Abstract
The rationale for the use of pulmonary arterial hypertension-targeted drugs in chronic thromboembolic pulmonary hypertension is based on four bundles of evidence, as follows: 1) the pathobiology of the disease, with a distal component of pre-capillary arteriopathy that is very similar to pulmonary arterial hypertension; 2) the inoperability of some patients, and the persistence or recurrence of pulmonary hypertension after pulmonary endarterectomy in others; 3) the short-term efficacy and safety of pulmonary arterial hypertension-targeted drugs in these patients; and 4) their potential effect on survival. Chronic thromboembolic pulmonary hypertension is essentially a surgical disease, curable by pulmonary endarterectomy, with acceptable procedural mortality in experienced centres. Patient selection for surgery is extremely complex and results in 30–50% of patients considered inoperable. A large clinical experience has been built up with endothelin receptor antagonists and phosphodiesterase-5 inhibitors, while evidence from controlled trials is running far behind schedule. More recently, a randomised controlled trial with the guanylate cyclase stimulator, riociguat, achieved its target and showed haemodynamic, as well as functional, improvements within 4 months of therapy. The place of this therapy in the therapeutic arsenal needs to be further defined, but should be strictly limited to inoperable patients.
Introduction
A large number of randomised controlled trials have clearly demonstrated the efficacy and safety of three classes of drugs in pulmonary arterial hypertension (PAH), namely the prostacyclin analogues (prostaglandin (PG) I2a), the endothelin receptor antagonists (ERA), and the phosphodiesterase-5 inhibitors (PDE5i). By contrast, chronic post-embolic pulmonary hypertension (CTEPH) has deserved less attention probably because it is essentially a surgical disease, curable by pulmonary endarterectomy (PEA). There is, however, a rationale for the use of PAH-targeted therapies in some patients with CTEPH.
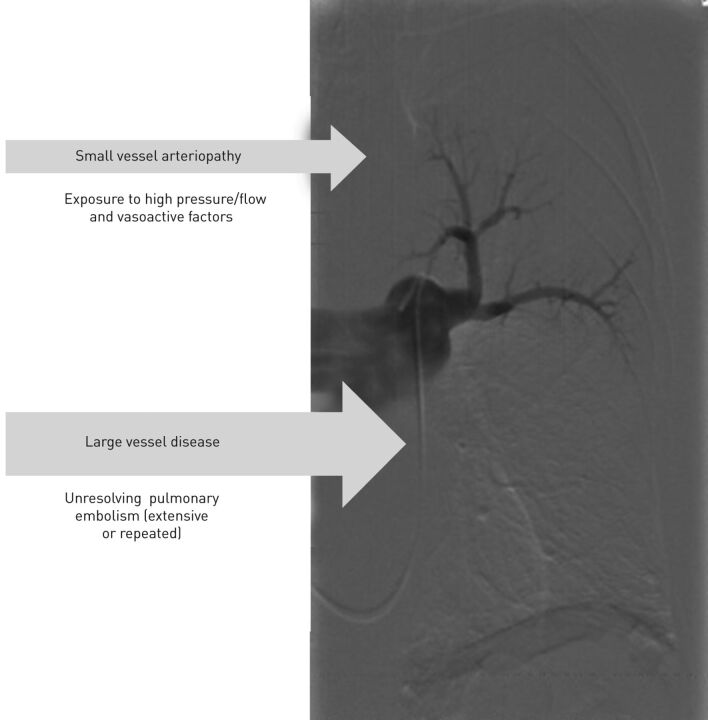
CTEPH is characterised by unresolved thromboemboli associated with fibrous stenosis obstructing the main, large, pulmonary arteries, in combination with distal arteriopathy of variable importance (fig. 1). Although a history of massive and/or recurrent episodes of acute pulmonary embolism had been documented in ∼75% of CTEPH patients [1], the persistence of obstructing intraluminal fibro-thrombotic material has not yet been elucidated. Because of the large cross-sectional area of the pulmonary vascular bed, with further possibility of recruitment and distension of vessels, symptoms appear usually lately during the disease process. Furthermore, because of the unspecific feature of symptoms, including dyspnoea at exercise and signs of right heart failure, CTEPH is notoriously underdiagnosed. It affects at least 15–19 patients per million inhabitants and carries a poor prognosis if left untreated [2].
Figure 1.
Characteristics of chronic post-embolic pulmonary hypertension.
Rationale for medical therapy in CTEPH
The histopathological examination of distal, precapillary pulmonary arteries of CTEPH patients shows vascular changes similar to those observed in patients with idiopathic PAH [3], which are induced by prolonged exposure to high flow and vasoactive factors. As observed in PAH, plasma levels of endothelin-1 are increased and correlate closely with the haemodynamic and clinical severity of the disease [4, 5]. If we all agree that surgery, by means of PEA, is the treatment of choice for CTEPH, a significant proportion of patients with CTEPH are not operable because of technical issues or comorbidities. Even patients who have been operated on can still display persistent pulmonary hypertension (PH) after surgery. In the large European/Canadian CTEPH registry, 17% of the patients had persistent PH (defined here as a mean pulmonary arterial pressure >25 mmHg at the last measurement in the intensive care unit), and at least 31% of the patients referred to the participating centres were considered inoperable, mainly because of thrombus inaccessibility, discrepancy between PH severity and morphological lesions, pulmonary vascular resistance (PVR) >1500 dyn·s−1·cm−5 and comorbidities [1]. In these patients, PAH-targeted therapies have been commonly used despite insufficient scientific evidence for regulatory approval of this indication.
Short-term effects of medical therapy
Open-label trials of 3–6-month duration, with the PGI2a epoprostenol and treprostinil, the PDE5i sildenafil, the ERA bosentan and the guanylate cyclase stimulator (sGC) riociguat, have shown improvements in haemodynamics (12–33% decrease in PVR) and exercise capacity (45–92-m increase in 6-min walking distance (6MWD)). These results are summarised in table 1. These different trials included mainly inoperable patients, and also patients with post-operative persistent PH, but did not include patients with comorbidities. Four, randomised, controlled trials included CTEPH patients (table 1). The first trial, with iloprost, did not meet the primary end-point in nonidiopathic PAH patients, and data specific to CTEPH group were not provided [8]. The second trial, with sildenafil, included only 19 patients and was consequently not powered to demonstrate a therapeutic effect [11]. PVR was improved by 28%, but 6MWD was unchanged. The first of the two large CTEPH-specific, 16-week duration, randomised controlled trials, the BENEFIT (Bosentan effects in inoperable forms of chronic thromboembolic pulmonary hypertension) study [16], included 157 patients (mean age 63 years, 6MWD 342 m and PVR 783 dyn·s−1·cm−5, at baseline) of whom 28% had been operated upon, and showed improvement of one of the co-primary end-points (PVR -24%; p<0.0001) in the group treated with bosentan. However, this effect was less important than in a previous study including PAH patients (PVR -46%; p<0.001) [19]. The other co-primary end-point, the 6MWD, was unchanged. Secondary end-points, such as N-terminal pro-brain natriuretic peptide (NT-proBNP) and Borg dyspnoea index, were also improved. The more recent CHEST-1 study [18], which included 261 patients (mean age 59 years, 6MWD 349 m, and PVR 785 dyn·s−1·cm−5, at baseline) of whom 27% had been operated on and had had riociguat administered for a similar duration of 16 weeks, with a randomisation of 2:1, showed improvement in 6MWD (46 m; p<0.0001), PVR, NT-proBNP, New York Heart Association functional class (NYHA FC) functional class, Borg dyspnoea index and quality of life. Riociguat was well tolerated, inducing mainly headache and dizziness as adverse events. Hypotension was observed in 9% of the patients, despite a cautious drug up-titration scheme. This makes riociguat the first drug to consistently demonstrate clinical efficacy in inoperable or persistent CTEPH, and it is currently being evaluated for approval by the American and European regulatory authorities. In both trials, inoperability was mandatory and needed to be confirmed by a panel of experts, who did not reach a consensus in a significant number of cases. Most frequently, this was related to poor imaging quality and emphasised the need for elaboration of objective operability criteria, for specific imaging guidelines and, even more importantly, for referral to expert surgeons. As patient characteristics and study duration were very similar in both large controlled trials, we have currently no convincing explanation for the paradoxical discordance between improvements in haemodynamics and exercise capacity in the BENEFIT trial. Usually, a 100 dyn·s−1·cm−5 decrease in PVR corresponds to a 20-m improvement in 6MWD, after surgery as well as under medical treatment. We can hypothesise either that the study was underpowered (with only 77 patients being treated in the BENEFIT study versus 173 in the CHEST-1 study) or that the greater mean age (by 4 years) could have prevented patients from recovering mobility within a 4-month period.
Table 1. Short-term effects of medical treatment in chronic post-embolic pulmonary hypertension.
| Treatment | Administration | First author [Ref.] | Study | Duration months | Patients n | NYHA-FC | 6MWD m | Effect m | PVR dyn·s−1·cm−5 | Effect % |
| Epoprostenol | i.v. | Cabrol [6] | 3 | 23 | III–IV | 280±112 | +66 | 29±7#,¶ | -21 | |
| Treprostinil | Subcutaneously | Skoro-Sajer [7] | 6 or 19 | 25 | III–IV | 260±111 | +59 | 924±347 | -13 | |
| Iloprost | Inhaled | Olschewski [8] | RCT | 3 | 57 | III–IV | NA | ns | NA | ns |
| Sildenafil | By mouth | Ghofrani [9] | 6 | 12 | ns | 312±30 | +54 | 1935±228+ | -30 | |
| Sildenafil | By mouth | Reichenberger [10] | 3 | 104 | II–IV | 310±11 | +51 | 863±38 | -12 | |
| Sildenafil | By mouth | Suntharalingam [11] | RCT | 3 | 19 | II–III | 339±58 | +18 (ns) | 734±363 | -27 |
| Bosentan | By mouth | Hoeper [12] | 3 | 19 | II–IV | 340±102 | +73 | 914±329 | -33 | |
| Bosentan | By mouth | Hughes [13] | 3 | 20 | II–IV | 262±106 | +45 | 1165±392# | -21 | |
| Bosentan | By mouth | Bonderman [14] | 6 | 16 | II–IV | 299±131 | +92 | 712±213 | NA | |
| Bosentan | By mouth | Seyfarth [15] | 6 | 12 | III | 319±85 | +72 | 1008±428 | NA | |
| Bosentan | By mouth | Jaïs [16] | RCT | 4 | 157 | II–III | 342±84 | +2 (ns) | 783 | -24 |
| Riociguat | By mouth | Ghofrani [17] | OL | 3 | 41 | II–III | 390 (330–441) | +55 | 691 (533–844) | -29 |
| Riociguat | By mouth | Ghofrani [18] | RCT | 4 | 261 | II–III | 349 | +46 | 785 | -31 |
Data are presented as mean±sd or median (interquartile range), unless otherwise stated. NYHA-FC: New York Heart Association functional class; 6MWD: 6-min walking distance; PVR: pulmonary vascular resistance; RCT: randomised controlled trial; OL: open label; NA: not available; ns: not significant. #: total PVR; ¶: in Woods Units·m2; +: in dyn·s−1·cm−5·m−2.
Long-term survival effects of medical therapy
Single-centre cohorts and the European/Canadian CTEPH registry also suggested an improved survival in inoperable patients treated with PAH-targeted drugs (table 2). In the Vienna cohort [7], 5-year survival of 50% was observed in treprostinil-treated patients, in comparison with 15% survival in historically untreated patients. In the Cambridge cohort [20], 5-year survival of medically treated patients was also >50%. In the previously mentioned CTEPH registry [21], 61% of the inoperable patients were treated with bosentan and/or sildenafil. Their survival was similar to the survival of untreated patients, while their functional and haemodynamic profile (88% versus 73% NYHA FC III–IV, 6MWD 300 m versus 340 m, PVR 778 dyn·s−1·cm−5 versus 480 dyn·s−1·cm−5) would suggest a worse outcome.
Table 2. Long-term survival outcomes in medically treated chronic post-embolic pulmonary hypertension patients.
| Treatment | Administration | First author [Ref.] | Patients n | NYHA-FC | 6MWD m | Survival % | |||
| Year 1 | Year 2 | Year 3 | Year 5 | ||||||
| Bosentan | Orally | Hughes [22] | 47 | II–IV | 291±116 | 96 | 90 | NA | NA |
| Bosentan | Orally | Seyfarth [15] | 12 | III | 319±85 | 100 | 100 | NA | NA |
| Epoprostenol | i.v. | Cabrol [6] | 27 | III–IV | 265±117 | 73 | 59 | 41 | NA |
| Treprostinil | Subcutaneously | Skoro-Sajer [7] | 25 | III–IV | 260±111 | 80 | 80 | 80 | 53 |
| Medical# | Suntharalingam [23] | 35 | NA | NA | 77 | NA | 53 | NA | |
| Medical¶ | Condliffe [20] | 148 | II–IV | 239±133 | 82 | NA | 75 | 55 | |
| Medical+ | Simonneau [21] | 275 | II–IV | 315 | 88 | 79 | 70 | NA | |
Data are presented as mean±sd, unless otherwise stated. NYHA-FC: New York Heart Association functional class; 6MWD: 6-min walking distance; NA: not available. #: 68% received medical treatment; ¶: 86% received medical treatment; +: 61% received medical treatment.
The therapeutic approach in CTEPH depends on the operability of the patient. The European/Canadian CTEPH registry clearly indicated a better survival in operated patients with a 3-year survival rate of 89% in comparison with 70% in inoperable patients with similar haemodynamics (PVR 728 dyn·s−1·cm−5 versus 676 dyn·s−1·cm−5). In single-centre cohorts, the difference seems even bigger at 5 years with a survival >75% in operated patients (table 3) and ∼50% in inoperable patients (table 2). However, as operability criteria are not easily and clearly defined, operability assessment differs from centre to centre and the percentage of operated patients increases with centre experience [26]. A large number of patients who could benefit from a curative PEA are currently treated medically in less experienced centres, which clearly impacts their survival.
Table 3. Long-term survival rate in operated chronic post-embolic pulmonary hypertension patients.
| Treatment | First author [Ref.] | Patients n | NYHA-FC | 6MWD m | Survival % | |||
| Year 1 | Year 2 | Year 3 | Year 5 | |||||
| PEA | Archibald [24] | 532 | ND | ND | 88 | 84 | 81 | 76 |
| PEA | Corsico [25] | 157 | II–IV | ND | NA | NA | NA | 84 |
| PEA | Condliffe [20] | 236 | II–IV | 243±133 | 88 | NA | 76 | 75 |
| PEA | Simonneau [21] | 404 | II–IV | 340 | 93 | 91 | 89 | NA |
Data are presented as mean±sd, unless otherwise stated. PEA: pulmonary endarterectomy; NYHA-FC: New York Heart Association functional class; 6MWD: 6-min walking distance; ND: not determined; NA: not available.
Patient selection for medical therapy
Inoperable patients
The main criteria for inoperability are as follows: 1) inaccessibility, with vascular obstruction distal to the segmental arteries and 2) discrepancy between PVR and vascular obstruction suggesting extensive small vessel arteriopathy. A cautious approach is also recommended in patients with very high PVR (>1200 dyn·s−1·cm−5) [27], and in patients with a history of splenectomy or ventriculoatrial shunt frequently associated with more distal disease and persistent post-operative PH [28].
Pre-treatment
When considering patients with very high PVR, some authors have proposed to pretreat patients with PAH-targeted drugs as a bridge to PEA. Epoprostenol [29–31] can reduce PVR pre-operatively, but has been suspected by surgeons to make resected material more friable and surgery more difficult. Furthermore, bosentan and sildenafil are currently prescribed in a large proportion of patients referred for PEA: up to 37% in the surgical centre of San Diego, CA, USA [31] and in 28% according to the CTEPH European/Canadian registry [1]. Globally, pretreatment delayed surgery and had no or even detrimental effect on PEA outcome [26, 31].
However, two small series suggest that patients with a mean pulmonary arterial pressure <30 mmHg receiving only anticoagulants have a favourable outcome, with 5-year survival of at least 90% [32, 33]. Such patients, in particular if they are old and poorly symptomatic (NYHA FC I–II), could benefit from conservative follow-up.
Persistent or recurrent PH
Concerning persistent post-operative PH, we are facing the lack of a common definition. Surgical series have reported 10–20% persistent PH; however, ∼50% of patients with normal pulmonary arterial pressure at rest have exercise-induced PH [34], and all patients have and maintain distal obstructive lesions (see post-PEA pulmonary angiographies and ventilation–perfusion scans) and have some degree of pre-capillary arteriopathy. Persistent PH can be defined differently at different time-points: immediately after cardiopulmonary bypass and in the intensive care unit by a PVR >500 dyn·s−1·cm−5, based on the observation that those patients have a worse survival [35] or 3–6 months after PEA by the usual pulmonary arterial pressure cut-off value of 25 mmHg. In the two previously mentioned large controlled trials [16, 18], patients with persistent post-operative PH were included if PVR was >300 dyn·s−1·cm−5 at >6 months after PEA.
New oral anticoagulants
Concerning the use of new oral anticoagulants (rivaroxaban, dabigatran and apixaban) in patients with CTEPH, we should be aware of drug interactions with ERA as well as with PDE5i, and should refrain from a systematic switch before more evidence is available. Bosentan, as cytochrome P450 3A4 (CYP3A4) inducer, can potentially reduce concentration of the factor Xa inhibitors, rivaroxaban and apixaban [36]. Moreover, PAH and CTEPH patients may also receive strong inhibitors of CYP3A4 and P-glycoprotein, such as azole-antimycotics and HIV protease inhibitors, causing a significant increase in rivaroxaban exposure that could increase the risk of bleeding [37].
Conclusions
PEA remains the first choice treatment for CTEPH with, by far, the best long-term outcome and acceptable mortality if performed in experienced centres. Therefore, all patients should be considered for surgery and evaluated in PEA centres. Delaying surgery in order to test the effect of PAH-targeted therapy is probably detrimental for the patient.
If surgery is impossible or the operative risk too high, or in the case of persistent PH after PEA, the use of PAH-targeted therapy seems appropriate. ERA and PDE5i are not approved for CTEPH, but a large clinical experience has built up that suggests improved survival. However, a recent controlled trial with the sGC riociguat showed improvements in exercise capacity and haemodynamics and the drug is currently under evaluation for approval in CTEPH.
Supplementary Material
Footnotes
Provenance: Publication of this peer-reviewed article was supported by the World Scleroderma Foundation, Switzerland (principal sponsor, European Respiratory Review issue 129).
Conflict of interest: Disclosures can be found alongside the online version of this article at err.ersjournals.com
References
- 1.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 2.Corris PA. National Audit of Pulmonary Hypertension. Leeds, Health and Social Care Information Centre, 2012. [Google Scholar]
- 3.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 1993; 103: 685–692. [DOI] [PubMed] [Google Scholar]
- 4.Langer F, Bauer M, Tscholl D, et al. Circulating big endothelin-1: an active role in pulmonary thromboendarterectomy? J Thorac Cardiovasc Surg 2005; 130: 1342–1347. [DOI] [PubMed] [Google Scholar]
- 5.Reesink HJ, Meijer RC, Lutter R, et al. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J 2006; 70: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 6.Cabrol S, Souza R, Jaïs X, et al. Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2007; 26: 357–362. [DOI] [PubMed] [Google Scholar]
- 7.Skoro-Sajer N, Bonderman D, Wiesbauer F, et al. Treprostinil for severe inoperable chronic thromboembolic pulmonary hypertension. J Thromb Haemost 2007; 5: 483–489. [DOI] [PubMed] [Google Scholar]
- 8.Olschewski H, Simonneau G, Galiè N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. [DOI] [PubMed] [Google Scholar]
- 9.Ghofrani HA, Schermuly RT, Rose F, et al. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2003; 167: 1139–1141. [DOI] [PubMed] [Google Scholar]
- 10.Reichenberger F, Voswinckel R, Enke B, et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Respir J 2007; 30: 922–927. [DOI] [PubMed] [Google Scholar]
- 11.Suntharalingam J, Treacy CM, Doughty NJ, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008; 134: 229–236. [DOI] [PubMed] [Google Scholar]
- 12.Hoeper MM, Kramm T, Wilkens H, et al. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest 2005; 128: 2363–2367. [DOI] [PubMed] [Google Scholar]
- 13.Hughes R, George P, Parameshwar J, et al. Bosentan in inoperable chronic thromboembolic pulmonary hypertension. Thorax 2005; 60: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonderman D, Nowotny R, Skoro-Sajer N, et al. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest 2005; 128: 2599–2603. [DOI] [PubMed] [Google Scholar]
- 15.Seyfarth HJ, Hammerschmidt S, Pankau H, et al. Long-term bosentan in chronic thromboembolic pulmonary hypertension. Respiration 2007; 74: 287–292. [DOI] [PubMed] [Google Scholar]
- 16.Jaïs X, D'Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 2008; 52: 2127–2134. [DOI] [PubMed] [Google Scholar]
- 17.Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J 2010; 36: 792–799. [DOI] [PubMed] [Google Scholar]
- 18.Ghofrani H, Grimminger F, Hoeper M, et al. Riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension: a randomized, double-blind, placebo-controlled study (CHEST-1). Chest 2012; 142: 1023A. [Google Scholar]
- 19.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001; 358: 1119–1123. [DOI] [PubMed] [Google Scholar]
- 20.Condliffe R, Kiely DG, Gibbs JSR, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008; 177: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 21.Simonneau G, Delcroix M, Lang I, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results of an international prospective registry comparing operated versus non operated patients. Am J Respir Crit Care Med 2013; 187: A5365. [Google Scholar]
- 22.Hughes RJ. The efficacy of bosentan in inoperable chronic thromboembolic pulmonary hypertension: a 1-year follow-up study. Eur Respir J 2006; 28: 138–143. [DOI] [PubMed] [Google Scholar]
- 23.Suntharalingam J, Machado RD, Sharples LD, et al. Demographic features, BMPR2 status and outcomes in distal chronic thromboembolic pulmonary hypertension. Thorax 2007; 62: 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archibald CJ, Auger WR, Fedullo PF, et al. Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med 1999; 160: 523–528. [DOI] [PubMed] [Google Scholar]
- 25.Corsico AG, D'armini AM, Cerveri I, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med 2008; 178: 419–424. [DOI] [PubMed] [Google Scholar]
- 26.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. [DOI] [PubMed] [Google Scholar]
- 27.Dartevelle P, Fadel E, Mussot S, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2004; 23: 637–648. [DOI] [PubMed] [Google Scholar]
- 28.Bonderman D, Skoro-Sajer N, Jakowitsch J, et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 2007; 115: 2153–2158. [DOI] [PubMed] [Google Scholar]
- 29.Nagaya N, Sasaki N, Ando M, et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Chest 2003; 123: 338–343. [DOI] [PubMed] [Google Scholar]
- 30.Bresser P, Fedullo PF, Auger WR, et al. Continuous intravenous epoprostenol for chronic thromboembolic pulmonary hypertension. Eur Respir J 2004; 23: 595–600. [DOI] [PubMed] [Google Scholar]
- 31.Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation 2009; 120: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 32.Riedel M, Stanek V, Widimsky J, et al. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81: 151–158. [DOI] [PubMed] [Google Scholar]
- 33.Lewczuk J, Piszko P, Jagas J, et al. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest 2001; 119: 818–823. [DOI] [PubMed] [Google Scholar]
- 34.Bonderman D, Martischnig AM, Vonbank K, et al. Right ventricular load at exercise is a cause of persistent exercise limitation in patients with normal resting pulmonary vascular resistance after pulmonary endarterectomy. Chest 2011; 139: 122–127. [DOI] [PubMed] [Google Scholar]
- 35.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg 2003; 76: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 36.Bertoletti L, Delavenne X, Montani D. Major bleeding with vitamin K antagonist anticoagulants in pulmonary hypertension. Eur Respir J 2013; 41: 872–878. [DOI] [PubMed] [Google Scholar]
- 37.Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013 [In press DOI: 10.1111/bcp.12075]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.