Abstract
2011 to 2012 has seen an explosion in published research in the field of pulmonary vascular disease, especially pulmonary hypertension. In conjunction with this research has been an explosion in clinical interest in treating pulmonary hypertension. This is possible because we now have three different generic classes of drug therapy: endothelin receptor antagonists, phosphodiesterase-5 inhibitors and prostacyclins. Clearly, however, we need to be careful that we are treating the correct disease with the correct drug and measuring the correct response. Herein, I will review the papers published over the last year that offer the most insight into the pathobiology, but also those that give us the clinical information we need in epidemiology, treatment and end-points so that we can treat this devastating group of disease.
Keywords: End points, epidemiology, pathobiology, pulmonary hypertension, treatment
The past year has been a dramatic one in pulmonary hypertension and there have been a number of advances in several fields. I shall try in this short review to outline some of the advances that really seemed important to me. Necessarily, this is a personal view of what has happened over the last year and it is important that people whose work is not mentioned do not feel aggrieved. We are all grateful for the tremendous research effort that has gone on in this field over the last 15 yrs. I have subdivided my review into a number of areas where I think there have been significant advances in 2011–2012.
I start with the classification of pulmonary arterial hypertension that was developed at the World Conference on Pulmonary Hypertension in Dana Point, CA, USA in 2008 (table 1) [1]. This is only to provide a reference for the various papers that will be mentioned under subsequent sections. It is important to also realise that the next World Conference in Pulmonary Hypertension will be held in Nice in 2013 and, thus, the classification may change again.
Table 1. Classification of pulmonary hypertension.
| 1 Pulmonary arterial hypertension |
| 1.1 Idiopathic |
| 1.2 Heritable |
| 1.2.1 Bone morphogenetic protein receptor type II |
| 1.2.2 Activin receptor-like kinase 1, endoglin (with or without hereditary haemorrhagic telangiectasia) |
| 1.2.3 Unknown |
| 1.3 Drug and toxin induced |
| 1.4 Associated pulmonary arterial hypertension |
| 1.4.1 Connective tissue disease |
| 1.4.2 HIV infection |
| 1.4.3 Portal hypertension |
| 1.4.4 Congenital heart disease |
| 1.4.5 Schistosomiasis |
| 1.4.6 Chronic haemolytic anaemia |
| 1.5 Persistent pulmonary hypertension of the newborn |
| 1′ Pulmonary veno-occlusive disease and/or pulmonary capillary haemangiomatosis |
| 2 Pulmonary hypertension due to left heart disease |
| 2.1 Systolic dysfunction |
| 2.2 Diastolic dysfunction |
| 2.3 Valvular disease |
| 3 Pulmonary hypertension due to lung diseases and/or hypoxia |
| 3.1 Chronic obstructive pulmonary disease |
| 3.2 Interstitial lung disease |
| 3.3 Other pulmonary diseases with mixed restrictive and obstructive pattern |
| 3.4 Sleep-disordered breathing |
| 3.5 Alveolar hypoventilation disorders |
| 3.6 Chronic exposure to high altitude |
| 3.7 Developmental abnormalities |
| 4 Chronic thromboembolic pulmonary hypertension |
| 5 Pulmonary hypertension with unclear and/or multifactorial mechanisms |
| 5.1 Haematological disorders: myeloproliferative disorders, splenectomy |
| 5.2 Systemic disorders: sarcoidosis, pulmonary Langerhans’ cell histiocytosis, lymphangioleiomyomatosis, neurofibromatosis, vasculitis |
| 5.3 Metabolic disorder: glycogen storage disease, Gaucher disease, thyroid disorders |
| 5.4 Others: tumoural obstruction, fibrosing mediastinitis, chronic renal failure on dialysis |
Reproduced and modified from [1] with permission from the publisher.
The areas in which I wish to make comment in the field of pulmonary arterial hypertension are: 1) epidemiology; 2) pathobiology; 3) treatment; and 4) end-points.
EPIDEMIOLOGY
Studying the epidemiology of pulmonary arterial hypertension is difficult for two reasons. First, a diagnosis can be difficult to make, particularly in the context of a patient with multisystem disorder. Secondly, patients with pulmonary arterial hypertension are not always referred to specialist centres that will record the main characteristics of the patient in a suitable database, which can later be interrogated to provide data about prevalence and incidence.
Registries
One of the best known registries is the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). The data come from a large number of pulmonary hypertension centres in the USA (although not all of them) and now encompass more than 3,000 patients. This very large database has given us important insights into pulmonary hypertension but the authors of the various papers associated with REVEAL will be the first to admit that it does not encompass the whole of the USA and includes patients in whom a diagnosis could have been made some time before, i.e. prevalent rather than incident patients.
The second major database is that of the French registry. It also contains a mixture of incident and prevalent patients and there have been recent analyses by our French colleagues to separate out the characteristics of patients with prevalent disease from those with incident disease. Interestingly, the French have shown that prognosis can be quite different, i.e. worse in those in the incident group than the prevalent group.
The latest registry to be described is the UK and Ireland registry [2]. The UK and Ireland registry would appear to have a number of particular features that will make it of interest. First, it is a registry of incident patients only. Secondly, all patients who enter the registry are treatment naïve. Thirdly, the registry has been open for 10 yrs and, therefore, it is possible to track the change in demographics of each cohort of patients as they come through the system. What Ling et al. [2] have shown is that there is an increasing incidence in age, body mass and comorbidities in the pulmonary hypertension population but there is no change in the duration of the disease prior to diagnosis or its severity at the time of diagnosis. This, of course, is disappointing, given the widespread publicity that is now given to pulmonary arterial hypertension at both international meetings and in journals. The most common causes of pulmonary hypertension in Europe and the USA are left heart disease and chronic hypoxic lung disease. However, these two aetiologies of pulmonary hypertension should not be treated by standard pulmonary hypertension therapy. Rather, the treatment should be aimed at the primary disorder. This can be difficult to do when the separation of pulmonary arterial disease from pulmonary venous and hypoxic lung disease can be so difficult. There have been good reviews of the diagnosis and management of pulmonary hypertension associated with left ventricular dysfunction [3–5]. Interestingly, some authors have shown that the phosphodiesterase-5 inhibitors sildenafil or tadalafil can improve pulmonary hypertension due to left ventricular diastolic dysfunction [6]. However, I believe that this remains experimental therapy and should not be used in normal circumstances.
Pulmonary hypertension in the developing world
In addition to the pulmonary arterial hypertension seen most commonly in Europe and the USA, there are new classifications of pulmonary arterial hypertension which, although mentioned in the Dana Point classification, are rarely seen in first-world countries. The most obvious of these are pulmonary hypertension associated with sickle cell disease and schistosomiasis [7]. It is clear that although pulmonary hypertension due to these diseases is rare in the western world, it is very common in the developing world and, in terms of worldwide health burden, these diseases are likely to be more important than idiopathic pulmonary arterial hypertension (IPAH).
Drug-induced pulmonary hypertension
A concern that does exist in the west is that some of the therapies we use for other conditions can actually cause pulmonary hypertension. The most famous class of drugs that has been linked with pulmonary hypertension is the anorexigens. Those anorexigens associated with pulmonary or cardiac disease are banned in most countries and, correspondingly, the incidence of pulmonary hypertension due to these drugs has fallen. However, there are other drugs that can cause pulmonary hypertension, of which the most interesting is probably dasatinib [8]. In this paper, the authors describe incident cases of dasatinib-associated pulmonary hypertension in the French pulmonary hypertension registry between November 2006 and September 2010. Nine cases were reported but no other cases were reported in association with other tyrosine kinase inhibitors. This is of great interest because, of course, imatinib, another tyrosine kinase inhibitor, is under trial at present for the treatment of pulmonary hypertension so, potentially, we have drugs that both cause and treat the condition belong to the same class.
PATHOBIOLOGY
The accepted traditional view of the pathobiology of pulmonary arterial disease is that there can be a genetic predisposition (e.g. BMPR2 mutation), which, when associated with a second hit such as an inflammatory disease, an environmental factor, hypoxia or other stress, can cause pulmonary arterial hypertension but there are a number of new concepts in development.
Circulating cells
In general, the pulmonary vascular remodelling that results and/or causes the pulmonary hypertension is thought to be a local event in the lungs. However, there is increasing evidence that circulating cells are important in the generation of the pulmonary arteriopathy and, in particular and most recently, there is evidence that circulating fibrocytes derived from the bone marrow occur in children and young adults with pulmonary hypertension. It appears that these mesenchymal progenitor cells are capable of regulating fibrogenesis in the pulmonary arteries. Their numbers correlate with clinical and haemodynamic variables [9, 10].
The oestrogen paradox
One feature of pulmonary arterial hypertension that is always of interest to epidemiologists and clinicians is the preponderance of females with the disease. This type of sex specificity has never really been explained but a number of groups are investigating the relationship of female sex hormones with pulmonary arterial hypertension. Unfortunately, this research has led to a paradox whereby, although pulmonary arterial hypertension is more frequent in females, oestrogen in itself appears to be strongly protective against the disease, at least in animal models. This was reviewed by Umar et al. [11]. They call this phenomenon the “oestrogen paradox” and, in their review, they investigate the different possible mechanisms.
Iron deficiency
Another aspect of the pathology of pulmonary hypertension, which has been prompted by clinical observation, is the relationship between iron deficiency and pulmonary arterial disease. It would appear that iron deficiency is increased in patients with IPAH. This relationship has been shown by Rhodes et al. [12], who confirmed that circulating transferrin levels are raised in IPAH patients, indicating significant iron deficiency. Interestingly, the presence of iron deficiency is associated with poor outcome from the disease. At present, the exact relationship between iron deficiency and the severity of pulmonary arterial hypertension is not understood but is the subject of ongoing research.
Inflammation
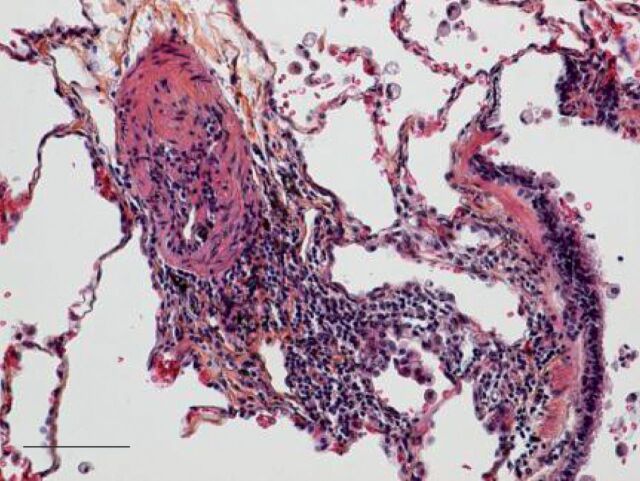
20 yrs ago, pulmonary arterial hypertension was assumed to be associated with vasculitis and there were a number of clinical trials of anti-inflammatory drugs such as cyclophosphamide in the treatment of pulmonary hypertension. Unfortunately, these trials were unsuccessful and the idea that inflammation was associated with pulmonary arterial hypertension was largely abandoned. However, the interest in this relationship has been rekindled and a number of laboratories are investigating the connection. This is reviewed by Price et al. [13]. While it is surprising that anti-inflammatory drugs have so little effect on pulmonary hypertension (with the important exception of systemic lupus erythematosus and some patients with mixed connective tissue disease), the ongoing interest is in whether the inflammation seen in pulmonary vessels in patients with pulmonary arterial hypertension is a primary cause of the arteriopathy or is a consequence of the other stimulating causes that are known to be aetiological, such as hypoxia, bowel disease, liver disease, etc. (fig. 1).
Figure 1.
Elastic staining of paraffin-embedded lung tissue. A pulmonary arterial lesion from a patient with idiopathic pulmonary arterial hypertension, illustrating the perivascular lymphocytic infiltrate (centre), a small pulmonary artery (left) and a bronchiole (right). Haematoxylin and eosin elastic stain. Scale bar=100 μm. Reproduced and modified from [13] with permission from the publisher.
Epigenetics
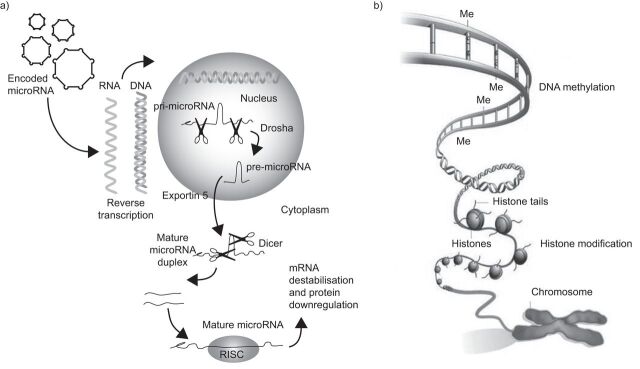
A whole new area of genetics that will clearly have a significant impact on our understanding of pulmonary hypertension and pulmonary vascular disease is epigenetics. This can be defined as a nuclear process that does not involve any change in the DNA structure but that results in a change in translation and, hence, protein production (fig. 2).
Figure 2.
a) Primary microRNA (pri-microRNA) is processed by Drosha in the nucleus to form pre-microRNA. The pre-microRNA enters the cytoplasm via exportin 5 where it is further processed by Dicer into a mature microRNA duplex. One strand of this duplex is incorporated into the RNA-induced silencing complex (RISC) where it binds to the target mRNA. The mRNA is destabilised and, consequently, the protein is downregulated. Reproduced and modified from [14] with permission from the publisher. b) The two main components of the epigenetic code are DNA methylation, in which methyl (Me) groups added to certain bases repress gene activity, and histone modification, where a combination of chemical modifications of the histone “tails” alter the activity of the DNA wrapped around them. Reproduced and modified from [15] with permission from the publisher.
There are four types of epigenetic regulation. These are histone modification, DNA methylation, noncoding RNAs and microRNAs. The area of epigenetics that has had the most impact on our thoughts about pulmonary hypertension is microRNAs. These are short, noncoding RNAs that usually function as negative regulators of gene expression. It has been discovered that the post-transcriptional regulation by microRNAs is critical for many aspects of vascular biology including the development of pulmonary vessels [16].
There are many papers on microRNAs in pulmonary hypertension but two papers stand out as being of fundamental significance. The first is by Courboulin et al. [17] who found that microRNA (miR)-204 expression was downregulated in pulmonary artery smooth muscle cells in both human and rodent disease. This downregulation seemed to correlate with pulmonary hypertension severity and related closely to the proliferative and anti-apoptotic phenotypes of pulmonary artery smooth muscle cells in patients with pulmonary hypertension. They also showed that if synthetic miR-204 was given to the lungs of animals with pulmonary arterial hypertension, this reduced the disease’s severity. This does suggest a high specific role of this particular microRNA in the understanding and possible treatment of this disease.
The second study was an examination of miR-17 and its role in lung and heart function in pulmonary hypertension [18]. The authors examined the role of microRNA inhibitors (called antagomirs) in experimental hypoxia and monocrotaline-induced pulmonary hypertension in rats. They found that antagonists to miR-17 and miR-21 reduced haemodynamics in these animals and also decreased pulmonary artery muscularisation. The antagomir to miR-17 also reduced right ventricular hypertrophy and improved right-sided haemodynamics.
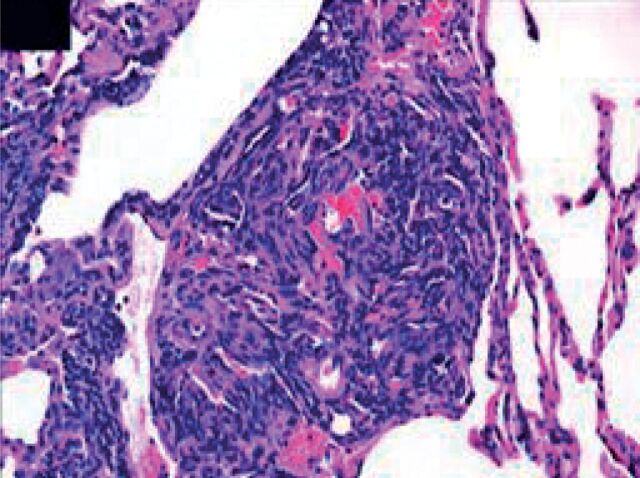
Plexiform lesion
The plexiform lesion, which is a complex abnormality of the endothelium, is thought to be pathognomonic of idiopathic pulmonary hypertension (fig. 3). Interestingly, although it is present in patients with IPAH, very few of the animal models will show this lesion, an exception being the sugen hypoxia model [20]. Two new studies have shown that long-term epoprostenol, which is perhaps the most powerful anti-pulmonary hypertension drug we have had, while showing clinical improvement, actually showed at death that the plexiform lesions had persisted despite this long-term therapy [21, 22].
Figure 3.
Plexiform lesion from a patient with severe pulmonary hypertension demonstrating the exuberant proliferation of cells that comprise the lumen of the small pulmonary artery. Multiple slit-like vascular spaces are all that remain of the original lumen (Haematoxylin-eosin). Reproduced from [19] with permission from the publisher.
This raises two possible explanations. First, it may be that the plexiform lesion, although pathognomonic of pulmonary arterial hypertension, is not actually an indicator of progression or deterioration. Although that it can be quite widespread, it can also be quite patchy in its development. Secondly, drugs such as prostacyclin work in ways other than reversal of the histopathology of the disease.
TREATMENT
Left ventricular dysfunction
As already stated, left ventricular dysfunction, both diastolic and systolic, are causes of pulmonary hypertension but treatment should be primarily aimed at the heart rather than trying to open up blood vessels. Park et al. [4] differentiated between passive and reactive pulmonary hypertension in the setting of left ventricular dysfunction. Reactive pulmonary hypertension is said to have occurred when there is a rise in pulmonary vascular resistance. It would appear that when this develops, the prognosis is a great deal worse. It does not, however, suggest that the treatment of the pulmonary venous hypertension should include pulmonary vasodilating drugs [3]. The one exception to this rule appears to be use of the phosphodiesterase-5 inhibitor sildenafil, which may help patients with left ventricular dysfunction [6]. However, further investigation is required before we can recommend this therapy in patients with this condition
Exercise therapy
For some years, we have known that a carefully graded exercise programme can provide significant benefit to patients with pulmonary arterial hypertension. Indeed, the benefits of doing this can be almost as great as those achieved with disease targeted pharmacological therapy. It has been shown again in patients with IPAH by a German group [23]. I believe this therapeutic modality deserves further examination because it is a great deal cheaper than other therapy and does not carry a risk of attendant side-effects.
Statins
There has been considerable interest in the use of statins to treat many conditions other than hypercholesterolaemia (the pleomorphic effect of statin therapy). This may include pulmonary hypertension. Some years ago, it was shown that statins can have a remarkable effect in animal models with pulmonary hypertension [24]. The first trial of a statin (simvastatin) in pulmonary arterial hypertension was negative [25]. This was a disappointing result for a clinical study but Carlin et al. [26] have looked at the various statins in animal models with pulmonary hypertension and showed that fluvastatin, unlike the others, will decrease pulmonary vascular cell proliferation in both acute and chronic hypoxic models at normal clinical doses. This raises the question of whether particular statins at particular doses will be effective rather than all statins.
Warfarin
For a number of years, clinicians have used anticoagulation as adjunctive treatment in idiopathic pulmonary hypertension. This use was never subjected to a double-blind clinical trial but reflects the results showing that warfarin alone would improve survival in idiopathic disease. Recent meta-analysis has shown no benefit from warfarin in systemic sclerosis or IPAH [27], so the question is now open as to whether or not these drugs are useful. It would certainly seem appropriate to conduct a proper double-blind, controlled trial.
Treating the right ventricle
In the past, we have always taken the view that the right ventricle fails because of the afterload it is facing, and although patients die of right heart failure, the treatment of pulmonary hypertension should be directed towards the pulmonary vasculature rather than the right ventricle. The reasoning behind this is that if the blood vessels can be opened up so that afterload is reduced, then the right ventricle will respond. A number of papers have suggested that this may not be the whole story.
First of all, a Dutch group [28] showed that right ventricular dysfunction can progress even in patients who appear to have responded to vasodilator therapy by reducing pulmonary vascular resistance. This makes the point that right ventricular function is more complex than simply a function of pulmonary vascular narrowing. At present, there are no trials of specific right ventricular therapy in pulmonary hypertension but a number of groups are considering the use of β-blockers in this condition (currently contraindicated because of their anti-chronotropic and anti-inotropic effects). Indeed, other therapies may also be relevant and we will need to see the results of further studies. Certainly, it would appear that the right ventricular dysfunction associated with connective tissue disease is due to primary myocardial damage as well as the pulmonary vascular abnormality. This suggests that at least in this particular type of pulmonary arterial hypertension, direct therapy of the right ventricle may be beneficial.
END-POINTS
Survival
Clearly, it is very important to have reliable measures of success or failure of treatment in patients with pulmonary hypertension. There has been considerable debate over the years as to the best end-points to be used both in the clinical trials of new therapy for pulmonary hypertension and also in following the patients in the clinic. The most important variable to be measured is, of course, survival and Humbert et al. [29] made the point that the survival of incident and prevalent patients is different.
This was followed up by the UK and Irish registry [2], which showed that in purely incident patients, there has been no difference in the overall unadjusted survival in IPAH over the decade 2001–2009. Interestingly, the authors showed that the comorbidities associated with IPAH had changed dramatically over that period and this was the reason for the poor survival rather than the fact that the treatments for pulmonary hypertension were not working. If the survival was adjusted for age and comorbidities, then the hazard ratio had improved by 100% over the same period.
6-min walk testing
The 6-min walk test is a simple test that has been used almost universally in drug trials as the best measure of the effect of new drugs for pulmonary arterial hypertension. Its usefulness has been accepted by both the US Food and Drug Administration and the European Medicines Agency. There are a number of difficulties with the 6-min walk test. First, it appears that the absolute test result is the relevant one and that the predicted test result has no benefits. This is counterintuitive given the obvious difference in the 6-min walk test between an athletic 6-foot-tall (1.8 m) male and an elderly 5-foot-tall (1.5 m) female. Secondly, there is a ceiling effect in the 6-min walk test such that patients who are young will often do well on the test and it is difficult to show the benefit of particular therapies. The view is that the test is a surrogate for pulmonary vascular and right ventricular function rather than a direct measure.
Recently, Gabler et al. [30] performed a meta-analysis of more than 2,000 patients to show that the threshold effect of the 6-min walk test was 42 m but the average improvement in drug trials was only 22 m. Therefore, they reasoned that the benefits of drugs were not fully explained by changes in the 6-min walk test. Clearly, we need better end-points to measure the effects of drugs on our patients and we would also like to get closer to the biology of the disease. In particular, it would be wonderful if we could image the small pulmonary arteries and show that drugs either improved or did not improve this histology. In the near future, there will also be better imaging of the effects of drugs on right ventricular function (measured by magnetic resonance imaging and other modalities).
Steps towards this have been made by Marsboom et al. [31] using lung and heart positron emission tomography; they showed that changes in fluorodeoxyglucose uptake could be seen both in the lung and in the heart in rats with experimental pulmonary hypertension. The feeling is that we are somewhat away from a patient test but it is encouraging that we are getting a little closer to the actual biology of the lesion because, ultimately, our treatments must improve this if we are to improve or have a significant effect on survival and morbidity in this most dreadful disease.
CONCLUSIONS
2011 and 2012 have been active and busy years for research in pulmonary arterial hypertension. In this article, I have tried to show how we now have new ideas about epidemiology; we have a progression of our ideas about pathobiology, and we are continually seeing new treatments that work by new modes of action and can be assessed by new end-points. I just hope that some of the answers to the questions raised above are answered in 2013.
Footnotes
Provenance
Submitted article, peer reviewed.
Statement of Interest
A. Peacock has received honoraria for speaking at meetings and assistance with travel from Actelion, Bayer, Eli Lilly, GSK, Novartis, Pfizer and United Therapeutics. He has received research grants from Actelion and Bayer, and is on advisory boards for Actelion, Bayer, Eli Lilly, GSK, Novartis and Pfizer.
REFERENCES
- 1.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54 Suppl., S43–S54. [DOI] [PubMed] [Google Scholar]
- 2.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology and survival of incident pulmonary arterial hypertension: results from the Pulmonary Hypertension Registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. [DOI] [PubMed] [Google Scholar]
- 3.de Jesus Perez VA, Haddad F, Zamanian RT. Diagnosis and management of pulmonary hypertension associated with left ventricular diastolic dysfunction. Pulm Circ 2012; 2: 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park MH, Mehra MR. Pulmonary hypertension. The great leveller. J Am Coll Cardiol 2012; 59: 232–234. [DOI] [PubMed] [Google Scholar]
- 5.Hassoun PM, Adnot S. Update in pulmonary vascular diseases 2011. Am J Respir Crit Care Med 2012; 185: 1177–1182. [DOI] [PubMed] [Google Scholar]
- 6.Guazzi M, Vicenzi M, Arena R, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124: 164–174. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca GHH, Souza R, Salemi VMC, et al. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J 2012; 39: 112–118. [DOI] [PubMed] [Google Scholar]
- 8.Montani D, Bergot E, Günther S, et al. Pulmonary arterial hypertension in patients treated by Dasatinib. Circulation 2012; 125: 2128–2137. [DOI] [PubMed] [Google Scholar]
- 9.Yeager ME, Nguyen CM, Belchenko DD, et al. Circulating fibrocytes are increased in children and young adults with pulmonary hypertension. Eur Respir J 2012; 39: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toshner MR, Morrell NW. The fibrocyte in pulmonary hypertension: we seek him here, we seek him there. Eur Respir J 2012; 39: 5–6. [DOI] [PubMed] [Google Scholar]
- 11.Umar S, Rabinovitch M, Eghbali M. Estrogen paradox in pulmonary hypertension. Am J Respir Crit Care Med 2012; 186: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes CJ, Howard LS, Busbridge M, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence outcomes, and mechanistic insights. J Am Coll Cardiol 2011; 58: 300–309. [DOI] [PubMed] [Google Scholar]
- 13.Price LC, Wort SJ, Perros F, et al. Inflammation in pulmonary arterial hypertension. Chest 2012; 141: 210–221. [DOI] [PubMed] [Google Scholar]
- 14.Thermo Scientific. shMIMIC Lentiviral microRNA. Supporting data, figure 1: over expression of mature microRNA results in gene modulation. www.thermoscientificbio.com/microrna/shmimic-lentiviral-microrna/.
- 15.Qui J. Epigenetics: Unfinished symphony. Nature 2006; 441: 143–145. [DOI] [PubMed] [Google Scholar]
- 16.Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res 2009; 104: 442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courboulin A, Paulin R, Giguère NJ, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011; 208: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pullamsetti SS, Doebele C, Fischer A, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Cell Mol Biol 2012; 185: 409–419. [DOI] [PubMed] [Google Scholar]
- 19.Cool CD, Groshong SD, Oakey J, et al. Pulmonary hypertension: cellular and molecular mechanisms. Chest 2005; 128: Suppl. 6, 565S–571S. [DOI] [PubMed] [Google Scholar]
- 20.Abe K, Toba M, Alzoubi A, et al. Tyrosine kinase inhibitors are potent acute pulmonary vasodilators in rats. Am J Respir Cell Mol Biol 2011; 45: 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stacher E, Graham BB, Hunt JM, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogoriler JE, Rich S, Archer SL, et al. Persistence of complex vascular lesions despite prolonged prostacyclin therapy of pulmonary arterial hypertension. Histopathology 2012; 61: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grünig E, Lichtblau M, Ehlken N, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J 2012; 40: 84–92. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura T, Vaszar LT, Faul JL, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 2003; 108: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins MR, Ali O, Bradlow W, et al. Simvastatin as a Treatment for Pulmonary Hypertension Trial (SiPHT). Am J Respir Crit Care Med 2010; 181: 1110–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlin C, Celnick DF, Pak O, et al. Low-dose fluvastatin reverses the hypoxic pulmonary adventitial fibroblast phenotype in experimental pulmonary hypertension. Am J Respir Cell Mol Biol 2012; 46: 1–9. [DOI] [PubMed] [Google Scholar]
- 27.Johnson SR, Granton JT, Tomlinson GA, et al. Warfarin in systemic sclerosis-associated and idiopathic pulmonary arterial hypertension. A Bayesian approach to evaluating treatment for uncommon disease. J Rheumatol 2012; 39: 276–285. [DOI] [PubMed] [Google Scholar]
- 28.van de Veerdonk MC, Kind T, Marcus JT. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2516–2517. [DOI] [PubMed] [Google Scholar]
- 29.Humbert M, Sitbon O, Yaïci A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010; 36: 549–555. [DOI] [PubMed] [Google Scholar]
- 30.Gabler NB, French B, Strom BL, et al. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial trials. Circulation 2012; 126: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsboom G, Wietholt C, Haney CR, et al. Lung 18F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 185: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]