genetic abnormalities in IPF and cancer
It is well known that methylation of tumour suppressor genes and/or hypomethylation of oncogenes has a fundamental role in carcinogenesis. These epigenetic alterations occurring in response to environmental exposures, tobacco smoke, diet or ageing have recently also been shown in IPF. In this disease, Rabinovich et al. [14] have recently shown that the global methylation pattern is altered and in some way similarly to lung cancer. In IPF, hypermethylation of the Thy-1 promoter region causes a reduced expression of the glycoprotein Thy-1, which is normally expressed by fibroblasts [15, 16]. In IPF, the loss of this molecule, which in cancer is associated with a more invasive behaviour of the disease, is linked to the transformation of fibroblasts into myofibroblasts within fibroblast foci. Interestingly, the pharmacological inhibition of the methylation of the Thy-1 gene may restore the expression of Thy-1, suggesting a novel therapeutic approach for this disease. Specific mutation of genes considered to be a key factor in the origin and progression of cancer [12–17], such as p53, fragile histidine triad, microsatellite instability and loss of heterozygosity have been observed in ∼50% of IPF patients and often described in the peripheral honeycomb areas characteristic of IPF [17–20]. Other mutations commonly associated with cancer initiation and development, such as telomere shortening and telomerase expression, have been described in familial and sporadic IPF [21–23]. More recently, the abnormal expression of mRNAs has also been associated with the pathogenesis of both cancer and IPF. mRNAs are short nonprotein-coding RNA strands that may regulate the expression of related target genes involved in the control of different processes linked to carcinogenesis, such as tumour growth, invasion and metastasis [24–26]. Recently, it has been shown that ∼10% of mRNAs are abnormally expressed in IPF, some of them, such as let-7, miR-29, miR-30 and miR-200, are significantly downregulated, while miR-155 and miR-21 are instead upregulated. In both cases, their changes are linked to groups of genes related to fibrosis and capable of intervening in epithelial-mesenchymal transition (EMT) induction, regulation of apoptosis and extracellular matrix. Interestingly, some of the mRNAs that appear to be involved in IPF increase the expression of TGF-β, which in turn causes their altered expression, thus creating a “vicious circle”. The involvement of mRNAs in the fibrotic process is supported by the observation that the miR-200 family members are downregulated in the lungs of mice with bleomycin-induced fibrosis and that restoration of miR-200 expression reverses lung fibrosis via the inhibition of the EMT induced by TGF-β [24]. The upregulation of miR-21 has also been related to bleomycin-induced fibrosis, whereas the administration of antisense nucleotides blocking miR-21 reduces the fibrotic effect exerted by bleomycin, even when treatment started a week after bleomycin administration [27, 28]. In addition to mRNAs, cell-free DNA is also present in the blood of cancer patients and is considered by some authors to be a potential diagnostic and prognostic biomarker of cancer [29]. It is noteworthy that Casoni et al. [30] showed an increase of free circulating DNA in patients affected by IPF compared with healthy subjects or patients with other fibrotic diffuse lung diseases, such as nonspecific pulmonary fibrosis.
Altered cell-to-cell communication in IPF and cancer
Metabolic and electrical coupling of cells is normally provided by intercellular channels that connect the cytoplasm of adjacent cells, allowing the passage of ions and small molecules. These channels, formed by proteins called connexins (Cxs), are essential for the synchronisation of cell activities, such as proliferation and tissue repair [31]. It has been shown that Cx43, the most represented connexin on fibroblasts, is involved in the reparative process that takes place during wound healing. The downregulation, induced by antisense oligodeoxynucleotides, of the expression of this Cx accelerates wound repair by increasing cell proliferation and migration of keratinocytes and fibroblasts at skin wound sites. Moreover, an in vitro model of fibroblast wound healing has demonstrated that the reduction of Cx43 is linked to increased expression of TGF-β, collagen production, myofibroblast differentiation and, hence, to a faster healing process. In different contexts, the increased fibroblast proliferation and differentiation induced by Cx43 downregulation might be responsible for the loss of fibroblast proliferative control that characterises abnormal repair and/or fibrosis. Indeed, fibroblasts from keloids and hypertrophic scars compared with normal skin tissue express significantly lower levels of Cx43 [32]. It is interesting that cancer, a condition marked by the loss of cell proliferative control, is often associated with a diminished expression of Cxs and to the reduction of intercellular communication. Cancer cell lines from mouse and human lung carcinoma have low or absent levels of Cx43 expression [33]. Moreover, the deletion of the Cx43 gene results in higher susceptibility to the insurgence of cancer [34]. However, the transfection of human lung carcinoma cell lines with Cx43 cDNA reduces the proliferative ability of these cells [35]. In gastric cancer, the expression of Cx43 is directly related to the degree of differentiation of cancer cells; the less differentiated and aggressive the cancer is the lower the expression of Cx43 [36]. We have shown that in primary lung fibroblasts from IPF patients there is a reduced expression of Cx43 and, by means of a dye-loading technique, we assessed gap junctional activity and showed a reduced intercellular communication in fibrotic fibroblasts compared with normal cells. The reduced cell-to-cell communication described in IPF fibroblasts is very similar to what has been described in cancer cells and may explain both the release from the restraint of contact-inhibition and uncontrolled proliferation that is present in these diseases [37].
The role of myofibroblasts in IPF and cancer
Abnormal wound healing and exaggerated myofibroblast activation are not specific features of IPF; other conditions including fibromatosis, inflammatory myofibroblastic tumours and myofibroblastic cancers, such as myofibromas and myofibroblastomas, are also characterised by uncontrolled proliferation of myofibroblasts [38]. In primary and metastatic cancers, TGF-β produced by cancer-derived epithelial cells is responsible for the emergence of myofibroblasts at the invasive front of the tumour and for protecting these cells from apoptosis. Myofibroblasts, encircling cancerous lesions, in turn produce additional TGF-β, inflammatory mediators and metalloproteinases that break apart the basement membrane of the surrounding tissues, thereby facilitating cancer invasiveness [39, 40]. In IPF, myofibroblasts, similarly to cancer cells, sustain their own growth through the autocrine production of the fibrogenic cytokines TGF-β and lose, at least in part, their ability to produce the anti-fibrotic prostaglandin E2 (PGE2) [41]. The controlling activity of PGE2 is further diminished by the reduced expression in IPF tissues of the E prostanoid receptor 2 [42, 43]. The capacity of cancer cells to infiltrate the surrounding tissue is strictly related to the expression of a series of molecules that are able to facilitate cancer cell invasiveness, including laminin, heat shock protein (HSP)27 and fascin [44–46]. Interestingly, in IPF it has been shown that epithelial cells surrounding fibroblast foci express large amounts of laminin, fascin and HSP27. These molecules are exclusively expressed by bronchiolar basal cells layered between luminal epithelial cells on the one side and myofibroblasts on the other [47]. The expression of molecules so involved in both cell migration and invasion in bronchiolar basal cells adjacent to myofibroblasts and at the same time facing the luminal epithelium is very reminiscent of what has already been described in cancer, where these molecules are expressed at the invasive front of carcinomas.
Abnormal activation of specific signalling pathways in IPF and cancer
The Wnt/β-catenin signalling pathway regulates the expression of molecules involved in tissue invasion, such as matrilysin, laminin and cyclin-D1, and most importantly is involved in a biologically relevant cross-talk with TGF-β. It is well known that this pathway is abnormally activated in several human cancers, including lung cancer, mesothelioma and desmoid tumours [48]. More recently, an activation of the Wnt/β-catenin pathway has also been described in different fibroproliferative disorders of the liver and kidney [49]. With regard to this, Chilosi et al. [50] have shown that the Wnt/β-catenin pathway is strongly activated in IPF lung tissues, as demonstrated by the presence of an intense immunoreactivity for β-catenin and a contemporary expression of high levels of two downstream genes of the Wnt/β-catenin pathway, cyclin-D1 and matrilysin. The Wnt pathway may also be activated by the fibrogenic cytokine TGF-β [51]. The transcription of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) target gene, induced by TGF-β, could also lead to a secondary activation of other signalling pathways, such as the phosphatidylinositol 3-kinase (PI3K)/Akt pathway that may regulate cell proliferation and apoptosis. We have demonstrated a role for the PI3K pathway in both proliferation and differentiation into myofibroblasts of normal human lung fibroblasts stimulated with TGF-β [52, 53]. More recently, we assessed the expression of class I PI3K p110 isoforms in IPF lung tissue, as well as in tissue-derived fibroblast cell lines also evaluating the effect of the selective inhibition of p110 isoforms on IPF fibroblast proliferation and fibrogenic activity. The expression of the p110γ isoform was increased in both IPF lung homogenates and ex vivo fibroblast cell lines. Myofibroblasts and bronchiolar basal cells in IPF lungs exhibited strong immunoreactivity for p110γ and a positive staining for the markers of proliferation, proliferating cell nuclear antigen and cyclin D1, within fibrolastic foci. Furthermore, both p110γ pharmacological inhibition and gene silencing were able to significantly inhibit proliferation, as well as α-smooth muscle actin expression in IPF fibroblasts. These data suggest that the PI3K p110γ isoform may have an important role in the pathogenesis of IPF and can be a specific pharmacological target [54]. In this regard, it has already been reported that oral administration of a p110γ inhibitor significantly prevents bleomycin-induced pulmonary fibrosis in rats [55]. These findings are also interesting, considering that in cancer the activation of the PI3K p110γ pathway is involved in lack of regulation of cell proliferation. Based on this knowledge, therapeutic inhibitors are being developed against the PI3K pathway, and their effect on tumour growth and survival in many cancers is being assessed [56].
Tyrosine kinases are key mediators of other signalling pathways involved in regulation of normal cellular processes, such as cell growth, differentiation, adhesion, motility and regulation of cell death. The activity of tyrosine kinase is mediated by specific transmembrane receptors, which mediate the activity of different ligands whose aberrant activity plays an important role in the development, progression and spread of several types of cancer [57]. More recently, the activity of these receptors has also been related to wound healing and fibrogenesis [58, 59]. Platelet-derived growth factor (PDGF), for instance, is a potent growth factor for fibroblasts in vitro, and there is enough evidence showing that fibrogenic mediators, such as TGF-β or basic fibroblast growth factor (FGF), have PDGF-dependent profibrotic activities. Indeed, PDGF protein and mRNA are increased in IPF and the inhibition of PDGF receptor attenuates the development of pulmonary fibrosis in an animal model of fibrosis induced by radiation. Irradiated mice showed higher levels of PDGF (A–D) isoforms and a prolonged life span after treatment with a PDGF receptor inhibitor [60, 61]. Recent evidence has shown that vascular endothelial growth factor (VEGF) and FGF, two other ligands of the tyrosine kinase receptor, are largely involved not only in carcinogenesis, but are also responsible for fibrogenesis. FGF receptors, present on epithelial cell and fibroblasts, mediate EMT and fibroblast transition into myofibroblasts, whereas VEGF, whose role on vascular remodelling in IPF is still debated, may indirectly promote cell survival and proliferation through the activation of ERK1/2 and PI3K. Elevated levels of VEGF mRNA, expressed by endothelial progenitor cells, have been measured in IPF patients, although plasma levels were no different between IPF patients and control subjects. The antifibrotic profile of the multiple inhibitors of tyrosine kinase receptor has been evaluated on fibroblasts in vitro and, more interestingly, in vivo in a rat model of bleomycin-induced fibrosis. The contemporary inhibition of PDGF receptors, VEGF receptors and FGF receptors resulted in significant attenuation of fibrosis, even though the inhibitory drug was administered 10 days after intratracheal instillation of bleomycin, suggesting a novel therapeutic approach for the treatment of IPF [62–65]. During the past few years, tyrosine kinase receptor inhibitors have been proposed, and used as potential targets for the treatment of nonsmall cell lung carcinoma and other cancers. More recently, based on increasing in vitro and in vivo evidence, combined VEGF receptor, FGF receptor and PDGF receptor inhibitors have entered phase II and phase III clinical trials with promising results for the treatment of IPF [66].
Conclusions
Although the 5-year survival of IPF is worse than the majority of cancers, the severity of this disease is still underestimated and its diagnosis is often made when the disease is already in advanced stages [6]. The current pathogenic depiction of IPF, although satisfactory from a speculative point of view, in practical terms has still not been able to support the development of valid diagnostic and prognostic biomarkers, and we are still far from effective therapeutic approaches capable of stopping the disease, possibly making it chronic, or even reversing the disease. To further complicate the matter, the inadequate attention given to this dreadful disease prevents sufficient awareness of IPF at a public, political and healthcare level. Conversely, the awareness of cancer as a potentially fatal disease is widespread and the need for supporting cancer research is accepted at any level of public opinion. During the past three to four decades this has led to an incredible improvement in the diagnostic and therapeutic strategies against this disease. The concept of IPF as a neo-proliferative disorder of the lung may help in meeting the urgent need for a better understanding of the pathogenesis of IPF by taking advantage of the great deal of knowledge that cancer biology may suggest. More importantly, the identification of common pathogenic pathways between the two diseases may stimulate new clinical trials with cancer drugs and with different combinations or different lines of drugs, as has been intensively explored in cancer. Furthermore, clinical trials in IPF could take advantage of the large experience of oncologists, following the cancer model of trials of new treatments by using progression-free survival, if not ideal, as a reasonable, logical and clinically meaningful end-point.
Supplementary Material
Footnotes
Provenance: Publication of this peer-reviewed article was supported by the World Scleroderma Foundation, Switzerland (principal sponsor, European Respiratory Review issue 129).
Conflict of interest: Disclosures can be found alongside the online version of this article at err.ersjournals.com
References
- 1.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001; 134: 136–151. [DOI] [PubMed] [Google Scholar]
- 2.Leslie KO. Idiopathic pulmonary fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung a unifying hypothesis regarding etiology and pathogenesis. Arch Pathol Lab Med 2012; 136: 591–600. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 4.Haddow A. Molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing? Adv Cancer Res 1972; 16: 181–234. [DOI] [PubMed] [Google Scholar]
- 5.Riss J, Khanna C, Koo S, et al. Cancers as wounds that do not heal: differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer Res 2006; 66: 7216–7224. [DOI] [PubMed] [Google Scholar]
- 6.Vancheri C, Failla M, Crimi N, et al. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 2010; 35: 496–504. [DOI] [PubMed] [Google Scholar]
- 7.Cool CD, Groshong SD, Rai PR, et al. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med 2006; 174: 654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira MR, Heim S. Cytogenetic analysis of tumor clonality. Adv Cancer Res 2011; 112: 127–149. [DOI] [PubMed] [Google Scholar]
- 9.Kotiligam D, Lazar AJ, Pollock RE, et al. Desmoid tumor: a disease opportune for molecular insights. Histol Histopathol 2008; 23: 117–126. [DOI] [PubMed] [Google Scholar]
- 10.Shah AA, Barfield ME, Kelsey CR, et al. Outcomes after surgical management of synchronous bilateral primary lung cancers. Ann Thorac Surg 2012; 93: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 11.Trousse D, Barlesi F, Loundou A, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg 2007; 133: 1193–1200. [DOI] [PubMed] [Google Scholar]
- 12.Kwast AB, Liu L, Roukema JA, et al. Increased risks of third primary cancers of non-breast origin among women with bilateral breast cancer. Br J Cancer 2012; 107: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuch B, Singer EA, Bratslavsky G. The surgical approach to multifocal renal cancers: hereditary syndromes, ipsilateral multifocality, and bilateral tumors. Urol Clin North Am 2012; 39: 133–148. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovich EI, Kapetanaki MG, Steinfeld I, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One 2012; 7: e33770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders YY, Kumbla P, Hagood JS. Enhanced myofibroblastic differentiation and survival in Thy-1(-) lung fibroblasts. Am J Respir Cell Mol Biol 2007; 36: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders YY, Pardo A, Selman M, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol 2008; 39: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwano K, Kunitake R, Kawasaki M, et al. p21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1996; 154: 477–483. [DOI] [PubMed] [Google Scholar]
- 18.Hojo S, Fujita J, Yamadori I, et al. Heterogeneous point mutations of the p53 gene in pulmonary fibrosis. Eur Respir J 1998; 12: 1404–1408. [DOI] [PubMed] [Google Scholar]
- 19.Uematsu K, Yoshimura A, Gemma A, et al. Aberrations in the fragile histidine triad (FHIT) gene in idiopathic pulmonary fibrosis. Cancer Res 2001; 61: 8527–8233. [PubMed] [Google Scholar]
- 20.Demopoulos K, Arvanitis DA, Vassilakis DA, et al. MYCL1, FHIT, SPARC, P16(INK4) and TP53 genes associated to lung cancer in idiopathic pulmonary fibrosis. J Cell Mol Med 2002; 6: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med 2008; 178: 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz de Leon A, Cronkhite JT, Katzenstein AL, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One 2010; 5: e10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T, Chung MJ, Ullenbruch M, et al. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest 2007; 117: 3800–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovat F, Valeri N, Croce CM. MicroRNAs in the pathogenesis of cancer. Semin Oncol 2011; 38: 724–733. [DOI] [PubMed] [Google Scholar]
- 25.Oak SR, Murray L, Herath A, et al. Micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One 2011; 6: e21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res 2011; 157: 191–199. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Banerjee S, de Freitas A, et al. Participation of miR-200 in pulmonary fibrosis. Am J Pathol 2012; 180: 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G, Friggeri A, Yang Y, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 2010; 207: 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11: 426–437. [DOI] [PubMed] [Google Scholar]
- 30.Casoni GL, Ulivi P, Mercatali L, et al. Increased levels of free circulating DNA in patients with idiopathic pulmonary fibrosis. Int J Biol Markers 2010; 25: 229–235. [PubMed] [Google Scholar]
- 31.Losa D, Chanson M, Crespin S. Connexins as therapeutic targets in lung disease. Expert Opin Ther Targets 2011; 15: 989–1002. [DOI] [PubMed] [Google Scholar]
- 32.Mori R, Power KT, Wang CM, et al. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci 2006; 119: 5193–5203. [DOI] [PubMed] [Google Scholar]
- 33.Cesen-Cummings K, Fernstrom MJ, Malkinson AM, et al. Frequent reduction of gap junctional intercellular communication and connexin43 expression in human and mouse lung carcinoma cells. Carcinogenesis 1998; 19: 61–67. [DOI] [PubMed] [Google Scholar]
- 34.Avanzo JL, Mesnil M, Hernandez-Blazquez FJ, et al. Increased susceptibility to urethane-induced lung tumors in mice with decreased expression of connexin43. Carcinogenesis 2004; 25: 1973–1982. [DOI] [PubMed] [Google Scholar]
- 35.Zhang ZQ, Zhang W, Wang NQ, et al. Suppression of tumorigenicity of human lung carcinoma cells after transfection with connexin43. Carcinogenesis 1998; 19: 1889–1894. [DOI] [PubMed] [Google Scholar]
- 36.Tang B, Peng ZH, Yu PW, et al. Expression and significance of Cx43 and E-cadherin in gastric cancer and metastatic lymph nodes. Med Oncol 2011; 28: 502–508. [DOI] [PubMed] [Google Scholar]
- 37.Trovato-Salinaro A, Trovato-Salinaro E, Failla M, et al. Altered intercellular communication in lung fibroblast cultures from patients with idiopathic pulmonary fibrosis. Respir Res 2006; 7: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fletcher CD. Myofibroblastic tumours: an update. Verh Dtsch Ges Pathol 1998; 82: 75–82. [PubMed] [Google Scholar]
- 39.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol 2004; 48: 509–517. [DOI] [PubMed] [Google Scholar]
- 40.Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer 2004; 45: Suppl. 2, S163–S175. [DOI] [PubMed] [Google Scholar]
- 41.Vancheri C, Sortino MA, Tomaselli V, et al. Different expression of TNF-alpha receptors and prostaglandin E(2) production in normal and fibrotic lung fibroblasts: potential implications for the evolution of the inflammatory process. Am J Respir Cell Mol Biol 2000; 22: 628–634. [DOI] [PubMed] [Google Scholar]
- 42.McAnulty RJ, Hernandez-Rodriguez NA, Mutsaers SE, et al. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-beta isoforms on fibroblast cell cultures. Biochem J 1997; 321: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore BB, Ballinger MN, White ES, et al. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J Immunol 2005; 174: 5644–5649. [DOI] [PubMed] [Google Scholar]
- 44.Moriya Y, Niki T, Yamada T, et al. Increased expression of laminin-5 and its prognostic significance in lung adenocarcinomas of small size. An immunohistochemical analysis of 102 cases. Cancer 2001; 91: 1129–1141. [DOI] [PubMed] [Google Scholar]
- 45.Garrido C, Schmitt E, Cande C, et al. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2003; 2: 579–584. [PubMed] [Google Scholar]
- 46.Pelosi G, Pastorino U, Pasini F, et al. Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer 2003; 88: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chilosi M, Zamo A, Doglioni C, et al. Migratory marker expression in fibroblast foci of idiopathic pulmonary fibrosis. Respir Res 2006; 7: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazieres J, He B, You L, et al. Wnt signaling in lung cancer. Cancer Lett 2005; 222: 1–10. [DOI] [PubMed] [Google Scholar]
- 49.Bowley E, O'Gorman DB, Gan BS. Beta-catenin signaling in fibroproliferative disease. J Surg Res 2007; 138: 141–150. [DOI] [PubMed] [Google Scholar]
- 50.Chilosi M, Poletti V, Zamo A, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 2003; 162: 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caraci F, Gili E, Calafiore M, et al. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res 2008; 57: 274–282. [DOI] [PubMed] [Google Scholar]
- 52.Tian B, Lessan K, Kahm J, et al. Beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem 2002; 277: 24667–24675. [DOI] [PubMed] [Google Scholar]
- 53.Conte E, Fruciano M, Fagone E, et al. Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I p110 isoforms. PLoS One 2011; 6: e24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conte E, Gili E, Fruciano M, et al. PI3K p110g overexpression in idiopathic pulmonary fibrosis lung tissue and fibroblast cells: in vitro effects of its inhibition. Lab Invest 2013; 93: 566–576. [DOI] [PubMed] [Google Scholar]
- 55.Wei X, Han J, Chen ZZ, et al. A phosphoinositide 3-kinasegamma inhibitor, AS605240 prevents bleomycin-induced pulmonary fibrosis in rats. Biochem Biophys Res Commun 2010; 397: 311–317. [DOI] [PubMed] [Google Scholar]
- 56.Guerreiro AS, Fattet S, Kulesza DW, et al. A sensitized RNA interference screen identifies a novel role for the PI3K p110gamma isoform in medulloblastoma cell proliferation and chemoresistance. Mol Cancer Res 2011; 9: 925–935. [DOI] [PubMed] [Google Scholar]
- 57.Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev 2010; 9: 956–970. [DOI] [PubMed] [Google Scholar]
- 58.Beyer C, Distler JH. Tyrosine kinase signaling in fibrotic disorders: translation of basic research to human disease. Biochim Biophys Acta 2013; 1832: 897–904. [DOI] [PubMed] [Google Scholar]
- 59.Hetzel M, Bachem M, Anders D, et al. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung 2005; 183: 225–237. [DOI] [PubMed] [Google Scholar]
- 60.Antoniades HN, Bravo MA, Avila RE, et al. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest 1990; 86: 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005; 201: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J 2007; 29: 976–985. [DOI] [PubMed] [Google Scholar]
- 63.Ando M, Miyazaki E, Ito T, et al. Significance of serum vascular endothelial growth factor level in patients with idiopathic pulmonary fibrosis. Lung 2010; 188: 247–252. [DOI] [PubMed] [Google Scholar]
- 64.Malli F, Koutsokera A, Paraskeva E, et al. Endothelial progenitor cells in the pathogenesis of idiopathic pulmonary fibrosis: an evolving concept. PLoS One 2013; 8: 53658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhee CK, Lee SH, Yoon HK, et al. Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice. Respiration 2011; 82: 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011; 365: 1079–1087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.