Abstract
Imaging plays a key role in the diagnosis and follow-up of acute respiratory distress syndrome (ARDS). Chest radiography, bedside lung ultrasonography and computed tomography scans can provide useful information for the management of patients and detection of prognostic factors. However, imaging findings are not specific and several possible differential diagnoses should be taken into account. Herein we will review the role of radiological techniques in ARDS, highlight the plain radiological and computed tomography findings according to the pathological stage of the disease (exudative, inflammatory and fibroproliferative), and summarise the main points for the differential diagnosis with cardiogenic oedema, which is still challenging in the acute stage.
Introduction
Acute respiratory distress syndrome (ARDS) is a type of acute diffuse inflammatory lung injury, characterised by increased permeability of the alveolar–capillary membrane with oedema, loss of aerated lung tissue, increased work of breathing and impaired gas exchange [1].
The first definition was assessed in 1994 by the American–European Consensus Conference, which defined ARDS as the acute onset of hypoxaemia (arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FIO2) ratio <200 mmHg), with bilateral infiltrates on chest radiograph, in the absence of left atrial hypertension [2]. However, over the years, this definition has led to the rise of several issues. First, the type of respiratory failure was defined to be “acute”, but a specific time frame was not specified. Secondly, the hypoxaemia criterion generated concerns because PaO2/FIO2 may vary with FIO2 and in response to other ventilatory settings. Finally, the chest radiograph criteria showed only moderate interobserver agreement. Therefore, its definition has been recently revised with the new Berlin definition [3, 4]. According to this latest definition, the diagnosis is based on the onset of hypoxaemia and of bilateral chest opacities within 1 week of a known risk factor. Respiratory failure is not fully explained by cardiac failure of fluid overload.
From the radiological point of view, the presence of bilateral opacities remains one of the hallmarks for diagnosis. However, it was explicitly recognised that these findings could be detected by computed tomography (CT) instead of radiography.
Another point of this recent revision consisted of the abolition of the term acute lung injury. This term previously identified a syndrome of acute and persistent lung inflammation with increased vascular permeability and included all patients with any gas exchange impairment, regardless of whether or not they reached the criteria of ARDS (PaO2/FIO2 <200 mmHg according to the old definition). It was considered confusing as most physicians included only subjects without ARDS (200 mmHg<PaO2/FIO2≤300 mmHg) in acute lung injury. To date, ARDS is classified into three levels (mild, moderate or severe) according to the severity of gas exchange impairment.
From the first description in 1967 [5], a number of prospective studies have demonstrated that ARDS is unfortunately not rare; ∼80 cases per year and 100 000 population [6]. Signs and symptoms are not specific (mainly dyspnoea, cyanosis, tachypnea and hypoxaemia) and mimic those of pulmonary oedema. The more common precipitating conditions are: pneumonia (pulmonary ARDS), aspiration of gastric contents, inhalational injury, contusion, vasculitis and near drowning. The indirect risk factors are: sepsis from an extrapulmonary source, non-cardiogenic shock, severe trauma, pancreatitis, extensive burns, drug overdose and multiple blood transfusions.
Pathologically, ARDS is characterised by diffuse alveolar damage (DAD) and evolves over 2 or 3 weeks through exudative, inflammatory and fibroproliferative phases. Superimposed cardiac failure, pneumonia, pulmonary embolism, ventilator-induced lung injury, malposition of tubes, central venous catheters and drainages or other conditions may suddenly worsen the clinical evolution. More rare complications are progressive lung fibrosis and pulmonary hypertension.
Although the death rate is declining (while still ∼50%), the long-term disability in survivors is considerable [4, 7–11].
Imaging plays a key role in the diagnosis and follow-up of ARDS and can provide prognostic information; however, imaging findings are not specific by themselves. Thus, the aim of the present article is to summarise the role of the main radiological techniques in ARDS, as well as the most common radiological findings in order to assess some diagnostic tools that could be useful for the clinical management and monitoring of these patients.
Imaging techniques in ARDS
Chest radiography
The role of chest radiography has been recognised since the first definition of ARDS. Lung opacities are bilateral, diffuse, patchy or homogeneous, involving at least three quadrants and cannot be fully explained by pleural effusion, atelectasis or nodules [4]. The most appropriate system of interpretation of chest radiographs is still not well defined and a wide interobserver agreement exists. Nevertheless, radiological findings are an integral part of the diagnosis. In fact, the Berlin definition statement underlined the limits of chest radiographs, recommending that chest radiograph criteria should be better clarified by creating a set of example radiographs [1, 3].
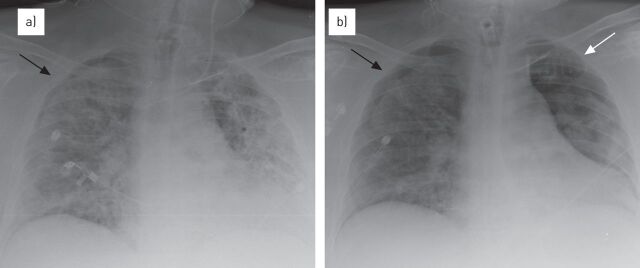
Chest radiography is also very useful to detect malposition of tubes and lines, and associated complications. The usefulness of a daily bedside chest radiograph has been analysed in a number of studies. Henschke et al. [12] prospectively analysed chest radiographs of >1100 intensive care unit (ICU) patients. On average, there were 0.7 radiographs per day per patient, 12% of endotracheal or tracheostomy tubes were malpositioned, 0.9% of central venous catheters were malpositioned, and significant interval changes were recorded in 44% of examinations after admission. Overall, new findings affecting patient management were detected in 65% of studies. The authors concluded that the use of daily bedside controls, albeit often technically limited, seemed to provide useful information in patients with multiple devices and provide rapidly evolving findings (fig. 1) [12].
Figure 1.
The role of chest radiographs in the follow-up of acute respiratory distress syndrome (ARDS). a) The serial evaluation of chest radiographs in ARDS and right pneumothorax (black arrow), b) allows the early detection of a left pneumothorax (white arrow) and the increase of the known right pneumothorax.
However, a more recent meta-analysis and a cluster-randomised, open-label crossover study concluded that systematic but unselective daily routine chest radiographs can probably be eliminated without increasing adverse outcomes in ICU adult patients [13, 14].
As these studies were performed in all patients admitted to an ICU, at present it is not clear what kind of patient population could really benefit from daily bedside radiographic controls [15], but this policy is still widely used, even in our department (Cardio-Thoracic-Vascular Dept, S. Orsola-Malpighi University, Bologna, Italy).
Chest radiography is still pivotal in the management of patients with ARDS and is still the focus of several studies. A recent study of Wallet et al. [16] showed that digital bedside chest radiography is able to predict a modification of recruited lung volume at different positive end-expiratory pressure (PEEP) levels through a change in density on digitally processed chest radiographs. Thus, especially with the advent of digitalisation, the role of chest radiography in ARDS does not seem to be outdated.
Computed tomography
The use of CT is increasing, notwithstanding the difficulties and risks of moving patients from and to the ICU, because CT has proven to be more accurate than chest radiography in detecting the underlying causes and complications of ARDS [17, 18].
Moreover, CT is crucial to understand the pathophysiology of ARDS and the complex interplay between lung parenchyma and mechanical ventilation [19], which is still the recognised cornerstone of treatment (though promising data about the effect of interferon-β has recently been published) [20].
First, CT quantitative assessment of poorly/normally aerated pulmonary regions has led to the discovery that the ARDS lung is not stiff but “small” (the so-called “baby lung”), and that the elasticity of the residual inflated lung is nearly normal. Secondly, CT proved that the acute exudative lesions in ARDS are not randomly distributed (as was thought on the basis of chest radiographs) but have a gravitationally dependent gradient, with more consolidation in the posterobasal regions, as a result of compressive gravitational forces [21].
The rationale for protective ventilation and prone positioning is based on these latter considerations. The lung protection strategy combines the use of higher levels of PEEP, to favour lung recruitment and reduce the cyclic recruitment/derecruitment by counteracting the compressive forces, and low tidal volume (VT), to prevent regional and global stress on the lung parenchyma. Indeed VT induces damaging stress only when the capacity of aerated and recruitable alveoli is insufficient to accept that volume, as in ARDS, where the “accessible” lung volume for ventilation is much reduced. For this reason, there is not an “absolute” safe value for VT (<6 mL·kg−1 predicted body weight). A dangerous value does not depend on the formula of expected healthy lung size (based on body weight), but on the actual size of aerated alveoli, which depends on the extension of alveolar flooding [22–24]. In the same way, the application of higher levels of PEEP could be potentially harmful in patients with low levels of recruitable lung.
However, the rationale of prone positioning is to improve ventilation to the dorsal areas of the lungs by reducing the vertical pressure gradient. The improvement of the ventilation/perfusion ratio is determined by positioning the ventilated alveoli in the dependent regions, where perfusion gravitationally predominates, and by decreasing the overdistension of ventral areas, with a reduction of alveolar wall injury. Indeed, prone positioning was demonstrated to enhance lung recruitment and to decrease alveolar instability at the same time [25, 26]. A significant improvement of PaO2/FIO2 has been shown by ventilating patients in the prone position [27]. Furthermore, a recent meta-analysis and a multicentre, randomised controlled trial have proved an improvement in survival by using a prone positioning approach [28, 29].
It follows that the knowledge of the capacity of the lung to become and remain recruited is essential. CT can carefully assess different aeration in lung regions through visual analysis or through the evaluation of the Hounsfield Unit in the single voxel. This allows the identification of four lung compartments: hyperinflated, normally aerated, poorly aerated and non-aerated [30].
For this reason, CT can be considered the gold standard to target PEEP and VT by assessing recruitment and hyperinflation [31, 32]. Furthermore, it can evaluate the percentage of potentially recruitable lung, which widely varies among patients with ARDS and is strongly associated with the response to PEEP [33], and the efficacy of the recruitment manoeuvres. In 2006, Gattinoni et al. [33] proposed a limited CT study (recruitment assessed by few slices scan per patient in inspiration and expiration at different PEEP values) to evaluate the efficacy of recruitment manoeuvres, identify the volume of atelectatic parenchyma and its response to ventilation, and distinguish atelectasis from consolidation. Lung recruitment manoeuvres improve oxygenation by expanding collapsed alveoli, without inducing excessive hyperinflation in ARDS patients. A recruitment manoeuvre during a CT scan gives morphological and functional information that could be useful in setting ventilatory parameters [34]. Modern multi-slice CT revealed the increase of poorly aerated lung as the main mechanism of a standard recruitment manoeuvre, also giving more precise information compared to single slice CT [35].
Lung ultrasonography
Nowadays, there is a great interest in the application of lung ultrasonography in the ICU. The main advantages of bedside lung ultrasonography include delaying or even avoiding transportation to the radiology unit (also preventing radiation exposure) and guiding life-saving therapies in extreme emergencies [36].
Ultrasound scanning was shown to be superior to auscultation and bedside chest radiography in the detection of the main lung pathologic entities in ARDS (pleural effusion, alveolar consolidations and alveolar interstitial syndrome), when considering CT as the reference for a correct diagnosis [37].
Lung ultrasonography can accurately demonstrate an “interstitial syndrome” which, in the appropriate clinical context, can be considered as suggestive of pulmonary oedema [38]. According to some authors, ultrasonography criteria to differentiate cardiogenic and permeability oedema could be detected [39]. ARDS tends to give rise to a more patchy involvement (with spared areas) and to inhomogeneous distribution of B-lines. Anterior subpleural consolidations may be seen, as well as reduced or even absent lung sliding or an irregular thickened pleural line [36]. The assessment of B-lines has also been proposed as a simple and semi-quantitative method to measure interstitial alveolar imbibition (i.e. as an index of extravascular lung water) [40]. However, even if lung ultrasonography is claimed as a bedside, radiation-free, highly reproducible technique with a short learning curve, in our opinion it requires learnt skills as for the interpretation of other imaging modalities.
In the follow-up of ARDS, today, bedside lung ultrasonography seems to offer a practical alternative to monitor recruitment efficacy and to rule out pneumothorax. Indeed, lung ultrasonography has been demonstrated to be superior to bedside chest radiography not only in the detection, but also in the follow-up, of pneumothorax after pleural drainage [36, 41]. Ultrasound was also able to show recruitment of lung parenchyma as PEEP was increased from 5 to 15 cmH2O with correlation to improvement of gas exchange [42].
Other techniques
Although the role of positron emission tomography in daily clinical practice is not likely to be feasible, it represents a precious research tool, the results of which could have an impact on our knowledge of ARDS.
In patients with ARDS, positron emission tomography with 2-fluoro-2-deoxy-d-glucose (FDG) revealed an increased metabolic activity, which interestingly also affects the normal aerated regions on the CT scan. This suggests that no pulmonary region is spared by the inflammatory process [43]. Moreover, recent studies focused on the possible influence of positron emission tomography on ventilation strategies. Indeed, Bellani et al. [44] found that the metabolic activity (i.e. FDG uptake) of aerated regions was linked to both plateau pressure and regional VT, whereas no association was found between cyclic recruitment/derecruitment and increased metabolic activity.
Magnetic resonance imaging of the lung is still rarely used and often limited by the low proton density of aerated lung tissue, as well as susceptibility effects at air-tissue interfaces and cardiac-respiratory artefacts. Nevertheless it could be of value when radiation exposure or iodinated contrast material is contraindicated. Experimental studies on animal models have revealed its efficacy in visualising and quantifying potential recruitable lung volume, as CT scans can be performed [45].
Recently, new techniques have also been proposed to assess changes in regional ventilation in patients with mechanical ventilatory support, such as synchrotron radiation CT and electrical impedance tomography [46]. However, few studies have been performed on this topic and their role in the clinical setting should be further clarified, especially with regard to electrical impedance tomography, the potential of which relies on the radiation-free bedside method [31].
Imaging findings in ARDS
As it is common in the current literature, we are going to outline the plain radiological and CT appearances according to the pathological stage of the disease, even if a certain grade of overlap can be expected [47–49].
Acute or exudative phase: first week
In the first 24–48 h after the initial damage, chest radiography may still appear normal (latent period), unless a pulmonary original lesion is observed (typically pneumonia).
Over the next 2 or 3 days, rapid deterioration occurs with increasing bilateral, patchy alveolar opacities progressing to diffuse consolidations, with a “white lung” appearance in the more severe cases (fig. 2) [50, 51]. Usually, lung volumes are reduced and air bronchograms are visible. Small pleural effusions are common and a diffuse “crazy paving” appearance can be documented. Distribution can be more peripheral or cortical than in cardiogenic oedema but a gravitational gradient is usually present, as demonstrated by CT. This observation suggests that atelectasis is an important factor in the inhomogeneous regional distribution of ARDS. Furthermore, this gravitational pattern can be helpful to exclude concomitant lung infections, as dependent atelectasis is more common in early ARDS without pneumonia [49, 52].
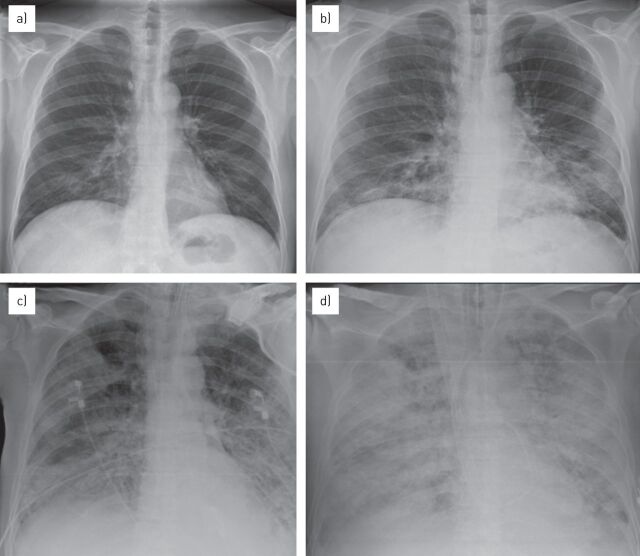
Figure 2.
Radiological evolution of acute respiratory distress syndrome over the first week in a 57-year-old male with non-Hodgkin’s lymphoma and H1N1 infection. a) On admission the radiography examination did not show any pathological findings. b) The next day some pulmonary consolidations appeared at the lower lobes. c, d) Over the next 2–3 days, a rapid deterioration of clinical and radiological conditions occurred with consolidations (c) progressing to diffuse alveolar involvement, with “white lung” appearance (d). The normal-sized heart and vascular structures help in the differential diagnosis of pulmonary oedema due to heart failure.
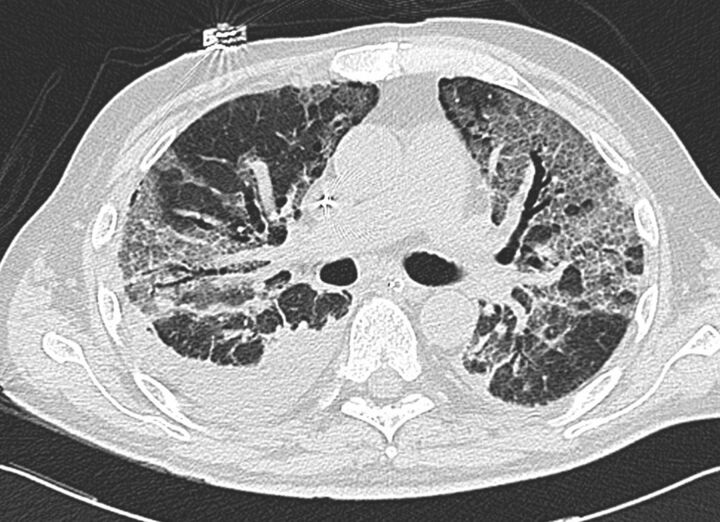
In the acute phase of ARDS, CT scans typically show a non-homogeneous distribution and a ventro-dorsal gradient of density, with more dense consolidations in the dependent regions, widespread ground-glass opacities and relatively normal or hyperinflated parenchyma (in case of mechanical ventilation) in non-dependent areas (fig. 3).
Figure 3.
Typical computed tomography features of acute respiratory distress syndrome showing: non-homogeneous distribution, a ventro-dorsal gradient of density, more dense consolidation in the dependent regions, widespread ground-glass opacities associated with thickening of interlobular septa (crazy paving), and pleural effusion.
Atypical ARDS, characterised by predominantly anterior consolidations in supine decubitus, can be observed in ∼5% of patients during the first stage of the disease, probably due to regional differences in ventilation [49].
The inhomogeneous appearance of lung opacification on CT is generally explained by the posterior compressive atelectasis caused by the weight of the overlying parenchyma. In fact, when the position of the patient is changed from supine to prone to improve oxygenation, by optimising lung recruitment and ventilation/perfusion matching, the density gradient suddenly changes in conformity with the position, the dorsal lung re-expands and the ventral zones tend to collapse [53].
CT has been considered instrumental in distinguishing whether the cause of the lesional oedema is pulmonary or extrapulmonary, which is useful for clinical management [54]. The typical CT presentation is more common in extrapulmonary ARDS, whereas in pulmonary ARDS the opacities tend to be asymmetrical, with non-dependent consolidations or ground-glass opacities and lung cysts. It must be kept in mind that the presence of consolidations in non-dependent areas or the appearance of a new area of consolidation may be a sign of both a pre-existing pneumonia and a new ventilation-associated pneumonia. Thus, in daily practice, trying to distinguish pulmonary ARDS from extrapulmonary ARDS on the basis of imaging findings alone can be a disappointing experience. However, an infective pneumonia is commonly missed because the airspace opacities due to ARDS obscure the associated pneumonia.
A more sound use of CT consists of the identification of complications, mainly due to high end-expiratory pressure during ventilation, in CT-guided drainage of loculated air collections [55] and in providing prognostic information. In patients with ARDS/DAD, CT predictors of mortality are >80% of lung involvement, enlargement of the right atrium or development of traction varicoid bronchiectasis [9, 56].
Intermediate or proliferative phase: second week
Reticular opacities may appear in the diffuse and persisting background of alveolar opacities; however, unless iatrogenic complications or superimposed pneumonia develop, the radiological findings are rather stable in this phase.
Coarse reticulations are not a reliable sign of fibrosis because they can subsequently resolve. In this phase, the extent of CT opacities (>80% of lung volume), along with the presence of bronchiectasis, honeycombing and signs of pulmonary hypertension (dilatation of pulmonary arteries and right ventricle) indicates early fibrosis and predicts mortality [56, 57]. Recurrent exudative episodes can occur, resulting in mixed radiological appearances [49].
Late or fibrotic phase: over 2 weeks
In surviving patients, pulmonary opacities tend to wane with unpredictable speed. The final result could be a normal looking lung or a pattern characterised by lung volume reduction and coarse reticulations.
More than 70% of patients present with CT abnormalities at 6 months follow-up [18]. Organisation, characterised by fibroblast proliferation, is a common and nearly universal response to lung injury whether it is focal or diffuse. Although there is a tendency to divide the types of organisation in different entities, the lung’s response to injury is quite limited, with a similar pattern regardless of the underlying cause [9]. While excessive fibroproliferation is clearly detrimental, an appropriate fibroproliferative response may have beneficial consequences in guiding lung repair; bronchoscopy with bronchoalveolar lavage as well as CT scan could both add useful information for the early detection of fibroproliferative activity [58].
In the later stages, CT generally demonstrates persistent ground-glass densities and reticulations, air cysts and bullae, mainly located in the ventral regions of the lung, more often than chest radiography.
More severe fibrosis has been reported in patients with pulmonary ARDS and prolonged mechanical ventilation with high PEEP. In these patients, fibrosis is typically more pronounced in the anterior, nondependent portions of the lung. The anterior location of fibrosis may be related to ventilator-induced lung injury due to mechanical ventilation trauma or oxygen toxicity, while the more dependent areas seem to be partially protected by atelectasis and consolidation (fig. 4) [9, 18]. However, recent studies have reported the “typical” distribution in only one-third of cases, while the majority shows a diffuse or, rarely, predominantly dorsal distribution [59].
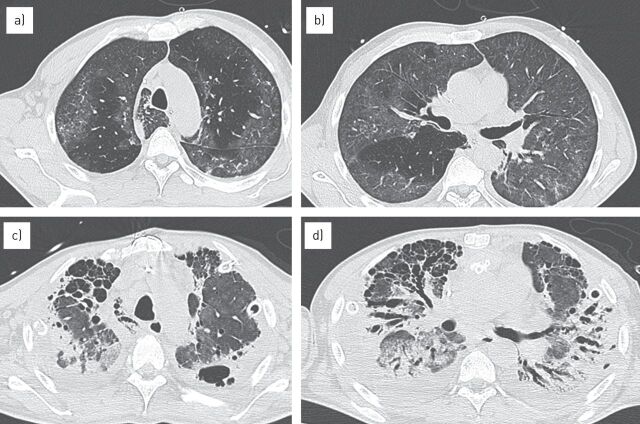
Figure 4.
Long-term evolution of acute respiratory distress syndrome. a, b) Computed tomography scan performed at admission demonstrated multiple and bilateral ground-glass opacities, which were inhomogeneous in distribution. c, d) After 2 months of recovery an advanced stage of pulmonary fibrosis could be detected, with honeycombing and bronchiectasis, which are more evident in the anterior lung regions.
Traction bronchiectasis is common and can develop in an early phase, indicating impending fibrosis and worsening prognosis. This finding is not completely specific because the reversibility of bronchial dilatation has also been described in the late stages of ARDS [60, 61].
Few studies have systematically evaluated high-resolution CT appearances of the lung after severe ARDS to determinate the correlation between radiological features and functional impairment. Masclans et al. [59] followed up 38 ARDS patients after 6 months. They found limitations in daily life activities in 40%, respiratory function abnormalities in 67% (with a predominant restrictive pattern), and CT alterations (mainly reticular) in 76% of patients. In most cases, the radiological abnormalities were limited to <25% of lung volume and the involvement of the anterior lung fields was predominant.
Wilcox et al. [62] were able to study 24 survivors at 5 years; 75% of them had abnormal findings on high-resolution CT scans, mainly minor and located in nondependent lung zones. No correlation was found with exercise and functional limitation experienced by the patients. Symptoms were considered more consistent with extrapulmonary muscle weakness than with structural lung disease [62].
Complications
Early signs of barovolutrauma often correspond to interstitial emphysema and subpleural cystic air spaces. Subsequently, imaging studies can demonstrate the development of pneumomediastinum, pneumothorax (often hypertensive in mechanically ventilated patients) and subcutaneous emphysema (fig. 5).
Figure 5.
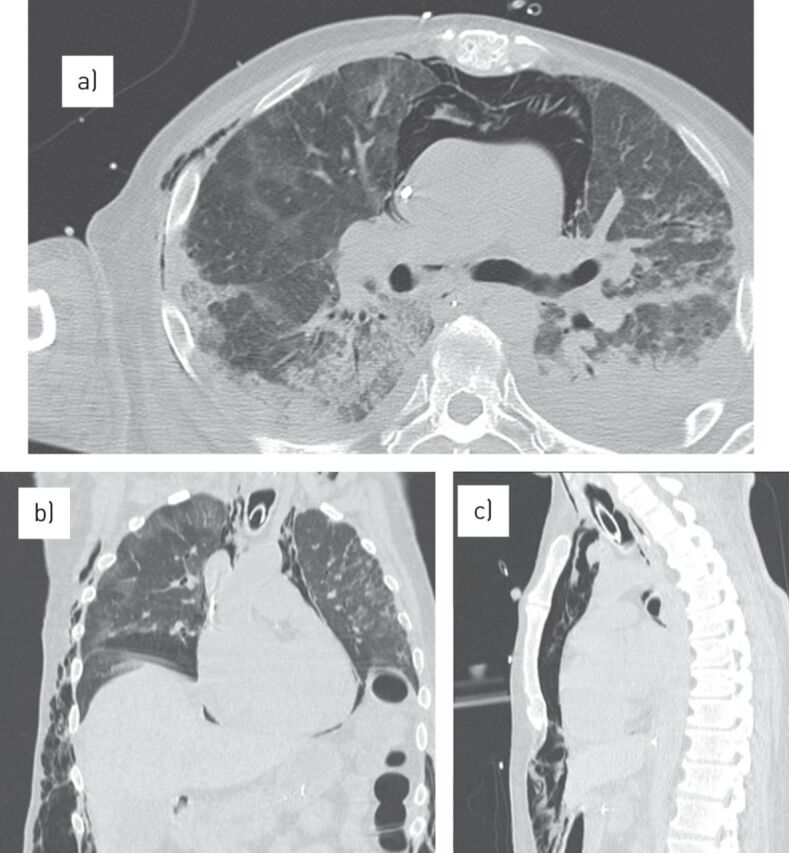
Complications of acute respiratory distress syndrome. a) Computed tomography scan showing a severe pneumomediastinum with subcutaneous emphysema. b, c) Multiplanar reconstructions in the b) coronal and c) sagittal plane better define their extension.
It is well known that CT studies can provide important information that the conventional bedside radiograph cannot supply [63, 64]. In case of a sudden and unexplained clinical deterioration or lack of improvement, an occult complication can often be detected by CT, such as pleural effusion, atelectasis, bullae, pneumothorax (especially when located in anterior lung regions) or necrotising pneumonia and lung abscesses [65].
In a retrospective study of 74 patients, CT yielded additional information in 66% of cases and had a direct impact on treatment in 22% [66].
Differential diagnosis: cardiogenic versus permeability oedema
The differential diagnosis of ARDS includes: cardiogenic pulmonary oedema, acute interstitial pneumonia, diffuse alveolar haemorrhage, acute eosinophilic pneumonia and lymphangitis carcinomatosa. However, the most challenging differential diagnosis is still between ARDS and cardiogenic oedema, especially in the acute phase.
The differential diagnosis between ARDS and cardiogenic oedema can be assessed on chest radiograph in typical cases [67, 68]. Cardiogenic oedema is usually characterised in its first stage by septal lines (more visible in the costophrenic angles), prominence of the upper lobe vessels, peribronchovascular haze and cuffing, widened vascular pedicle of the mediastinum and pleural effusion [49]. Nevertheless, the phase of interstitial oedema is often undetected. If an alveolar oedema occurs, it appears as an airspace opacity with gravitational or perihilar distribution. Sometimes the distribution is not uniform, mainly due to a gravitational effect in patients lying on one side or affected by emphysema, concomitant infections, pulmonary embolism or other more rare conditions.
Acute (“flash”) congestive cardiac failure with oedema generally produces a radiographic pattern of patchy or “bat wing” alveolar opacities with peripheral sparing. A bat wing distribution can be observed in <10% of cases of acute pulmonary oedema but also in different situations; for example, in opportunistic lung infections or diffuse alveolar haemorrhage [49].
Consequently, the clinical evolution, in addition to the absence of heart enlargement, septal lines or pleural effusion and the abrupt appearance of alveolar opacities, all favour ARDS. However, in the daily clinical practice, the differential diagnosis between cardiogenic and injury lung oedema can be even more challenging than previously reported.
According to Aberle et al. [69], radiographic criteria allow a correct identification of up to 87% of patients with cardiogenic oedema but of only 60% of those with increased permeability oedema. This limited accuracy could be explained by several reasons: 1) oedema may not be visible until the lung water increases by 30%; and 2) features once considered more typical of cardiogenic oedema can be commonly found in ARDS, including a widened vascular pedicle, pleural effusion, peribronchial cuffs and septal lines. A patchy peripheral distribution of alveolar opacities is slightly more specific for ARDS (50% of cases), but can also be found in cardiac failure (13%). First, from the radiological point of view, different conditions can mimic pulmonary oedema. Secondly, a significant interobserver error and technical variability need to be considered. Finally, acute exacerbation of a pre-existing interstitial pneumonia may further complicate the radiological presentation.
A radiographic scoring system was used by Rocker et al. [70] to distinguish pulmonary oedema of renal, cardiac or capillary origin in 51 patients. Using the final clinical diagnosis as the gold standard, sensitivity and specificity in cardiac oedema were 46% and 84%, in renal patients were 63% and 86%, and in ARDS were 89% and 33%, respectively. The radiographic scoring system failed to distinguish between different kinds of oedema but was relatively more sensitive (albeit nonspecific) in the assessment of ARDS [70].
Besides, from a general point of view, the old division of lung oedema into two simple categories (cardiogenic–hydrostatic and non-cardiogenic increased permeability) is outdated and no longer adequate, even if time honoured [71]. A more reasonable classification should now require at least four categories [49]: 1) hydrostatic oedema; 2) ARDS (permeability oedema caused by DAD); 3) permeability oedema without alveolar damage (such as heroin-induced oedema, oedema following cytokine administration and high-altitude oedema); and 4) mixed forms (such as neurogenic oedema, reperfusion and re-expansion oedema, oedema following lung transplantation, etc.).
In a recent study, Figueroa-Casas et al. [72] evaluated the diagnostic accuracy of chest radiography to identify bilateral pulmonary infiltrates compatible with the diagnosis of ARDS, using CT as the reference standard. The sensitivity of chest radiographs was 73%, specificity was 70%, and positive and negative predictive values were 88% and 47%, respectively. Sensitivity was significantly lower for focal disease distribution compared with diffuse distribution. The authors were able to conclude that the accuracy of bedside radiography is limited due to under-recognition of the syndrome, particularly when opacities are not diffusely distributed, and over diagnosis [72]. Thus, chest radiography is not always able to provide a correct diagnosis and other diagnostic tools should be taken into account.
Plasma levels of B-type natriuretic peptide (BNP) can be helpful, because a BNP >500 pg·mL−1 indicates that heart failure is likely. When BNP levels are <100 pg·mL−1, heart failure is unlikely; however, high BNP levels can also be found in acute pulmonary embolism, cor pulmonale and pulmonary hypertension. Intermediate levels are considered unreliable. Besides, an important prospective study by Levitt et al. [73] demonstrated that BNP levels drawn within 48 h of admission to ICU do not reliably distinguish ARDS from cardiogenic oedema, do not correlate with invasive haemodynamic measurements and do not track predictably with changes in volume status on consecutive daily measurements. BNP levels cannot reliably distinguish cardiogenic from non-cardiogenic oedema compared with an adjudicated blinded diagnosis by experts [73]. However, BNP levels are significantly associated with mortality, as they probably reflect concomitant pulmonary hypertension and cardiac dysfunction [74].
Differences between cardiac and ARDS pulmonary oedema have been shown by the evaluation of extravascular lung water. Indeed, patients with ARDS were found to have higher extravascular lung water, while their hydrostatic component (i.e. pulmonary artery occlusion pressure) was much lower. Moreover, for a given hydrostatic pressure, extravascular lung water is greater in patients with ARDS compared to patients with cardiogenic oedema [75].
Transthoracic echocardiography is often limited by technical constraints and cannot always rule out cardiogenic oedema because it is not sensitive enough in the case of diastolic dysfunction. However, according to the Berlin definition, the cardiogenic origin of oedema can be reasonably excluded by the evaluation of cardiac function using echocardiography.
Pulmonary artery catheterisation, when feasible and technically adequate, can still be considered the gold standard reference and is able to definitely exclude or confirm a cardiogenic oedema (when the wedge pressure is >18 mmHg in the acute presentation). As ARDS may coexist with hydrostatic oedema, pulmonary artery catheterisation should mainly be considered for ruling out cardiogenic oedema if no risk factors for ARDS are identified [3].
In a recent study by Komiya et al. [76], the authors reported a rather high accuracy of chest CT in the differential diagnosis between acute cardiogenic and permeability oedema. In our opinion, the applied criteria are not as clear and these results need to be further confirmed with more extensive studies.
Thus, a reliable method to distinguish cardiac from ARDS oedema or to evaluate the hydrostatic component in ARDS patients still does not exist.
Conclusion
Although the criteria for the diagnosis of ARDS have evolved over time, along with the progress of technology and scientific knowledge, the core principles have remained remarkably similar over the past 45 years. The most recent definition of ARDS [77] is still based on the physiological and radiological findings described in the the seminal paper in 1967 by Ashbaugh et al. [5].
As the science progresses, further refinements are now required and actively pursued, even from the radiological viewpoint, as ARDS is probably a non-homogeneous combination of different disorders sharing some clinical and radiological abnormalities, but probably based on different genetic susceptibility and requiring the development of novel biomarkers and targeted therapies.
Up to what point radiology will be able to follow and integrate clinical evolution of ARDS is not clear; it will be a difficult task, but certainly a worthwhile endeavour.
Footnotes
Previous articles in the Series. No. 1: Guérin C. Prone ventilation in acute respiratory distress. Eur Respir Rev 2014; 23: 249–257. No. 2: Finney SJ. Extracorporeal support for patients with acute respiratory distress syndrome. Eur Respir Rev 2014; 23: 379–389.
Provenance: Submitted article, peer-reviewed.
Conflict of interest: None declared.
References
- 1.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38: 1537–1582. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, et al. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149: 818–824. [DOI] [PubMed] [Google Scholar]
- 3.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome. The Berlin definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 4.Fanelli V, Vlachou A, Ghannadian S, et al. Acute respiratory distress syndrome. New definition, current and future therapeutic options. J Thorac Dis 2013; 5: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967; 2: 319–323. [DOI] [PubMed] [Google Scholar]
- 6.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007; 369: 1553–1565. [DOI] [PubMed] [Google Scholar]
- 8.Rubinowitz A, Siegel M, Tocino I. Thoracic imaging in ICU. Crit Care Clin 2007; 3: 539–573. [DOI] [PubMed] [Google Scholar]
- 9.Kligerman S, Franks T, Galvin J. Organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics 2013; 33: 1951–1975. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Itoh T, Sasaki Y, et al. Diagnostic imaging of idiopathic adult respiratory distress syndrome (ARDS)/diffuse alveolar damage (DAD). Histopathological correlation with radiological imaging. Clin Imaging 1996; 20: 1–7. [DOI] [PubMed] [Google Scholar]
- 11.Obadina ET, Torrealba JR, Kanne JP. Acute pulmonary injury: high-resolution CT and histopathological spectrum. Br J Radiol 2013; 86: 20120614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henschke C, Pasternack G, Schroeder S, et al. Bedside chest radiography: diagnostic efficacy. Radiology 1983; 149: 23–26. [DOI] [PubMed] [Google Scholar]
- 13.Oba Y, Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology 2010; 255: 386–395. [DOI] [PubMed] [Google Scholar]
- 14.Hejblum G1, Chalumeau-Lemoine L, Ioos V, et al. Comparison of routine and on demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet 2009; 374: 1687–1693. [DOI] [PubMed] [Google Scholar]
- 15.Milne ENC. Imaging expertise in critical care units. Radiology 2010; 256: 1013. [DOI] [PubMed] [Google Scholar]
- 16.Wallet F, Delannoy B, Haquin A, et al. Evaluation of recruited lung volume at inspiratory plateau pressure with PEEP using bedside digital chest X-ray in patients with acute lung injury/ARDS. Respir Care 2013; 58: 416–423. [DOI] [PubMed] [Google Scholar]
- 17.Mazzei MA, Guerrini S, Cioffi Squitieri N, et al. Role of computed tomography in the diagnosis of acute lung injury/acute respiratory distress syndrome. Recenti Prog Med 2012; 103: 459–464. [DOI] [PubMed] [Google Scholar]
- 18.Sheard S, Rao P, Devaraj A. Imaging of acute respiratory distress syndrome. Respir Care 2012; 57: 607–612. [DOI] [PubMed] [Google Scholar]
- 19.Fan E, Villar J, Slutsky A. Novel approaches to minimize ventilator-induced lung injury. BMC Med 2013; 11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellingan G, Maksimow M, Howell DC, et al. The effect of intravenous interferon-β-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: an open-label study. Lancet Respir Med 2014; 2: 98–107. [DOI] [PubMed] [Google Scholar]
- 21.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med 2005; 31: 776–784. [DOI] [PubMed] [Google Scholar]
- 22.Marini JJ. Lower tidal volume for everyone: principle or prescription? Intensive Care Med 2013; 39: 3–5. [DOI] [PubMed] [Google Scholar]
- 23.Protti A, Cressoni M, Santini A, et al. Lung stress and strain during mechanical ventilation. Any safe threshold? Am J Respir Crit Care Med 2011; 183: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 24.Fan E, Villar J, Slutsky AS. Novel approaches to minimize ventilator induced lung injury. BMC Medicine 2013; 11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galiatsou E, Kostanti E, Svarna E, et al. Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med 2006; 174: 187–197. [DOI] [PubMed] [Google Scholar]
- 26.Cornejo RA, Díaz JC, Tobar EA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2013; 188: 440–448. [DOI] [PubMed] [Google Scholar]
- 27.Abroug F, Ouanes-Besbes L, Elatrous S, et al. The effect of prone positioning in acute respiratory distress syndrome or acute lung injury: a meta-analysis. Areas of uncertainty and recommendations for research. Intensive Care Med 2008; 34: 1002–1011. [DOI] [PubMed] [Google Scholar]
- 28.Gattinoni L, Carlesso E, Taccone P, et al. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol 2010; 76: 448–454. [PubMed] [Google Scholar]
- 29.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368: 2159–2168. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L, Caironi P, Pelosi P, et al. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 2001; 164: 1701–1711. [DOI] [PubMed] [Google Scholar]
- 31.Luecke T, Corradi F, Pelosi P. Lung imaging for titration of mechanical ventilation. Curr Opin Anesthesiol 2012; 25: 131–140. [DOI] [PubMed] [Google Scholar]
- 32.Pelosi P, Rocco PR, de Abreu MG. Use of computed tomography scanning to guide lung recruitment and adjust positive–end expiratory pressure. Curr Opin Crit Care 2011; 17: 268–274. [DOI] [PubMed] [Google Scholar]
- 33.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006; 354: 1775–1786. [DOI] [PubMed] [Google Scholar]
- 34.Bugedo G, Bruhn A, Hernandez G, et al. Lung computed tomography during a lung recruitment maneuver in patients with acute lung injury. Intensive Care Med 2003; 29: 218–225. [DOI] [PubMed] [Google Scholar]
- 35.Henzler D, Mahnken AH, Wildberger JE, et al. Multislice computed tomography to determine the effects of a recruitment maneuver in experimental lung injury. Eur Radiol 2006; 16: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 36.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38: 577–591. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenstein D, Goldstein D, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anestesthesiology 2004; 100: 9–15. [DOI] [PubMed] [Google Scholar]
- 38.Gardelli G, Feletti F, Nanni A, et al. Chest ultrasonography in ICU. Respir Care 2012; 57: 773–781. [DOI] [PubMed] [Google Scholar]
- 39.Koegelenberg CF, von Groote-Bidlingmaier F, Bolliger CT. Transthoracic ultrasonography for the respiratory physician. Respiration 2012; 84: 337–350. [DOI] [PubMed] [Google Scholar]
- 40.Jambrik Z, Monti S, Coppola V, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardio 2004; 93: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 41.Galbois A, Ait-Oufella H, Baudel JL, et al. Pleural ultrasound compared with chest radiographic detection of pneumothorax resolution after drainage. Chest 2010; 138: 648–655. [DOI] [PubMed] [Google Scholar]
- 42.Stefanidis K, Dimopoulos S, Tripodaki ES, et al. Lung sonography and recruitment in patients with early acute respiratory distress syndrome: a pilot study. Crit Care 2011; 15: R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellani G, Messa C, Guerra L, et al. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-d-glucose PET/CT study. Crit Care Med 2009; 37: 2216–2222. [DOI] [PubMed] [Google Scholar]
- 44.Bellani G, Guerra L, Musch G, et al. Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med 2011; 183: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudolph A, Markstaller K, Gast KK, et al. Visualization of alveolar recruitment in a porcine model of unilateral lung lavage using 3He-MRI. Acta Anaesthesiol Scand 2009; 53: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 46.Shi C, Boehme S, Hartmann EK, et al. Novel technologies to detect atelectotrauma in the injured lung. Exp Lung Res 2011; 37: 18–25. [DOI] [PubMed] [Google Scholar]
- 47.Desai SR, Wells AU, Rubens MB, et al. Acute respiratory distress syndrome. CT abnormalities at long term follow up. Radiology 1999; 210: 29–35. [DOI] [PubMed] [Google Scholar]
- 48.Desai SR. Acute respiratory distress syndrome. Imaging of the injured lung. Clin Radiol 2002; 57: 8–17. [DOI] [PubMed] [Google Scholar]
- 49.Gluecker T, Capasso P, Schnyder P, et al. Clinical and radiologic features of pulmonary edema. Radiographics 1999; 19: 1507–1531. [DOI] [PubMed] [Google Scholar]
- 50.Aberle DR, Brown K. Radiologic considerations in the adult respiratory distress syndrome. Clin Chest Med 1990; 11: 737–754. [PubMed] [Google Scholar]
- 51.Eisenhuber E, Schaefer-Prokop C, Prosch H, et al. Bedside chest radiography. Respir Care 2012; 57: 427–443. [DOI] [PubMed] [Google Scholar]
- 52.Winer-Muram HT, Steiner RM, Gurney JW, et al. Ventilator-associated pneumonia in patients with adult respiratory distress syndrome: CT evaluation. Radiology 1998; 208: 193–199. [DOI] [PubMed] [Google Scholar]
- 53.Gattinoni L, Pelosi P, Vitale G, et al. Body position changes redistribute lung computed-tomography density in patients with acute respiratory failure. Anesthesiology 1991; 74: 15–23. [DOI] [PubMed] [Google Scholar]
- 54.Goodman LR, Fumagalli R, Tagliabue P, et al. Adult respiratory distress syndrome due to pulmonary and extrapulmonary causes. CT, clinical, and functional correlations. Radiology 1999; 213: 545–552. [DOI] [PubMed] [Google Scholar]
- 55.Chon KS, vanSonnenberg E, D’Agostino HB, et al. CT-guided catheter drainage of loculated thoracic air collections in mechanically ventilated patients with acute respiratory distress syndrome. AJR Am J Roentgenol 1999; 173: 1345–1350. [DOI] [PubMed] [Google Scholar]
- 56.Chung JH, Kradin RL, Greene RE, et al. CT predictors of mortality in pathology confirmed ARDS. Eur Radiol 2011; 21: 730–737. [DOI] [PubMed] [Google Scholar]
- 57.Ichikado K, Suga M, Muranaka H, et al. Prediction of prognosis for acute respiratory distress syndrome with thin section CT: validation in 44 cases. Radiology 2006; 238: 321–329. [DOI] [PubMed] [Google Scholar]
- 58.Burnham EL, Janssen WJ, Riches DW, et al. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J 2014; 43: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masclans JR, Roca O, Munoz X, et al. Quality of life, pulmonary function, and tomographic scan abnormalities after ARDS. Chest 2011; 139: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 60.Howling SJ, Evans TW, Hansell DM. The significance of bronchial dilatation on CT in patients with adult respiratory distress syndrome. Clin Radiol 1998; 53: 105–109. [DOI] [PubMed] [Google Scholar]
- 61.Mineo G, Ciccarese F, Modolon C, et al. Post-ARDS pulmonary fibrosis in patients with H1N1 pneumonia: role of follow-up CT. Radiol Med 2012; 117: 185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilcox ME, Patsios D, Murphy G, et al. Radiologic outcomes at 5 years after severe ARDS. Chest 2013; 143: 920–926. [DOI] [PubMed] [Google Scholar]
- 63.Caironi P, Carlesso E, Gattinoni L. Radiological imaging in acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med 2006; 27: 404–415. [DOI] [PubMed] [Google Scholar]
- 64.Caironi P, Langer T, Gattinoni L. Acute lung injury/acute respiratory distress syndrome pathophysiology: what we have learned from computed tomography scanning. Curr Opin Crit Care 2008; 14: 64–69. [DOI] [PubMed] [Google Scholar]
- 65.Gattinoni L, Caironi P, Valenza F, et al. The role of CT-scan studies for the diagnosis and therapy of acute respiratory distress syndrome. Clin Chest Med 2006; 27: 559–570. [DOI] [PubMed] [Google Scholar]
- 66.Tagliabue M, Casella TC, Zincone GE, et al. CT and chest radiography in the evaluation of adult respiratory distress syndrome. Acta Radiol 1994; 35: 230–234. [PubMed] [Google Scholar]
- 67.Milne ENC, Pistolesi M, eds. Reading the Chest Radiograph. A Physiologic Approach. St Louis, Mosby Year Book, 1993. [Google Scholar]
- 68.Pistolesi M, Miniati M, Ravelli V, et al. Injury versus hydrostatic lung edema. Detection by chest X-ray. Ann N Y Acad Sci 1982; 384: 364–380. [DOI] [PubMed] [Google Scholar]
- 69.Aberle DR, Wiener-Kronish J, Webb W, et al. Hydrostatic versus increased permeability pulmonary edema. Diagnosis based on radiographic criteria in critically ill patients. Radiology 1988; 168: 73–79. [DOI] [PubMed] [Google Scholar]
- 70.Rocker GM, Rose DH, Manhire AR, et al. The radiographic differentiation of pulmonary edema. Br J Radiol 1989; 62: 582–586. [DOI] [PubMed] [Google Scholar]
- 71.Ketai LH, Godwin JD. A new view of pulmonary edema and acute respiratory distress syndrome. J Thorac Imaging 1998; 13: 147–171. [PubMed] [Google Scholar]
- 72.Figueroa-Casas JB, Brunner N, Dwivedi AK, et al. Accuracy of chest radiograph to identify bilateral pulmonary infiltrates consistent with the diagnosis of acute respiratory distress syndrome using computed tomography as reference standard. J Crit Care 2013; 28: 352–357. [DOI] [PubMed] [Google Scholar]
- 73.Levitt JR, Vinayak AG, Gehlbach BK, et al. Diagnostic utility of B-type natriuretic peptide in critically ill patients with pulmonary edema: a prospective cohort study. Crit Care 2008; 12: R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fowler RA, Adhikari NKJ, Scales DC, et al. Update in critical care 2008. Am J Respir Crit Care Med 2009; 179: 743–758. [DOI] [PubMed] [Google Scholar]
- 75.Sakka SG. Extravascular lung water in ARDS patients. Minerva Anestesiol 2013; 79: 274–284. [PubMed] [Google Scholar]
- 76.Komiya K, Ishii H, Murakami J, et al. Comparison of chest computed tomography features in the acute phase of cardiogenic pulmonary edema and acute respiratory distress syndrome on arrival at the emergency department. J Thorac Imaging 2013; 28: 322–328. [DOI] [PubMed] [Google Scholar]
- 77.Thompson BT, Moss M. A new definition for the acute respiratory distress syndrome. Semin Respir Crit Care Med 2013; 34: 441–447. [DOI] [PubMed] [Google Scholar]